Abstract
The intestinal epithelium undergoes a rapid turnover in addition to rapid exfoliation in response to bacterial infection, thus acting as an intrinsic defense against microbial intruders. It has long been questioned how mucosal pathogens can circumvent the intestinal defense systems. Our recent discovery of a bacterial ploy used by Shigella provided us with fresh insight. Shigella delivers OspE via the type III secretion system during multiplication within epithelial cells. This effector protein reinforces epithelial adherence to the basement membrane by interacting with integrin-linked kinase (ILK), a unique intracellular Ser/Thr kinase that links the cell-adhesion receptors, integrin, and growth factors to the actin cytoskeleton. The interaction between OspE and ILK increased formation of focal adhesions (FAs) and surface levels of β1-integrin, while suppressing phosphorylation of FAK and paxillin, thus suppressing rapid turnover of FAs, reducing cell motility and promoting cell adhesion to extracellular matrix. The impact of this OspE-ILK interplay was demonstrated both in vitro and in vivo by infecting polarized epithelial cell monolayers and guinea pig colons with Shigella possessing or lacking the ospE gene. The findings thus establish a new class of virulence-associated factors, and provide new insight into the functioning of the intestinal barrier and bacterial strategies for circumventing it.
Key words: effectors, epithelial cells, focal adhesion, integrin-linked kinase, shigella, type III secretion system
The intestinal epithelium is exposed to both dietary and environmental antigens, and many bacteria use the intestinal epithelium as an infectious foothold. Therefore, the epithelium has multiple innate defense mechanisms that protect the intestine from microbial intruders. The integrity of the epithelium and the constant renewal of epithelial cells are critical anti-bacterial defense mechanisms. Although the intestinal mucosa is a tightly sealed monolayer, it exists in a highly dynamic state. Epithelial cells are generated from stem cells at the bottom of crypts and then migrate upward to the tip of the villi, undergoing proliferation, differentiation, maturation and apoptosis, ultimately peeling off into the lumen.1,2 In addition to this basal level of renewal, the epithelium can also turnover in response to various stimuli, including immune disorders, gut microbiota, and pathogens.3–6 Cliffe et al. observed that the large intestines of mice infected with Trichuris muris, a cecal-dwelling parasitic nematode that actively penetrates the mice intestinal epithelium, underwent accelerated epithelial cell turnover to expel the nematode through an IL-13- and CXCL10-dependent process.4 In addition, Buchon et al. demonstrated that Drosophila gut epithelial cells turned over in response to epithelial cell damage induced by a Gram-negative bacterium. Furthermore, this epithelial turnover occurred through stimulation of the JAK-STAT and JNK pathways in intestinal stem cells.5,7 Mysorekar et al. showed that BMP 4 signaling upregulates epithelial renewal in the urinary tract in response to uropathogenic Escherichia coli (UPEC) infection.6 These studies clearly indicate that epithelial renewal helps maintain intestinal homeostasis and limit the colonization of pathogens.
Nevertheless, mucosal pathogenic bacteria such as Shigella and Helicobacter pylori possess countermeasures that circumvent this intestinal defense and exploit the intestinal epithelium as a replicative niche. We recently found that Shigella and H. pylori can modulate rapid epithelial turnover and prolong the life span of epithelial cells during infection.8,9 Shigella can dampen intestinal epithelial turnover by interfering with the cell cycle progression of progenitor cells. Shigella uses its type III secretion system (T3SS) to deliver the IpaB effector into cells, which contributes to colonization of the intestine.8 H. pylori dampens gut epithelial cell renewal by delivering the anti-apoptotic effector protein CagA into cells via its type IV secretion system (T4SS). CagA stimulates the production of prosurvival factors, including phospho-ERK and the antiapoptotic protein MCL-1. CagA was also shown to contribute to increased bacterial colonization of the superficial gut epithelium in Mongolian gerbils.9 These studies demonstrate that mucosal pathogens use special tactics to circumvent rapid epithelial cell turnover in order to promote colonization.
