The authors describe a novel signaling pathway for PLD in nontransfected, primary cells of the rat lacrimal gland, where Rho and ROCK1 activate PLD1. Formation of this signaling complex results in the downstream activation of PKC, MEK, and ERK in a novel Ras-, Raf-, Pyk2- and cSrc-independent pathway that attenuates cholinergic agonist-stimulated protein secretion.
Abstract
Purpose.
A prior study showed that cholinergic agonists activate phospholipase D (PLD). The purpose of this study was to determine whether cholinergic agonists use the PLD pathway to alter protein secretion and to identify the molecular signaling components of this pathway in rat lacrimal gland acini.
Methods.
Rat lacrimal gland acini were isolated by collagenase digestion. Presence and localization of PLD1 and -2 were determined by immunofluorescence and Western blot experiments. Acini were incubated with adenoviruses overnight or the inhibitors 1-butanol, Y-27632, or C3 exotoxin before stimulation with the cholinergic agonist carbachol (Cch, 10−4 M) for 5 minutes. Western blot analysis was performed for 20 minutes, and protein secretion was measured spectrophotometrically. Activation of ERK, MEK, Pyk2, Ras, and Raf was determined by Western blot analysis.
Results.
1-Butanol increased Cch-stimulated protein secretion and decreased ERK activity. Incubation with catalytically inactive PLD1, but not catalytically inactive mutant PLD2 adenovirus, also increased Cch-stimulated protein secretion and decreased ERK activity. Inhibition of Rho with C3 exotoxin and a dominant negative Rho adenovirus and inhibition of ROCK with Y-27632 inhibited Cch-stimulated PLD1 activity, increased protein secretion, and decreased ERK activity. The association of PLD1 and ROCK increased with Cch stimulation, as determined by immunoprecipitation. PMA-stimulated ERK activity was also inhibited by 1-butanol. 1-Butanol had no effect on Cch-stimulated Pyk2, Ras, and Raf activity, but decreased MEK activity.
Conclusions.
Cholinergic agonists activate PLD1 through Rho and ROCK, which in turn activate MEK and ERK, which attenuate protein secretion in freshly isolated epithelial cells.
The lacrimal gland is an exocrine gland and is responsible for secretion of proteins, water, and electrolytes that cover and protect the cornea and conjunctiva to ensure clear vision.1 Hyposecretion from the lacrimal gland of both proteins and fluid leads to dry eye disease, which has no cure or treatment. For effective treatments to be designed, it is imperative to investigate how lacrimal gland secretion is regulated under nonpathologic conditions. This type of study will provide potential targets for new treatments for dry eye.
Because less secretion leads to ocular surface disease, secretion from the lacrimal gland is tightly regulated via neural control. Efferent, sensory nerves in the cornea are the mechanism by which an afferent pathway via parasympathetic and sympathetic nerves in the lacrimal gland is activated. We have found in freshly isolated, nontransformed lacrimal gland acinar cells that cholinergic agonists are potent stimuli of protein secretion and activate the signaling cascade, which involves activation of protein kinase C (PKC). PKC stimulates Pyk2 and p60Src to activate Ras, Raf, mitogen-activated protein kinase (MEK), and extracellular signal-regulated kinase 1/2 (ERK 1/2).2 Interestingly, in the short term (within 20 minutes), activation of ERK attenuates agonist-stimulated protein secretion.2 In addition, we have determined that cholinergic agonists activate phospholipase D (PLD), although neither the isoform of PLD nor the functional effect of PLD activation has been investigated.3
PLD is a ubiquitously expressed enzyme that cleaves phosphatidylcholine to generate choline and phosphatidic acid (PA). PA is a well-known signaling molecule that can be converted to diacylglycerol (DAG), known to activate PKC.4 PLD has been implicated in a variety of cellular processes, including secretion, and its activity is under the control of neurotransmitters, growth factors, and cytokines.4 In mammalian cells, there are two isoforms of PLD: PLD1 and -2. It has been reported that the small GTPase Rho and phospholipids activate recombinant PLD1, while regulation of PLD2 is less understood and appears to be constitutively active in many cell types.5
The signaling pathways, both upstream and downstream of PLD activation, are complex. PLD can be activated upstream by cPKCs through a direct interaction of PKC with PLD.4,6 Downstream signaling pathways of PLD are monitored by the production of PA. PA can signal through its generation of DAG and lysophosphatidic acid. Production of DAG by activation of PLD can activate PKC.6 Thus, PKC can be either upstream or downstream of PLD.
The signaling molecules recruited by PA include Raf-1 and SOS, which couple to the extracellular signal-regulated kinase 1/2 (ERK 1/2, also known as p42/p44 MAPK) cascade. In turn, ERK can induce cell proliferation, differentiation, and exocytosis. ERK activation has generally been attributed to PLD2, although it may depend on cell type, stimulus, and the function being measured. In contrast, exocytosis and secretion are generally attributed to PLD1.
Rho is a family of small GTP-binding proteins that can also activate several effector molecules. One such molecule is Rho-associated kinase (ROCK).7 The two isoforms, ROCK1 and -2, appear to regulate cell growth, migration, apoptosis, and exocytosis, primarily by acting on the actin cytoskeleton.8,9 However, ROCK can also regulate cellular functions independent of its effect on the cytoskeleton.9
In the present study, we investigated the PLD pathway in the lacrimal gland and identified a novel signaling pathway for PLD. In these cells, Rho and ROCK1 activate PLD1, but not PLD2, in response to cholinergic agonists causing their association with one another. Formation of this signaling complex results in the downstream activation of MEK and ERK, but the activation is independent of the signaling molecules Ras, Raf, Pyk2, and cSrc, which usually activate ERK. Activation of ERK then attenuates cholinergic agonist-stimulated protein secretion.
Materials and Methods
Antibodies against PLD1, PLD2, and PLD1 phosphorylated on Thr147; c-Raf phosphorylated on Ser338; Pyk2 phosphorylated on Tyr402; and MEK1/2 phosphorylated on Ser217/221 were from Cell Signaling Technology (Danvers, MA), and antibodies to ERK 2, ERK 1/2 phosphorylated on Tyr 202/204, MEK, and mouse secondary antibody conjugated to horseradish peroxidase (HRP) were from Santa Cruz Biotechnology (Santa Cruz, CA). The antibody to ROCK1 was from Epitomics, Inc. (Burlingame, CA). Antibody against α-smooth muscle actin was from Sigma-Aldrich (St. Louis, MO). Ras activity assay kits, an antibody to total Raf-1 and rabbit secondary antibody conjugated to HRP were purchased from Millipore (Chemicon, Temecula, CA). Cell-permeable exoenzyme C3 transferase from Clostridium botulinum was obtained from Cytoskeleton (Denver, CO). Y-27632 was purchased from EMD (Madison, WI). Dominant negative Rho adenovirus was the kind gift of John A. Williams, University of Michigan.10,11 Catalytically inactive PLD1 and -2 and vector control adenoviruses were generated as described previously.12–15
Immunohistochemistry
All experiments conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Schepens Eye Research Institute Animal Care and Use Committee. Whole lacrimal glands from male Sprague-Dawley rats (Taconic Farms, Germantown, NY) were fixed by immersion in 4% formaldehyde diluted in phosphate-buffered saline (PBS; 145 mM NaCl, 7.3 mM Na2HPO4, and 2.7 mM NaH2PO4 [pH 7.2]) for 4 hours at 4°C. The glands were incubated overnight at 4°C in 30% sucrose dissolved in PBS and the tissue was frozen in optimal cutting temperature embedding compound. Cryostat sections (6 μm) were placed on gelatin-coated slides, air dried for 2 hours, and rinsed in TBS (10 mM Tris-HCl [pH 8.0] and 150 mM NaCl). Nonspecific binding sites were blocked in TBS containing 10% normal goat serum, 1% bovine serum albumin (BSA), and 0.2% Triton X-100. The sections were then incubated with the primary antibody for 2 hours at 1:100 dilution at room temperature in a humidified chamber. The secondary antibodies, conjugated to either FITC or Cy3 (Jackson ImmunoResearch Laboratories) were applied at 1:150 dilution for 1 hour at room temperature. Coverslips were mounted with a medium consisting of glycerol and paraphenylenediamine. In negative control experiments, the primary antibody was omitted. The sections were viewed by microscope (Eclipse E80i; Nikon, Tokyo, Japan), and micrographs were taken with a digital camera (Spot; Diagnostic Instruments, Inc., Sterling Heights, MI).
