Abstract
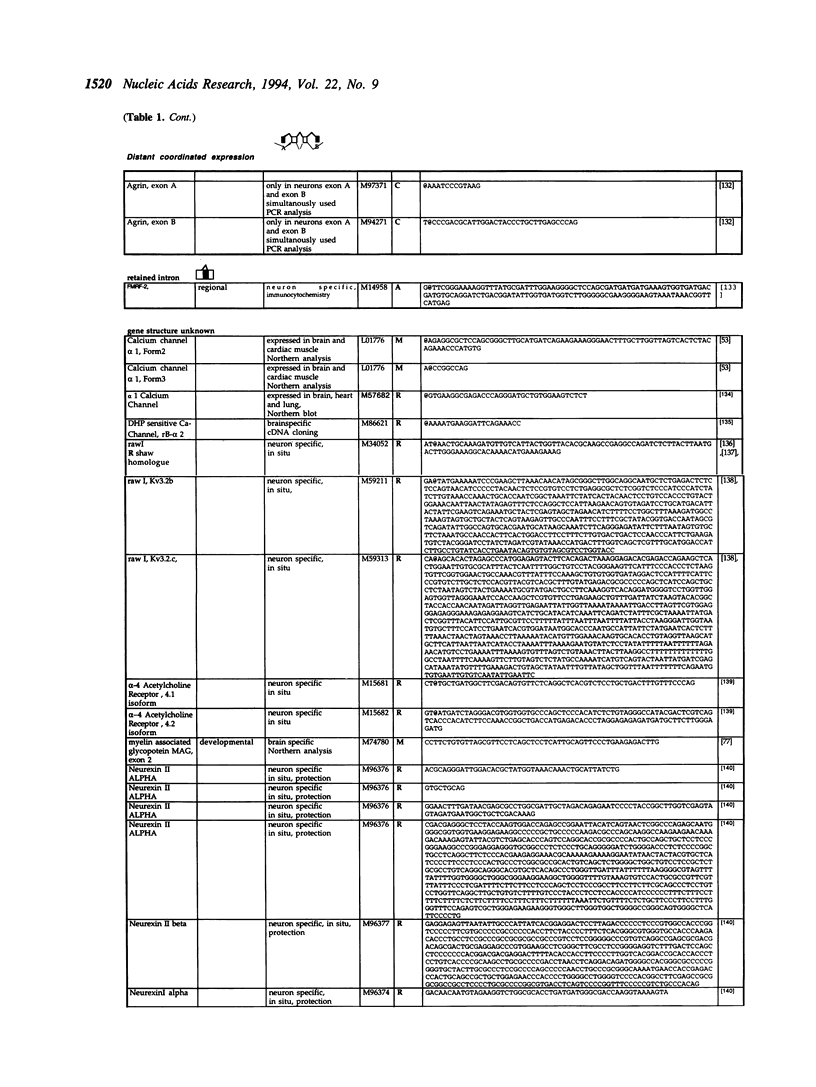
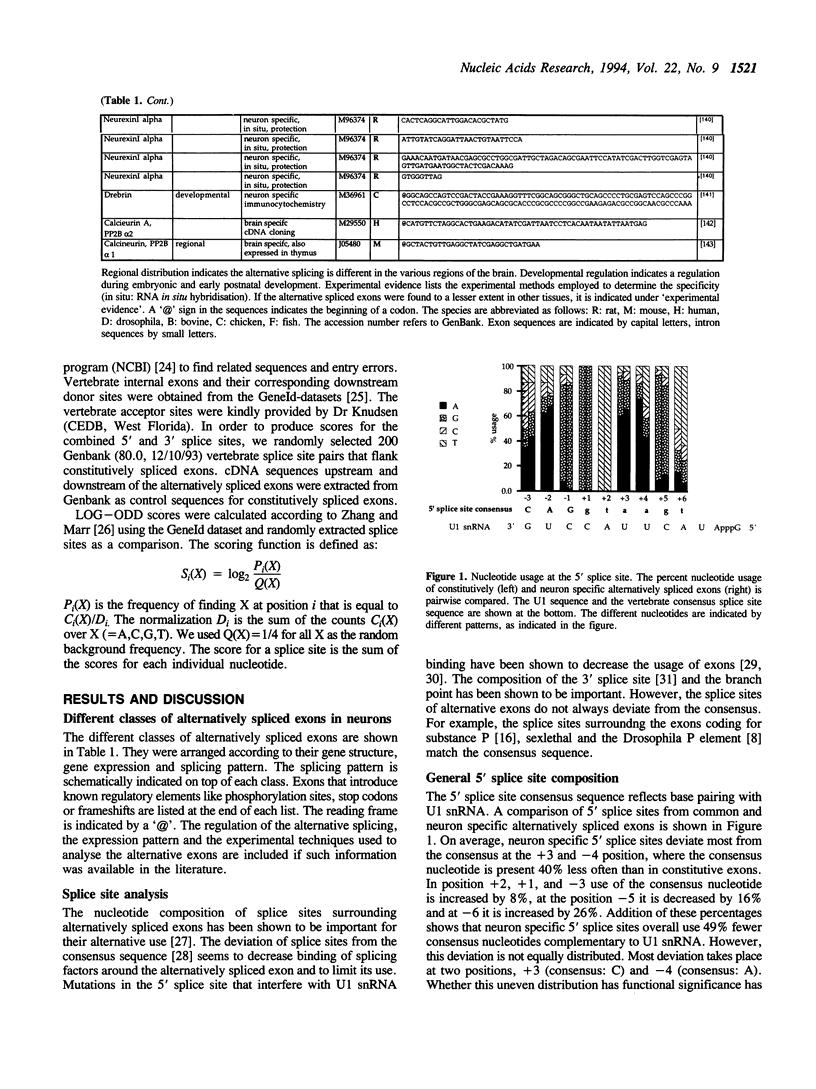
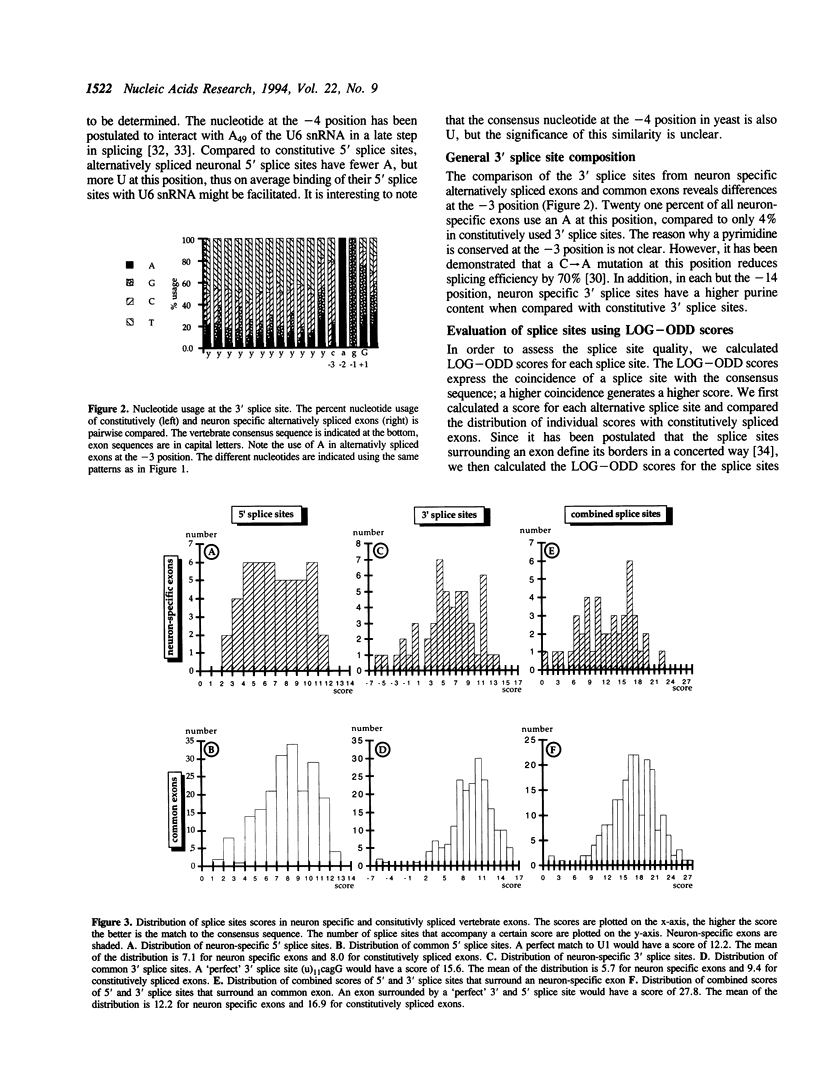
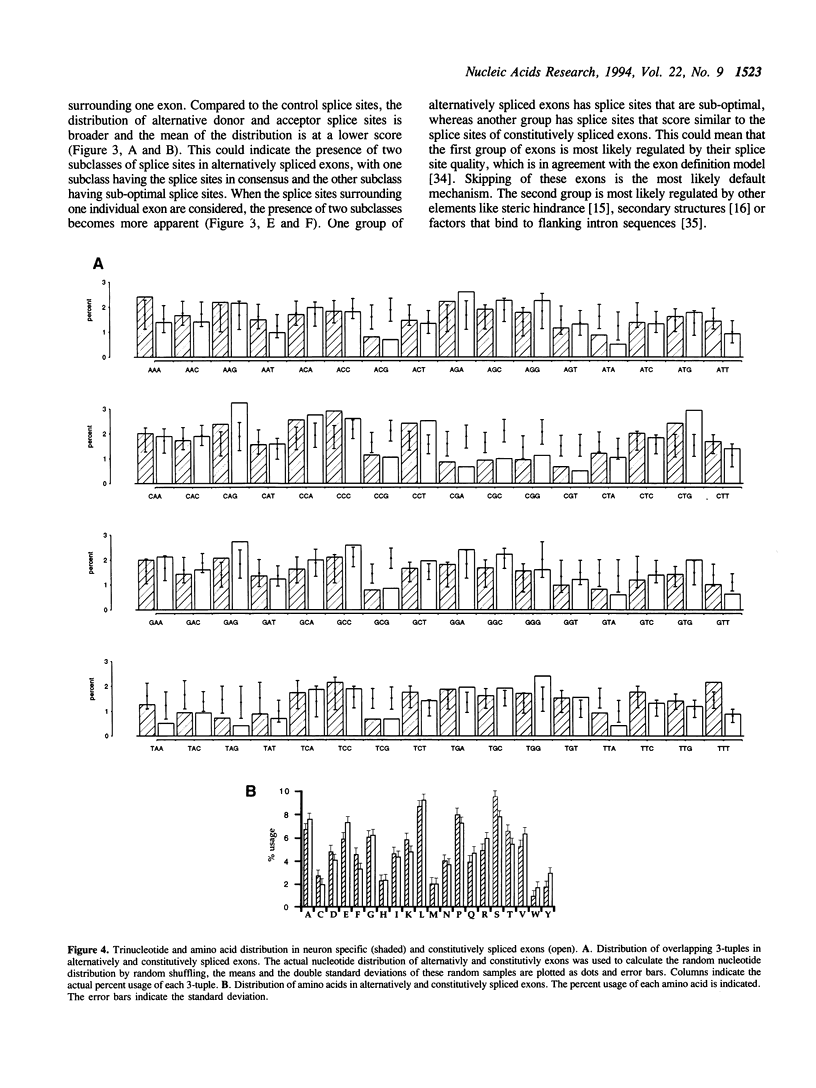
Alternative splicing is an important regulatory mechanism to create protein diversity. In order to elucidate possible regulatory elements common to neuron specific exons, we created and statistically analysed a database of exons that are alternatively spliced in neurons. The splice site comparison of alternatively and constitutively spliced exons reveals that some, but not all alternatively spliced exons have splice sites deviating from the consensus sequence, implying diverse patterns of regulation. The deviation from the consensus is most evident at the -3 position of the 3' splice site and the +4 and -3 position of the 5' splice site. The nucleotide composition of alternatively and constitutively spliced exons is different, with alternatively spliced exons being more AU rich. We performed overlapping k-tuple analysis to identify common motifs. We found that alternatively and constitutively spliced exons differ in the frequency of several trinucleotides that cannot be explained by the amino acid composition and may be important for splicing regulation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adema G. J., van Hulst K. L., Baas P. D. Uridine branch acceptor is a cis-acting element involved in regulation of the alternative processing of calcitonin/CGRP-l pre-mRNA. Nucleic Acids Res. 1990 Sep 25;18(18):5365–5373. doi: 10.1093/nar/18.18.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebi M., Hornig H., Padgett R. A., Reiser J., Weissmann C. Sequence requirements for splicing of higher eukaryotic nuclear pre-mRNA. Cell. 1986 Nov 21;47(4):555–565. doi: 10.1016/0092-8674(86)90620-3. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Amara S. G., Evans R. M., Rosenfeld M. G. Calcitonin/calcitonin gene-related peptide transcription unit: tissue-specific expression involves selective use of alternative polyadenylation sites. Mol Cell Biol. 1984 Oct;4(10):2151–2160. doi: 10.1128/mcb.4.10.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara S. G., Jonas V., Rosenfeld M. G., Ong E. S., Evans R. M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982 Jul 15;298(5871):240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Andreadis A., Brown W. M., Kosik K. S. Structure and novel exons of the human tau gene. Biochemistry. 1992 Nov 3;31(43):10626–10633. doi: 10.1021/bi00158a027. [DOI] [PubMed] [Google Scholar]