In addition to rapid turnover, the epithelium uses another intrinsic defense system, the exfoliation of infected epithelial cells, to quickly expel colonized bacterium. For example, UPEC infection of the urinary bladder causes rapid sloughing of epithelial cells.3,10 Similarly, gonococci infection of the fallopian tubes induces epithelial cell detachment.11 Intriguingly, bladder epithelial cells on the luminal surface undergo only a low level of basal turnover; however, exfoliated urotherial cells are occasionally detected in the urine of UPEC-infected mice and patients, suggesting that epithelial exfoliation occurs in response to bacterial infection.12 Mulvey et al. provided direct evidence that bladder cells undergo exfoliation and apoptosis-like cell death upon bacterial infection. UPEC strains possessing type I pili cause host DNA fragmentation and caspase activation in infected epithelial cells.10 Despite host cell exfoliation, Neisseria gonorrhoeae and UPEC are highly successful in colonizing epithelial cells, suggesting that these pathogens have mechanisms to circumvent these host defenses. Muenzner et al. recently reported that N. gonorrhoeae, N. meningitidis, Haemophilus influenzae and Moraxella catarrhalis, known as carcinoembryonic antigen-related cell adhesion molecule (CECAM)-binding bacteria, induce enhanced expression of CD105 in host epithelial cells.13 CD105 is a member of the TGF-β1 receptor family that has been linked to cell migration and the organization of cell adhesion sites. Therefore, upregulation of CD105 expression allows these pathogens to counteract the bacterial-induced detachment of epithelial cells.
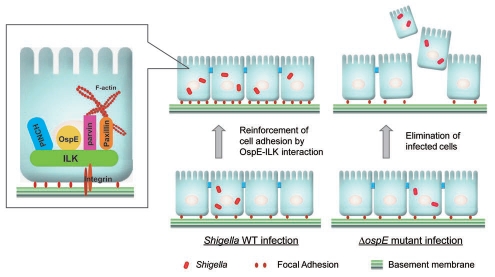
We recently found that the Shigella-encoded effector, OspE, prevents intestinal epithelial cell detachment, and demonstrated that OspE activity is pivotal for promoting bacterial colonization of the epithelium in both in vitro and in vivo infectious systems.14 Shigella invades the colonic epithelium through M-cells and subsequently infects resident macrophages, where it disseminates into the cytoplasm and replicates. This process leads to macrophage cell death. After being releasing from these killed macrophages, the bacteria invade the surrounding epithelial cells, migrate to the cytoplasm where they multiply and then disseminate into neighboring cells, eventually leading to epithelial cell death. Thereby, strong intestinal inflammation and the resulting destruction of epithelial integrity caused by repeated host cell death during the course of infection are the prominent pathogenic features of bacillary dysentery.15 The OspE effector delivered from intracellular Shigella via its TTSS targets a host protein, integrinlinked kinase (ILK), and reinforces host cell adherence to the basement membrane (Fig. 1).14 ILK was originally identified as a 59-kDa protein that binds to the cytoplasmic domain of β1-integrin, and has a C-terminus that has significant homology with Ser/Thr kinases.16 Later studies reported that ILK localizes at focal adhesions and modulates cell adhesion, proliferation, and focal adhesion formation by interacting with numerous proteins, including β1-, β3-integrin, ILK-associated phosphatase (ILKAP), MIg-2/Kindlin-2, α-parvin, β-parvin, PINCH-1, PINCH-2 and paxillin.17 ILK plays key roles in connecting integrins to actin stress fibers, modulating the assembly of focal adhesions (FAs), and transducing various cellular signals through integrins in response to extracellular matrix assembly.16
Figure 1.
A proposed model of Shigella tactics that reinforce the adherence of infected epithelial cells to the basement membrane through the OspE-ILK interaction.
