A new method for visualizing and quantifying the breakdown of outer blood–retina barrier in rodents was developed. With this method, a substantial leakage of macromolecules through the outer blood–retina barrier was observed in diabetic and ischemic animals.
Abstract
Purpose.
The outer blood–retina barrier (BRB) separates the neural retina from the choroidal vasculature, which is responsible for approximately 80% of blood supplies in the eye. To determine the significance of outer BRB breakdown in diabetic retinopathy, the outer BRB–specific leakage of macromolecules in diabetic and ischemic rodents was investigated.
Methods.
Diabetes and ischemia were induced in rodents by streptozotocin and oxygen-induced retinopathy, respectively. Diabetic and ischemic rodents were injected intravenously with fluorescein isothiocyanate (FITC)-dextran. The outer BRB–specific leakage in diabetic and ischemic rodents was visualized by fluorescent microscopy.
Results.
A microscopic imaging assay was developed to examine outer BRB breakdown. The outer BRB–specific leakage of fluorescent macromolecules was visualized in diabetic and ischemic rodents. Substantial leakages of macromolecules through the outer BRB in diabetic and ischemic rodents were detected with this assay. The number of severe outer BRB leakage sites is inversely proportional to the size of macromolecules. Significant depletion of occludin in the RPE of ischemic and diabetic rodents was also observed.
Conclusions.
For the first time, a microscopic imaging assay for directly visualizing macromolecules leaked through the outer BRB in rodents was developed. Using this assay, the authors demonstrated the significance of outer BRB breakdown in diabetes and ischemia, which will have implications to the understanding, diagnosis, and treatment of diabetic macular edema and other ocular diseases with outer BRB defects. The microscopic imaging assay established in this study will likely be very useful to the development of drugs for macular edema.
Aunique feature about the human retina is the presence of two blood–retina barriers (BRBs), the inner and outer BRBs that are formed by tight junctions between adjacent endothelial or retinal pigment epithelial (RPE) cells. While the inner BRB has been well studied due to its apparent relevance to diabetic retinopathy and retinopathy of prematurity, little is known about the biology and pathophysiology of the outer BRB. The outer BRB separates the neural retina from a network of fenestrated vessels called choriocapillaris, which is the major blood supplier for the neural retina. The outer BRB plays many essential roles in the maintenance of normal physiological processes in the retina, through the transport of nutrients, water, and ions, and the removal of metabolic wastes (for review, see Ref. 1). A major outer BRB function is to participate in phagocytosis, a process that requires the daily renewal and removal of approximately 10% of lipid-rich photoreceptor outer segment discs.2 Thus, the outer BRB plays a crucial role in the transport and recycle of fatty acids,3 such as docosahexaenoic acid, which is a major component of photoreceptors and is important to retinal function.4 Since the retina is the most active tissue metabolically, the transport of glucose and lactose as major energy sources through glucose and monocarboxylate transporters in the RPE is important to visual function.5,6 Likewise, the transport of retinoids from the blood circulation to the retina through the RPE is essential to a normal visual cycle.7 These active transports are fueled by the Na+-K+-ATPase, located in the apical side of the RPE.1 Finally, the outer BRB is also responsible for the removal of excessive water in the subretinal space to the choriocapillaris, which is driven by the transport of Cl− and K+.1,8
The diabetes-induced outer BRB dysfunction has been observed in humans and animals.9–12 Morphologic changes in the RPE can be readily detected in diabetic animals,13,14 which is reflected by an alteration in c-wave of electroretinography.15–17 Biochemical changes related to many pathways also occur in the human RPE in early diabetic patients.18 Despite these observations, the contribution of outer BRB dysfunction in diabetic retinopathy is almost neglected. A major hurdle for an insufficient progress in this area is the difficulty to measure the outer BRB–specific leakage experimentally, as existing methods cannot clearly distinguish the macromolecules leaked through the outer BRB and what appeared to be the overwhelming inner BRB–specific leakage under pathologic conditions. Consequently the extent and significance of outer BRB–specific leakage to the pathology of macular edema and other BRB diseases are unclear. Therefore, we recently developed a fluorescent microscopic imaging assay for visualizing and quantifying the outer BRB–specific leakage in diabetic and ischemic rodents after intravenous injection of fluorescent macromolecules. This report summarizes our study on the development of the methodology and visualization/detection of outer BRB breakdown in diabetic and ischemic rodents.