- Bateson A. N., Lasham A., Darlison M. G. gamma-Aminobutyric acidA receptor heterogeneity is increased by alternative splicing of a novel beta-subunit gene transcript. J Neurochem. 1991 Apr;56(4):1437–1440. doi: 10.1111/j.1471-4159.1991.tb11443.x. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., Kennedy M. B. Deduced primary structure of the beta subunit of brain type II Ca2+/calmodulin-dependent protein kinase determined by molecular cloning. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1794–1798. doi: 10.1073/pnas.84.7.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham P. M., Chou T. B., Mims I., Zachar Z. On/off regulation of gene expression at the level of splicing. Trends Genet. 1988 May;4(5):134–138. doi: 10.1016/0168-9525(88)90136-9. [DOI] [PubMed] [Google Scholar]
- Black D. L. Activation of c-src neuron-specific splicing by an unusual RNA element in vivo and in vitro. Cell. 1992 May 29;69(5):795–807. doi: 10.1016/0092-8674(92)90291-j. [DOI] [PubMed] [Google Scholar]
- Black D. L. Does steric interference between splice sites block the splicing of a short c-src neuron-specific exon in non-neuronal cells? Genes Dev. 1991 Mar;5(3):389–402. doi: 10.1101/gad.5.3.389. [DOI] [PubMed] [Google Scholar]
- Branlant C., Krol A., Ebel J. P., Lazar E., Haendler B., Jacob M. U2 RNA shares a structural domain with U1, U4, and U5 RNAs. EMBO J. 1982;1(10):1259–1265. doi: 10.1002/j.1460-2075.1982.tb00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck L. B., Bigelow J. M., Axel R. Alternative splicing in individual Aplysia neurons generates neuropeptide diversity. Cell. 1987 Oct 9;51(1):127–133. doi: 10.1016/0092-8674(87)90017-1. [DOI] [PubMed] [Google Scholar]
- Bulleit R. F., Bennett M. K., Molloy S. S., Hurley J. B., Kennedy M. B. Conserved and variable regions in the subunits of brain type II Ca2+/calmodulin-dependent protein kinase. Neuron. 1988 Mar;1(1):63–72. doi: 10.1016/0896-6273(88)90210-3. [DOI] [PubMed] [Google Scholar]
- Carter M. S., Krause J. E. Structure, expression, and some regulatory mechanisms of the rat preprotachykinin gene encoding substance P, neurokinin A, neuropeptide K, and neuropeptide gamma. J Neurosci. 1990 Jul;10(7):2203–2214. doi: 10.1523/JNEUROSCI.10-07-02203.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. H., Olson M. O. A single gene codes for two forms of rat nucleolar protein B23 mRNA. J Biol Chem. 1989 Jul 15;264(20):11732–11737. [PubMed] [Google Scholar]
- Chaudhari N., Hahn W. E. Genetic expression in the developing brain. Science. 1983 May 27;220(4600):924–928. doi: 10.1126/science.6189184. [DOI] [PubMed] [Google Scholar]
- Chikaraishi D. M., Deeb S. S., Sueoka N. Sequence complexity of nuclear RNAs in adult rat tissues. Cell. 1978 Jan;13(1):111–120. doi: 10.1016/0092-8674(78)90142-3. [DOI] [PubMed] [Google Scholar]
- Claverie J. M., Sauvaget I., Bougueleret L. K-tuple frequency analysis: from intron/exon discrimination to T-cell epitope mapping. Methods Enzymol. 1990;183:237–252. doi: 10.1016/0076-6879(90)83017-4. [DOI] [PubMed] [Google Scholar]
- Collett J. W., Steele R. E. Identification and developmental expression of Src+ mRNAs in Xenopus laevis. Dev Biol. 1992 Jul;152(1):194–198. doi: 10.1016/0012-1606(92)90170-l. [DOI] [PubMed] [Google Scholar]
