Abstract
As stored blood ages intraerythrocytic energy sources are depleted resulting in reduced structural integrity of the membrane. Thus, stored red cells become less deformable and more fragile as they age. This fragility leads to release of cell-free hemoglobin and formation of microparticles, sub-micron hemoglobin-containing vesicles. Upon transfusion, it is likely that additional hemolysis and microparticle formation occurs due to breakdown of fragile red blood cells. Release of cell-free hemoglobin and microparticles leads to increased consumption of nitric oxide (NO), an important signaling molecule that modulates blood flow, and may promote inflammation. Stored blood may also be deficient in recently discovered blood nitric oxide synthase activity. We hypothesize that these factors play a potential role in the blood storage lesion.
The Storage lesion
The storage lesion refers to changes in red cells during storage. Over time, glucose in stored blood is consumed, levels of 2,3-diphosphoglycerate (DPG) and ATP decrease, while potassium levels increase.1-9 As a result, there is red cell membrane loss during storage that leads to substantial changes in rheological properties.10-15 This loss of red cell integrity results in hemolysis and formation of microparticles4,16-19 that may contribute to complications associated with transfusion. Several studies have found that transfusions using older blood are associated with adverse clinical outcomes20-27. It should be noted, however, that others have not found these types of associations.9,28-34 Although the impact of transfusion of old blood is a matter of debate, the fact that transfusion represents one of the most common medical therapies suggests that further large-scale study of its impact is warranted, and that the mechanisms involved should be elucidated. In this mini-review, we suggest how disturbance of nitric oxide homeostasis and its consequences may underlie, to some extent, the storage lesion. An overview of the mechanisms we propose to be involved are shown in Figure 1.35
Figure 1.
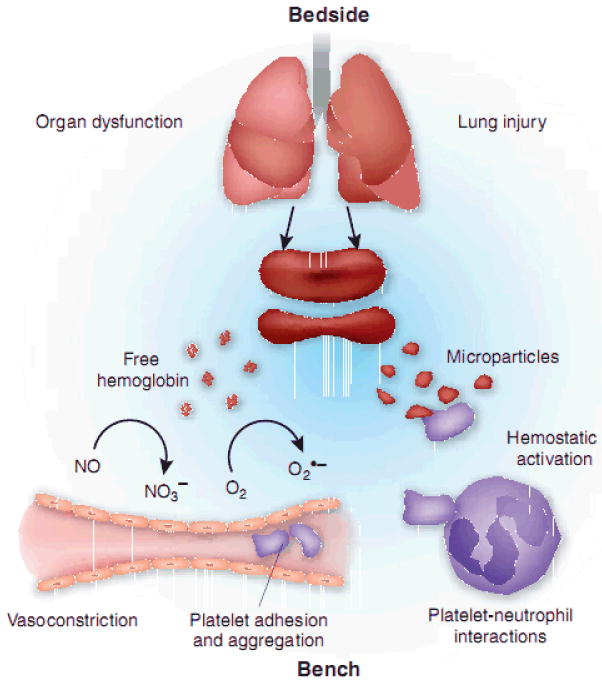
Proposed mechanisms contributing to the storage lesion. Reprinted by permission from Macmillan Publishers Ltd:Nature Medicine (16(4):381-2), copyright (2010). Red cell breakdown leads to release of cell-free hemoglobin and red cell microparticles. These scavenge NO which leads to vasoconstriction, platelet activation and adhesion, and inflammatory pathways.
Nitric Oxide
Nitric oxide (NO) is a neutral, radical molecule that has several important roles in physiological signaling. NO is the endothelial-derived relaxing factor (EDRF); it is made in endothelial cells and plays a major role in controlling blood flow by effecting smooth muscle relaxation adjacent to the blood vessels.36-39 It is made by endothelial nitric oxide synthase (eNOS) from arginine and diffuses to the smooth muscle where it activates soluble guanylyl cyclase to produce cGMP, initiating a signaling cascade leading to vasodilation. In addition, via this ENOS, the two other isoforms (inducible NOS and neuronal NOS), or other mechanisms of formation, it plays a role in homeostasis through inhibition of platelet aggregation, acts a toxic agent in host defense, decreases expression of adhesion molecules, and has anti-oxidant properties.40-42 More recently, a red blood cell NOS has been discovered.43 Importantly then, NO is seen to contribute to many functions that could be linked to the storage lesion including blood flow, inflammation, and thrombosis.