Material and Methods
Animal Treatment
Mice with C57B6 genetic background were used to examine the diabetes- and ischemia-induced outer BRB breakdown. Brown Norway rats were used to investigate the diabetes-induced outer BRB leakage. Animal studies were conducted according to the ARVO statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee at the University of Oklahoma Health Sciences Center. Ischemia was induced with the oxygen-induced retinopathy (OIR) by placing newborn mice in 75% oxygen from postnatal day (P)7 to P12, and room air afterward until P17, as described previously.19,20 Diabetes was induced by intraperitoneal injection of freshly made streptozotocin solution (55 mg/kg body weight in 10 mM citrate buffer [pH 4.5]) daily for 5 days, as described previously.21 Animals with blood glucose level above 300 mg/dL were considered diabetic.
Analysis of Outer BRB Leakage with FITC-Dextran
OIR and diabetic rodents were anesthetized and injected intravenously with FITC-dextran of various molecular weights (0.5 g/kg body weight; Sigma, St. Louis, MO) in phosphate buffered saline (PBS). The animals were killed 1 minute after injection unless otherwise indicated. The eyes were frozen and embedded in OCT media immediately. Serial sections (6 μm) of whole eyes were cut sagittally, through the cornea and parallel to the optic nerve. The sections were kept “dry” immediately after the cutting. Slides were then observed and imaged using a fluorescence microscope with a digital camera (Olympus IX71; Olympus, Center Valley, PA). Six sagitally cut serial cryosections (60 μm apart) from each eye were used for quantifying the frequency of severe leakage sites. Slides from four eyes were used to calculate the relative ratio of outer BRB leakage. Commercial Imaging software (Photoshop 7.0; Adobe, San Jose, CA), which has been widely used for various quantifications in our laboratory,21,22 was used to estimate the relative intensity of BRB–specific leakages. In this procedure, the digital color or single-channel pictures were converted to grayscale images. By using the free-hand lasso tool provided by the software, the area for outer BRB–specific leakage (AoBRB) was defined as the total pixels between the edge of the RPE and the other board of this leakage (the lower white line in Figs. 1D; see also Figs. 3A, 3C). Likewise, the area for inner BRB–specific leakage (AiBRB) was defined as the total pixels between the inner limiting membrane and the other board of the leakage (the upper white line and the edge of the leakage in Figs. 1D; see also Figs. 3A, 3C). The fluorescent intensity (I) of a BRB-specific leakage was calculated by deducting its average fluorescent intensity with that of background fluorescence (IB, average fluorescent intensity in non-leakage area). The amount of leakage (L) from a specific BRB was estimated by multiplying the fluorescent intensity (I) with its corresponding area (A). The relative ratio (R) of the outer or inner BRB–specific leakage was estimated by dividing its leakage (L) with that contributed by both BRBs. The following are the mathematic equations:
Figure 1.
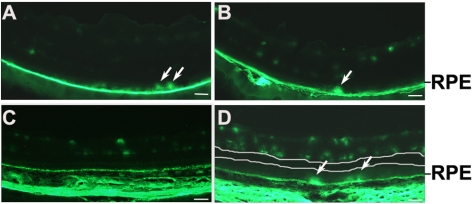
Detection of diabetes-induced outer BRB breakdown in rodents. (A, B) Fluorescent microscopic images of retinal sections from 12-month-old diabetic mice injected with 10 kDa (A) and 40 kDa (B) FITC-dextran. (C, D) Fluorescent microscopic images of retinal sections from 9-month-old non-diabetic controls (C) and diabetic rats (D) injected with 10 kDa FITC-dextran. All images were obtained from animals killed 1 minute after injecting FITC-dextran. Upper white line in (D): a board for inner BRB–specific leakage. Lower white line in (D): a board for outer BRB–specific leakage. Outer BRB leakage was visible in diabetic rodents. Arrows: severe leakage sites. Scale bar, 50 μm.
Figure 3.