- Cooper T. A. In vitro splicing of cardiac troponin T precursors. Exon mutations disrupt splicing of the upstream intron. J Biol Chem. 1992 Mar 15;267(8):5330–5338. [PubMed] [Google Scholar]
- Coussens L., Rhee L., Parker P. J., Ullrich A. Alternative splicing increases the diversity of the human protein kinase C family. DNA. 1987 Oct;6(5):389–394. doi: 10.1089/dna.1987.6.389. [DOI] [PubMed] [Google Scholar]
- Cyr J. L., Pfister K. K., Bloom G. S., Slaughter C. A., Brady S. T. Molecular genetics of kinesin light chains: generation of isoforms by alternative splicing. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10114–10118. doi: 10.1073/pnas.88.22.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Toso R., Sommer B., Ewert M., Herb A., Pritchett D. B., Bach A., Shivers B. D., Seeburg P. H. The dopamine D2 receptor: two molecular forms generated by alternative splicing. EMBO J. 1989 Dec 20;8(13):4025–4034. doi: 10.1002/j.1460-2075.1989.tb08585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danoff S. K., Ferris C. D., Donath C., Fischer G. A., Munemitsu S., Ullrich A., Snyder S. H., Ross C. A. Inositol 1,4,5-trisphosphate receptors: distinct neuronal and nonneuronal forms derived by alternative splicing differ in phosphorylation. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2951–2955. doi: 10.1073/pnas.88.7.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delsert C. D., Rosenfeld M. G. A tissue-specific small nuclear ribonucleoprotein and the regulated splicing of the calcitonin/calcitonin gene-related protein transcript. J Biol Chem. 1992 Jul 25;267(21):14573–14579. [PubMed] [Google Scholar]
- Duilio A., Zambrano N., Mogavero A. R., Ammendola R., Cimino F., Russo T. A rat brain mRNA encoding a transcriptional activator homologous to the DNA binding domain of retroviral integrases. Nucleic Acids Res. 1991 Oct 11;19(19):5269–5274. doi: 10.1093/nar/19.19.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishburn C. S., Belleli D., David C., Carmon S., Fuchs S. A novel short isoform of the D3 dopamine receptor generated by alternative splicing in the third cytoplasmic loop. J Biol Chem. 1993 Mar 15;268(8):5872–5878. [PubMed] [Google Scholar]
- Forry-Schaudies S., Hughes S. H. The chicken tropomyosin 1 gene generates nine mRNAs by alternative splicing. J Biol Chem. 1991 Jul 25;266(21):13821–13827. [PubMed] [Google Scholar]
- Fu X. D., Katz R. A., Skalka A. M., Maniatis T. The role of branchpoint and 3'-exon sequences in the control of balanced splicing of avian retrovirus RNA. Genes Dev. 1991 Feb;5(2):211–220. doi: 10.1101/gad.5.2.211. [DOI] [PubMed] [Google Scholar]
- Fujita N., Sato S., Kurihara T., Inuzuka T., Takahashi Y., Miyatake T. Developmentally regulated alternative splicing of brain myelin-associated glycoprotein mRNA is lacking in the quaking mouse. FEBS Lett. 1988 May 23;232(2):323–327. doi: 10.1016/0014-5793(88)80762-2. [DOI] [PubMed] [Google Scholar]
