Animal studies with chromoblastomycosis (CBM) were initiated together with the first descriptions of the disease by Medlar in 1915.1 He inoculated Fonsecaea pedrosoi into guinea pigs, rats, and mice, producing a “non-progressive” granulomatous reaction, sometimes with abscesses, from which it was possible to re-isolate and culture the fungus.
Thereafter, a series of experiments were done with different animals, varying from guinea pigs and rabbits,2 to subcutaneous inoculation of rhesus monkeys, which did not result in lesions even after six months of observation.3
In 1945, Professor Azulay performed experiments in which F. pedrosoi was delivered via intratesticular and intraperitoneal inoculation, showing that it was possible to produce testicular but not peritoneal lesions, with a granulomatous reaction containing sclerotic cells.4 Professor Azulay also demonstrated that it was not necessary to sensitize animals with several inoculations to reproduce the disease, as thought by Gomes and Pessoa.5
From the 1950s until today, different animal models for CBM have been proposed, but unfortunately, no simple suitable model was available to reliably reproduce CBM in animals, mimicking that in humans. In 1966, the first case of CBM was described in a horse,6 and from that time forward, CBM was found in different animals ranging from frogs to dogs, indicating that it infects both humans and animals.
In 1966, the first investigations of CBM immunology were performed with precipitating antibodies,7 followed by immunodiffusion and immunoelectrophoresis. The results revealed that some strains isolated from nature were identical and shared common antigens with the recognized human pathogens F. pedrosoi, Phialophora verrucosa and Cladophialophora carrionii.8
Subsequently, Kurita combined animal experiments with cellular immunology to show that intravenous injection of yeast-like cells of F. pedrosoi results in inflammatory lesions in different organs,9 and Ibrahim-Granet et al. showed that rabbit IgG produces a 50–60% inhibition of fungal growth.10 Tsuneto et al. later carried out human studies, indicating that HLA-A29 can cause susceptibility to CBM.11 Animal models, immunology, and HLA studies point to individual susceptibilities in determining infection, a fact that can be corroborated by the finding of high levels of antibodies against C. carrionii in a Falcón State (Venezuela) population with a few cases of CBM.12
In 1989, Ahrens et al. demonstrated that different mice, some immunodeficient, present CBM lesions for about two weeks, and then these lesions evolve to spontaneous cure. Nude mice, however, develop CBM lesions that can become systemic or can resolve two months after the adoptive transfer of lymphocytes,13 indicating the importance of these cells in controlling CBM infection.
In the last two decades, molecular biology techniques provided novel information about the cellular and molecular biology of the fungus. New immunology tools, such as immunohistochemistry, expanded the data available regarding fungus-cell interactions in vivo, and new in vitro immunology experiments were performed with cultured cells, either from established cultures or from freshly isolated cells. The results of experiments performed using these techniques have improved our knowledge about CBM fungus interactions with immune cells.
In 1994, Esterre et al. described the production of TNFα and TGFβ by lesional macrophages,14 while Rozental et al. demonstrated that fungal adherence to activated macrophages triggers the respiratory burst, but does not kill the fungus,15 reinforcing the notion that lymphocytes are essential for fungal killing.
In 2003, D'Ávila et al. demonstrated that patients with verrucous lesions have a Th1 response, while patients with atrophic lesions have a Th2 response.16 In the following year, Alviano et al. showed the importance of melanin to the induction of the immune response in CBM.17 In 2005, Mazo et al. demonstrated the presence of Th2 cytokines in patients with a severe form of CBM, and Th1 cytokines in patients with a mild form of the disease.18 In 2006, the same group showed that the absence of CD4+ T cells induces a more severe form of the disease during experimental infection in mice.19
Although all of these data suggest a great importance of the host in the immune response and, consequently, lend insight to the clinical picture in humans, whether the response depends on the host, the species or the strain variations among CBM etiologic agents remains unknown. Recently, the Netherland CBS team headed by De Hoog demonstrated new cladistic species of the Fonsecaea genus that cause CBM,20 and it is now necessary to try to link these new species with the different clinical pictures found in CBM patients.
