Abstract
Hsp90 is and environmentally contingent molecular chaperone that influences the form and function of diverse signal transducers. Here we discuss our recent findings that Hsp90 regulates the morphogenetic transition from yeast to filamentous forms required for virulence of the most prevalent fungal pathogen of humans, Candida albicans, and does so via cAMP-PKA signaling. This transition is normally regulated by environmental cues that are contingent upon elevated temperature to relieve Hsp90-mediated repression of the morphogenetic program. Intriguingly, Hsp90 inhibition induces filamentation independent of the canonical PKA transcription factor Efg1, in striking similarity to a select set of morphogenetic stimuli. Further investigation will determine the downstream transcription factors through which Hsp90 regulates morphogenesis and the precise mechanism of Hsp90's interaction with the cAMP-PKA pathway. C. albicans is one of many fungal species that undergo a morphological transition in a temperature-dependent manner, thus Hsp90's capacity to govern this key developmental program may provide insight into morphogenesis of diverse organisms.
Key words: Candida albicans, fungal morphogenesis, temperature sensing, Hsp90, cAMP-PKA
Precise coordination of sensing and response to environmental cues is imperative for the survival of all organisms. In the leading fungal pathogen of humans, Candida albicans, the capacity to sense environmental signals and undergo morphological transitions is tightly linked to its virulence. In C. albicans, the morphological transition between yeast and filamentous growth states is regulated by diverse environmental cues, including nutrient limitation, pH, CO2, serum and temperature (Fig. 1A).1–3 Elevated temperature of 37°C is critical for C. albicans to undergo morphogenesis under most conditions (Fig. 1A), yet until recently, little was understood about the cellular signaling underpinning this temperature dependence. Numerous signal transduction pathways have been implicated in C. albicans morphogenesis, including the mitogen-activated protein kinase (MAPK) pathway and the cAMP-protein kinase A (PKA) pathway.1–3 Recently, we demonstrated that the molecular chaperone Hsp90 orchestrates temperature-dependent morphogenesis in C. albicans in a manner that is contingent on cAMP-PKA signaling.4
Figure 1.
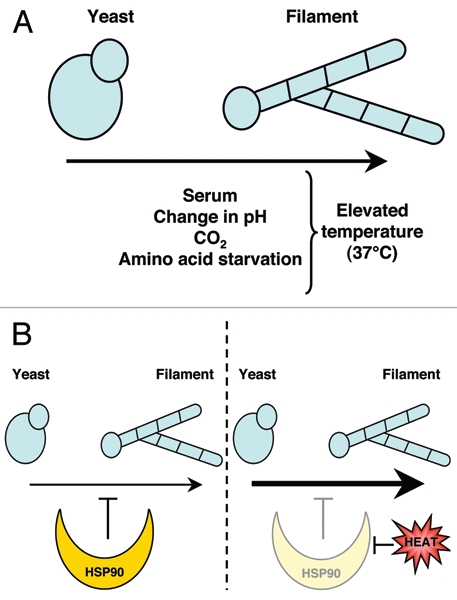
Temperature-dependent morphogenesis in C. albicans. (A) in C. albicans, the morphological transition between yeast and filamentous growth (hyphal and pseudohyphal) is regulated by many different environmental cues, including exposure to serum, change in pH, CO2, and amino acid starvation. Elevated temperature of 37°c is required for C. albicans to undergo morphogenesis under these conditions. (B) Hsp90 exerts a repressive effect on the yeast to filament morphological transition. When Hsp90 function is compromised, for instance by elevated temperature, the yeast to filament transition is induced.
Hsp90 is an essential molecular chaperone that responds to environmental cues and regulates the form and function of its client proteins. Many Hsp90 client proteins are regulators of cellular signaling, such as kinases and transcription factors, which dwell in incompletely folded or aggregation-prone states.5–7 As a heat shock protein, Hsp90 is induced under conditions of stress, such as increased temperature; however, global problems in protein folding that occur at elevated temperature can overwhelm Hsp90 chaperone function. Therefore, as a thermally responsive chaperone that regulates key signal transducers, Hsp90 is uniquely poised to govern temperature-dependent traits, such morphogenesis in C. albicans.