We identified ILK as an OspE binding partner using a GST pull-down assay and MS spectrometric analysis. Furthermore, we confirmed that the interaction between OspE and ILK is specific by an immunoprecipitation assay with other major FA components, such as paxillin, vinculin, talin, FAK and β1-integrin, all of which were unable to bind to OspE. To establish that the interaction between OspE and ILK is functionally important for FA formation, several assays were performed. ILK-deficient mouse embryonic fibroblasts (MEFs) and MEFs re-expressing different ILK constructs, such as WT-ILK, K220M-ILK (kinase domain-inactive mutant) and S343D-ILK (constitutively active kinase domain), were co-transfected with GFP-OspE, and the effects of OspE and the different ILK constructs on FA formation and colocalization were tested. In the presence of a functional ILK (WT-ILK and S343D-ILK), OspE could colocalize at FAs, while in the absence of a functional ILK (K220M-ILK) OspE was dispersed within the cytoplasm, indicating that the subcellular localization of OspE at FAs requires a functional ILK. Previous studies indicated that ILK can be recruited to FAs through a mechanism that requires binding to additional adaptors such as paxillin, which binds both ILK, α-parvin and β-parvin.16 Furthermore, a membrane-targeted form of ILK strengthens FA formation.18 These reports prompted us to determine whether the OspE-ILK interaction could influence the subcellular localization of ILK in host cells, such as NIH3T3 and HeLa cells. We found that the level of membrane-retained ILK greatly increased in the presence of OspE, suggesting that OspE-ILK forms a complex that is associated with the cytoplasmic membrane and stabilizes FAs through an unknown mechanism.
In response to extracellular environmental changes, FAs in mammalian cells undergo assembly and disassembly through integrins that connect the extracellular matrix (ECM) to the cytoskeleton.19,20 Using the nocodazole-washout method, it was determined that the OspE-ILK interaction had no effect on FA assembly, but rather interfered with FA disassembly. Since FA disassembly is accompanied by FAK and paxillin phosphorylation, we investigated the effect of the OspE-ILK interaction on the extent of FAK(Y397) phosphorylation. The results indicated that although FAK(Y397) phosphorylation and paxillin phosphorylation levels were higher in the presence of a functional ILK, these phosphorylation levels were significantly reduced upon OspE expression. Furthermore, surface levels of β1-integrin were increased upon OspE expression. To directly demonstrate a functional consequence of the OspE-ILK interaction on cell surface β1-integrin levels, cells were seeded on fibronectin-coated coverslips and wound closure was monitored. The motility of cells expressing ILK with an intact kinase domain was significantly reduced in the presence of OspE compared with that in the absence of OspE. These data further supported the notion that the OspE-ILK complex interferes with FA disassembly, thus stabilizing FAs and reinforcing cell adhesion to the ECM.
Intriguingly, OspE-mediated bacterial manipulation of ILK during infection seems to be widespread among other enteric bacterial pathogens. OspE cognate effector proteins from other pathogens, including EPEC, EHEC, Citrobacter rodentium and Salmonella, are functionally interchangeable with Shigella OspE in their ability to reinforce FAs.14 To ensure the impact of OspE activity during Shigella infection of the intestinal epithelium, we generated a series of OspE mutants and identified OspE(W68A) as a single OspE point mutant that was unable to bind to ILK and localize to FAs. Importantly, this tryptophan 68 (Trp68) residue of OspE is a highly conserved amino acid among the OspE family proteins encoded by Shigella, EPEC, EHEC, C. rodentium, S. typhimurium and S. enteritidis. To confirm an in vivo role for OspE, guinea pigs were intrarectally inoculated with 1 × 109 cfu of Shigella (wild-type and mutant strains), which is a reliable animal model to evaluate Shigella pathogenesis. Inoculating the distal colon with a wild-type Shigella strain resulted in severe inflammation and intestinal hemorrhagic diarrhea, whereas these pathogenic features were not prominent after inoculation with the ospE deletion mutant. Importantly, at 24 h post inoculation, the colonization rate of the ospE deletion mutant was reduced compared with that of the wild-type strain. These results propose a model in which Shigella secures an infectious foothold by reinforcing the adherence of infected epithelial cells to the basement membrane through the OspE-ILK interaction (Fig. 1).