In some experiments, freshly isolated cells were placed on slides and air dried. They were then fixed in 4% formaldehyde in PBS for 2 hours at 4°C before preservation in 30% sucrose in PBS for 2 hours at 4°C. The cells were blocked and permeabilized in PBS containing 10% normal goat serum, 1% bovine serum albumin (BSA), and 0.2% Triton X-100 for 45 minutes at room temperature. They were then incubated with phalloidin (Sigma-Aldrich) directly conjugated to FITC at 1:1000 dilution for 1 hour.
Preparation of Rat Lacrimal Gland Acini
Lacrimal glands were trimmed, fragmented, and incubated with collagenase CLSIII (100 U/mL) in Krebs-Ringer bicarbonate (KRB) buffer (119 mM NaCl, 4.8 mM KCl, 1 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, and 25 mM NaHCO3) supplemented with 10 mM HEPES, 5.5 mM glucose (KRB-HEPES), and 0.5% BSA (pH 7.4). Lobules were subjected to gentle pipetting through tips of decreasing diameter, filtered through nylon mesh (150-μm pore size), and centrifuged briefly. The pellet was washed twice by centrifugation (50g, 2 minutes) through a 4% BSA solution made in KRB-HEPES. The dispersed acini were allowed to recover for 60 minutes in fresh KRB-HEPES buffer containing 0.5% BSA.
Western Blot Analysis
Acini were preincubated with or without inhibitors or adenoviruses for the indicated time before stimulation with the cholinergic agonist carbachol (Cch, 10−4 M diluted in KRB-HEPES) for 5 minutes. The cells were sonicated in RIPA buffer (9.1 mM dibasic sodium phosphate, 1.7 mM monobasic sodium phosphate, 150 mM NaCl [pH 7.4], 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS with phenylmethylsulfonyl fluoride 100 μg/mL, aprotinin 30 μL/mL, and sodium orthovanadate 100 nM). Proteins in the supernatant were separated by SDS-PAGE on a 10% gel. Separated proteins were transferred onto nitrocellulose membranes, which were blocked overnight at 4°C in 5% nonfat dried milk in TBS containing 0.05% Tween-20 (TBST) and then incubated with the primary antibody for 2 hours at room temperature or overnight at 4°C. After the membranes were washed three times in TBST, they were incubated with horseradish peroxidase (HRP)–conjugated secondary antibody. Immunoreactive bands were detected by enhanced chemiluminescence.
Mouse monoclonal antibodies to phosphorylated (active ERK) and total ERK were used at 1:500 and 1:1000 dilution, respectively. Rabbit polyclonal antibodies against activated and total PLD, pyk2, and MEK were used at 1:250 dilution, and antibodies against activated Raf and total Raf were used at 1:2000 dilution. Secondary antibodies were used at either 1:1000 (mouse) or 1:5000 (rabbit) dilution.
Measurement of Peroxidase Secretion
Lacrimal gland acini were incubated in duplicate in 0.5% BSA-KRB-HEPES buffer for 20 minutes at 37°C in the absence or presence of the cholinergic agonist Cch (10–4 M). Inhibitors were added 15 minutes before the addition of Cch. All inhibitors were diluted in either KRB-HEPES or DMSO (0.01% final concentration). After a brief centrifugation, supernatant was collected, and the pellet was sonicated in 10 mM Tris-HCl. Peroxidase activity was measured in duplicate in both the supernatant and the pellet fraction with a fluorogenic substrate (Amplex Red; Invitrogen, Carlsbad, CA). Oxidation of this reagent by peroxidase in the presence of hydrogen peroxide produces a highly fluorescent molecule, resorufin. The amount of fluorescence was quantified by a fluorescence microplate reader (model FL600; Bio-Tek, Winooski, VT) with an excitation wavelength of 530 nm and an emission wavelength of 590 nm. Peroxidase secretion, an index of lacrimal gland protein secretion, was expressed as the percentage of peroxidase secreted into the medium (supernatant) compared with total peroxidase in the cells (pellet) plus the medium.
Immunoprecipitation Experiments
Lacrimal gland acini were incubated for 0 to 60 seconds with Cch (10–4 M). To terminate incubation, we centrifuged the acini, discarded the supernatant, and added ice-cold RIPA buffer. The homogenate was centrifuged at 20,000g for 30 minutes, and the supernatant was incubated overnight at 4°C on a rocker platform in the presence of the immunoprecipitating antibody. Protein A-agarose was added for 2 hours and incubated at 4°C. The immunoprecipitate was collected by brief centrifugation, washed three times with RIPA buffer, and resuspended in Laemmli sample buffer. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes.
Data Presentation and Statistical Analysis
Data are expressed as the ratio of increase over the basal value, which was standardized to 1.0. The results are expressed as the mean ± SEM. Data were analyzed by Student's t-test. P < 0.05 was considered statistically significant.
Results
Presence and Localization of PLD
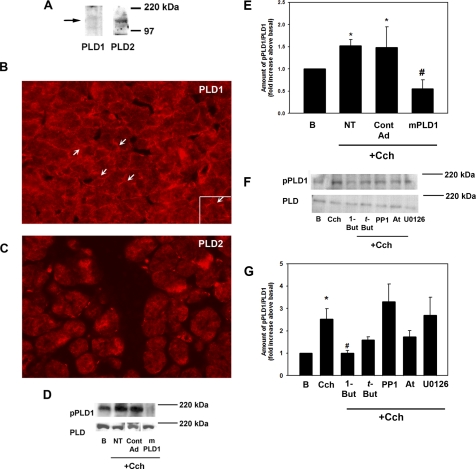
The presence of PLD in the lacrimal gland was confirmed by Western blot analysis with antibodies directed against PLD1 and -2. Both PLD1 and -2 were detected as single bands at 120 kDa (Fig. 1A). The lacrimal gland is composed of polarized acinar cells surrounding ducts. The localization of PLD1 and -2 in nontransformed cells was determined by incubating sections of lacrimal gland with the same PLD antibodies used in the Western blot experiments. PLD1 was present in the cytosol and basolateral membranes of the acinar cells (Fig. 1B, arrows). PLD2 has a localization similar to that of PLD1, as it was present in the cytosol and basolateral membranes of acinar cells (Fig. 1C). Thus, both PLD1 and -2 are present in the lacrimal gland.
Figure 1.