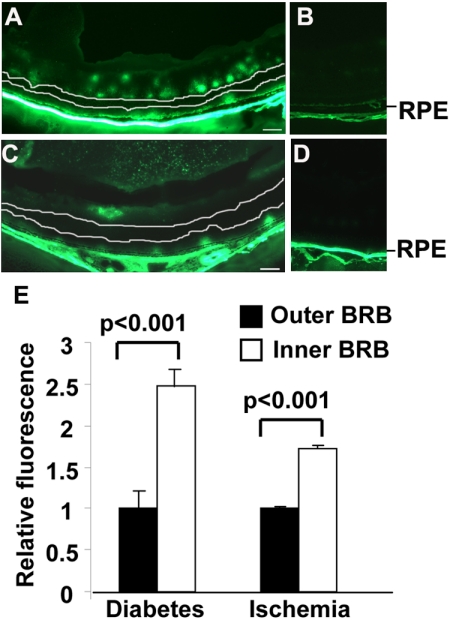
Significance of outer BRB–specific leakage in diabetic and ischemic mice. All images were obtained in animals killed 1 minute after injecting 10 kDa FITC-dextran. (A, B) Fluorescent images of retinal sections from 12-month-old diabetic mice and age-matched non-diabetic controls. (C, D) Fluorescent images of retinal sections from P17 OIR mice and age-matched room air controls. (E) Relative ratios of FITC-dextran leaked through each BRB. Scale bar, 50 μm. Inner BRB–specific leakage: fluorescence between the upper white line and the inner limiting membrane in (A, C). Outer BRB–specific leakage: fluorescence between the lower white line and the RPE in (A, C). Substantial outer BRB–specific leakages were detected in diabetic and ischemic mice.
Immunohistochemistry
The cornea and lens were removed from dissected eyes and eyecups were then transferred to ice-cold PBS. The RPE/choroid was dissected and fixed in 4% paraformaldehyde at 4°C for 1 hour. After washed with PBS at room temperature for 30 minutes, the RPE/choroid was blocked with 5% (wt/vol) bovine serum albumin (BSA) with 1% Triton in PBS at room temperature for 1 hour and was then incubated with rabbit polyclonal antibody against occludin (1:100 dilution, Invitrogen, Carlsbad, CA) at 4°C overnight. Secondary detections were performed by incubating Alexa Fluor 488 (or 586) goat anti-rabbit IgG (1:500 dilution; Invitrogen) at room temperature for 1 hour. Images were captured with fluorescent or confocal microscopy. For each group, at least six samples were examined.
Statistical Analysis
Quantitative results were expressed as the mean ± SEM. Student's t-test was used to determine statistical significance. P < 0.05 was considered significant.
Results
Diabetes-Induced Outer BRB Leakage
As 10 kDa FITC-dextran was not capable of passing through the outer BRB in normal rodents (Fig. 1C), 10 kDa and 40 kDa FITC-dextran was used to evaluate the diabetes-induced outer BRB breakdown with fluorescent microscopy. The FITC-dextran was intravenously injected to mice 12 months after the onset of diabetes. The bright FITC-dextran dots leaked through the outer BRB were clearly visible (arrows in Figs. 1A, 1B). Since rat is a more sensitive species to the diabetes-induced vascular leakage, we also examined whether the diabetes-induced outer BRB leakage occurred in STZ-injected rats. As predicted, the outer BRB–specific leakage was observed in rats 9 months after onset of diabetes (Fig. 1D). In addition, images with sufficient fluorescent intensity demonstrated the boundary (the lower white line in Fig. 1D) of outer BRB–specific leakage and the leaked FITC-dextran within this area was clearly visible. These results unequivocally demonstrated that a substantial outer BRB–specific leakage of macromolecules, at least to the size of 40 kDa proteins, occurred in diabetic rodents.