- Fujita N., Sato S., Kurihara T., Kuwano R., Sakimura K., Inuzuka T., Takahashi Y., Miyatake T. cDNA cloning of mouse myelin-associated glycoprotein: a novel alternative splicing pattern. Biochem Biophys Res Commun. 1989 Dec 29;165(3):1162–1169. doi: 10.1016/0006-291x(89)92724-1. [DOI] [PubMed] [Google Scholar]
- Galas D. J., Eggert M., Waterman M. S. Rigorous pattern-recognition methods for DNA sequences. Analysis of promoter sequences from Escherichia coli. J Mol Biol. 1985 Nov 5;186(1):117–128. doi: 10.1016/0022-2836(85)90262-1. [DOI] [PubMed] [Google Scholar]
- Giros B., Martres M. P., Pilon C., Sokoloff P., Schwartz J. C. Shorter variants of the D3 dopamine receptor produced through various patterns of alternative splicing. Biochem Biophys Res Commun. 1991 May 15;176(3):1584–1592. doi: 10.1016/0006-291x(91)90469-n. [DOI] [PubMed] [Google Scholar]
- Giros B., Sokoloff P., Martres M. P., Riou J. F., Emorine L. J., Schwartz J. C. Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature. 1989 Dec 21;342(6252):923–926. doi: 10.1038/342923a0. [DOI] [PubMed] [Google Scholar]
- Glencorse T. A., Bateson A. N., Darlison M. G. Sequence of the chicken GABAA receptor gamma 2-subunit cDNA. Nucleic Acids Res. 1990 Dec 11;18(23):7157–7157. doi: 10.1093/nar/18.23.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Crowther R. A. Cloning of a big tau microtubule-associated protein characteristic of the peripheral nervous system. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1983–1987. doi: 10.1073/pnas.89.5.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Jakes R., Rutherford D., Crowther R. A. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989 Oct;3(4):519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Potier M. C., Ulrich J., Crowther R. A. Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J. 1989 Feb;8(2):393–399. doi: 10.1002/j.1460-2075.1989.tb03390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D., Deneris E., Luyten W., Kochhar A., Patrick J., Heinemann S. Members of a nicotinic acetylcholine receptor gene family are expressed in different regions of the mammalian central nervous system. Cell. 1987 Mar 27;48(6):965–973. doi: 10.1016/0092-8674(87)90705-7. [DOI] [PubMed] [Google Scholar]
- Grima B., Lamouroux A., Boni C., Julien J. F., Javoy-Agid F., Mallet J. A single human gene encoding multiple tyrosine hydroxylases with different predicted functional characteristics. Nature. 1987 Apr 16;326(6114):707–711. doi: 10.1038/326707a0. [DOI] [PubMed] [Google Scholar]
- Guerini D., Klee C. B. Cloning of human calcineurin A: evidence for two isozymes and identification of a polyproline structural domain. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9183–9187. doi: 10.1073/pnas.86.23.9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guigó R., Knudsen S., Drake N., Smith T. Prediction of gene structure. J Mol Biol. 1992 Jul 5;226(1):141–157. doi: 10.1016/0022-2836(92)90130-c. [DOI] [PubMed] [Google Scholar]