PLD1 is present in freshly isolated epithelial cells and is activated by cholinergic agonists. (A) The presence of PLD1 and -2 was determined by Western blot analysis. (B) Localization of PLD1. Arrows: basolateral membranes. (C) Localization of PLD2. Cells were transduced overnight with no addition (NT), a vector control (Cont AD), or an adenovirus containing a gene for catalytically inactive mutant (m)PLD1 before stimulation with Cch (10−4 M) for 5 minutes. The amount of phosphorylated PLD1 was determined by Western blot analysis. (D) A representative blot. The blot is from a single gel in which the lanes have been rearranged for ease of comparison. Three experiments were analyzed by densitometry and are shown in (E). Cells were preincubated with 1-but (0.3%), t-but (0.3%), the M3 muscarinic receptor antagonist atropine (At, 10−4 M), the c-Src inhibitor PP1 (10−5 M), or the MEK inhibitor U0126 (10−6 M) for 10 (PP1) or 30 (U0126) minutes before stimulation with the cholinergic agonist Cch (10−4 M) for 5 minutes, and the amount of phosphorylated PLD1 was determined by Western blot analysis. (F) A representative blot. Four experiments were analyzed by densitometry and are shown in (G). Data are the mean ± SEM. *Significant difference from no addition (B); #significant difference from Cch alone. Magnification: (B, C) ×200; (B, inset) ×400.
Effect of Cch on PLD1 Activity
To confirm that cholinergic agonists activate PLD, we stimulated freshly isolated acini with Cch (10−4 M) for 5 minutes. PLD1 activity was determined by Western blot analysis with an antibody against PLD1 phosphorylated on Thr147, which has been shown to be phosphorylated when PLD1 is activated.16,17 As shown in Figure 1D (lanes 1 and 2), Cch (NT) increased the phosphorylation of PLD1 over basal. When the blots were analyzed by densitometry, Cch significantly increased activation of PLD1 (1.5 ± 0.1-fold above basal; Fig. 1E).
A second method was used to determine whether Cch activates PLD1. Acini were transduced overnight with an adenovirus containing a gene for catalytically inactive mutant PLD1 (mPLD1) or a vector control before Western blot analysis with the antibody directed against activated PLD1 (Fig. 1D). This mPLD adenovirus has been shown to attenuate cell migration, wound healing, and phosphorylation of the PDGF receptor β.13,14 The amount of activated PLD1 in nontransduced acini incubated with Cch (10−4 M) for 5 minutes was not significantly different from that in acini incubated with the empty vector control (1. 5 ± 0.1- and 1.5 ± 0.5-fold above basal, respectively). mPLD1 (108 pfu) decreased the amount of active PLD1 to 0.6 ± 0.2-fold above basal (Fig. 1E). Thus, Cch activates PLD1 in nontransformed epithelial cells.
In a third method of determining whether Cch activates PLD1, we took advantage of the unique reaction wherein PLD preferentially catalyzes the transphosphatidylation of a primary alcohol over water, to produce the metabolically inert phosphatidylalcohol.18 Acini were incubated with 0.3% 1-butanol (1-but, diluted in KRB), which uncouples PLD from its pathway, or tertiary butanol (t-but), which is not capable of being used by PLD, diluted in KRB19 for 15 minutes before incubation with Cch (10−4 M) for 5 minutes. In these experiments, Cch significantly increased PLD activity (to 2.5 ± 0.3-fold above basal). This activity was completely inhibited by 1-but but not t-but (Figs. 1F, 1G). Thus, Cch stimulates PLD1 activity.
In the lacrimal gland, Cch binds to and activates the M3 muscarinic receptor.20 To ensure that PLD1 activation by carbachol is receptor mediated, we preincubated acini for 10 minutes with the specific muscarinic antagonist atropine (10−4 M, dissolved in KRB buffer), before Cch stimulation for 5 minutes. Atropine inhibited PLD1 activation by 61%, to 1.7 ± 0.3-fold above basal (Figs. 1F, 1G), although the decrease was not significant. These data imply that muscarinic receptors in freshly isolated cells are coupled to PLD1.
Cch also activates p60Src, leading to phosphorylation of ERK. To determine whether PLD is downstream of p60Src and ERK signaling or in a parallel signaling pathway, we preincubated cells with p60 Src inhibitor PP1 (10−5 M) or the MEK inhibitor U0126 (10−6 M) for 30 minutes before stimulation with Cch for 5 minutes. Both inhibitors were dissolved in DMSO, and the amount of DMSO (0.01%) was the same in all conditions. Neither PP1 nor U0126 had any effect on PLD1 activation (Figs. 1F, 1G). This indicates that PLD1 is either upstream of both p60Src and ERK or in a separate signaling pathway.
Effect of PLD1 Activation on Protein Secretion and ERK Activation
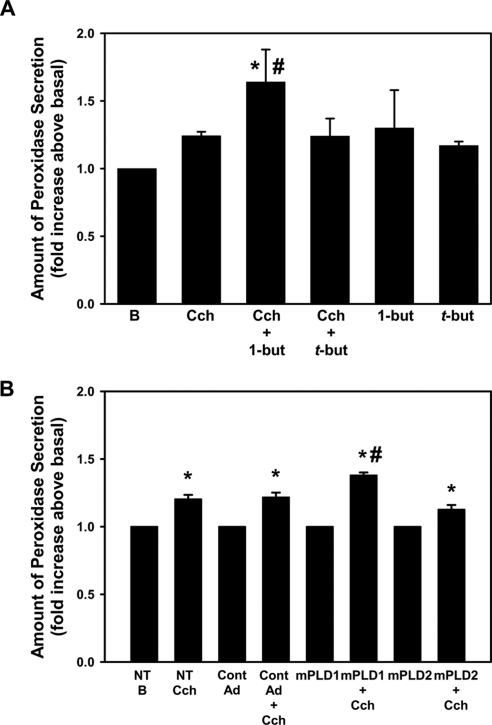
To determine whether PLD1 is involved in lacrimal gland protein secretion, we pretreated freshly isolated lacrimal gland acini for 15 minutes with 0.3% 1-but or t-but, which is not capable of being used by PLD, for 15 minutes before incubating them with Cch (10−4 M) for 20 minutes. Cch significantly increased protein secretion (to 1.2 ± 0.03-fold above basal). Uncoupling PLD1 from its signaling pathway with 1-but significantly increased protein secretion above that seen with Cch alone (to 1.6 ± 0.2-fold above basal), while t-but had no effect on Cch-stimulated secretion (Fig. 2A). 1-But and t-but alone had no effect on basal protein secretion.
Figure 2.
Inhibition of PLD increases cholinergic agonist-stimulated protein secretion. (A) Freshly isolated cells were preincubated for 15 minutes with either 0.3% 1-but or t-but before stimulation with the cholinergic agonist Cch (Cch, 10−4 M) for 20 minutes. The amount of protein secretion was measured. Data are the mean ± SEM from six independent experiments. (B) Cells were transduced overnight with no addition (NT), a vector control (Cont AD), an adenovirus containing a gene for catalytically inactive mutant PLD1 (mPLD1), or catalytically inactive mutant PLD2 (mPLD2) before stimulation with Cch (Cch 10−4 M) for 20 minutes. Data are the mean ± SEM from three independent experiments. *Significant difference from no addition (B, basal); #significant difference from Cch alone.
Similar results were obtained when freshly isolated cells were incubated overnight with an adenoviruses containing catalytically inactive PLD1 (mPLD1), PLD2 (mPLD2), or a control adenovirus (Cont AdV). In the absence of adenoviruses, Cch (10−4 M) significantly increased protein secretion after 20 minutes (to 1.2 ± 0.03-fold above basal). Incubation with either Cont AdV or mPLD2 had no effect on Cch-stimulated secretion (Fig. 2B). However, mPLD1 adenovirus increased Cch-stimulated secretion to 1.4 ± 0.03-fold above basal (Fig. 2B). None of the adenoviruses had an effect on basal secretion (data not shown). Thus PLD1, but not PLD2, is activated by cholinergic agonists and attenuates protein secretion in freshly isolated cells.