Ischemia-Induced Outer BRB Leakage
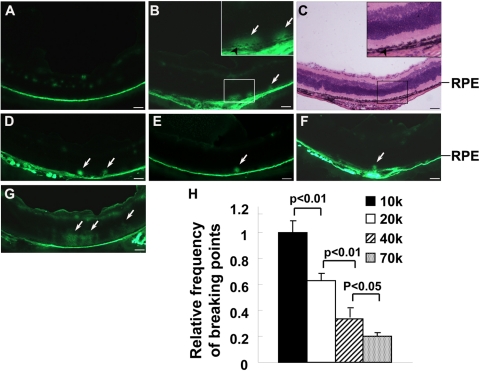
Since retinal ischemia is a major consequence of diabetes and ischemic animals can be generated in a shorter time, we performed more detailed experiments in OIR mice. The OIR mice were generated by placing newborn mice in 75% oxygen from P7 to P12 and room air afterward. The OIR-treated mice were anesthetized and injected intravenously with FITC-dextran at P17. The dot-like FITC-dextran leaked through the outer BRB was clearly visible (Fig. 2B). The leaked FITC-dextran diffused toward the vitreous if the mice were killed 20 minutes after the injection (Fig. 2G). By using six sagitally cut serial cryosections (60 μm apart), we quantified the number of severe leakage sites in OIR model. With increasing in molecular weight (10 k, 20 k, 40 k, or 70 kDa), the number of severe leakage sites decreased in the ischemic retina (Figs. 2B, 2D–F, 2H). These results suggest that the outer BRB–specific leakage of macromolecules occurred in a size-dependent manner under ischemic conditions.
Figure 2.
Detection of ischemia-induced outer BRB breakdown in mice. All images were obtained from animals killed 1 minute after injecting FITC-dextran, except that in (G). (A, B) Fluorescent microscopic images of retinal sections from P17 room air controls (A) and OIR mice (B) injected with 10 kDa FITC-dextran. (C) Light microscopic image of H&E-stained identical retinal section as shown in (B). (D–F) Fluorescent microscopic images of retinal sections from P17 OIR mice injected with 20 (D), 40 (E), or 70 kDa (F) FITC-dextran. (G) Fluorescent microscopic image of retinal sections from P17 OIR mice killed 20 minutes after injection of 10 kDa FITC-dextran. Arrows: severe leakage sites. Scale bar, 50 μm. (H) Statistical analysis for relative frequency of severe outer BRB leakage sites in P17 OIR mice injected with FITC-dextran of various molecular weights. Error bar: SEM. Frequencies of severe outer BRB leakage sites were inversely proportional to the size of FITC-dextran.
Significance of Outer BRB Breakdown under Diabetic and Ischemic Conditions
To evaluate the contribution of the outer BRB breakdown to overall vascular leakage under diabetic and ischemic conditions, we examined the relative fluorescent intensity in retinal sections from diabetic and ischemic animals 1 minute after intravenous injection of FITC-dextran. As the leaked FITC-dextran was not able to diffuse completely at this time, the boundary of each BRB-specific leakage was detectable (Figs. 3A, 3C). The total fluorescence intensity of the inner BRB–specific leakage (between the upper white line and the inner limiting membrane in Figs. 3A, 3C) and the outer BRB–specific leakage (between the lower white line and the RPE in Figs. 3A, 3C) in diabetic and ischemic mice were used to estimate the significance of leakage through each BRB. The relative ratio of leakage of the outer versus inner BRB was 1:2.48 in diabetic mice and 1:1.73 in ischemic mice, suggesting that a substantial outer BRB–specific leakage occurred in diabetic and ischemic mice.
Diabetes and Ischemia-Induced Breakdown of Tight Junctions in the RPE
To determine the mechanism of outer BRB breakdown in diabetic and ischemic rodents, we examined the integrity of tight junctions in the RPE of diabetic and ischemic mice with immunostaining. Substantial depletion of occludin, a tight-junction protein, was observed in the RPE flat-mounts of diabetic or ischemic mice (Fig. 4). These results suggest that the diabetes- and ischemia-induced outer BRB leakage is a consequence of the breakdown of tight junctions in the outer BRB.
Figure 4.