- Guo W., Mulligan G. J., Wormsley S., Helfman D. M. Alternative splicing of beta-tropomyosin pre-mRNA: cis-acting elements and cellular factors that block the use of a skeletal muscle exon in nonmuscle cells. Genes Dev. 1991 Nov;5(11):2096–2107. doi: 10.1101/gad.5.11.2096. [DOI] [PubMed] [Google Scholar]
- Hampson R. K., La Follette L., Rottman F. M. Alternative processing of bovine growth hormone mRNA is influenced by downstream exon sequences. Mol Cell Biol. 1989 Apr;9(4):1604–1610. doi: 10.1128/mcb.9.4.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmler A. Structure of the bovine tau gene: alternatively spliced transcripts generate a protein family. Mol Cell Biol. 1989 Apr;9(4):1389–1396. doi: 10.1128/mcb.9.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata M., Saito N., Kono M., Tanaka C. Differential expression of the beta I- and beta II-PKC subspecies in the postnatal developing rat brain; an immunocytochemical study. Brain Res Dev Brain Res. 1991 Oct 21;62(2):229–238. doi: 10.1016/0165-3806(91)90170-n. [DOI] [PubMed] [Google Scholar]
- Hollmann M., Boulter J., Maron C., Beasley L., Sullivan J., Pecht G., Heinemann S. Zinc potentiates agonist-induced currents at certain splice variants of the NMDA receptor. Neuron. 1993 May;10(5):943–954. doi: 10.1016/0896-6273(93)90209-a. [DOI] [PubMed] [Google Scholar]
- Horowitz D. S., Krainer A. R. Mechanisms for selecting 5' splice sites in mammalian pre-mRNA splicing. Trends Genet. 1994 Mar;10(3):100–106. doi: 10.1016/0168-9525(94)90233-x. [DOI] [PubMed] [Google Scholar]
- Hortsch M., Bieber A. J., Patel N. H., Goodman C. S. Differential splicing generates a nervous system-specific form of Drosophila neuroglian. Neuron. 1990 May;4(5):697–709. doi: 10.1016/0896-6273(90)90196-m. [DOI] [PubMed] [Google Scholar]
- Huang J. P., Tang C. J., Kou G. H., Marchesi V. T., Benz E. J., Jr, Tang T. K. Genomic structure of the locus encoding protein 4.1. Structural basis for complex combinational patterns of tissue-specific alternative RNA splicing. J Biol Chem. 1993 Feb 15;268(5):3758–3766. [PubMed] [Google Scholar]
- Hui A., Ellinor P. T., Krizanova O., Wang J. J., Diebold R. J., Schwartz A. Molecular cloning of multiple subtypes of a novel rat brain isoform of the alpha 1 subunit of the voltage-dependent calcium channel. Neuron. 1991 Jul;7(1):35–44. doi: 10.1016/0896-6273(91)90072-8. [DOI] [PubMed] [Google Scholar]
- Jackson A. P., Parham P. Structure of human clathrin light chains. Conservation of light chain polymorphism in three mammalian species. J Biol Chem. 1988 Nov 15;263(32):16688–16695. [PubMed] [Google Scholar]
- Jackson A. P., Seow H. F., Holmes N., Drickamer K., Parham P. Clathrin light chains contain brain-specific insertion sequences and a region of homology with intermediate filaments. Nature. 1987 Mar 12;326(6109):154–159. doi: 10.1038/326154a0. [DOI] [PubMed] [Google Scholar]