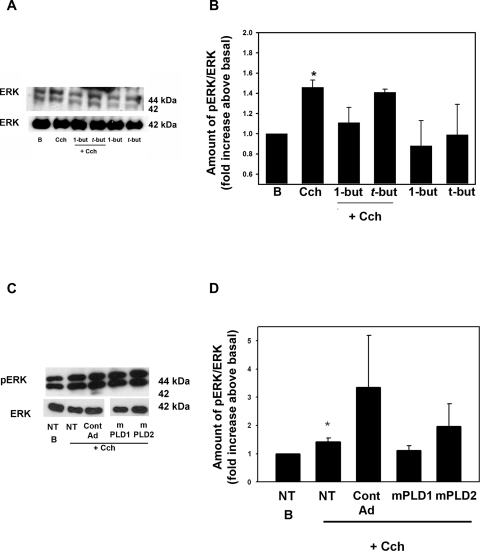
As we had found that activation of ERK negatively modulates Cch-stimulated protein secretion,2 we wanted to determine whether PLD activates ERK to account for the increase seen in Cch-stimulated secretion. Cch-stimulated ERK activity was determined by Western blot analysis (Fig. 3A). Cch stimulated ERK activity to 1.5 ± 0.1-fold above basal (Figs. 3A, 3B). In the presence of 1-but, Cch-stimulated ERK activity decreased to 1.1 ± 0.2-fold above basal (Figs. 3A, 3B). In the presence of t-but, Cch-stimulated ERK activity was not different from the ERK activity stimulated by Cch alone. 1-But and t-but alone had no effect on basal ERK activity. These data indicate that the muscarinic activation of PLD1 attenuates protein secretion by activating ERK.
Figure 3.
Inhibition of PLD blocks cholinergic agonist-stimulated ERK activity. Cells were incubated with no additions (B, basal), Cch (10−4), Cch + 1-butanol (1-but) and t-butanol (t-but). (A) The amount of activated ERK was determined by Western blot analysis. (B) The results of seven independent experiments. Cells were transduced overnight with no addition (NT), a vector control (Cont AD), an adenovirus containing a gene for catalytically inactive mutant PLD1 (mPLD1) or catalytically inactive mutant PLD2 (mPLD2) before stimulation with Cch (10−4 M) for 5 minutes. The amount of phosphorylated ERK was determined by Western blot analysis (C). (D) Results of three experiments analyzed by densitometry. Data are the mean ± SEM. *Significant difference from no addition (B, no addition).
Experiments with butanol were confirmed by using mPLD1. Acini were incubated overnight with 109 pfu of mPLD1, mPLD2, or vector control adenovirus. ERK activity was determined via Western blot analysis after Cch stimulation (10−4 M for 5 minutes, Fig. 3C). In acini treated with vector control, Cch increased ERK activity by 1.4 ± 0.1- and 3.3 ± 1.9-fold above basal in nontransduced acini and acini transduced with vector control adenovirus (Fig. 3D). mPLD1 decreased ERK activity to 1.1 ± 0.2-fold above basal. In contrast, mPLD2 did not decrease Cch-stimulated ERK activity (Fig. 3D). Thus PLD1, but not PLD2, is responsible for Cch-stimulated activation of ERK.
Effect of Inhibition of Rho on PLD1 and ERK Activation and Protein Secretion
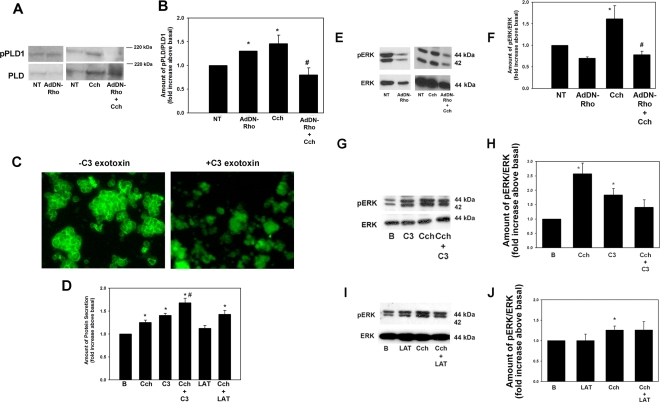
We next determined the upstream effectors of PLD1. It is known that PLD1 can be activated by the small GTPase Rho and its associated kinase ROCK. Therefore, we determined the effects of an adenovirus containing a dominant negative Rho gene (AdDN-Rho) on PLD1 activity. Freshly prepared acinar cells were again incubated overnight with the AdDN-Rho (109 pfu) and stimulated with Cch (10−4 M) for 5 minutes. The effect on activated PLD1 was determined with Western blot analysis with the antibody directed against phosphorylated (activated) PLD1 (Fig. 4A). In cells incubated overnight in the in the absence of Ad-DNRho, Cch significantly increased PLD1 activation (to 1.5 ± 0.2-fold above basal). This level was significantly decreased (to 0.8 ± 0.2-fold of basal) by AdV-DNRho (Fig. 4B). Interestingly, overnight incubation with the AdDN-Rho also significantly increased PLD1 phosphorylation (to 1.3 ± 0.01-fold above basal).
Figure 4.
Inhibition of Rho increases cholinergic-agonist-stimulated protein secretion and inhibits cholinergic agonist-stimulated ERK activity. Freshly isolated cells were transduced overnight with an adenovirus containing a gene for dominant negative Rho (AdDN-Rho) before stimulation with Cch (10−4 M) for 5 minutes. (A) The amount of phosphorylated (p)PLD and PLD was determined by Western blot analysis. (B) Results of seven experiments analyzed by densitometry. Cells were also preincubated for 30 minutes with cell-permeable C3 exotoxin (4 μg/mL). The actin cytoskeleton was detected by phalloidin conjugated to FITC (C). Cells were pretreated with C3 exotoxin or latrunculin (10−5 M) before stimulation with the cholinergic agonist Cch (10−4 M) for 5 minutes. (D) The amount of protein secretion was determined. Data are the mean ± SEM of results from four independent experiments. The amount of activated ERK was also determined by Western blot analysis after treatment with (E) AdDN-Rho, (G) C3 exotoxin, and (I) latrunculin. Results of seven (F, AdDN-Rho), three (H, C3), and five (J, LAT) independent experiments. Data are mean ± SEM *Significant difference from Cch; #significant difference from Cch.
In addition to activating PLD1, Rho has been linked to reorganization of the actin cytoskeleton.21 We used a cell-permeable form of the exoenzyme C3 Transferase from Clostridium botulinum which ADP ribosylates and inactivates Rho. To determine whether Rho is inactivated with treatment of the C3 exotoxin, cells were incubated with 4 μg/mL of exotoxin dissolved in KRB-HEPES for 30 minutes, placed on slides, air-dried, and fixed. They were then incubated with FITC-conjugated phalloidin, which binds specifically to F-actin. Before treatment with C3 exotoxin, the acinar cells had intense staining with phalloidin, and individual cells within the acinus were clearly outlined. This staining was completely abolished by C3 exotoxin, indicating that the exotoxin is effective at this concentration and preincubation time (Fig. 4C).
To determine the effect of inhibition of Rho on Cch-stimulated protein secretion, we incubated cells with C3 exotoxin 4 μg/mL for 30 minutes before stimulation with Cch (10−4 M) for 20 minutes and measured peroxidase secretion. Cch significantly increased protein secretion (to 1.3 ± 0.05-fold above basal; Fig. 4D). C3 exotoxin alone also significantly increased secretion (to 1.4 ± 0.04-fold above basal). Incubation with both C3 exotoxin and Cch significantly increased secretion above Cch (to 1.7 ± 0.1-fold above basal; Fig. 4D).