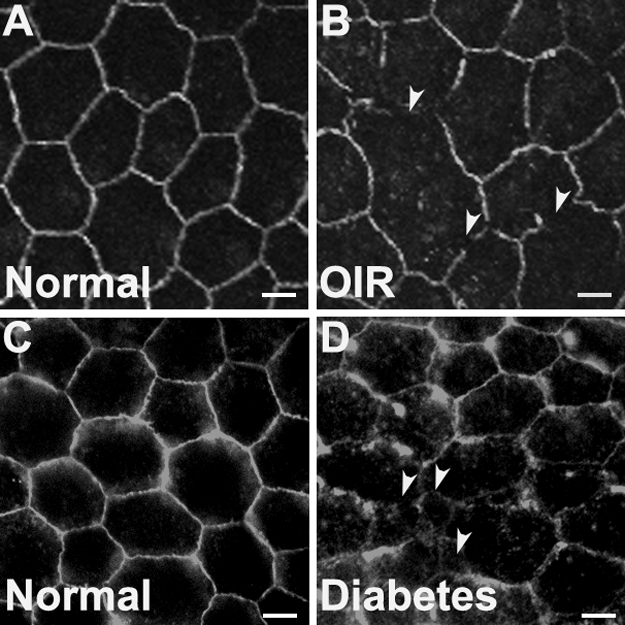
Integrity of tight junctions in the RPE of ischemic and diabetic mice. (A, B) Immunostaining for occludin in P17 normal and OIR mice. (C, D) Immunostaining for occludin in 1-year-old non-diabetic and diabetic mice. Scale bars, 10 μm. Arrowheads: depletion of occludin in the outer BRB. Ischemia and diabetes induced significant depletion of occludin in the RPE.
Discussion
The existence of two BRBs reflects the nature and complexity of the retina and is necessary to keep a homeostatic retinal microenvironment. The outer BRB separates the neural retina from choroidal vasculature that is responsible for approximately 80% of oxygen supplies in the eye. Although many independent studies have demonstrated the outer BRB breakdown in diabetes and ischemia,9–18 its significance in diabetic retinopathy has not been widely accepted. Such an unfortunate situation is largely due to a lack of appropriate methods that can be used to define the diabetes-induced outer BRB–specific leakage. With a goal to elucidate the cellular mechanisms of the diabetes-induced outer BRB breakdown, we developed an assay that allowed the visualization and quantification of RPE barrier leakage in diabetic and ischemic rodents. A fluorescent spot of FITC-dextran leaked through the outer BRB may correspond to a severely damaged leakage site. To our knowledge, this is the first direct visual evidence that demonstrated distinguishable macromolecules leaked through the outer BRB in diabetic and ischemic rodents. In diabetic and OIR mice, the retina is highly hypoxic, which causes the over-expression of hypoxia-inducible factor (HIF)-1α and vascular endothelial growth factor (VEGF).19,21 Up-regulation of VEGF signaling pathway ultimately leads to the loss of barrier function and tight-junction integrity in the outer BRB,23–25 which is reflected in the depletion of the occludin in the diabetic/ischemic RPE (Fig. 4). This is likely the cause of the outer BRB–specific leakage. As a byproduct of our study, we were able to estimate the relative amount of the outer BRB–specific leakage in diabetic and ischemic rodents, which represents the significance of the diabetes- and ischemia-induced outer BRB leakage. The diabetes-induced FITC-dextran leaked through the outer BRB, the fluorescence within the boundary of the outer BRB–specific leakage of macromolecules (Figs. 3A, 3C, 3E), was substantial compared to that of the inner BRB–specific leakage. This result suggests that a lower level of diabetes-induced outer BRB leakage occurs evenly across the whole retina. Our estimated ratio of the diabetes-induced outer BRB–specific leakage versus inner BRB–specific leakage with 10 kDa FITC-dextran was comparable to that determined with fluorescein in a confocal fluorescence microscopy assay.26 Taken together, the diabetes-induced outer BRB breakdown may play a more significant role in the overall disease pathology than what is appreciated at present time.