The bacterial stratagem to reinforce epithelial adherence appears to be universal among various enteric pathogens, despite the fact that their modes of infection differ; Shigella and Salmonella invade epithelial cells, while EPEC, EHEC and C. rodentium intimately attach to the epithelial cell surface. Nevertheless, as mentioned above, they share functionally and structurally similar OspE family proteins as T3SS-secreted effectors, strongly suggesting that OspE cognates are important players in bacterial infection. In this regard, it is worth discussing when epithelial cell exfoliation occurs during bacterial infection. Recent studies have indicated that despite the presence of extracellular-derived ligands or cytosolic-derived ligands (effectors, toxins, peptidoglycan or flagellin) that are delivered via various bacterial transport systems, such as T3SS, T4SS, T5SS and T6SS, or spontaneously released from bacteria, the ligands are recognized by various pattern-recognition receptors such as Toll-like receptors and NOD-like receptors, leading to the activation of various cell inflammatory and cell death signaling pathways such as the activation of inflammasomes.21,22 In addition, perturbation of the actin cytoskeleton by pharmacological agents or stimulation of actin polymerization by bacterial effectors can stimulate innate immune responses via caspase-1 activation.23,24 Indeed, host cells seem to have systems that sense actin cytoskeleton disruption and lead to inflammasome activation.25 Although the mechanisms underlying bacteria-induced inflammatory cell death are not fully understood, these studies suggest that host cell signaling pathways that are stimulated by bacterial effectors or ligands during infection cause epithelial cell detachment and cell death.22,26 We therefore assume that bacteria need OspE potentially together with other effector(s) to coordinately interfere with infected cell death and detachment. In the case of Shigella, the lack of OspE activity has serious implications not only for intracellular bacterial multiplication but also intercellular spreading. When OspE activity is blocked in Shigella, the bacterium is unable to efficiently disseminate into surrounding epithelial cells and fails to develop plaques in the epithelial monolayer. Because these plaques are hallmarks of successful bacterial intracellular multiplication and cell-to-cell spreading, this phenotype indicates that OspE acts as an essential protein that ensures an infectious foothold. Although bacterial strategies greatly vary, as mentioned above, similar mechanisms that prevent cell detachment were also indentified in N. gonorrhoeae, N. meningitidis, Haemophilus influenzae and Moraxella catarrhalis.13 Therefore, the bacterial strategy to reinforce host cell adherence to extracellular matrices may be a universal countermeasure against host cell detachment that occurs in response to bacterial infection of epithelial cells. Therefore, the findings in our recent studies add new information that expands the complexity of our knowledge on how bacteria employ strategies to preserve their replicative niches during infection.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/10486
References
- 1.Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–1925. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 3.Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc Natl Acad Sci USA. 2000;97:8829–8835. doi: 10.1073/pnas.97.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cliffe LJ, Humphreys NE, Lane TE, Potten CS, Booth C, Grencis RK. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science. 2005;308:1463–1465. doi: 10.1126/science.1108661. [DOI] [PubMed] [Google Scholar]
