SD-OCT was used to measure retinal thickness in mice and to quantitate the reduction in retinal thickness over time in response to optic nerve crush.
Abstract
Purpose.
To investigate the longitudinal effect of optic nerve crush injury in mice by measuring retinal thickness with spectral-domain optical coherence tomography (SD-OCT).
Methods.
Optic nerves of one eye from each C57Bl/6 mouse were crushed under direct visualization for 3 seconds, 1 mm posterior to the globe. The optic nerve head (ONH) was imaged with SD-OCT (1.5 × 1.5 × 2.0 mm scan) before the surgical intervention and repeated subsequently for up to 32 days postinjury. A cohort of mice not exposed to the nerve crush procedure served as control. En face SD-OCT images were used to manually align subsequent scans to the baseline en face image. Total retinal thickness (TRT) (along a sampling band with radii 0.33–0.42 mm centered on the ONH) from each follow-up day was automatically quantified for global and sectoral measurements using custom software. Linear mixed-effects models with quadratic terms were fitted to compare TRT of nerve-crushed and control eyes over time.
Results.
Eleven eyes from 11 nerve crush mice (baseline age 76 ± 11.8 days) and eight eyes from four healthy mice (baseline age 64 ± 0 days) were included. The control eyes showed a small, gradual, and consistent TRT increase throughout follow-up. Nerve-crushed eyes showed an initial period of thickening, followed by thinning and slight rebound after day 21. The decrease in thickness observed after the early thickening resolved was statistically significantly different from the control eyes (P < 0.05 for global and sectoral measurements).
Conclusions.
SD-OCT can be used to quantitatively monitor changes in retinal thickness in mice over time.
Retinal ganglion cell (RGC) death induced by optic nerve crush injury has been used as a model to investigate axonal degeneration of the central nervous system in mice.1–4 In adult mice, approximately 60% of RGCs are lost within 3 weeks after optic nerve crush injury, as determined by histology.1,4
Ophthalmic imaging techniques such as confocal scanning laser ophthalmoscopy (CSLO) and optical coherence tomography (OCT) can provide quantitative and detailed structural information from the retina and optic nerve head (ONH) region. In vivo images of RGCs in mice and rats after optic nerve crush have been acquired using retrograde labeling and CSLO.5–7 Recently, Leung et al.8 demonstrated the use of CSLO with Thy-1 cyan fluorescent protein expressing transgenic mice to monitor RGCs in vivo after nerve crush. The authors in these studies were able to observe the loss of RGCs in the same eyes over time, contrary to previous experiments using histology that required several different animals at each time point.
OCT allows for noninvasive, in vivo imaging of ocular structures and has been used to obtain optical cross sections of the mouse retina.9–12 Time-domain OCT9,10 and spectral-domain OCT (SD-OCT)13–17 imaging have been used to observe retinal thinning in mouse models of retinal degeneration. One study used SD-OCT to image axotomized rats and manually obtained measurements of retinal layers in six cross-sectional images.18 This approach, however, is prone to subjective bias both in the selection of individual cross-sections and in the manual segmentation of retinal layers. To fully benefit from the high resolution and rapid scanning provided by SD-OCT, it would be desirable to obtain automated measurements from the area of interest. The goal of this study, therefore, was to investigate the longitudinal effect of optic nerve crush injury on total retinal thickness (TRT) in adult mice using automated segmentation analysis of SD-OCT images.
Methods
This experiment was approved by the University of Pittsburgh's Institutional Animal Care and Use Committee and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Animals
Healthy adult male C57Bl/6 mice (Jackson Laboratory, Bar Harbor, ME) were used in this study. Mice were maintained in the University of Pittsburgh Animal Facility with a 12-hour light-dark cycle and had free access to water and standard laboratory feed. Control animals were not exposed to any surgical procedure, whereas nerve crush animals had one optic nerve that was crushed.
Nerve Crush Procedure
Animals were anesthetized with an intraperitoneal injection of ketamine (80 mg/kg; Ketaject; Phoenix Pharmaceuticals, St. Joseph, MI) and xylazine (5 mg/kg, Xyla-ject; Phoenix Pharmaceuticals). An incision was made in the conjunctiva at the limbus, and then the sub-Tenon's space was bluntly dissected posteriorly. The muscle cone was entered and the optic nerve exposed. The optic nerve was crushed with fine forceps for 3 seconds, approximately 1 mm posterior to the globe, under direct visualization. Care was taken not to damage surrounding blood vessels.