Furthermore, we do not know which form of the CBM fungus penetrates the skin to produce disease: hyphae, conidia, or sclerotic cells. In 2004, we demonstrated the presence of F. pedrosoi hyphae and conidia responsible for disease in one patient on the thorns of Mimosa pudica.21 We subsequently observed sclerotic cells, which are very similar to lesional cells, inside M. pudica thorns (Fig. 1). Thus, we remain uncertain which fungal form is responsible for initiating the disease and whether the host or the fungus are responsible for quite different clinical forms of the disease, such as cutaneous diffuse CBM22 or cutaneous localized annular CBM.23
Figure 1.
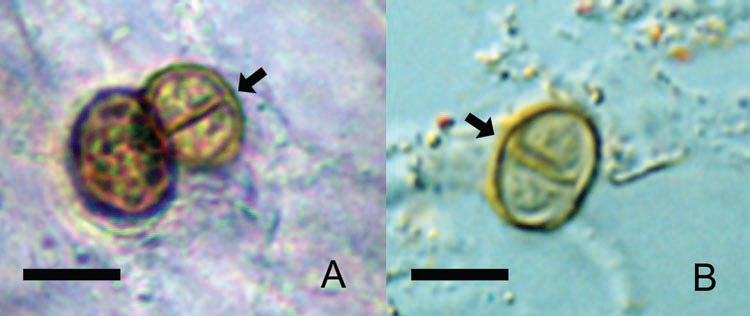
Sclerotic cells can be isolated from human lesions and from plants. Typical round, thick-walled, brown color, septated (arrows) sclerotic cells after direct mycological examination from human lesion (A) or from Mimosa pudica thorns (B). scale bars: 10 µm.
In order to work with sclerotic cells in vitro, we searched for simple media to induce differentiation from conidia into sclerotic cells, but there were no options other than Butterfield's, with which a period of 20 days was required to transform conidia into sclerotic cells in vitro. After a few unsuccessful attempts to produce sclerotic cells from conidia using Butterfield's media, we decided to produce a new media (which we now have in powder form) that permits the transformation of F. pedrosoi conidia into sclerotic cells in no more than two days.24 In fact, the media was also found to work for C. carrionii (personal communication, Dr. Hamid Badali, CBS). Using this media, we were able to demonstrate that F. pedrosoi conidia, but not sclerotic cells, inhibit CD40 and B7-2 expression in Langerhans cells from Balb/c mice, indicating that the form of the fungus is important for the type of response elicited during the first fungal-cell interactions.25
Regarding this issue of virulence, Machado et al. presented new data employing multiple-site inoculation of F. pedrosoi conidia in different mouse strains, as well as in some knockout mice.26 They showed an equal increase in footpad volume after inoculation when viable or non-viable fungal cells were inoculated some days earlier into the peritoneum, indicating that delayed-type hypersensitivity (DTH) is involved in lesion maintenance in mice.
Another important point is that oral immunization results in less footpad inflammation, with the same granulomatous reaction,26 which corroborates our previous results demonstrating that conidia, but not sclerotic cells, are capable of downregulating costimulatory molecules on Langerhans cells,25 likely resulting in less inflammation. Interestingly, CD4KO, CD8KO and IL-10KO all presented the same tissue pattern, with a granulomatous reaction and the presence of giant cells, indicating the high capacity of these black fungi to stimulate histiocytes.26 Along these lines, we have induced giant cells quite similar to those found in vivo (Fig. 2) via the interaction of sclerotic cells generated in vitro24 with mouse peritoneal macrophages.
Figure 2.
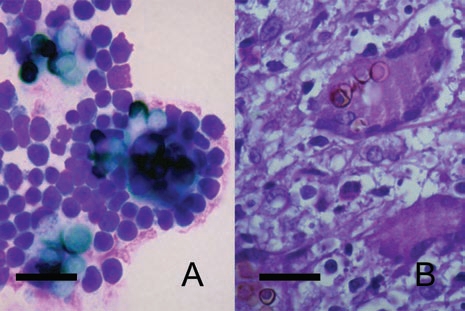
In vitro-generated sclerotic cells can stimulate the formation of Langhans giant cells in vitro. Langhans giant cells are observed 24 hours after interaction with balb/c mice peritoneal macrophages (A). Morphologically similar giant cells are present in the histopathology of lesional skin (B). Scale bars: 50 µm.