Nitric Oxide Scavenging by Hemoglobin
Nitric oxide reacts with hemoglobin in a reaction that is rate-limited by diffusion to the heme group within hemoglobin,44-46
| (1) |
This dioxygenation reaction occurs when oxygenated hemoglobin reacts with NO to form methemoglobin (FeIII) and nitrate, and effectively destroys NO activity. Nitric oxide can also bind to a ferrous, vacant heme but once it comes off it is likely to undergo dioxygenation, so this pathway would be a poor mechanism in itself to preserve NO bioactivity. In 1994, Lancaster pointed out that given the rate of the dioxygenation reaction and the large amount of hemoglobin in blood, NO could not possibly act as the EDRF; it would undergo too much dioxygenation.47 It has been subsequently determined, however, that the degree of NO dioxygenation that one would predict based on the amount of hemoglobin present is much less due to the fact that NO reacts with red cell encapsulated hemoglobin much more slowly than when the hemoglobin is free in solution or plasma.48-58 In vitro measurements where NO is mixed with red cells or hemoglobin show that red cells react up to 1000 times slower than free hemoglobin, 48,49,58 primarily due to the reaction becoming rate-limited by the time it takes for NO to diffuse to the red cell through “an unstirred layer.”58 In addition, a finite permeability of the red cell membrane to NO may play a role.53 In vivo, a major contribution of reduced NO scavenging by red cells is thought to be due to the cell-free zone, where blood flow leads to a pressure gradient that pushes red cells to the center of vessels and away from the endothelium where NO is made.50,52,59 Regardless of the relative contribution of these mechanisms, it is important to point out that they all breakdown upon hemolysis.60
Pathology associated with RBC breakdown
Given the many important functions of NO, it is not surprising that diminished NO bioavailability contributes to pathology in many diseases. Many of these, including atherosclerosis, obesity, diabetes, peripheral artery disease and coronary artery disease in general, result from endothelial dysfunction that is often due to reduced NO synthesis by NOS.61-65 Besides reduced production, NO bioavailability can also be reduced by increased consumption; one way for this reduction to occur results from scavenging by cell-free hemoglobin that is released upon hemolysis.66 As noted above, cell-free hemoglobin reacts with NO much faster than that encapsulated in a red cell. Nitric oxide scavenging has been a major contributor to pathological consequences of many blood substitutes that involve hemoglobin-based oxygen carriers.67-70 In these cases millimolar amounts of hemoglobin were infused. One might think that in other conditions, such as hemolytic anemias like sickle cell disease, the amount of cell-free hemoglobin present is too low to substantially affect NO bioavailabilty in the background of red cell encapsulated hemoglobin (normally about 10 mM in heme). However, in studies initially performed in patients with sickle cell disease, we found that the presence of even low micromolar amounts of cell-free hemoglobin in patients with sickle cell disease results in diminished blood flow response from NO.71 That small concentrations of cell-free hemoglobin can affect blood-flow and have pathological consequences was subsequently confirmed in a canine hemolysis model,72 and computational simulations suggest that as little as one micromolar cell-free hemoglobin (concentration in terms of heme, so in fact 0.25 micromolar hemoglobin tetramer) can substantially reduce NO bioavailabilty73. There is increasing evidence from transgenic animals, large animal and human epidemiological studies supporting a role for hemolysis in the pathobiology of sickle cell disease and other hemolytic anemias.72,74-80 Despite this evidence, the fact that micromolar amounts of cell-free hemoglobin can affect NO bioavailability to an extent to which pathological consequences occur is not universally accepted.81 Although we recognize that the extent of the impact of low amounts of hemolysis remains controversial, we feel the evidence strongly suggests the impact is substantial,80 and that continuing research on this front is necessary. Moreover, the observation that larger amounts of hemolysis (resulting in tens of micromolar hemoglobin) leads to pathological consequences is not contested.81
Red cell breakdown and the storage lesion
Loss of red cell integrity during storage results in hemolysis and formation of microparticles.4,16-19 There have been several studies that documented hemolysis as a function of time during storage. The levels of extracellular hemoglobin reported in the literature range from 28 μM (in heme) after 35 days of storage in citrate phosphate dextrose adenine (CPDA)4 to 80 μM after 50 days of storage17. We recently reported similar findings.82 Transfusion of just one unit of blood with this much hemolysis would result in plasma levels exceeding those of steady state sickle cell disease. As expected, we showed that NO consumption by the non-erythrocytic (plasma) fraction of older stored blood is dramatically greater than that from blood stored only one week and NO consumption is directly proportional to the extent of NO consumption.82
In addition to release of cell-free molecular hemoglobin, red cell breakdown leads to formation of hemoglobin-containing microparticles. In fact, when measuring cell-free hemoglobin in blood, no efforts are usually taken to separate microparticles from cell-free hemoglobin. In fact, in at least one case, the majority of hemoglobin in the supernatant after sedimentation of stored blood was found to be in the form of microparticles.18 Due to their small size, we propose that microparticles will scavenge NO to a similar extent as cell-free hemoglobin. The extent to which external diffusion of NO to hemoglobin reduces the rate of NO scavenging depends on the average distance between vesicle (red blood cells or microparticles). Taking a red cell with an equivalent spherical radius of 3 μm and a microparticle with a radius of 0.075 μm, the time for NO to diffuse to the red cell would be roughly 2000 times longer on the average than to the microparticle.* In addition, due to their small size, it is unlikely that shear stress will result in removing microparticles from the cell-free zone. Any reduction in NO permeability that may exist in red blood cells could be absent or diminished in microparticles as the physical membrane barrier to NO entry is thought to be due to the underlying spectrin and other cytoskeleton proteins83 and some of these are absent in microparticles 19. Thus, all three mechanisms that contribute to reduced NO scavenging by red blood cells compared to cell-free hemoglobin are likely to be absent or lessened for microparticles. Importantly, the effects of microparticles on NO bioavailability could be more extreme than those of cell-free hemoglobin as microparticles are not cleared by haptoglobin.