SD-OCT Imaging
Before each session, mice were anesthetized with an intraperitoneal injection of ketamine and xylazine to prevent large movements during SD-OCT image acquisition. Pupils were dilated using a topically applied drop of tropicamide (1%; Falcon Pharmaceuticals, Fort Worth, TX). To neutralize corneal optical power and focus the SD-OCT beam onto the retina, a thin glass coverslip was applied to the cornea. Hydroxymethylcellulose ophthalmic demulcent solution (Goniosol, 2.5%; Akon, Buffalo Grove, IL) was used to preserve corneal hydration and couple the coverslip to the cornea. Mice were secured on a custom stage that allowed for free rotation to acquire images centered on the ONH.19 All images were acquired within 20 minutes from the onset of anesthesia to avoid a reduction of signal intensity, which can be caused by the occurrence of reversible cataract.20
Volumetric images centered on the ONH were acquired using SD-OCT (Bioptigen, Inc., Research Triangle Park, NC). All SD-OCT images consisted of a 250 × 250 A-scan array; there were 250 A-scans per B-scan, 250 B-scan frames, and 1024 samplings/A-scan in depth. Four repeated A-scans were collected and averaged at each location to improve the signal-to-noise ratio. This corresponds to a volume of approximately 1.5 × 1.5 × 2.0 mm at the surface of the coverslip. A baseline SD-OCT scan was obtained before the nerve crush procedure in each nerve-crushed eye; the first imaging session was used as baseline in the control eyes. Follow-up scans were obtained at multiple days, up to 32 days post–nerve crush in both groups.
SD-OCT Image Analysis
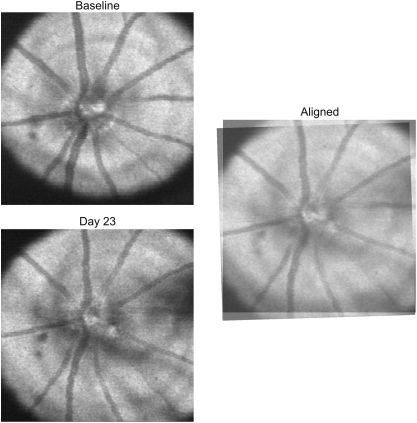
Since mice were rotated freely on our stage, and eyes could be oriented differently from scan to scan, we manually aligned SD-OCT en face images (Fig. 1), the details of which were previously described.19 Briefly, baseline SD-OCT en face images acquired in a given eye were used as reference to align all follow-up images, based on landmarks such as blood vessels and the optic nerve, and the coordinates of rotation and translation for each scan relative to reference were used to align thickness and quality measurements as described below. This method for image registration does not distort or magnify the image and uses only translation and rotation with respect to the optic nerve head.
Figure 1.
SD-OCT en face image from 23 days post–nerve crush, manually aligned to baseline by rotating and translating
SD-OCT Image Analysis: Automated Segmentation
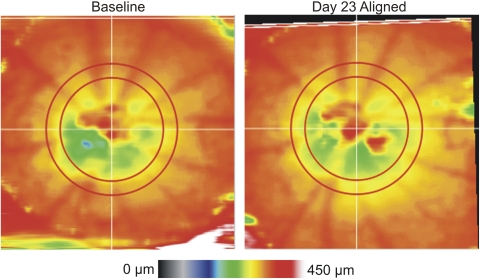
OCT en face images were used to manually define the ONH margin for each eye; this was done on all images by one experienced operator (MG). The geometric center of the ONH was used as a center point for subsequent analysis. Custom automated segmentation software, which was previously described21 but modified for SD-OCT, was used to detect the internal limiting membrane and retinal pigment epithelium. These boundaries defined the anterior and posterior borders used for quantification of TRT. All TRT measurements within a sampling band with radii of 55 to 70 pixels (0.33–0.42 mm at the surface of the coverslip; the sampling band and a map of TRT can be seen in Fig. 2), centered on the ONH, were obtained. TRT measurements were obtained directly from the volume within the sampling band and did not contain any interpolated values. Scans were subsequently rotated and translated based on the coordinates obtained from manual image alignment, and global and sectoral average TRTs were computed. These aligned TRT measurements were used to assess retinal thickness changes over time.