We discovered that compromising Hsp90 function induces a transition from yeast to filamentous growth in C. albicans, even in the absence of external cues.4 Our results support the model that Hsp90 is a key temperature sensor that governs C. albicans morphogenesis such that elevated temperature is required to relieve Hsp90-mediated repression of the morphogenetic program (Fig. 1B). When Hsp90 function is compromised, by elevated temperature or by specific genetic or pharmacological perturbation, the yeast to filament transition is induced via the cAMP-PKA pathway.4 Consistent with the association between morphogenetic plasticity and virulence, depletion of C. albicans Hsp90 attenuates virulence of the fungus in a murine model of systemic disease.4
Strikingly, although morphogenesis induced by Hsp90 inhibition depends on upstream inputs from the cAMP-PKA pathway, such as the GTPase Ras1, the adenylyl cyclase Cdc35 and the PKA complex itself, this morphogenetic program occurs independent of Efg1,4 the canonical transcription factor of the PKA pathway. Efg1 is a member of the APSES family of transcriptional regulators, which is unique to the fungal kingdom. This family includes C. albicans proteins Efg1 and Efh1, which have roles in filamentation,8 as well as the Saccharomyces cerevisiae regulators Phd1 and Sok2, which are known to regulate the pseudohyphal transition.9,10 In C. albicans, Efg1 is often considered the key transcriptional regulator of morphogenesis and is required for the yeast to filament transition induced by numerous cues including serum, pH and glucose starvation.11–14 That Hsp90-mediated morphogenesis occurs independent of Efg1, suggests either that parallel signaling pathways or alternate transcription factor(s) downstream of PKA signaling function to regulate this yeast to filament transition. Other factors thought to act downstream of PKA signaling in C. albicans include Flo8, Sfl1 and Tec1.15–17
Despite the central role of Efg1 in C. albicans morphogenesis, certain stimuli will induce filamentation in an Efg1-independent manner. For instance, filamentation stimulated by solid medium containing serum or by macrophage ingestion occurs independent of Efg1.13 In this instance, the MAPK transcription factor Cph1 has been implicated as the other factor involved in filamentation, as deletion of both Efg1 and Cph1 blocks filamentation under these conditions. Further, although Efg1 has been linked to the expression of filament-specific transcripts in vitro, many of these transcripts are expressed independent of Efg1 in an in vivo intestinal tract model of C. albicans colonization.18
Notably, certain morphogenetic stimuli demonstrate a strikingly similar response pattern to inhibition of Hsp90 in that they are dependent on upstream inputs of the cAMP-PKA pathway, but are not dependent on Efg1. For instance, depletion of the cell cycle regulatory polo-like kinase Cdc5 induces filamentation that is strictly dependent on Cdc35, but not on Efg1.19 The same holds true for filamentation induced in the presence of the DNA synthesis inhibitor hydroxyurea.19 Additionally, depletion of the DNA-damage checkpoint regulator Rad52 triggers filamentation in a Cdc35-dependent and Efg1-independent manner.20 In these cases, the identities of the additional pathways or transcription factors involved in regulating filamentation remain enigmatic.19
Regardless of the downstream transcriptional regulator involved, current data suggest that Hsp90 represses cAMP-PKA signaling.4 Conceivably, there are three broad models that could explain this regulation (Fig. 2). First, Hsp90 could interact with a positive regulator of the pathway, and maintain it in an inactive conformation until Hsp90 function is compromised. Precedent for Hsp90 maintaining proteins in an inactive, but active-competent state has been well established for specific client proteins, including the heat shock factor Hsf1.21,22 In relation to the PKA pathway, this could involve Hsp90 interacting with the positive catalytic subunits of PKA, Tpk1 or Tpk2 (Fig. 2A). Second, Hsp90 might stabilize a negative regulator of the cAMP-PKA pathway, such that inhibition of Hsp90 would lead to loss of function of the negative regulator. Precedent for this regulation comes from client proteins such as the protein phosphatase calcineurin and the tyrosine kinase v-src, which both exhibit dependence on Hsp90 for function.23,24 In the PKA pathway, this could involve Hsp90 interacting with the negative regulatory subunit of PKA, Bcy1 (Fig. 2B). Third, Hsp90 might interact indirectly with the cAMP-PKA pathway (Fig. 2C) via another regulator such as Gcn4, which is known to regulate morphogenesis and metabolic response in C. albicans by stimulating the cAMP-PKA pathway.25 Of course, more complex models are possible where Hsp90 influences cAMP-PKA signaling and morphogenesis via multiple client proteins.