- Kaneda N., Kobayashi K., Ichinose H., Kishi F., Nakazawa A., Kurosawa Y., Fujita K., Nagatsu T. Isolation of a novel cDNA clone for human tyrosine hydroxylase: alternative RNA splicing produces four kinds of mRNA from a single gene. Biochem Biophys Res Commun. 1987 Aug 14;146(3):971–975. doi: 10.1016/0006-291x(87)90742-x. [DOI] [PubMed] [Google Scholar]
- Katz R. A., Skalka A. M. Control of retroviral RNA splicing through maintenance of suboptimal processing signals. Mol Cell Biol. 1990 Feb;10(2):696–704. doi: 10.1128/mcb.10.2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. L., Kim H., Lee P., King R. G., Chin H. Rat brain expresses an alternatively spliced form of the dihydropyridine-sensitive L-type calcium channel alpha 2 subunit. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3251–3255. doi: 10.1073/pnas.89.8.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid R. L., Giri P. R., Higuchi S., Tamura J., Dixon S. C., Marietta C. A., Amorese D. A., Martin B. M. Cloning and characterization of molecular isoforms of the catalytic subunit of calcineurin using nonisotopic methods. J Biol Chem. 1990 Jul 5;265(19):11312–11319. [PubMed] [Google Scholar]
- Kirchhausen T., Scarmato P., Harrison S. C., Monroe J. J., Chow E. P., Mattaliano R. J., Ramachandran K. L., Smart J. E., Ahn A. H., Brosius J. Clathrin light chains LCA and LCB are similar, polymorphic, and share repeated heptad motifs. Science. 1987 Apr 17;236(4799):320–324. doi: 10.1126/science.3563513. [DOI] [PubMed] [Google Scholar]
- Kofuji P., Wang J. B., Moss S. J., Huganir R. L., Burt D. R. Generation of two forms of the gamma-aminobutyric acidA receptor gamma 2-subunit in mice by alternative splicing. J Neurochem. 1991 Feb;56(2):713–715. doi: 10.1111/j.1471-4159.1991.tb08209.x. [DOI] [PubMed] [Google Scholar]
- Kojima N., Kato Y., Shirao T., Obata K. Nucleotide sequences of two embryonic drebrins, developmentally regulated brain proteins, and developmental change in their mRNAs. Brain Res. 1988 Nov;464(3):207–215. doi: 10.1016/0169-328x(88)90027-7. [DOI] [PubMed] [Google Scholar]
- Kuhse J., Kuryatov A., Maulet Y., Malosio M. L., Schmieden V., Betz H. Alternative splicing generates two isoforms of the alpha 2 subunit of the inhibitory glycine receptor. FEBS Lett. 1991 May 20;283(1):73–77. doi: 10.1016/0014-5793(91)80557-j. [DOI] [PubMed] [Google Scholar]
- Kuo H. C., Nasim F. H., Grabowski P. J. Control of alternative splicing by the differential binding of U1 small nuclear ribonucleoprotein particle. Science. 1991 Mar 1;251(4997):1045–1050. doi: 10.1126/science.1825520. [DOI] [PubMed] [Google Scholar]