Figure 2.
TRT map from baseline and 23 days post-nerve crush, with the post-crush thickness map aligned to baseline using the coordinates obtained from en face image alignment. The red concentric circles indicate the boundaries of the measurement sampling region. An overall thinning can be discerned on day 23 by observing the broadening of the yellow region on the TRT map compared with baseline.
SD-OCT Image Analysis: Scan Quality
SD-OCT en face images were subjectively checked to ensure that there was consistent image quality across the scan, and that there were no areas of shadowing from media opacities within the TRT sampling band. In addition, we measured the quality index, as previously described,22 along every A-scan in the data volume.
Statistical Analysis
Linear mixed effects models with polynomial (quadratic) terms were fitted to describe global and sectoral (temporal, superior, nasal, inferior quadrants) TRT of nerve crush and control eyes over time, with scan quality index included as a covariate in the analysis. This mixed effects analysis accounted for the repeated measurements over time and the use of both eyes from some animals. All analysis was conducted using freely available programming software (R Language and Environment for Statistical Computing program; http://www.R-project.org). An alpha level of 0.05 was used as a cutoff for statistical significance.
Results
Eight eyes from four healthy control mice (baseline age 64 ± 0 days) and 11 eyes from 11 nerve crush mice (baseline age 76 ± 11.8 days) were included in this study. All control eyes were scanned at baseline and eight consecutive sessions over the course of 31 days, and nerve-crushed eyes were followed for up to 32 days (average time of follow-up, 29.2 ± 4.0 days; average number of sessions, 9.9 ± 0.7). In the control eyes, 4% (3/72) of global and 2% (7/288) of quadrant TRT measurements were excluded based on our quality criteria. These segments were excluded because of algorithm failure caused by a low signal. In the nerve-crushed eyes, a total of 39% (43/109) of global and 29% (126/436) of quadrant TRT measurements were excluded. There was a higher incidence of poor signal quality caused by shadowing from media opacities in nerve-crushed eyes, which caused algorithm failure.
Mean TRT across all eyes at baseline was 299.7 ± 8.3 μm. TRT showed an initial period of thickening, followed by a steep decrease and slight increase after day 21 in the nerve-crushed eyes (Fig. 3, bottom panel). There was a statistically significant positive quadratic term in nerve-crushed eyes (P < 0.05 for global and sectoral measurements), but not in control eyes, which can be attributed to the initial phase of thickening. Control eyes showed a slight but statistically significant increase in slope over time (P < 0.05 for global and sectoral measurements). The fitted models for the nerve crush group showed a statistically significant difference from control for both linear and quadratic components (P < 0.05) for global and sectoral measurements. Image quality did not significantly affect TRT measurements for control or nerve-crushed eyes, except for the superior quadrant in nerve-crushed eyes (P < 0.05).
Figure 3.
Top: Individual total retinal thickness (TRT) measurements, each eye represented by a different color; bottom: mean global TRT over time for control and nerve-crushed eyes. The solid line represents mean thickness, and dashed lines represent the SD.
To eliminate the potential confounding effect of the initial TRT increase on the analysis, we performed a post hoc analysis using mixed effects modeling with TRT measurements acquired on or after day 6, once the transient thickness increase resolved. Global and all sectoral measurements from nerve-crushed eyes had a statistically significant negative slope (P < 0.05). There was a statistically significant positive quadratic term for global measurements as well as temporal, nasal, and inferior quadrants (P < 0.05), and the quality index was not a statistically significant covariate in any sector. There was no statistically significant quadratic term in the control eyes, but there was a statistically significant slight linear increase in thickness over time in global measurements as well as superior and inferior quadrants (P < 0.05).