Figure 2.
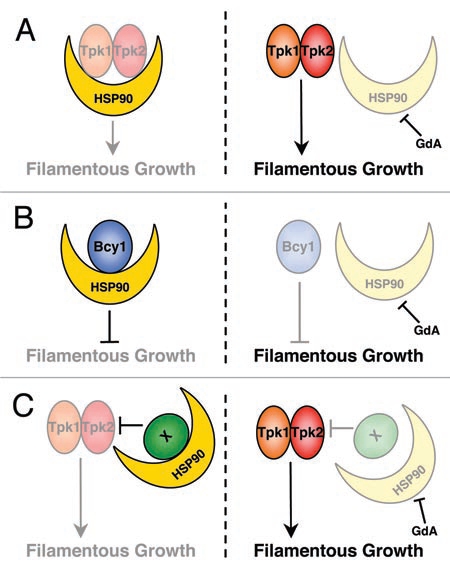
Models for Hsp90's interaction with the PKA pathway. (A) Hsp90 could interact with a positive regulator of the cAMP-PKA pathway and maintain it in an inactive, but active competent state. This could involve Hsp90 interacting the positive catalytic subunits of PKA, Tpk1 or Tpk2. (B) Hsp90 could stabilize a negative regulator of the cAMP-PKA pathway, such that inhibiting Hsp90 would destabilize the negative regulator. This could involve Hsp90 interacting the negative regulatory subunit of PKA, Bcy1. (C) Hsp90 might interact indirectly with the cAMP-PKA pathway. This could occur via a positive regulator such as Tpk1 or Tpk2, or by a negative regulator. More complex models are possible, where Hsp90 could regulate cAMP-PKA signaling via multiple client proteins.
Although the precise model of Hsp90 regulation of C. albicans morphogenesis remains elusive, our results suggest that Hsp90 likely influences activation of the cAMP-PKA cascade at the level of the PKA complex itself.4 We found that while the quorum-sending molecules farnesol and dodecanol block the morphogenetic response to serum,26 they do not block the response to Hsp90 inhibition.4 Serum has been shown to directly stimulate Cdc35,27 suggesting that Hsp90 is likely to regulate morphogenesis via an effector downstream of Cdc35. Notably, deletion of upstream regulators of PKA block the morphogenetic response to Hsp90 inhibition, suggesting that PKA activation remains dependent on upstream input. This is consistent with ras1 null mutants being defective in response to serum-induced filamentation, despite the fact that serum stimulates a factor downstream of Ras1.27 Further, our findings that deleting Ira2, Pde1 or Pde2 does not phenocopy Hsp90 inhibition suggest that Hsp90 does not stabilize these negative regulators upstream of PKA.4 As the efg1 mutant still filaments in response to Hsp90 inhibition, we propose a model in which Hsp90 inhibition leads to activation of the PKA complex itself and induction of filamentous growth.