In addition to reductions in NO bioavailability that could result due to increased NO scavenging by cell-free hemoglobin and microparticles, NO bioavailability may be reduced due to decreased red cell or blood NOS. This reduction could be due to oxidative stress (oxidation of tetrahydrobiopterin and arginine deficiency) with red cell storage that is associated with functional uncoupling of the NOS protein. NO synthase uncoupling results in formation of superoxide from the NOS rather than NO. It is also possible that NO bioavailability is reduced due to infusion of stored, red cells from diminished NOS activity that is otherwise present in other blood cells. Future work is needed to explore this possibility.
Effects of Inflammation
Experimental evidence from murine studies suggests that transfusion of stored red cells can augment inflammation by various mechanisms.84,85 We have previously shown that transfusion of red cells increased both systemic and lung inflammatory responses in endotoxemic mice to promote chemokine-mediated neutrophil accumulation and lung injury that were storage- and red cell-dependent.85 Hod and colleagues have also shown that transfusion of stored red cells elicits systemic inflammatory cytokine responses related to the ingestion of membrane-encapsulated hemoglobin (Hb) by the mononuclear phagocyte system.84 While human red cell units (PRBC) show increased hemolysis86 and microparticle formation87 with storage duration, little is known regarding the effects of free Hb and membrane-encapsulated Hb in the form of microparticles in altering inflammation in humans following transfusion. Our recent findings show that red cell microparticles in banked blood units demonstrate inflammatory chemokine binding and release ligand upon interaction with platelets in vitro.87 Whether direct interactions between red cell microparticles and platelets actually occur to propagate or amplify inflammation in vivo is not currently known, although, insights from sickle cell disease (a disease characterized by hemolysis, reduced NO bioavailability, and inflammation) invoke a relationship between red cell microparticles and platelets.
Indeed, sickle cell disease shows increased circulating red cell microparticles expressing surface phosphatidylserine with thrombin generating potential88 that trigger activation of the alternative complement pathway89. Platelet activation, an increasingly recognized component and propagator of persistent inflammation as observed in rheumatoid arthritis90 or acute lung injury/acute respiratory distress syndrome91, is negatively regulated by endothelial NO, ADPase, and PGI2. Thus, in diseases of reduced NO bioavailability such as in hemolytic states, platelet activation is a characteristic feature as it has been observed for sickle cell disease92 and paroxysmal nocturnal hemoglobinuria93. We suggest that transfusion of stored red blood may elicit a state of reduced NO bioavailability through the release of cell free Hb and microparticles and contribute to persistent inflammation and injury in susceptible hosts. However, other components of red cell breakdown may be involved to induce or amplify inflammation. Heme and free iron, the breakdown products of hemoglobin, may be involved in not only inflammation but also elicit pro-oxidant, cytotoxic effects.84,86,94
The epidemiological studies demonstrating an association between red cell transfusion and potentially worse outcomes were conducted in patients with underlying traumatic injury, the critically ill95, or following cardiac surgery requiring support with cardiopulmonary bypass pump27. These findings invite the possibility that an underlying systemic inflammatory response may predispose individuals to transfusion-associated complications. The only completed prospective, randomized controlled trial examining red cell transfusion and outcomes in the critically ill was an efficacy trial of transfusion using a restrictive versus liberal strategy95. In this study, a subset of patients, those younger and less ill in the ICU with stable anemia, showed a possibly superior outcome with less blood transfusions95. However, the etiology of this association is still unclear and whether hemolysis-related breakdown of the red cell during storage perpetuates ongoing inflammation following transfusion remains to be determined. In a recent study, Larsen and colleagues showed the deleterious effects of hemolysis during severe sepsis syndrome and implicated a central role for free heme in promoting death in patients96. Low serum concentrations of hemopexin, the counter-regulatory molecule that binds to free heme, predicted multiple organ failure and death in septic shock patients96. Thus, hemolysis is an increasingly recognized feature of severe sepsis, either directly caused by blood-borne pathogens or through microangiopathic hemolytic anemia from disseminated intravascular coagulation that often accompanies severe sepsis. Red cell transfusion may exacerbate inflammation in susceptible hosts through hemolysis and contribute to microvascular perturbations by reducing NO bioavailabiity, promoting platelet activation, inducing pro-oxidant effects and cytotoxicity through release of red cell breakdown products.
Planned Approach
Figure 2 summarizes our general hypotheses and approach. In Aim 1 of our proposed work we plan to examine the effects of blood storage on red cell integrity and how this affects NO bioavailabilty. We will look at NO deformability and fragility as a function of time during storage while monitoring hemolysis and microparticle formation. We will test our hypothesis that microparticles scavenge NO similarly to cell-free hemoglobin using time-resolved absorption spectroscopies and computational modeling in a similar manner to the approaches we have used in the past.58,73,97 As red cell fragility increases during storage, we hypothesize that additional red cell breakdown occurs during and after transfusion. We will then test this hypothesis and the effects on NO bioavailabilty by examining blood flow and other physiological parameters upon infusion of older vs newer stored blood in humans and animals using methods similar to those previously reported.71,72
Figure 2.