- Lai C., Brow M. A., Nave K. A., Noronha A. B., Quarles R. H., Bloom F. E., Milner R. J., Sutcliffe J. G. Two forms of 1B236/myelin-associated glycoprotein, a cell adhesion molecule for postnatal neural development, are produced by alternative splicing. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4337–4341. doi: 10.1073/pnas.84.12.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigueur A., La Branche H., Kornblihtt A. R., Chabot B. A splicing enhancer in the human fibronectin alternate ED1 exon interacts with SR proteins and stimulates U2 snRNP binding. Genes Dev. 1993 Dec;7(12A):2405–2417. doi: 10.1101/gad.7.12a.2405. [DOI] [PubMed] [Google Scholar]
- Le Bourdellès B., Horellou P., Le Caer J. P., Denèfle P., Latta M., Haavik J., Guibert B., Mayaux J. F., Mallet J. Phosphorylation of human recombinant tyrosine hydroxylase isoforms 1 and 2: an additional phosphorylated residue in isoform 2, generated through alternative splicing. J Biol Chem. 1991 Sep 15;266(26):17124–17130. [PubMed] [Google Scholar]
- Lees-Miller J. P., Goodwin L. O., Helfman D. M. Three novel brain tropomyosin isoforms are expressed from the rat alpha-tropomyosin gene through the use of alternative promoters and alternative RNA processing. Mol Cell Biol. 1990 Apr;10(4):1729–1742. doi: 10.1128/mcb.10.4.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff S. E., Evans R. M., Rosenfeld M. G. Splice commitment dictates neuron-specific alternative RNA processing in calcitonin/CGRP gene expression. Cell. 1987 Feb 13;48(3):517–524. doi: 10.1016/0092-8674(87)90202-9. [DOI] [PubMed] [Google Scholar]
- Lesser C. F., Guthrie C. Mutations in U6 snRNA that alter splice site specificity: implications for the active site. Science. 1993 Dec 24;262(5142):1982–1988. doi: 10.1126/science.8266093. [DOI] [PubMed] [Google Scholar]
- Levy J. B., Dorai T., Wang L. H., Brugge J. S. The structurally distinct form of pp60c-src detected in neuronal cells is encoded by a unique c-src mRNA. Mol Cell Biol. 1987 Nov;7(11):4142–4145. doi: 10.1128/mcb.7.11.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillycrop K. A., Latchman D. S. Alternative splicing of the Oct-2 transcription factor RNA is differentially regulated in neuronal cells and B cells and results in protein isoforms with opposite effects on the activity of octamer/TAATGARAT-containing promoters. J Biol Chem. 1992 Dec 15;267(35):24960–24965. [PubMed] [Google Scholar]
- Lorenzi M. V., Trinidad A. C., Zhang R., Strauss W. L. Two mRNAs are transcribed from the human gene for choline acetyltransferase. DNA Cell Biol. 1992 Oct;11(8):593–603. doi: 10.1089/dna.1992.11.593. [DOI] [PubMed] [Google Scholar]
- Luneau C. J., Williams J. B., Marshall J., Levitan E. S., Oliva C., Smith J. S., Antanavage J., Folander K., Stein R. B., Swanson R. Alternative splicing contributes to K+ channel diversity in the mammalian central nervous system. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3932–3936. doi: 10.1073/pnas.88.9.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luneau C., Wiedmann R., Smith J. S., Williams J. B. Shaw-like rat brain potassium channel cDNA's with divergent 3' ends. FEBS Lett. 1991 Aug 19;288(1-2):163–167. doi: 10.1016/0014-5793(91)81026-5. [DOI] [PubMed] [Google Scholar]