Our study provides the foundation from which to dissect the cellular circuitry through which C. albicans employs an environmentally responsive chaperone to couple temperature change with a key morphogenetic program. C. albicans represents one of many fungal species that undergo a morphological transition in a temperature-dependent manner. Intriguingly, the systemic dimorphic fungi, including Histoplasma capsulatum and Paracoccidioides brasiliensis, exhibit the reverse transition. These fungi grow as yeast at elevated temperature within their human hosts, but in filamentous forms at ambient temperature in the environment.28 This raises the tantalizing possibility that Hsp90 may regulate temperature-dependent morphogenesis in other pathogenic fungi. Both H. capsulatum and P. brasiliensis have known Hsp90 homologs29 and it has been demonstrated that P. brasiliensis differentially expresses many heat shock proteins, including Hsp90, during its morphological transition.30 Dissecting the mechanisms by which Hsp90 regulates temperature-dependent developmental transitions in diverse species is poised to reveal how environmental response programs have been rewired throughout evolution.
Acknowledgements
R.S.S. is supported by an NSERC Postgraduate Scholarship and L.E.C. by a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund, by a Canada Research Chair in Microbial Genomics and Infectious Disease, and by Canadian Institutes of Health Research Grant MOP-86452.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/10320
References
- 1.Brown AJP, Argimon S, Gow NAR. Signal Transduction and Morphogenesis in Candida albicans. In: Howard RJ, Gow NAR, editors. Biology of the Fungal Cell. Second Edition. Berlin: Springer-Verlag; 2007. pp. 167–194. [Google Scholar]
- 2.Sudbery P, Gow N, Berman J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004;12:317–324. doi: 10.1016/j.tim.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Whiteway M, Oberholzer U. Candida morphogenesis and host-pathogen interactions. Curr Opin Microbiol. 2004;7:350–357. doi: 10.1016/j.mib.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro RS, Uppuluri P, Zaas AK, Collins C, Senn H, Perfect JR, et al. Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signaling. Curr Biol. 2009;19:621–629. doi: 10.1016/j.cub.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- 6.Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 7.Wandinger SK, Richter K, Buchner J. The Hsp90 chaperone machinery. J Biol Chem. 2008;283:18473–18477. doi: 10.1074/jbc.R800007200. [DOI] [PubMed] [Google Scholar]
- 8.Doedt T, Krishnamurthy S, Bockmuhl DP, Tebarth B, Stempel C, Russell CL, et al. APSES proteins regulate morphogenesis and metabolism in Candida albicans. Mol Biol Cell. 2004;15:3167–3180. doi: 10.1091/10.1091/mbc.E03-11-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gimeno CJ, Fink GR. Induction of pseudohyphal growth by overexpression of PHD1, a Saccharomyces cerevisiae gene related to transcriptional regulators of fungal development. Mol Cell Biol. 1994;14:2100–2112. doi: 10.1128/mcb.14.3.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward MP, Gimeno CJ, Fink GR, Garrett S. SOK2 may regulate cyclic AMP-dependent protein kinase-stimulated growth and pseudohyphal development by repressing transcription. Mol Cell Biol. 1995;15:6854–6863. doi: 10.1128/mcb.15.12.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bockmuhl DP, Ernst JF. A potential phosphorylation site for an A-type kinase in the Efg1 regulator protein contributes to hyphal morphogenesis of Candida albicans. Genetics. 2001;157:1523–1530. doi: 10.1093/genetics/157.4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Barkani A, Kurzai O, Fonzi WA, Ramon A, Porta A, Frosch M, Muhlschlegel FA. Dominant active alleles of RIM101 (PRR2) bypass the pH restriction on filamentation of Candida albicans. Mol Cell Biol. 2000;20:4635–4647. doi: 10.1128/mcb.20.13.4635-4647.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 14.Stoldt VR, Sonneborn A, Leuker CE, Ernst JF. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 1997;16:1982–1991. doi: 10.1093/emboj/16.8.