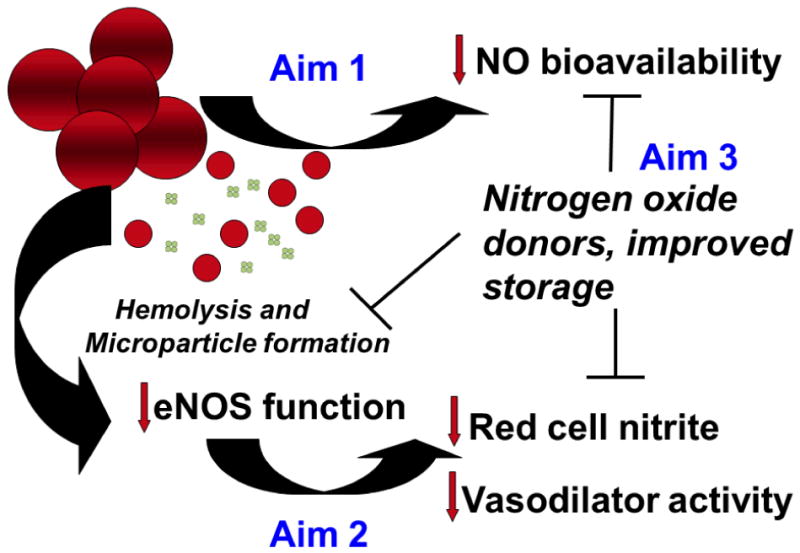
General hypothesis and approach. Loss of red cell integrity during storage or upon transfusion (to be studied in Aim 1) results in release of free hemoglobin and red cell microparticles which scavenge nitric oxide leading to deleterious effects including susceptibility to platelet activation, inflammation, and poor control of blood flow. This loss of NO bioavailability may also be exacerbated by loss of red cell NOS activity, to be studied as part of Aim 2. In order to counteract these effects, in Aim 3, we will explore ways to reduce red cell breakdown during storage, reduce NO scavenging when there is red cell breakdown, and compensate for loss of NO activity using various donor substances.
In Aim 2 of our proposed work, we plan to examine the role of the red cell NOS in the storage lesion. We will first examine the functionality using NOS knockout mice specifically in the endothelium or blood. The source of the blood NOS will be determined by immunodepleting platelets and removing leukocytes. After determining the contribution of blood NOS to NO homeostasis, we will examine the extent to which this activity is reduced in blood storage.
The last phase of our project focuses on therapeutics. We aim to explore mechanisms to both decrease NO scavenging and increase its production upon and post transfusion. To decrease NO scavenging we will explore preservation solutions with additives that decrease hemolysis while also examining additives that can neutralize the NO scavenging ability of cell-free Hb by preferential oxidation. Increased NO production may be accomplished by inclusion of additives that can be converted to nitric oxide in the blood such as nitrite98, and compounds that may increase blood NOS activity will also be explored. By increasing NO bioavailaibility, inflammatory consequences of transfusion of old, stored blood will also be reduced.
Acknowledgments
This work was supported by NIH grantHL098032.
Dr. Gladwin has received grant support in the form of a Collaborative Research and Development agreement between the US government and IKARIA Pharmaceuticals.
Footnotes
JL declares no conflict of interest.
The time for diffusion is taken as x2/D where x is the distance between the vesicles and D is the diffusion constant. The distance is given as, where r is the radius and Hct is the hematocrit. Here it is assumed that the concentration of hemoglobin inside red cells is the same as in microparticles. The calculation is performed for equivalent hematocrits.
References
- 1.Dern RJ, Brewer GJ, Wiorkowski JJ. Studies on the preservation of human blood. II. The relationship of erythrocyte adenosine triphosphate levels and other in vitro measures to red cell storageability. J Lab Clin Med. 1967;69:968–78. [PubMed] [Google Scholar]
- 2.Silliman CC, Clay KL, Thurman GW, et al. Partial Characterization of Lipids That Develop During the Routine Storage of Blood and Prime the Neutrophil Nadph Oxidase. Journal of Laboratory and Clinical Medicine. 1994;124(5):684–94. [PMC free article] [PubMed] [Google Scholar]
- 3.Beutler E, Kuhl W. Volume Control of Erythrocytes During Storage - the Role of Mannitol. Transfusion. 1988;28(4):353–7. doi: 10.1046/j.1537-2995.1988.28488265266.x. [DOI] [PubMed] [Google Scholar]
- 4.Latham JT, Bove JR, Weirich FL. Chemical and Hematologic Changes in Stored Cpda-1 Blood. Transfusion. 1982;22(2):158–9. doi: 10.1046/j.1537-2995.1982.22282177126.x. [DOI] [PubMed] [Google Scholar]
- 5.Greenwalt TJ, Sostok CZ, Dumaswala UJ. Studies in Red-Blood-Cell Preservation .2. Comparison of Vesicle Formation, Morphology, and Membrane-Lipids During Storage in as-1 and Cpda-1. Vox Sanguinis. 1990;58(2):90–3. doi: 10.1111/j.1423-0410.1990.tb02068.x. [DOI] [PubMed] [Google Scholar]
- 6.Chanutin A, Curnish RR. Effect of organic phosphates on the O2 equilibrium of carboxypeptidase digests of human hemoglobin. Archives of Biochemistry and Biophysics. 1968;123:163–5. doi: 10.1016/0003-9861(68)90114-8. [DOI] [PubMed] [Google Scholar]
- 7.Beutler E, Meul A, Wood LA. Depletion and regeneration of 2,3-diphosphoglyceric acid in stored red blood cells. Transfusion. 1969;9:109–15. doi: 10.1111/j.1537-2995.1969.tb05527.x. [DOI] [PubMed] [Google Scholar]
- 8.Bunn HF, May MH, Kocholaty WF, Shields CE. Hemoglobin Function in Stored Blood. Journal of Clinical Investigation. 1969;48:311–21. doi: 10.1172/JCI105987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein HG, Spahn DR, Carson JL. Transfusion Medicine 1 - Red blood cell transfusion in clinical practice. Lancet. 2007;370(9585):415–26. doi: 10.1016/S0140-6736(07)61197-0. [DOI] [PubMed] [Google Scholar]
- 10.Berezina TL, Zaets SB, Morgan C, et al. Influence of storage on red blood cell rheological properties. Journal of Surgical Research. 2002;102(1):6–12. doi: 10.1006/jsre.2001.6306. [DOI] [PubMed] [Google Scholar]