Discussion
In this study, we demonstrated that quantification of longitudinal TRT changes is possible in untreated mice and after optic nerve crush using OCT with automated segmentation. We were able to follow a cohort of mice over time and therefore did not have to kill mice at each time point to obtain structural information. This enables a true longitudinal assessment as opposed to attempting to extract a longitudinal effect from multiple cross-sectional data as in the case of histologic sectioning. In addition, this can substantially reduce the number of animals to be used in such experiments.
The initial phase of retinal thickening that was seen in the mice with nerve-crushed eyes may be due to an inflammatory reaction causing transient edema. It is possible that this finding has not been reported previously because the increase of ∼25 μm in TRT is difficult to detect clinically. Histologic studies investigated retinal response to nerve-crush–observed mice at later time points and therefore missed early inflammation.1,2,4 In addition, dehydration and tissue shrinkage that occurs while processing tissue for histology may prevent edematous changes from being readily apparent. This highlights the advantages of noninvasive cross-sectional in vivo tissue assessment.
Although our crush procedure consisted of a single 3-second crush, the duration of the crush may affect the severity of damage and the persistence of early retinal thickening. It is possible that exposure to a longer crush may induce a larger response, but we chose to limit the length of the crush to assess the utility of this imaging approach and to avoid complete axotomy or blood vessel damage.
Previous reports have shown a decrease in retinal ganglion cell count of ∼60% by 3 weeks post-lesion.1,4 Although our results showed a decrease in thickness over time, our method is currently limited to measurements of the entire thickness of the retina. Hence, the exact axial location of changes within the retina is not known. It is possible that segmenting the RGC or retinal nerve fiber layer may show an even more noticeable decrease in thickness. However, at this stage, automated retinal nerve fiber layer thickness measurements are unreliable because the retinal nerve fiber layer in mice is especially thin.
Another limitation of this study is that it is possible that exact sectoral boundary locations could vary from mouse to mouse because of the rotation of mice on the stage. We could correct for rotation in follow-up images for a given mouse using the method described above, but this adjustment is less reliable across mice. For this reason, we chose not to assess clock hour measurements and to include only global and quadrant thicknesses in the analysis. Global measurements would not be affected by this limitation because all measurements within the band are compared.
We observed that more sectors from nerve-crushed eyes had to be excluded because of poor image quality than from control eyes. The media opacities leading to poor image quality were often due to corneal opacities, suggesting that eyes that received the nerve crush injury were more sensitive to damage from the coverslip used to focus on the retina. This may be due to corneal dehydration caused by the extended time of recovery from anesthesia after surgery, or because surgery leaves an irregular conjunctiva, inhibiting proper hydration and tear film coverage.
We observed a slight linear increase in retinal thickness in control eyes over the course of this experiment. The significant positive quadratic term in the nerve-crushed eyes indicates that there was a rebound in thickness those eyes as well. Previous studies have shown that the mouse eye23,24 and retina25 are still growing out to at least 15 weeks after birth. The follow-up period in our study lasted to an age of 15 weeks, which may explain the increase in retinal thickness in control eyes and the rebound in thickness in nerve-crushed eyes. The rebound in TRT in nerve-crushed eyes may also be due to retinal response to inflammation, such as gliosis; however, this is simply conjecture at this point. Another potential explanation for the gradual thickening might be related to scan quality. OCT studies in humans demonstrated that an increase in signal quality leads to an increase in thickness measurements.26–29 However, image quality was included as a covariate in our mixed effects model but did not have a statistically significant effect. Nevertheless, it should be noted that our quality criteria excluded images that were of poor quality, which might explain the discrepancy between our findings and those reported previously.
We did not include the contralateral eyes of the nerve crush mice as a control in this experiment because there is evidence that there may be change occurring in those eyes.30–32 Future studies measuring retinal thickness in the contralateral eyes are required to determine whether they undergo structural changes that are detectable with OCT.
In conclusion, we demonstrated the ability of OCT to detect retinal thinning in vivo after nerve crush. Using OCT to monitor retinal thickness in the mouse optic nerve crush model may be valuable for evaluating the pathophysiology of disease as well as testing the efficacy of treatment because the same animals can be followed over time.
Acknowledgments
The authors thank Christopher Leung (Department of Ophthalmology and Visual Sciences, The Chinese University of Hong Kong, Hong Kong, P.R. China) for assistance with the optic nerve crush technique.