Furthermore, CD8KO mice displayed more pronounced lesions than CD4KO, with no signs of healing after almost six months of follow-up.26 These results are apparently inconsistent with recently published data demonstrating that the absence of CD4+ cells induces a more severe form of the disease, in comparison to CD8KO mice.19 However, if we consider DTH responsible for more pronounced CBM lesions in CD8KO animals, the results would be consistent with less DTH response found in CD4KO mice.19 In fact, both works show a higher foot pad thickness increase in CD8KO mice, when compared to CD4KO. Altogether, these data indicate that we are only beginning to understand CBM immunopathology, and more research is necessary to increase our knowledge, with the goal of resolving disease in difficult-to-treat CBM patients.
Acknowledgements
C.G.S. is an Associate Professor at the Instituto de Ciências Biológicas, Universidade Federal do Pará, and has research grants from Conselho Nacional de Pesquisa (CNPQ), and Secretaria de Ciência, Tecnologia e Insumos Estratégicos do Ministério da Saúde do Brasil and scholarships for students, received from Fundação de Amparo à Pesquisa do Estado do Pará (FAPESPA), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and CNPQ.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/10169
References
- 1.Medlar EM. A cutaneous infection caused by a new fungus, Phialophora verrucosa, with a study of the fungus. J Med Res. 1915;32:506. [PMC free article] [PubMed] [Google Scholar]
- 2.Pedroso A, Gomes JM. Sobre quatro casos de dermatite verrucosa produzidos por Phialophora verrucosa. An Paul Med Cir. 1920;11:53–61. [Google Scholar]
- 3.Simson WF, Harrington C, Barnetson J. Chromoblastomycosis: a report of six cases. J Path Bact. 1943;45:191–198. [Google Scholar]
- 4.Azulay RD. Experimental studyes on chromoblastomycosis. J Invest Dermatol. 1945;6:281–292. doi: 10.1038/jid.1945.26. [DOI] [PubMed] [Google Scholar]
- 5.Gomes JM, Pessoa S. Reprodução experimental de dermatite verrucosa. Brasil Medico. 1929;43:255–257. [Google Scholar]
- 6.Simpson JG. A case of chromoblastomycosis in a horse. Vet Med Small Anim Clin. 1966;61:1207–1209. [PubMed] [Google Scholar]
- 7.Buckley HR, Murray IG. Precipitating antibodies in chromomycosis. Sabouraudia. 1966;5:78–80. doi: 10.1080/00362176785190121. [DOI] [PubMed] [Google Scholar]
- 8.Velasquez LF, Restrepo A. Chromomycosis in the toad (Bufo marinus) and a comparison of the etiologic agent with fungi causing human chromomycosis. Sabouraudia. 1975;13:1–9. doi: 10.1080/00362177585190021. [DOI] [PubMed] [Google Scholar]
- 9.Kurita N. Cell-mediated immune responses in mice infected with Fonsecaea pedrosoi. Mycopathologia. 1979;68:9–15. doi: 10.1007/BF00490385. [DOI] [PubMed] [Google Scholar]
- 10.Ibrahim-Granet O, de Bievre C, Jendoubi M. Immunochemical characterization of antigens and growth inhibition of Fonsecaea pedrosoi by species-specific IgG. J Med Microbiol. 1988;26:217–222. doi: 10.1099/00222615-26-3-217. [DOI] [PubMed] [Google Scholar]
- 11.Tsuneto LT, Arce-Gomez B, Petzl-Erler ML, Queiroz-Telles F. HLA-A29 and genetic susceptibility to chromoblastomycosis. J Med Vet Mycol. 1989;27:181–185. doi: 10.1080/02681218980000241. [DOI] [PubMed] [Google Scholar]
- 12.Yegres F. Cromomicosis por Cladosporium carrionii en criadores de caprinos del estado Fálcon. Investigacíon Clínica. 1985;26:235–246. [Google Scholar]
- 13.Ahrens J, Graybill JR, Abishawl A, Tio FO, Rinaldi MG. Experimental murine chromomycosis mimicking chronic progressive human disease. Am J Trop Med Hyg. 1989;40:651–658. doi: 10.4269/ajtmh.1989.40.651. [DOI] [PubMed] [Google Scholar]