- 11.Haradin AR, Weed RI, Reed CF. Changes in Physical Properties of Stored Erythrocytes. Transfusion. 1969;9:229–35. doi: 10.1111/j.1537-2995.1969.tb04929.x. [DOI] [PubMed] [Google Scholar]
- 12.d'Almeida MS, Jagger J, Duggan M, et al. A comparison of biochemical and functional alterations of rat and human erythrocytes stored in CPDA-1 for 29 days: implications for animal models of transfusion. Transfusion Medicine. 2000;10(4):291–303. doi: 10.1046/j.1365-3148.2000.00267.x. [DOI] [PubMed] [Google Scholar]
- 13.Izzo P, Manicone A, Spagnuolo A, et al. Erythrocytes stored in CPD SAG-mannitol: evaluation of their deformability. Clinical Hemorheology and Microcirculation. 1999;21(3-4):335–9. [PubMed] [Google Scholar]
- 14.Card RT, Mohandas N, Perkins HA, Shohet SB. Deformability of Stored Red-Blood-Cells - Relationship to Degree of Packing. Transfusion. 1982;22(2):96–101. doi: 10.1046/j.1537-2995.1982.22282177134.x. [DOI] [PubMed] [Google Scholar]
- 15.Card RT, Mohandas N, Mollison PL. Relationship of Post-Transfusion Viability to Deformability of Stored Red-Cells. British Journal of Haematology. 1983;53(2):237–40. doi: 10.1111/j.1365-2141.1983.tb02016.x. [DOI] [PubMed] [Google Scholar]
- 16.Aubuchon JP, Estep TN, Davey RJ. The Effect of the Plasticizer Di-2-Ethylhexyl Phthalate on the Survival of Stored Rbcs. Blood. 1988;71(2):448–52. [PubMed] [Google Scholar]
- 17.Salzer U, Zhu R, Luten M, et al. Vesicles generated during storage of red cells are rich in the lipid raft marker stomatin. Transfusion. 2008;48(3):451–62. doi: 10.1111/j.1537-2995.2007.01549.x. [DOI] [PubMed] [Google Scholar]
- 18.Greenwalt TJ, McGuinness CG, Dumaswala UJ. Studies in Red-Blood-Cell Preservation .4. Plasma Vesicle Hemoglobin Exceeds Free Hemoglobin. Vox Sanguinis. 1991;61(1):14–7. doi: 10.1111/j.1423-0410.1991.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 19.Lutz HU, Liu SC, Palek J. Release of Spectrin-Free Vesicles from Human Erythrocytes During Atp Depletion .1. Characterization of Spectrin-Free Vesicles. Journal of Cell Biology. 1977;73(3):548–60. doi: 10.1083/jcb.73.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tinmouth A, Fergusson D, Yee IC, Hebert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46(11):2014–27. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 21.Zallen G, Offner PJ, Moore EE, et al. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. American Journal of Surgery. 1999;178(6):570–2. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 22.Moore FA, Moore EE, Sauaia A. Blood transfusion - An independent risk factor for postinjury multiple organ failure. Archives of Surgery. 1997;132(6):620–5. [PubMed] [Google Scholar]
- 23.Marik PE, Sibbald WJ. Effect of Stored-Blood Transfusion on Oxygen Delivery in Patients with Sepsis. Jama-Journal of the American Medical Association. 1993;269(23):3024–9. [PubMed] [Google Scholar]
- 24.Offner PJ, Moore EE, Biffl WL, et al. Increased rate of infection associated with transfusion of old blood after severe injury. Archives of Surgery. 2002;137(6):711–6. doi: 10.1001/archsurg.137.6.711. [DOI] [PubMed] [Google Scholar]
- 25.Purdy FR, Tweeddale MG, Merrick PM. Association of mortality with age of blood transfused in septic ICU patients. Canadian Journal of Anaesthesia-Journal Canadien D Anesthesie. 1997;44(12):1256–61. doi: 10.1007/BF03012772. [DOI] [PubMed] [Google Scholar]
- 26.Leal-Noval SR, Jara-Lopez I, Garcia-Garmendia JL, et al. Influence of erythrocyte concentrate storage time on postsurgical morbidity in cardiac surgery patients. Anesthesiology. 2003;98(4):815–22. doi: 10.1097/00000542-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. New England Journal of Medicine. 2008;358(12):1229–39. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 28.Walsh TS, McArdle F, McLellan SA, et al. Does the storage time of transfused red blood cells influence regional or global indexes of tissue oxygenation in anemic critically ill patients? Critical Care Medicine. 2004;32(2):364–71. doi: 10.1097/01.CCM.0000108878.23703.E0. [DOI] [PubMed] [Google Scholar]
- 29.Klein HG. Getting older is not necessarily getting better. Anesthesiology. 2003;98(4):807–8. doi: 10.1097/00000542-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Adamson JW. New blood, old blood, or no blood? New England Journal of Medicine. 2008;358(12):1295–6. doi: 10.1056/NEJMe0800520. [DOI] [PubMed] [Google Scholar]
- 31.Vamvakas EC, Carven JH. Length of storage of transfused red cells and postoperative morbidity in patients undergoing coronary artery bypass graft surgery. Transfusion. 2000;40(1):101–9. doi: 10.1046/j.1537-2995.2000.40010101.x. [DOI] [PubMed] [Google Scholar]
- 32.de Watering LV, Lorinser J, Versteegh M, et al. Effects of storage time of red blood cell transfusions on the prognosis of coronary artery bypass graft patients. Transfusion. 2006;46(10):1712–8. doi: 10.1111/j.1537-2995.2006.00958.x. [DOI] [PubMed] [Google Scholar]