The outer BRB breakdown results in the leakage of blood-contents and influx of osmolytes that should lead to accumulating fluid in the subretinal space (edema) and exudative retinal detachment. Clinically, patients with diabetic macular edema, soft drusen (the cause of wet age-related macular degeneration), and chorioretinopathy (CSC) are treated with sub-threshold photocoagulation.27 The major effect of such a treatment is to induce RPE cell migration, which is responsible for the healing of RPE layer and ultimately results in the disappearance of macular edema, soft drusen, and the leakage (for CSC) in these patients. Our study provides a cellular mechanism for these diseases and non-retinal neovascular leakage in diabetic maculopathy in which the outer BRB breakdown is implicated.12
In this study, we observed that the FITC-dextran leaked through the outer BRB to outer retina in rodents (Fig. 2G). This result is in agreement with previous observations that the blood-contents leaked from the outer BRB are cleared through the vitreous.19,28–30 Therefore, the cumulative effect of outer BRB breakdown on the development of a disease may be larger than what is reflected in cryosections. However, such an effect can only be evaluated by measuring the dynamics of outer BRB–specific leakage in live animals, which is a limitation of our assay.
At present, fundus fluorescein angiography (FFA) is a major clinical diagnosis for the pathologic breakdown of the outer BRB. One such a pathologic condition is CSC. Conceptually, our observation demonstrating leaked dot-like fluorescent spots is similar to that identified by the FFA in mild CSC. However, the leakage from retinal vessels was apparently visible when the FITC-dextran was injected into diabetic animals (Fig. 1D). Since the inner and outer BRBs are interconnected to the fluidal retina and a major direction of the blood-contents leaked specifically through the outer BRB is cleared via the vitreous (Fig. 2G),28–30 the macromolecules leaked through the outer-BRB could be misidentified as the inner BRB leakage during their clearance. Unfortunately, this situation may have caused an inflated significance for the inner BRB leakage and discounted importance for the outer BRB leakage in diabetic retinopathy and other BRB diseases. Therefore, current FFA technology may not be suitable for detecting the outer BRB–specific leakage, as the fluorescent material leaked specifically through the retinal vessels may obscure the leakage from the outer BRB in rodents.
The key to the success of our methodology is to avoid any unnecessary disturbance of leaked fluorescent macromolecules within slides. As rat is a more widely used species for screening therapeutic agents for diabetes-induced vascular leakage, our imaging assay will not only be beneficial to mechanistic studies for outer BRB functions but also useful to the screen of therapeutic agents for various ocular diseases with defects in the outer BRB. The identification of clear boundaries for the inner or outer BRB–specific leakage may be the basis for the development of more accurate quantification in the future. Although we were able to use the relative intensity of FITC-dextran to estimate the significance of outer BRB–specific leakage, new technologies that distinguish barrier-specific leakage are required. For diabetic animals, our calculation did not exclude the bright dot-like fluorescent spots that were mainly comprised of intact vessels (Fig. 3), which inflated the significance of inner BRB–specific leakage. On the other hand, the leaked fluorescence detected outside the retina immediately after the injection of FITC-dextran might be contributed by the endothelial leakage, which may also discount the extent of inner BRB leakage in our study. In addition, a measurement of fluorescence intensity for a particular BRB in retinal sections was a reflection of the leakage at the time of animal death. The rate of leakage may depend on the flow/pressure of endothelial capillaries or fenestrated choriocapillaris. Finally, measurements of cumulative leakage (Fig. 2G) may not be accurate, as both the inner and outer BRB–specific leakage were cleared through the vitreous and the readout of inner BRB at a given time may be smaller than the actual value. For these reasons, we are not in a position to conclude that the estimated ratio of inner versus outer BRB–specific leakage in our study is completely quantitative. Nevertheless, our study may be a starting point for developing new imaging technologies for clinical and pre-clinical diagnosis and classification of ocular diseases with outer BRB defects.
Breakdown of outer BRB was observed with indirect measurement, previously.9,31 Due to a lack of direct assessment, outer BRB breakdown under pathologic conditions, such as that in diabetic retinopathy, has not been considered as a serious matter in the field. Our study clarifies a dilemma in the field by allowing the visualization of outer BRB–specific leakage in rodents under pathologic conditions, which is not a widely accepted concept at present. The assay established in our study can also be used to evaluate the severity of outer BRB breakdown and to yield qualitative information about the significance of outer BRB leakage under a pathologic condition in which both BRBs are compromised. With the development of tissue-specific gene expression tools for the RPE and animal models that could potentially be used to manipulate their RPE barrier,32–34 we are confident that the significance of the outer BRB breakdown in macular edema and other BRB diseases will be recognized appropriately by the field in the near future.