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao F, Lane S, Raniga PP, Lu Y, Zhou Z, Ramon K, et al. The Flo8 transcription factor is essential for hyphal development and virulence in Candida albicans. Mol Biol Cell. 2006;17:295–307. doi: 10.1091/mbc.E05-06-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Su C, Mao X, Cao F, Chen J. Roles of Candida albicans Sfl1 in hyphal development. Eukaryot Cell. 2007;6:2112–2121. doi: 10.1128/EC.00199-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schweizer A, Rupp S, Taylor BN, Rollinghoff M, Schroppel K. The TEA/ATTS transcription factor CaTec1p regulates hyphal development and virulence in Candida albicans. Mol Microbiol. 2000;38:435–445. doi: 10.1046/j.1365-2958.2000.02132.x. [DOI] [PubMed] [Google Scholar]
- 18.White SJ, Rosenbach A, Lephart P, Nguyen D, Benjamin A, Tzipori S, et al. Self-regulation of Candida albicans population size during GI colonization. PLoS Pathog. 2007;3:184. doi: 10.1371/journal.ppat.0030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bachewich C, Thomas DY, Whiteway M. Depletion of a polo-like kinase in Candida albicans activates cyclase-dependent hyphal-like growth. Mol Biol Cell. 2003;14:2163–2180. doi: 10.1091/mbc.02-05-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andaluz E, Ciudad T, Gomez-Raja J, Calderone R, Larriba G. Rad52 depletion in Candida albicans triggers both the DNA-damage checkpoint and filamentation accompanied by but independent of expression of hypha-specific genes. Mol Microbiol. 2006;59:1452–1472. doi: 10.1111/j.1365-2958.2005.05038.x. [DOI] [PubMed] [Google Scholar]
- 21.Guo Y, Guettouche T, Fenna M, Boellmann F, Pratt WB, Toft DO, et al. Evidence for a mechanism of repression of heat shock factor 1 transcriptional activity by a multichaperone complex. J Biol Chem. 2001;276:45791–45799. doi: 10.1074/jbc.M105931200. [DOI] [PubMed] [Google Scholar]
- 22.Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94:471–480. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
- 23.Singh SD, Robbins N, Zaas AK, Schell WA, Perfect JR, Cowen LE. Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathog. 2009;5:1000532. doi: 10.1371/journal.ppat.1000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y, Singer MA, Lindquist S. Maturation of the tyrosine kinase c-src as a kinase and as a substrate depends on the molecular chaperone Hsp90. Proc Natl Acad Sci USA. 1999;96:109–114. doi: 10.1073/pnas.96.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tripathi G, Wiltshire C, Macaskill S, Tournu H, Budge S, Brown AJ. Gcn4 co-ordinates morphogenetic and metabolic responses to amino acid starvation in Candida albicans. EMBO J. 2002;21:5448–5456. doi: 10.1093/emboj/cdf507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis-Hanna A, Piispanen AE, Stateva LI, Hogan DA. Farnesol and dodecanol effects on the Candida albicans Ras1-cAMP signalling pathway and the regulation of morphogenesis. Mol Microbiol. 2008;67:47–62. doi: 10.1111/j.1365-2958.2007.06013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu XL, Lee RT, Fang HM, Wang YM, Li R, Zou H, et al. Bacterial peptidoglycan triggers Candida albicans hyphal growth by directly activating the adenylyl cyclase Cyr1p. Cell Host Microbe. 2008;4:28–39. doi: 10.1016/j.chom.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Klein BS, Tebbets B. Dimorphism and virulence in fungi. Curr Opin Microbiol. 2007;10:314–319. doi: 10.1016/j.mib.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burnie JP, Carter TL, Hodgetts SJ, Matthews RC. Fungal heat-shock proteins in human disease. FEMS Microbiol Rev. 2006;30:53–88. doi: 10.1111/j.1574-6976.2005.00001.x. [DOI] [PubMed] [Google Scholar]
- 30.Goldman GH, dos Reis Marques E, Duarte Ribeiro DC, de Souza Bernardes LA, Quiapin AC, Vitorelli PM, et al. Expressed sequence tag analysis of the human pathogen Paracoccidioides brasiliensis yeast phase: identification of putative homologues of Candida albicans virulence and pathogenicity genes. Eukaryot Cell. 2003;2:34–48. doi: 10.1128/EC.2.1.34-48.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