Footnotes
Supported by NIH Grants T32-EY017271, NIH R21-EY019092, NIH R01-EY013178, and P30-EY008098; Eye and Ear Foundation, Pittsburgh, PA; and Research to Prevent Blindness, New York, NY.
Disclosure: M.L. Gabriele, None; H. Ishikawa, None; J.S. Schuman, Carl Zeiss Meditec (F), Bioptigen (F), P; Y. Ling, None; R.A. Bilonick, None; J.S. Kim, None; L. Kagemann, None; G. Wollstein, None
Presented at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, May 2010.
References
- 1. Allcutt D, Berry M, Sievers J. A quantitative comparison of the reactions of retinal ganglion cells to optic nerve crush in neonatal and adult mice. Brain Res. 1984;318:219–230 [DOI] [PubMed] [Google Scholar]
- 2. Allcutt D, Berry M, Sievers J. A qualitative comparison of the reactions of retinal ganglion cell axons to optic nerve crush in neonatal and adult mice. Brain Res. 1984;318:231–240 [DOI] [PubMed] [Google Scholar]
- 3. Castano A, Bell MD, Perry VH. Unusual aspects of inflammation in the nervous system: Wallerian degeneration. Neurobiol Aging. 1996;17:745–751 [DOI] [PubMed] [Google Scholar]
- 4. Li Y, Schlamp CL, Nickells RW. Experimental induction of retinal ganglion cell death in adult mice. Invest Ophthalmol Vis Sci. 1999;40:1004–1008 [PubMed] [Google Scholar]
- 5. Thanos S, Indorf L, Naskar R. In vivo FM: using conventional fluorescence microscopy to monitor retinal neuronal death in vivo. Trends Neurosci. 2002;25:441–444 [DOI] [PubMed] [Google Scholar]
- 6. Higashide T, Kawaguchi I, Ohkubo S, Takeda H, Sugiyama K. In vivo imaging and counting of rat retinal ganglion cells using a scanning laser ophthalmoscope. Invest Ophthalmol Vis Sci. 2006;47:2943–2950 [DOI] [PubMed] [Google Scholar]
- 7. Cordeiro MF, Guo L, Luong V, et al. Real-time imaging of single nerve cell apoptosis in retinal neurodegeneration. Proc Natl Acad Sci U S A. 2004;101:13352–13356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leung CK, Lindsey JD, Crowston JG, Lijia C, Chiang S, Weinreb RN. Longitudinal profile of retinal ganglion cell damage after optic nerve crush with blue-light confocal scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci. 2008;49:4898–4902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Horio N, Kachi S, Hori K, et al. Progressive change of optical coherence tomography scans in retinal degeneration slow mice. Arch Ophthalmol. 2001;119:1329–1332 [DOI] [PubMed] [Google Scholar]
- 10. Li Q, Timmers AM, Hunter K, et al. Noninvasive imaging by optical coherence tomography to monitor retinal degeneration in the mouse. Invest Ophthalmol Vis Sci. 2001;42:2981–2989 [PubMed] [Google Scholar]
- 11. Kocaoglu OP, Uhlhorn SR, Hernandez E, et al. Simultaneous fundus imaging and optical coherence tomography of the mouse retina. Invest Ophthalmol Vis Sci. 2007;48:1283–1289 [DOI] [PubMed] [Google Scholar]
- 12. Srinivasan VJ, Ko TH, Wojtkowski M, et al. Noninvasive volumetric imaging and morphometry of the rodent retina with high-speed, ultrahigh-resolution optical coherence tomography. Invest Ophthalmol Vis Sci. 2006;47:5522–5528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fischer MD, Tanimoto N, Beck SC, et al. Structural and functional phenotyping in the cone-specific photoreceptor function loss 1 (cpfl1) mouse mutant: a model of cone dystrophies. Adv Exp Med Biol. 2010;664:593–599 [DOI] [PubMed] [Google Scholar]
- 14. Maeda A, Golczak M, Maeda T, Palczewski K. Limited roles of Rdh8, Rdh12, and Abca4 in all-trans-retinal clearance in mouse retina. Invest Ophthalmol Vis Sci. 2009;50:5435–5443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ruggeri M, Wehbe H, Jiao S, et al. In vivo three-dimensional high-resolution imaging of rodent retina with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2007;48:1808–1814 [DOI] [PubMed] [Google Scholar]