To determine whether Rho is involved in Cch-stimulated ERK activity, we preincubated freshly isolated cells with the AdDN-Rho overnight before stimulation with Cch (10−4 M) for 5 minutes. The amount of activated ERK was determined as described. In the absence of AdDN-Rho, Cch significantly stimulated ERK activity (to 1.6 ± 0.3-fold above basal; Figs. 4E, 4F). AdDN-Rho significantly decreased Cch-stimulated ERK (to 0.8 ± 0.1-fold). Unlike PLD1, incubation with AdDN-Rho alone had no significant effect on ERK activity.
Freshly isolated cells were also incubated with C3 exotoxin (4 μg/mL for 30 minutes) before stimulation with Cch (10−4 M) for 5 minutes, and the amount of ERK activity was determined. Cch significantly increased ERK phosphorylation (to 2.6 ± 0.4-fold above basal; Figs. 4G, 4H). C3 exotoxin alone also significantly increased ERK phosphorylation (by 1.8 ± 0.2-fold above basal). In cells treated with both C3 exotoxin and Cch, the amount of phosphorylated ERK was significantly decreased from Cch alone (to 1.4 ± 0.3-fold above basal; Fig. 4H).
We mimicked the effects of disruption of actin in a Rho-independent manner on Cch-stimulated secretion. Cells were incubated with or without latrunculin B, dissolved in DMSO, which is known to prevent polymerization of actin.21 All conditions contained 0.01% DMSO. As shown in Figure 4D, a 30-minute preincubation with latrunculin 10−5 M did not have a significant effect on Cch-stimulated protein secretion (1.4 ± 0.1-fold above basal). When Cch-stimulated ERK activity was measured after incubation with latrunculin, there was no effect on ERK stimulation (Figs. 4I, 4J).
These data imply that cholinergic agonists activate PLD1 through stimulation of Rho, but the effects seen with C3 exotoxin could occur through the ROCK pathway rather than through the actin cytoskeleton.
Presence and Localization of ROCK1
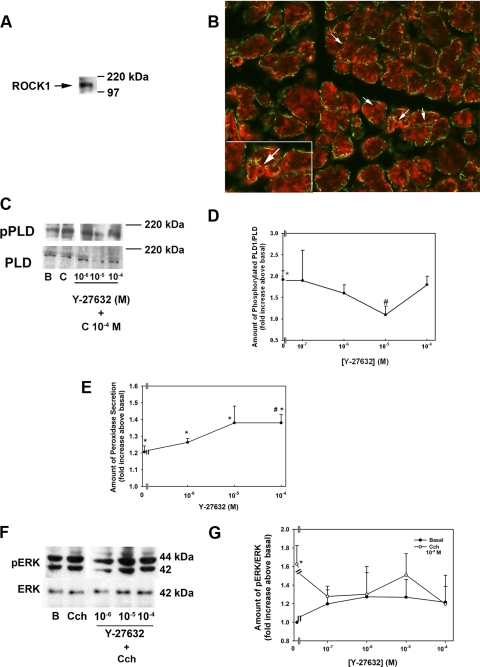
One possible mechanism by which Rho exerts its effect is the activation of ROCK.7 To confirm the presence of ROCK1 in the lacrimal gland, we performed Western blot analysis with antibodies directed against ROCK1. ROCK1 was detected as a single band at 160 kDa (Fig. 5A). To determine the localization of ROCK1 in nontransformed cells, we incubated sections of lacrimal gland with the same ROCK1 antibody used in the Western blot experiments. Similar to PLD1, ROCK1 (stained red) was present in the cytosol and plasma membranes of the acinar cells, as indicated by arrows (Fig. 5B). It did not appear to be present in the myoepithelial cells that surround the acini (indicated by green staining with α smooth muscle actin).
Figure 5.
ROCK1 is present in freshly isolated epithelial cells and inhibits cholinergic agonist-stimulated PLD activation. (A) Blot showing the presence of ROCK1. (B) Localization of ROCK1. Red: ROCK; green: smooth muscle actin. Arrows: membranes of acinar cells. Cells were preincubated with the ROCK inhibitor Y-27632 (10−7–10−4 M) for 20 minutes before stimulation with the cholinergic agonist Cch (C, 10−4 M) for 5 minutes, and the amount of phosphorylated PLD1 was determined by Western blot analysis (C). (D) Four experiments analyzed by densitometry. (E) The amount of protein secretion was determined. The amount of activated ERK was also determined by Western blot analysis (F). (G) Results of seven independent experiments. All results are expressed as the mean ± SEM. *Significant difference from no addition (B, basal); #significant difference from Cch. Magnification: (B) ×200; (B, inset) ×400.
Effect of ROCK on PLD1 Activity, Protein Secretion, and ERK Activity
To determine whether ROCK1 is involved in Cch-stimulated PLD activation, we preincubated freshly isolated cells with the ROCK inhibitor Y-27632 (10−7–10−4 M, dissolved in KRB-HEPES) for 20 minutes before stimulating them with Cch (10−4 M) for 5 minutes. As shown in Figure 5C, Cch-stimulated PLD phosphorylation was inhibited by Y-27632. When four independent experiments were analyzed, Cch significantly increased PLD1 phosphorylation (1.9 ± 0.2-fold above basal; Fig. 5D). Y-27632 at 10−5 M inhibited PLD phosphorylation (to 1.1 ± 0.2-fold above basal; Fig. 5D).
As activation of PLD attenuates Cch-stimulated protein secretion, inhibition of ROCK should increase Cch-stimulated protein secretion. To test this hypothesis, we preincubated freshly isolated cells with Y-27632 10−6, 10−5, and 10−4 M for 20 minutes before adding Cch (10−4 M) for 20 minutes. The amount of peroxidase was measured. Cch significantly increased protein secretion (to 1.2 ± 0.04 above no addition). Cch plus Y-27632, at all concentrations, significantly increased protein secretion above Cch alone. Secretion was increased to 1.3 ± 0.02, 1.4 ± 0.2, and 1.4 ± 0.01 above Cch at 10−6, 10−5, and 10−4 M. The increase by Y-27632 at 10−4 M was significantly increased above Cch alone (Fig. 5E).
PLD activation also leads to ERK phosphorylation; therefore, we also determined the effect of inhibition of ROCK with Y-27632 on ERK activation. Cch (10−4 M) increased the activation of ERK (Fig. 5F). In six independent experiments, Cch significantly increased ERK activation (to 1.6 ± 0.2-fold above basal). Y-27632 decreased Cch-stimulated ERK activity at 10−7, 10−6, 10−5, and 10−4 M to 1.3 ± 0.1, 1.3 ± 0.3, 1.5 ± 0.2, and 1.2 ± 0.3-fold above basal, respectively (Fig. 5G). Y-27632 alone at any concentration did not have a significant effect on ERK activation. These data, taken together, imply that cholinergic agonists use ROCK1 along with Rho to activate ERK, which attenuates secretion.
Coimmunoprecipitation of ROCK1 and PLD1
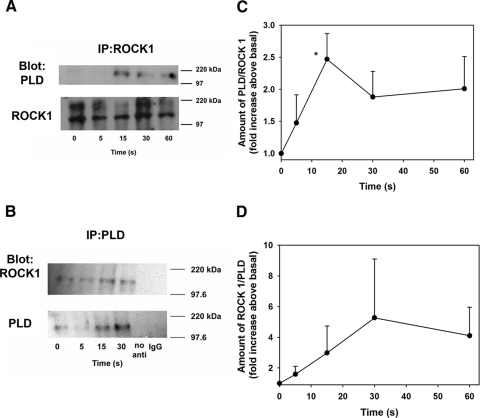
To determine whether ROCK1 and PLD1 become associated when stimulated with Cch, we incubated cells with Cch (10−4 M) for 0 to 60 seconds. In a first set of experiments, ROCK1 antibody was used for immunoprecipitation, the amount of PLD1 associated with ROCK1 increased in a time-dependent manner after addition of Cch. Cch increased the association of PLD1 with ROCK1 to 1.5 ± 0.4, 2.5 ± 0.4, 1.9 ± 0.4, and 2.0 ± 0.5-fold above time 0. The increase at 15 seconds was significantly greater than at time 0 (Figs. 6A, 6B).