- 5.Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009;23:2333–2344. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mysorekar IU, Isaacson-Schmid M, Walker JN, Mills JC, Hultgren SJ. Bone morphogenetic protein 4 signaling regulates epithelial renewal in the urinary tract in response to uropathogenic infection. Cell Host Microbe. 2009;5:463–475. doi: 10.1016/j.chom.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Iwai H, Kim M, Yoshikawa Y, Ashida H, Ogawa M, Fujita Y, et al. A Bacterial Effector Targets Mad2L2, an APC Inhibitor, to Modulate Host Cell Cycling. Cell. 2007;130:611–623. doi: 10.1016/j.cell.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 9.Mimuro H, Suzuki T, Nagai S, Rieder G, Suzuki M, Nagai T, et al. Helicobacter pylori dampens gut epithelial self-renewal by inhibiting apoptosis, a bacterial strategy to enhance colonization of the stomach. Cell Host Microbe. 2007;2:250–263. doi: 10.1016/j.chom.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, Hultgren SJ. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 11.Apicella MA, Ketterer M, Lee FK, Zhou D, Rice PA, Blake MS. The pathogenesis of gonococcal urethritis in men: confocal and immunoelectron microscopic analysis of urethral exudates from men infected with Neisseria gonorrhoeae. J Infect Dis. 1996;173:636–646. doi: 10.1093/infdis/173.3.636. [DOI] [PubMed] [Google Scholar]
- 12.McTaggart LA, Rigby RC, Elliott TS. The pathogenesis of urinary tract infections associated with Escherichia coli, Staphylococcus saprophyticus and S. epidermidis. J Med Microbiol. 1990;32:135–141. doi: 10.1099/00222615-32-2-135. [DOI] [PubMed] [Google Scholar]
- 13.Muenzner P, Rohde M, Kneitz S, Hauck CR. CEACAM engagement by human pathogens enhances cell adhesion and counteracts bacteria-induced detachment of epithelial cells. J Cell Biol. 2005;170:825–836. doi: 10.1083/jcb.200412151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim M, Ogawa M, Fujita Y, Yoshikawa Y, Nagai T, Koyama T, et al. Bacteria hijack integrin-linked kinase to stabilize focal adhesions and block cell detachment. Nature. 2009;459:578–582. doi: 10.1038/nature07952. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa M, Handa Y, Ashida H, Suzuki M, Sasakawa C. The versatility of Shigella effectors. Nat Rev Microbiol. 2008;6:11–16. doi: 10.1038/nrmicro1814. [DOI] [PubMed] [Google Scholar]
- 16.Legate KR, Montanez E, Kudlacek O, Fassler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- 17.McDonald PC, Fielding AB, Dedhar S. Integrin-linked kinase—essential roles in physiology and cancer biology. J Cell Sci. 2008;121:3121–3132. doi: 10.1242/jcs.017996. [DOI] [PubMed] [Google Scholar]
- 18.Boulter E, Grall D, Cagnol S, Van Obberghen-Schilling E. Regulation of cell-matrix adhesion dynamics and Rac-1 by integrin linked kinase. FASEB J. 2006;20:1489–1491. doi: 10.1096/fj.05-4579fje. [DOI] [PubMed] [Google Scholar]
- 19.Webb DJ, Parsons JT, Horwitz AF. Adhesion assembly, disassembly and turnover in migrating cells—over and over and over again. Nat Cell Biol. 2002;4:E97–E100. doi: 10.1038/ncb0402-e97. [DOI] [PubMed] [Google Scholar]
- 20.Vicente-Manzanares M, Choi CK, Horwitz AR. Integrins in cell migration—the actin connection. J Cell Sci. 2009;122:199–206. doi: 10.1242/jcs.018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 22.Vance RE, Isberg RR, Portnoy DA. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe. 2009;6:10–21. doi: 10.1016/j.chom.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kustermans G, El Mjiyad N, Horion J, Jacobs N, Piette J, Legrand-Poels S. Actin cytoskeleton differentially modulates NFkappaB-mediated IL-8 expression in myelomonocytic cells. Biochem Pharmacol. 2008;76:1214–1228. doi: 10.1016/j.bcp.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Muller AJ, Hoffmann C, Galle M, Van Den Broeke A, Heikenwalder M, Falter L, et al. The S. typhimurium effector SopE induces caspase-1 activation in stromal cells to initiate gut inflammation. Cell Host Microbe. 2009;6:125–136. doi: 10.1016/j.chom.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Waite AL, Schaner P, Hu C, Richards N, Balci-Peynircioglu B, Hong A, et al. Pyrin and ASC colocalize to cellular sites that are rich in polymerizing actin. Exp Biol Med (Maywood) 2009;234:40–52. doi: 10.3181/0806-RM-184. [DOI] [PubMed] [Google Scholar]
- 26.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]