- 33.Edgren G, Kamper-Jorgensen M, Eloranta S, et al. Duration of red blood cell storage and survival of transfused patients (CME) Transfusion. 50(6):1185–95. doi: 10.1111/j.1537-2995.2010.02583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Triulzi DJ, Yazer MH. Clinical studies of the effect of blood storage on patient outcomes. Transfusion and Apheresis Science. 43(1):95–106. doi: 10.1016/j.transci.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 35.Lee JS, Gladwin MT. Bad Blood The risks of red cell storage. Nature Medicine. 2010;16(4):381–2. doi: 10.1038/nm0410-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furchgott RF, Zawadzki JV. The Obligatory Role of Endothelial-Cells in the Relaxation of Arterial Smooth-Muscle by Acetylcholine. Nature. 1980;288(5789):373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 37.Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-Derived Relaxing Factor from Pulmonary-Artery and Vein Possesses Pharmacological and Chemical-Properties Identical to Those of Nitric-Oxide Radical. Circulation Research. 1987;61(6):866–79. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- 38.Katsuki S, Arnold W, Mittal C, Murad F. Stimulation of Guanylate Cyclase by Sodium Nitroprusside, Nitroglycerin and Nitric-Oxide in Various Tissue Preparations and Comparison to Effects of Sodium Azide and Hydroxylamine. Journal of Cyclic Nucleotide Research. 1977;3(1):23–35. [PubMed] [Google Scholar]
- 39.Palmer RMJ, Ferrige AG, Moncada S. Nitric-Oxide Release Accounts for the Biological-Activity of Endothelium-Derived Relaxing Factor. Nature. 1987;327(6122):524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt PM, Schramm M, Schroder H, et al. Identification of residues crucially involved in the binding of the heme moiety of soluble guanylate cyclase. Journal of Biological Chemistry. 2004;279(4):3025–32. doi: 10.1074/jbc.M310141200. [DOI] [PubMed] [Google Scholar]
- 41.Patel RP, McAndrew J, Sellak H, et al. Biological aspects of reactive nitrogen species. Biochimica Et Biophysica Acta-Bioenergetics. 1999;1411(2-3):385–400. doi: 10.1016/s0005-2728(99)00028-6. [DOI] [PubMed] [Google Scholar]
- 42.Ignarro LJ. Nitric Oxide Biology and Pathobiology. San Diego: Academic press; 2000. [Google Scholar]
- 43.Kleinbongard P, Schulz R, Rassaf T, et al. Red blood cells express a functional endothelial nitric oxide synthase. Blood. 2005;107:2943–51. doi: 10.1182/blood-2005-10-3992. [DOI] [PubMed] [Google Scholar]
- 44.Doyle MP, Hoekstra JW. Oxidation of Nitrogen-Oxides by Bound Dioxygen in Hemoproteins. Journal of Inorganic Biochemistry. 1981;14(4):351–8. doi: 10.1016/s0162-0134(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 45.Eich RF, Li TS, Lemon DD, et al. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35(22):6976–83. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 46.Herold S, Exner M, Nauser T. Kinetic and mechanistic studies of the NO center dot-mediated oxidation of oxymyoglobin and oxyhemoglobin. Biochemistry. 2001;40(11):3385–95. doi: 10.1021/bi002407m. [DOI] [PubMed] [Google Scholar]
- 47.Lancaster JR. Simulation of the Diffusion and Reaction of Endogenously Produced Nitric-Oxide. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(17):8137–41. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carlsen E, Comroe JH. The rate of uptake of Carbon Monoxide and of Nitric Oxide by normal and human erythrocytes and experimentally produced spherocytes. Journal of General Physiology. 1958;42(1):83–107. doi: 10.1085/jgp.42.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coin JT, Olson JS. Rate of Oxygen-Uptake by Human Red Blood-Cells. Journal of Biological Chemistry. 1979;254(4):1178–90. [PubMed] [Google Scholar]
- 50.Butler AR, Megson IL, Wright PG. Diffusion of nitric oxide and scavenging by blood in the vasculature. Biochimica Et Biophysica Acta-General Subjects. 1998;1425(1):168–76. doi: 10.1016/s0304-4165(98)00065-8. [DOI] [PubMed] [Google Scholar]
- 51.Vaughn MW, Kuo L, Liao JC. Estimation of nitric oxide production and reaction rates in tissue by use of a mathematical model. American Journal of Physiology-Heart and Circulatory Physiology. 1998;43(6):H2163–H76. doi: 10.1152/ajpheart.1998.274.6.H2163. [DOI] [PubMed] [Google Scholar]
- 52.Liao JC, Hein TW, Vaughn MW, et al. Intravascular flow decreases erythrocyte consumption of nitric oxide. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(15):8757–61. doi: 10.1073/pnas.96.15.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaughn MW, Huang KT, Kuo L, Liao JC. Erythrocytes possess an intrinsic barrier to nitric oxide consumption. Journal of Biological Chemistry. 2000;275(4):2342–8. doi: 10.1074/jbc.275.4.2342. [DOI] [PubMed] [Google Scholar]