Acknowledgments
The authors thank Jian-xing Ma, Juanjuan Wang, and Bin Zhang for helpful suggestions; Meili Zhu for technical assistance; and Yuwei Le for English editing.
Footnotes
Supported by NIH Grants R01EY20900, P20RR17703, P20RR024215, and P30EY12190; American Diabetes Association Grant 1-10-BS-94; Beckman Initiative for Macular Research Grant 1003; Foundation Fighting Blindness Grant BR-CMM-0808-0453-UOK; Oklahoma Center for Advancement of Science and Technology Contract HR09-058; and Unrestricted Research Awards from Hope for Vision and Research to Prevent Blindness.
Disclosure: H.-Z. Xu, None; Y.-Z. Le, None
References
- 1. Simo R, Villarroel M, Corraliza L, Hernandez C, Garcia-Ramirez M. The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier–implications for the pathogenesis of diabetic retinopathy. J Biomed Biotechnol. 2010;190724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969;42:392–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gordon WC, Rodriguez de Turco EB, Bazan NG. Retinal pigment epithelial cells play a central role in the conservation of docosahexaenoic acid by photoreceptor cells after shedding and phagocytosis. Curr Eye Res. 1992;11:73–83 [DOI] [PubMed] [Google Scholar]
- 4. Benolken RM, Anderson RE, Wheeler TG. Membrane fatty acids associated with the electrical response in visual excitation. Science. 1973;182:1253–1254 [DOI] [PubMed] [Google Scholar]
- 5. Philp NJ, Ochrietor JD, Rudoy C, Muramatsu T, Linser PJ. Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/basigin-null mouse. Invest Ophthalmol Vis Sci. 2003;44:1305–1311 [DOI] [PubMed] [Google Scholar]
- 6. Kumagai AK, Glasgow BJ, Pardridge WM. GLUT1 glucose transporter expression in the diabetic and nondiabetic human eye. Invest Ophthalmol Vis Sci. 1994;35:2887–2894 [PubMed] [Google Scholar]
- 7. Saari JC. Biochemistry of visual pigment regeneration: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2000;41:337–348 [PubMed] [Google Scholar]
- 8. Hamann S. Molecular mechanisms of water transport in the eye. Int Rev Cytol. 2002;215:395–431 [DOI] [PubMed] [Google Scholar]
- 9. Vinores SA, Gadegbeku C, Campochiaro PA, Green WR. Immunohistochemical localization of blood-retinal barrier breakdown in human diabetics. Am J Pathol. 1989;134:231–235 [PMC free article] [PubMed] [Google Scholar]
- 10. Kirber WM, Nichols CW, Grimes PA, Winegrad AI, Laties AM. A permeability defect of the retinal pigment epithelium. Occurrence in early streptozocin diabetes. Arch Ophthalmol. 1980;98:725–728 [DOI] [PubMed] [Google Scholar]
- 11. Vinores SA, Derevjanik NL, Ozaki H, Okamoto N, Campochiaro PA. Cellular mechanisms of blood-retinal barrier dysfunction in macular edema. Doc Ophthalmol. 1999;97:217–228 [DOI] [PubMed] [Google Scholar]
- 12. Weinberger D, Fink-Cohen S, Gaton DD, Priel E, Yassur Y. Non-retinovascular leakage in diabetic maculopathy. Br J Ophthalmol. 1995;79:728–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aizu Y, Katayama H, Takahama S, Hu J, Nakagawa H, Oyanagi K. Topical instillation of ciliary neurotrophic factor inhibits retinal degeneration in streptozotocin-induced diabetic rats. Neuroreport. 2003;14:2067–2071 [DOI] [PubMed] [Google Scholar]
- 14. Aizu Y, Oyanagi K, Hu J, Nakagawa H. Degeneration of retinal neuronal processes and pigment epithelium in the early stage of the streptozotocin-diabetic rats. Neuropathology. 2002;22:161–170 [DOI] [PubMed] [Google Scholar]
- 15. Pautler EL, Ennis SR. The effect of induced diabetes on the electroretinogram components of the pigmented rat. Invest Ophthalmol Vis Sci. 1980;19:702–705 [PubMed] [Google Scholar]