Figure 6.
ROCK1 and PLD1 associate upon cholinergic agonist stimulation. Freshly isolated cells were stimulated with the cholinergic agonist Cch (10−4 M) for 5 to 60 seconds. (A) Immunoprecipitation with a ROCK1 antibody and Western blot analysis was performed with PLD and ROCK1 antibody. (B) Results of three independent experiments. (C) Immunoprecipitation with a PLD antibody and Western blot analysis was performed with ROCK1 and PLD antibody. (D) Results of three independent experiments All results are expressed as the mean ± SEM. *Significant difference from Cch.
Consistent with these experiments, when PLD1 was used as the immunoprecipitating antibody, ROCK1 association with PLD1 also increased to 1.6 ± 0.5-, 3.0 ± 1.7-, 5.3 ± 3.8-, and 4.1 ± 1.9-fold above the time 0 level (Figs. 6C, 6D). These results show that cholinergic agonist stimulation causes a complex of ROCK1 and PLD1 to form.
PLD1 Activates ERK in a Pyk2-, Ras-, and Raf-Independent Manner
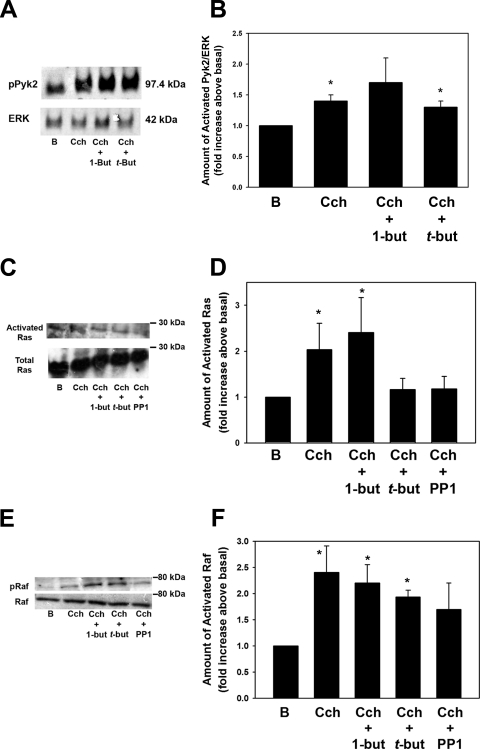
We have shown that Cch activates ERK via Pyk2 and Src, leading to activation of the Ras-Raf-MEK-ERK signaling cascade.2 To determine whether PLD1 initiates this cascade, we investigated the effect of Cch-stimulated Pyk2, Ras, and Raf in cells preincubated with 1-but and t-but. Pyk2 activity was determined using an antibody directed against Pyk2 phosphorylated on Tyr402, whereas Ras activity was determined with a Ras activation kit. Raf activity was determined with an antibody against Raf phosphorylated on Ser338. Similar to previous reports in the lacrimal gland,22 a 5-minute incubation with Cch (10−4 M) increased Pyk2 activity by 1.3-fold above basal (Fig. 7A). Incubation with 1-but did not alter Cch-stimulated Pyk2 activity (Fig. 7B).
Figure 7.
PLD activation of ERK occurs in a Pyk2-, Ras-, and Raf-independent manner. Freshly isolated acini were preincubated with 0.3% 1-but or t-but for 15 minutes before stimulation with the cholinergic agonist Cch (10−4 M) for 5 minutes. The amount of phosphorylated Pyk2 was determined by Western blot analysis (A). (B) Results of six independent experiments. (C) The amount of activated Ras was measured with a Ras-activation kit according to the manufacturer's instructions. (D) The mean ± SEM of results in nine independent experiments. (E) The amount of phosphorylated Raf was determined by Western blot analysis. (F) The mean ± SEM of results of four independent experiments. *Significant difference from basal (B).
In addition, Cch significantly increased Ras activity (Fig. 7C; by 2.0 ± 0.6 times basal), which, similar to Pyk2, was not significantly affected by incubation with 1-but, although t-but decreased Cch-stimulated Ras activity (Fig. 7D). As a control,2 a 30-minute preincubation with PP1 (10−5 M), an Src inhibitor, decreased Ras activity by 87% (to 1.2 ± 0.3 times basal; Fig. 7D).
Similarly to Pyk2 and Ras, Cch significantly stimulated Raf phosphorylation (by 2.4 ± 0.5-fold above basal; Figs. 7E, 7F). Phosphorylation was not significantly decreased by preincubation with 1-but or t-but. However, PP1 did significantly decrease Raf activity (by 60% to 1.7 ± 0.5 times basal; Fig. 7F). These results indicate that while Cch activates Ras and Raf and ultimately ERK through Pyk2/Src, PLD is not upstream of these enzymes and activates ERK via a different pathway.
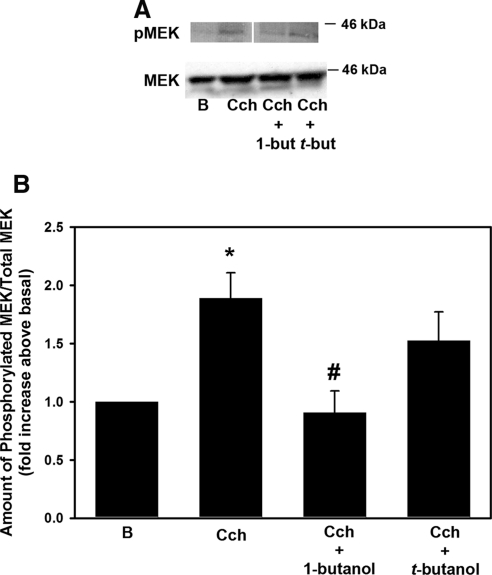
PLD Increases MEK 1/2 Activity
U0126 is a well-known and specific inhibitor of MEK, the kinase immediately upstream of ERK that is responsible for activation of ERK. While it is well established that inhibition of MEK completely abolishes Cch-stimulated ERK activation2 in the lacrimal gland, it is not known whether PLD activates MEK. To this end, freshly isolated acini were preincubated with 0.3% 1-but for 15 minutes before stimulation with Cch (10−4 M) for 5 minutes. The amount of MEK 1/2 phosphorylated on Ser217/221 was determined by Western blot analysis (Fig. 8A). Cch significantly increased MEK phosphorylation (to 1.9 ± 0.2-fold above basal; Fig. 8B). 1-But completely abolished Cch-stimulated MEK phosphorylation, but t-but had no effect (Fig. 8B). This finding indicates that cholinergic agonist stimulation of PLD causes phosphorylation, and thus activation, of MEK which in turn activates ERK.
Figure 8.
PLD activates MEK. Freshly isolated acini were preincubated with either 0.3% 1-but or t-but for 15 minutes before stimulation with the cholinergic agonist Cch (10−4 M) for 5 minutes. (A) The amount of phosphorylated MEK was determined by Western blot analysis. The blot is from a single gel in which the lanes have been rearranged for ease of comparison. (B) The mean ± SEM of results in six independent experiments. *Significant difference from basal (B); #significance from Cch alone.