- 54.Vaughn MW, Huang KT, Kuo L, Liao JC. Erythrocyte consumption of nitric oxide: Competition experiment and model analysis. Nitric Oxide-Biology and Chemistry. 2001;5(1):18–31. doi: 10.1006/niox.2000.0328. [DOI] [PubMed] [Google Scholar]
- 55.Liu XP, Samouilov A, Lancaster JR, Zweier JL. Nitric oxide uptake by erythrocytes is primarily limited by extracellular diffusion not membrane resistance. Journal of Biological Chemistry. 2002;277(29):26194–9. doi: 10.1074/jbc.M201939200. [DOI] [PubMed] [Google Scholar]
- 56.Huang KT, Han TH, Hyduke DR, et al. Modulation of nitric oxide bioavailability by erythrocytes. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(20):11771–6. doi: 10.1073/pnas.201276698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu XP, Miller MJS, Joshi MS, et al. Diffusion-limited reaction of free nitric oxide with erythrocytes. Journal of Biological Chemistry. 1998;273(30):18709–13. doi: 10.1074/jbc.273.30.18709. [DOI] [PubMed] [Google Scholar]
- 58.Azarov I, Huang KT, Basu S, et al. Nitric oxide scavenging by red blood cells as a function of hematocrit and oxygenation. J Biol Chem. 2005;280(47):39024–8032. doi: 10.1074/jbc.M509045200. [DOI] [PubMed] [Google Scholar]
- 59.Vaughn MW, Kuo L, Liao JC. Effective diffusion distance of nitric oxide in the microcirculation. American Journal of Physiology-Heart and Circulatory Physiology. 1998;43(5):H1705–H14. doi: 10.1152/ajpheart.1998.274.5.H1705. [DOI] [PubMed] [Google Scholar]
- 60.Kim-Shapiro DB, Schechter AN, Gladwin MT. Unraveling the Reactions of Nitric Oxide, Nitrite, and Hemoglobin in Physiology and Therapeutics. Arterioscler Thromb Vasc Biol. 2006;26:697–705. doi: 10.1161/01.ATV.0000204350.44226.9a. [DOI] [PubMed] [Google Scholar]
- 61.Kevil CG, Patel RP. S-Nitrosothiol biology and therapeutic potential in metabolic disease. Current Opinion in Investigational Drugs. 2010;11(10):1127–34. [PMC free article] [PubMed] [Google Scholar]
- 62.Knott AB, Bossy-Wetzel E. Impact of nitric oxide on metabolism in health and age-related disease. Diabetes Obesity & Metabolism. 2010;12:126–33. doi: 10.1111/j.1463-1326.2010.01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shimokawa H, Tsutsui M. Nitric oxide synthases in the pathogenesis of cardiovascular disease. Pflugers Archiv-European Journal of Physiology. 2010;459(6):959–67. doi: 10.1007/s00424-010-0796-2. [DOI] [PubMed] [Google Scholar]
- 64.Virdis A, Ghiadoni L, Giannarelli C, Taddei S. Endothelial dysfunction and vascular disease in later life. Maturitas. 2010;67(1):20–4. doi: 10.1016/j.maturitas.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 65.Ferrari CKB, Franca EL, Honorio-Franca AC. Nitric oxide, health and disease. Journal of Applied Biomedicine. 2009;7(4):163–73. [Google Scholar]
- 66.Rother RP, B L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293(13):1653–62. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 67.Hess JR, Macdonald VW, Brinkley WW. Systemic and Pulmonary-Hypertension after Resuscitation with Cell-Free Hemoglobin. Journal of Applied Physiology. 1993;74(4):1769–78. doi: 10.1152/jappl.1993.74.4.1769. [DOI] [PubMed] [Google Scholar]
- 68.Lee R, Neya K, Svizzero TA, Vlahakes GJ. Limitations of the Efficacy of Hemoglobin-Based Oxygen-Carrying Solutions. Journal of Applied Physiology. 1995;79(1):236–42. doi: 10.1152/jappl.1995.79.1.236. [DOI] [PubMed] [Google Scholar]
- 69.Vogel WM, Dennis RC, Cassidy G, et al. Coronary Constrictor Effect of Stroma-Free Hemoglobin-Solutions. American Journal of Physiology. 1986;251(2):H413–H20. doi: 10.1152/ajpheart.1986.251.2.H413. [DOI] [PubMed] [Google Scholar]
- 70.Murray JA, Ledlow A, Launspach J, et al. The Effects of Recombinant Human Hemoglobin on Esophageal Motor Function in Humans. Gastroenterology. 1995;109(4):1241–8. doi: 10.1016/0016-5085(95)90584-7. [DOI] [PubMed] [Google Scholar]
- 71.Reiter CD, Wang XD, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nature Medicine. 2002;8(12):1383–9. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 72.Minneci PC, D K, Zhi H, Yuen PS, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115(12):3409–17. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jeffers A, Gladwin MT, Kim-Shapiro DB. Computation of plasma hemoglobin nitric oxide scavenging in hemolytic anemias. Free Radical Biology and Medicine. 2006;41(10):1557–65. doi: 10.1016/j.freeradbiomed.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin - A novel mechanism of human disease. Jama-Journal of the American Medical Association. 2005;293(13):1653–62. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 75.Gladwin MT. Unraveling the hemolytic subphenotype of sickle cell disease. Blood. 2005;106(9):2925–6. [Google Scholar]