- 14.Esterre P, Lortat-Jacob H, Sainte-Marie D, Pradinaud R, Grimaud JA. The potential role of cytokines in the immunopathology of chromoblastomycosis. J Mycol Méd. 1994;4:145–148. [Google Scholar]
- 15.Rozental S, Alviano CS, de Souza W. The in vitro susceptibility of Fonsecaea pedrosoi to activated macrophages. Mycopathologia. 1994;126:85–91. doi: 10.1007/BF01146200. [DOI] [PubMed] [Google Scholar]
- 16.d'Avila SC, Pagliari C, Duarte MI. The cell-mediated immune reaction in the cutaneous lesion of chromoblastomycosis and their correlation with different clinical forms of the disease. Mycopathologia. 2003;156:51–60. doi: 10.1023/a:1022948329193. [DOI] [PubMed] [Google Scholar]
- 17.Alviano DS, Franzen AJ, Travassos LR, Holandino C, Rozental S, Ejzemberg R, et al. Melanin from Fonsecaea pedrosoi induces production of human antifungal antibodies and enhances the antimicrobial efficacy of phagocytes. Infect Immun. 2004;72:229–237. doi: 10.1128/IAI.72.1.229-237.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazo FGV, Da Gloria DS, Ferreira KS, Marques SG, Goncalves AG, Vagner de Castro Lima Santos et al. Cytokines and lymphocyte proliferation in patients with different clinical forms of chromoblastomycosis. Microbes Infect. 2005;7:708–713. doi: 10.1016/j.micinf.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Teixeira de Sousa MG, Ghosn EE, Almeida SR. Absence of CD4+ T cells impairs host defence of mice infected with Fonsecaea pedrosoi. Scand J Immunol. 2006;64:595–600. doi: 10.1111/j.1365-3083.2006.01846.x. [DOI] [PubMed] [Google Scholar]
- 20.Najafzadeh MJ, Gueidan C, Badali H, Van Den Ende AH, Xi L, De Hoog GS. Genetic diversity and species delimitation in the opportunistic genus Fonsecaea. Med Mycol. 2009;47:17–25. doi: 10.1080/13693780802527178. [DOI] [PubMed] [Google Scholar]
- 21.Salgado CG, da Silva JP, Diniz JA, da Silva MB, da Costa PF, Teixeira C, et al. Isolation of Fonsecaea pedrosoi from thorns of Mimosa pudica, a probable natural source of chromoblastomycosis. Rev Inst Med Trop Sao Paulo. 2004;46:33–36. doi: 10.1590/s0036-46652004000100006. [DOI] [PubMed] [Google Scholar]
- 22.Salgado CG, da Silva JP, da Silva MB, da Costa PF, Salgado UI. Cutaneous diffuse chromoblastomycosis. Lancet Infect Dis. 2005;5:528. doi: 10.1016/S1473-3099(05)70195-X. [DOI] [PubMed] [Google Scholar]
- 23.Salgado CG, da Silva MB, Yamano SS, Salgado UI, Diniz JA, da Silva JP. Cutaneous localized annular chromoblastomycosis. J Cutan Pathol. 2009;36:257–261. doi: 10.1111/j.1600-0560.2008.01025.x. [DOI] [PubMed] [Google Scholar]
- 24.da Silva MB, da Silva JP, Sirleide Pereira YS, Salgado UI, Diniz JA, Salgado CG. Development of natural culture media for rapid induction of Fonsecaea pedrosoi sclerotic cells in vitro. J Clin Microbiol. 2008;46:3839–3841. doi: 10.1128/JCM.00482-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.da Silva JP, da Silva MB, Salgado UI, Diniz JA, Rozental S, Salgado CG. Phagocytosis of Fonsecaea pedrosoi conidia, but not sclerotic cells caused by Langerhans cells, inhibits CD40 and B7-2 expression. FEMS Immunol Med Microbiol. 2007;50:104–111. doi: 10.1111/j.1574-695X.2007.00239.x. [DOI] [PubMed] [Google Scholar]
- 26.Machado AP, Silva MRR, Fischman O. Prolonged infection by Fonsecaea pedrosoi after antigenic costimulation at different sites in experimental murine chromoblastomycosis. Virulence. 2010;1:29–36. doi: 10.4161/viru.1.1.9920. [DOI] [PMC free article] [PubMed] [Google Scholar]