Discussion
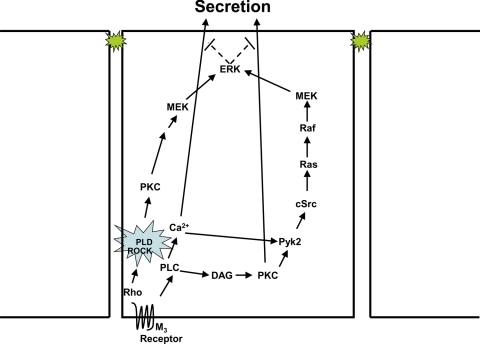
In the present study, we identified a novel signaling pathway activated by cholinergic agonists in freshly isolated, nontransformed epithelial cells. Binding of cholinergic agonists to M3 muscarinic receptors activates the small GTPase Rho upstream of PLD1 in the lacrimal gland (Fig. 9). This results in the association of ROCK1 with PLD1 and the subsequent activation of PLD1. PLD1 initiates a signaling cascade that culminates in activation of ERK in a unique Pyk2, cSrc, Ras, and Raf-independent, but MEK-dependent manner (Fig. 9). Activation of ERK attenuates cholinergic agonist-stimulated exocytosis. This pathway differs from previously described pathways, in that PLD1 rather than PLD2 activates ERK, PLD1 is upstream rather than downstream of ERK, PLD1 and ERK activation inhibits rather than stimulates exocytosis, and PLD1 does not activate Raf-1 but does activate MEK to stimulate ERK activity.
Figure 9.
The PLD signaling pathway in primary epithelial cells. Activation of M3 muscarinic receptors stimulates Rho to cause the association of ROCK1 and PLD1. This stimulates MEK and ERK and attenuates protein secretion.
We previously showed that cholinergic agonists stimulated the activity of PLD in freshly isolated lacrimal gland epithelial cells,3 while the isoform of PLD was not identified. We have now identified that PLD1 is activated by cholinergic agonists as determined by an increase in phosphorylation of PLD1. Activation of PLD1 by cholinergic agonists leads to activation of ERK and attenuation of exocytosis. Cholinergic activation of PLD2 does not appear to play a role in protein secretion or activation of ERK and was not investigated further.
It is well known that PLD1 can be activated by many molecules, including the small GTPase Rho23 and its associated kinase ROCK.9 Indeed, purified PLD1 has been shown to bind to purified RhoA in the presence of GTP.23 In freshly isolated cells, the association of ROCK1 and PLD1 increased with cholinergic agonist stimulation, implying that Rho, ROCK1, and PLD are in a single signaling complex or in the same subcellular location, which has been suggested to confer specificity to the signaling pathways.24
In the lacrimal gland, cholinergic agonists also activated ERK with Pyk2 and c-Src.2,22 Inhibition of c-Src with PP1 resulted in an approximately 50% reduction in ERK activity, implying that at least one additional pathway activates ERK independent of Pyk2/cSrc.22 Taking advantage of the transphosphatidyl reaction in which PLD preferentially catalyzes the transphosphatidylation of a primary alcohol over water to uncouple PLD from its signaling components, we showed that incubation with 1-but also decreased ERK activity by approximately 50%. As PP1 had no effect on PLD activity, it is unlikely that PLD1 is downstream of Pyk2 and c-Src. Thus, it seems likely that cholinergic agonists use at least two pathways to activate ERK: PLD in a Ras- and Raf-independent manner and Pyk2/cSrc in a Ras- and Raf-dependent manner.
The stimulation of PLD1 by cholinergic agonists most likely plays a key role in the regulation of protein secretion from the lacrimal gland as cholinergic agonists are a major stimuli of this response. PLD activity has also been shown to play key roles in exocytosis in a variety of tissues and cell lines, including insulin from pancreatic β-cells, catecholamines from chromaffin cells, and von Willebrand factor from endothelial cells.25–27 In lacrimal gland disease, overactivation of the PLD1 pathway or its components could block cholinergic agonist stimulation of secretion leading to dry eye disease. Thus inhibition of PLD1, ROCK, MEK, or ERK could be developed as a treatment for dry eye. In many cell types, PKC plays an important role in stimulation of PLD activity.4,28 Regulation of PLD by PKC is not completely understood but appears to be a result of a direct interaction between the two molecules. Indeed, the C terminus of PKCα has been shown to bind to and activate PLD1 in cells overexpressing PLD through a direct interaction and not by phosphorylation.28 The antibody used in this study to detect activated PLD is directed against Thr147, a site shown to be phosphorylated by PKCα.29 PKCβ isoforms can also stimulate PLD1.30 The lacrimal gland contains at least four PKC isoforms.31 In these cells, with specific inhibitors of PKCα, -δ, and -ε, we determined that cholinergic agonists activate PKCα, -δ, and -ε to stimulate protein secretion.32 PKC also activates Pyk2, cSrc, and ERK to attenuate protein secretion in these cells.22 In addition, cholinergic agonist-stimulated ERK activation was inhibited by nearly 80% by two PKC inhibitors.22
Rho and RhoGTPAse are well-known regulators of actin assembly. Previous studies in the exocrine pancreas demonstrated that activation of Rho potentiates protein secretion.10 In this study, we show that inhibition of Rho with C3 exotoxin increases protein secretion above that seen with agonist alone, similar to glutamate secretion by astrocytes.33 It has been shown in the lacrimal gland that disruption of actin with jasplakinolide, cytochalasin D, or latrunculin does not decrease cholinergic agonist-stimulated protein secretion.34 It has been hypothesized that the actin filaments near the apical membrane act as a physical barrier to keep the vesicles separated from the plasma membrane. Stimulation rearranges actin and allows fusion of vesicles. In the present study, disruption of actin with latrunculin had no effect on Cch-stimulated ERK activity, unlike inhibition of Rho with C3 exotoxin. As regulation of actin filaments is a highly complex process, it is possible that Rho acts on pools of actin different from those affected by latrunculin.
Interestingly, PLD1 activated ERK in unique Pyk2-, Ras-, and Raf-independent manner, as 1-but did not have any effect on cholinergic-stimulated Pyk2, Ras, or Raf activity. As the MEK inhibitor U0126 completely abolished Cch-stimulated ERK phosphorylation,2 the interaction between PLD and ERK activation must occur at the level of MEK1/2. The inhibition of phosphorylation of MEK by Cch with 1-but confirms this. It is not known how the interaction between PLD and MEK occurs. Multiple MEK kinases (MEKK), in addition to Raf, have been identified that lead to the activation of ERK, including MEKK1, -2, -3, and -4,.35 It is possible that a MEK kinase other than Raf is coupled to the PLD1 signaling pathway.
In conclusion, we showed in freshly isolated cells that cholinergic agonists cause an increase in the association of PLD1, Rho, and ROCK1, which in turn activates MEK/ERK in a Pyk2-, Ras- and Raf-independent manner. Activation of ERK then attenuates protein secretion.
Footnotes
Supported by National Institutes of Health Grant EY 06177 (DAD).