- 76.Kato GJ, McGowan VR, Machado RF, et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension and death in patients with sickle cell disease. Blood. 2006;107:2279–85. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nolan VG, Wyszynski DF, Farrer LA, Steinberg MH. Hemolysis-associated priapism in sickle cell disease. Blood. 2005;106(9):3264–7. doi: 10.1182/blood-2005-04-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. New England Journal of Medicine. 2004;350(9):886–95. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 79.Morris CR, Kato GJ, Poijakovic M, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. Jama-Journal of the American Medical Association. 2005;294(1):81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gladwin MT, Barst RJ, Castro OL, et al. Pulmonary hypertension and NO in sickle cell. Blood. 2010;116(5):852–4. doi: 10.1182/blood-2010-04-282095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bunn HF, Nathan DG, Dover GJ, et al. Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood. 2010;116(5):687–92. doi: 10.1182/blood-2010-02-268193. [DOI] [PubMed] [Google Scholar]
- 82.Gladwin MT, Kim-Shapiro DB. Storage lesion in banked blood due to hemolysis-dependent disruption of nitric oxide homeostasis. Current Opinion in Hematology. 2009;16(6):515–23. doi: 10.1097/MOH.0b013e32833157f4. [DOI] [PubMed] [Google Scholar]
- 83.Han TH, Liao JC. Erythrocyte nitric oxide transport reduced by a submembrane cytoskeletal barrier. Biochimica Et Biophysica Acta-General Subjects. 2005;1723(1-3):135–42. doi: 10.1016/j.bbagen.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 84.Hod EA, Zhang N, Sokol SA, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115(21):4284–92. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mangalmurti NS, Xiong Z, Hulver M, et al. Loss of red cell chemokine scavenging promotes transfusion-related lung inflammation. Blood. 2009;113(5):1158–66. doi: 10.1182/blood-2008-07-166264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jeney V, Balla J, Yachie A, et al. Pro-oxidant and cytotoxic effects of circulating heme. Blood. 2002;100(3):879–87. doi: 10.1182/blood.v100.3.879. [DOI] [PubMed] [Google Scholar]
- 87.Xiong Z, Cavaretta J, Qu L, et al. Red blood cell microparticles show altered inflammatory chemokine binding and release ligand upon interaction with platelets. Transfusion. doi: 10.1111/j.1537-2995.2010.02861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Westerman M, Pizzey A, Hirschman J, et al. Microvesicles in haemoglobinopathies offer insights into mechanisms of hypercoagulability, haemolysis and the effects of therapy. British Journal of Haematology. 2008;142(1):126–35. doi: 10.1111/j.1365-2141.2008.07155.x. [DOI] [PubMed] [Google Scholar]
- 89.Wang RH, Phillips G, Jr, Medof ME, Mold C. Activation of the alternative complement pathway by exposure of phosphatidylethanolamine and phosphatidylserine on erythrocytes from sickle cell disease patients. J Clin Invest. 1993;92(3):1326–35. doi: 10.1172/JCI116706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boilard E, Nigrovic PA, Larabee K, et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 327(5965):580–3. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.George JN, Pickett EB, Saucerman S, et al. Platelet surface glycoproteins. Studies on resting and activated platelets and platelet membrane microparticles in normal subjects, and observations in patients during adult respiratory distress syndrome and cardiac surgery. J Clin Invest. 1986;78(2):340–8. doi: 10.1172/JCI112582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Villagra J, Shiva S, Hunter LA, et al. Platelet activation in patients with sickle disease, hemolysis-associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood. 2007;110(6):2166–72. doi: 10.1182/blood-2006-12-061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.del Conde I, Cruz MA, Zhang H, et al. Platelet activation leads to activation and propagation of the complement system. Journal of Experimental Medicine. 2005;201(6):871–9. doi: 10.1084/jem.20041497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wagener FA, Eggert A, Boerman OC, et al. Heme is a potent inducer of inflammation in mice and is counteracted by heme oxygenase. Blood. 2001;98(6):1802–11. doi: 10.1182/blood.v98.6.1802. [DOI] [PubMed] [Google Scholar]
- 95.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 96.Larsen R, Gozzelino R, Jeney V, et al. A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med. 2(51):51ra71. doi: 10.1126/scitranslmed.3001118. [DOI] [PubMed] [Google Scholar]
- 97.Azarov I, He XJ, Jeffers A, et al. Rate of nitric oxide scavenging by hemoglobin bound to haptoglobin. Nitric Oxide-Biology and Chemistry. 2008;18(4):296–302. doi: 10.1016/j.niox.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cosby K, Partovi KS, Crawford JH, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nature Medicine. 2003;9(12):1498–505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]