- 16. MacGregor LC, Matschinsky FM. Experimental diabetes mellitus impairs the function of the retinal pigmented epithelium. Metabolism. 1986;35:28–34 [DOI] [PubMed] [Google Scholar]
- 17. Rimmer T, Linsenmeier RA. Resistance of diabetic rat electroretinogram to hypoxemia. Invest Ophthalmol Vis Sci. 1993;34:3246–3252 [PubMed] [Google Scholar]
- 18. Decanini A, Karunadharma PR, Nordgaard CL, Feng X, Olsen TW, Ferrington DA. Human retinal pigment epithelium proteome changes in early diabetes. Diabetologia. 2008;51:1051–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bai Y, Ma JX, Guo J, et al. Muller cell-derived VEGF is a significant contributor to retinal neovascularization. J Pathol. 2009;219:446–454 [DOI] [PubMed] [Google Scholar]
- 20. Smith LE, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111 [PubMed] [Google Scholar]
- 21. Wang J, Xu X, Elliott MH, Le Y. Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010;59:2297–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Le Y. Computer-assisted semi-quantitative analysis of choroidal density. Adv Exp Med Biol. 2010;664:211–216 [DOI] [PubMed] [Google Scholar]
- 23. Hartnett ME, Lappas A, Darland D, McColm JR, Lovejoy S, D'Amore PA. Retinal pigment epithelium and endothelial cell interaction causes retinal pigment epithelial barrier dysfunction via a soluble VEGF-dependent mechanism. Exp Eye Res. 2003;77:593–599 [DOI] [PubMed] [Google Scholar]
- 24. Ghassemifar R, Lai CM, Rakoczy PE. VEGF differentially regulates transcription and translation of ZO-1alpha+ and ZO-1alpha– and mediates trans-epithelial resistance in cultured endothelial and epithelial cells. Cell Tissue Res. 2006;323:117–125 [DOI] [PubMed] [Google Scholar]
- 25. Ablonczy Z, Crosson CE. VEGF modulation of retinal pigment epithelium resistance. Exp Eye Res. 2007;85:762–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Do carmo A, Ramos P, Reis A, Proença R, Cunha-vaz JG. Breakdown of the inner and outer blood retinal barrier in streptozotocin-induced diabetes. Exp Eye Res. 1998;67:569–575 [DOI] [PubMed] [Google Scholar]
- 27. Roider J, Brinkmann R, Wirbelauer C, Laqua H, Birngruber R. Subthreshold (retinal pigment epithelium) photocoagulation in macular diseases: a pilot study. Br J Ophthalmol. 2000;84:40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Googe JM, Hirose T, Apple DJ, Melgen S. Vitreous hemorrhage secondary to age-related macular degeneration. Survey Ophthalmol. 1987;32:123–130 [DOI] [PubMed] [Google Scholar]
- 29. el Baba F, Jarrett WH, 2nd, Harbin TS, Jr, et al. Massive hemorrhage complicating age-related macular degeneration. Clinicopathologic correlation and role of anticoagulants. Ophthalmology. 1986;93:1581–1592 [DOI] [PubMed] [Google Scholar]
- 30. Takeuchi A, Kricorian G, Marmor MF. Albumin movement out of the subretinal space after experimental retinal detachment. Invest Ophthalmol Vis Sci. 1995;36:1298–1305 [PubMed] [Google Scholar]
- 31. Tso MO, Cunha-Vaz JG, Shih CY, Jones CW. Clinicopathologic study of blood-retinal barrier in experimental diabetes mellitus. Arch Ophthalmol. 1980;98:2032–2040 [DOI] [PubMed] [Google Scholar]
- 32. Le YZ, Zheng W, Rao PC, et al. Inducible expression of cre recombinase in the retinal pigmented epithelium. Invest Ophthalmol Vis Sci. 2008;49:1248–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Le YZ, Bai Y, Zhu M, Zheng L. Temporal requirement of RPE-derived VEGF in the development of choroidal vasculature. J Neurochem. 2010;112:1584–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Le YZ. Conditional gene targeting: dissecting the cellular mechanisms of retinal degenerations. J Ophthalmol. 2011:806783. [DOI] [PMC free article] [PubMed] [Google Scholar]