Disclosure: R.R. Hodges, None; E. Guilbert, None; M.A. Shatos, None; V. Natarajan, None; D.A. Dartt, None
References
- 1. Hodges RR, Dartt DA. Regulatory pathways in lacrimal gland epithelium. Int Rev Cytol. 2003;231:129–196 [DOI] [PubMed] [Google Scholar]
- 2. Ota I, Zoukhri D, Hodges RR, et al. Alpha 1-adrenergic and cholinergic agonists activate MAPK by separate mechanisms to inhibit secretion in lacrimal gland. Am J Physiol Cell Physiol. 2003;284(1):C168–C178 [DOI] [PubMed] [Google Scholar]
- 3. Zoukhri D, Dartt DA. Cholinergic activation of phospholipase D in lacrimal gland acini is independent of protein kinase C and calcium. Am J Physiol. 1995;268:C713–C720 [DOI] [PubMed] [Google Scholar]
- 4. Exton JH. Regulation of phospholipase D. FEBS Lett. 2002;531(1):58–61 [DOI] [PubMed] [Google Scholar]
- 5. Cockcroft S. Signalling roles of mammalian phospholipase D1 and D2. Cell Mol Life Sci. 2001;58(11):1674–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kanaho Y, Funakoshi Y, Hasegawa H. Phospholipase D signalling and its involvement in neurite outgrowth. Biochim Biophys Acta. 2009;1791(9):898–904 [DOI] [PubMed] [Google Scholar]
- 7. Schmandke A, Schmandke A, Strittmatter SM. ROCK and Rho: biochemistry and neuronal functions of Rho-associated protein kinases. Neuroscientist. 2007;13(5):454–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lecuona E, Ridge K, Pesce L, et al. The GTP-binding protein RhoA mediates Na,K-ATPase exocytosis in alveolar epithelial cells. Mol Biol Cell. 2003;14(9):3888–3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol. 2006;290(3):C661–C668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bi Y, Page SL, Williams JA. Rho and Rac promote acinar morphological changes, actin reorganization, and amylase secretion. Am J Physiol Gastrointest Liver Physiol. 2005;289(3):G561–G570 [DOI] [PubMed] [Google Scholar]
- 11. Bi Y, Williams JA. A role for Rho and Rac in secretagogue-induced amylase release by pancreatic acini. Am J Physiol Cell Physiol. 2005;289(1):C22–C32 [DOI] [PubMed] [Google Scholar]
- 12. Usatyuk PV, Gorshkova IA, He D, et al. Phospholipase D-mediated activation of IQGAP1 through Rac1 regulates hyperoxia-induced p47phox translocation and reactive oxygen species generation in lung endothelial cells. J Biol Chem. 2009;284(22):15339–15352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang L, Cummings R, Zhao Y, et al. Involvement of phospholipase D2 in lysophosphatidate-induced transactivation of platelet-derived growth factor receptor-beta in human bronchial epithelial cells. J Biol Chem. 2003;278(41):39931–39940 [DOI] [PubMed] [Google Scholar]
- 14. Gorshkova I, He D, Berdyshev E, et al. Protein kinase C-epsilon regulates sphingosine 1-phosphate-mediated migration of human lung endothelial cells through activation of phospholipase D2, protein kinase C-zeta, and Rac1. J Biol Chem. 2008;283(17):11794–11806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang L, Cummings R, Usatyuk P, et al. Involvement of phospholipases D1 and D2 in sphingosine 1-phosphate-induced ERK (extracellular-signal-regulated kinase) activation and interleukin-8 secretion in human bronchial epithelial cells. Biochem J. 2002;367:751–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim Y, Han JM, Han BR. Phospholipase D1 is phosphorylated and activated by protein kinase C in caveolin-enriched microdomains within the plasma membrane. J Biol Chem. 2000;275(18):13621–13627 [DOI] [PubMed] [Google Scholar]
- 17. Zeniou-Meyer M, Liu Y, Begle A, et al. The Coffin-Lowry syndrome-associated protein RSK2 is implicated in calcium-regulated exocytosis through the regulation of PLD1. Proc Natl Acad Sci U S A. 2008;105(24):8434–8439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown HA, Henage LG, Preininger AM, et al. Biochemical analysis of phospholipase D. Methods Enzymol. 2007;434:49–87 [DOI] [PubMed] [Google Scholar]
- 19. Hu T, Exton JH. 1-Butanol interferes with phospholipase D1 and protein kinase Calpha association and inhibits phospholipase D1 basal activity. Biochem Biophys Res Commun. 2005;327(4):1047–1051 [DOI] [PubMed] [Google Scholar]
- 20. Lemullois M, Rossignol B, Mauduit P. Immunolocalization of myoepithelial cells in isolated acini of rat exorbital lacrimal gland: cellular distribution of muscarinic receptors. Biol Cell. 1996;86(2–3):175–181 [DOI] [PubMed] [Google Scholar]
- 21. Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16(10):522–529 [DOI] [PubMed] [Google Scholar]
- 22. Hodges RR, Rios JD, Vrouvlianis J, Ota I, Zoukhri D, Dartt DA. Roles of protein kinase C, Ca2+, Pyk2, and c-Src in agonist activation of rat lacrimal gland p42/p44 MAPK. Invest Ophthalmol Vis Sci. 2006;47(8):3352–3359 [DOI] [PubMed] [Google Scholar]
- 23. Jenkins GM, Frohman MA. Phospholipase D: a lipid centric review. Cell Mol Life Sci. 2005;62(19–20):2305–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O'Neill E, Kolch W. Conferring specificity on the ubiquitous Raf/MEK signalling pathway. Br J Cancer. 2004;90(2):283–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Disse J, Vitale N, Bader MF, et al. Phospholipase D1 is specifically required for regulated secretion of von Willebrand factor from endothelial cells. Blood. 2009;113(4):973–980 [DOI] [PubMed] [Google Scholar]
- 26. Hughes WE, Elgundi Z, Huang P, et al. Phospholipase D1 regulates secretagogue-stimulated insulin release in pancreatic beta-cells. J Biol Chem. 2004;279(26):27534–27541 [DOI] [PubMed] [Google Scholar]
- 27. Vitale N, Chasserot-Golaz S, Bader MF. Regulated secretion in chromaffin cells: an essential role for ARF6-regulated phospholipase D in the late stages of exocytosis. Ann N Y Acad Sci. 2002;971:193–200 [DOI] [PubMed] [Google Scholar]
- 28. Hu T, Exton JH. Mechanisms of regulation of phospholipase D1 by protein kinase Calpha. J Biol Chem. 2003;278(4):2348–2355 [DOI] [PubMed] [Google Scholar]
- 29. Exton JH. Regulation of phospholipase D. Biochim Biophys Acta. 1999;1439(2):121–133 [DOI] [PubMed] [Google Scholar]
- 30. Conricode KM, Smith DL, Burns DJ, et al. Phospholipase D activation in fibroblast membranes by the alpha and beta isoforms of protein kinase C. FEBS Lett. 1994;342(2):149–153 [DOI] [PubMed] [Google Scholar]
- 31. Zoukhri D, Hodges RR, Willert S, et al. Immunolocalization of lacrimal gland PKC isoforms: effect of phorbol esters and cholinergic agonists on their cellular distribution. J Membr Biol. 1997;157(2):169–175 [DOI] [PubMed] [Google Scholar]
- 32. Zoukhri D, Hodges RR, Sergheraert C, et al. Lacrimal gland PKC isoforms are differentially involved in agonist-induced protein secretion. Am J Physiol. 1997;272:C263–C269 [DOI] [PubMed] [Google Scholar]
- 33. Holtje M, Hofman F, Lux R, et al. Glutamate uptake and release by astrocytes are enhanced by Clostridium botulinum C3 protein. J Biol Chem. 2008;283(14):9289–9299 [DOI] [PubMed] [Google Scholar]
- 34. da Costa SR, Yarber FA, Zhang L, et al. Microtubules facilitate the stimulated secretion of beta-hexosaminidase in lacrimal acinar cells. J Cell Sci. 1998;111:1267–1276 [DOI] [PubMed] [Google Scholar]
- 35. Schlesinger TK, Fanger GR, Yujiri T, et al. The TAO of MEKK. Front Biosci. 1998;3:D1181–D1186 [DOI] [PubMed] [Google Scholar]