- 16. Huber G, Beck SC, Grimm C, et al. Spectral domain optical coherence tomography in mouse models of retinal degeneration. Invest Ophthalmol Vis Sci. 2009;50:5888–5895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim KH, Puoris'haag M, Maguluri GN, et al. Monitoring mouse retinal degeneration with high-resolution spectral-domain optical coherence tomography. J Vis. 2008;8(1):17.1–11 [DOI] [PubMed] [Google Scholar]
- 18. Bai Y, Xu J, Brahimi F, Zhuo Y, Sarunic MV, Saragovi HU. An agonistic anti-TrkB mAb, but not BDNF, causes sustained TrkB activation, delays RGC death, and protects the retinal structure in optic nerve axotomy and in glaucoma. Invest Ophthalmol Vis Sci. 2010;51:4722–4731 [DOI] [PubMed] [Google Scholar]
- 19. Gabriele ML, Ishikawa H, Schuman JS, et al. Reproducibility of spectral-domain optical coherence tomography total retinal thickness measurements in mice. Invest Ophthalmol Vis Sci. 2010;51:6519–6523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Calderone L, Grimes P, Shalev M. Acute reversible cataract induced by xylazine and by ketamine-xylazine anesthesia in rats and mice. Exp Eye Res. 1986;42:331–337 [DOI] [PubMed] [Google Scholar]
- 21. Ishikawa H, Stein DM, Wollstein G, Beaton S, Fujimoto JG, Schuman JS. Macular segmentation with optical coherence tomography. Invest Ophthalmol Vis Sci. 2005;46:2012–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stein DM, Ishikawa H, Hariprasad R, et al. A new quality assessment parameter for optical coherence tomography. Br J Ophthalmol. 2006;90:186–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Puk O, Dalke C, Favor J, de Angelis MH, Graw J. Variations of eye size parameters among different strains of mice. Mamm Genome. 2006;17:851–857 [DOI] [PubMed] [Google Scholar]
- 24. Zhou G, Williams RW. Mouse models for the analysis of myopia: an analysis of variation in eye size of adult mice. Optom Vis Sci. 1999;76:408–418 [DOI] [PubMed] [Google Scholar]
- 25. Schmucker C, Schaeffel F. A paraxial schematic eye model for the growing C57BL/6 mouse. Vision Res. 2004;44:1857–1867 [DOI] [PubMed] [Google Scholar]
- 26. Stein DM, Wollstein G, Ishikawa H, Hertzmark E, Noecker RJ, Schuman JS. Effect of corneal drying on optical coherence tomography. Ophthalmology. 2006;113:985–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Velthoven ME, van der Linden MH, de Smet MD, Faber DJ, Verbraak FD. Influence of cataract on optical coherence tomography image quality and retinal thickness. Br J Ophthalmol. 2006;90:1259–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu Z, Huang J, Dustin L, Sadda SR. Signal strength is an important determinant of accuracy of nerve fiber layer thickness measurement by optical coherence tomography. J Glaucoma. 2009;18:213–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vizzeri G, Bowd C, Medeiros FA, Weinreb RN, Zangwill LM. Effect of signal strength and improper alignment on the variability of stratus optical coherence tomography retinal nerve fiber layer thickness measurements. Am J Ophthalmol. 2009;148:249–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bodeutsch N, Siebert H, Dermon C, Thanos S. Unilateral injury to the adult rat optic nerve causes multiple cellular responses in the contralateral site. J Neurobiol. 1999;38:116–128 [DOI] [PubMed] [Google Scholar]
- 31. Panagis L, Thanos S, Fischer D, Dermon CR. Unilateral optic nerve crush induces bilateral retinal glial cell proliferation. Eur J Neurosci. 2005;21:2305–2309 [DOI] [PubMed] [Google Scholar]
- 32. Macharadze T, Goldschmidt J, Marunde M, et al. Interretinal transduction of injury signals after unilateral optic nerve crush. Neuroreport. 2009;20:301–305 [DOI] [PubMed] [Google Scholar]