- Ma W. J., Holz R. W., Uhler M. D. Expression of a cDNA for a neuronal calcium channel alpha 1 subunit enhances secretion from adrenal chromaffin cells. J Biol Chem. 1992 Nov 15;267(32):22728–22732. [PubMed] [Google Scholar]
- Malosio M. L., Grenningloh G., Kuhse J., Schmieden V., Schmitt B., Prior P., Betz H. Alternative splicing generates two variants of the alpha 1 subunit of the inhibitory glycine receptor. J Biol Chem. 1991 Feb 5;266(4):2048–2053. [PubMed] [Google Scholar]
- Mardon H. J., Sebastio G., Baralle F. E. A role for exon sequences in alternative splicing of the human fibronectin gene. Nucleic Acids Res. 1987 Oct 12;15(19):7725–7733. doi: 10.1093/nar/15.19.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R. A., Erwin C. R., Donelson J. E. Analysis of 5' flanking sequences and intron-exon boundaries of the rat prolactin gene. J Biol Chem. 1981 Oct 25;256(20):10524–10528. [PubMed] [Google Scholar]
- Maycox P. R., Link E., Reetz A., Morris S. A., Jahn R. Clathrin-coated vesicles in nervous tissue are involved primarily in synaptic vesicle recycling. J Cell Biol. 1992 Sep;118(6):1379–1388. doi: 10.1083/jcb.118.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister G., Amara S. G., Lerner M. R. Tissue-specific expression and cDNA cloning of small nuclear ribonucleoprotein-associated polypeptide N. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5296–5300. doi: 10.1073/pnas.85.14.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack T., Vega-Saenz de Miera E. C., Rudy B. Molecular cloning of a member of a third class of Shaker-family K+ channel genes in mammals. Proc Natl Acad Sci U S A. 1991 May 1;88(9):4060–4060. doi: 10.1073/pnas.88.9.4060-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. C., Wang Y., Filer D. Identification by sequence analysis of a second rat brain cDNA encoding the dopamine (D2) receptor. Biochem Biophys Res Commun. 1990 Jan 15;166(1):109–112. doi: 10.1016/0006-291x(90)91917-h. [DOI] [PubMed] [Google Scholar]
- Milner R. J., Sutcliffe J. G. Gene expression in rat brain. Nucleic Acids Res. 1983 Aug 25;11(16):5497–5520. doi: 10.1093/nar/11.16.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M., Kobayashi M., Asou H., Uyemura K. Molecular cloning of cDNA encoding the rat neural cell adhesion molecule L1. Two L1 isoforms in the cytoplasmic region are produced by differential splicing. FEBS Lett. 1991 Sep 2;289(1):91–95. doi: 10.1016/0014-5793(91)80915-p. [DOI] [PubMed] [Google Scholar]
- Monsma F. J., Jr, McVittie L. D., Gerfen C. R., Mahan L. C., Sibley D. R. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989 Dec 21;342(6252):926–929. doi: 10.1038/342926a0. [DOI] [PubMed] [Google Scholar]
- Montmayeur J. P., Bausero P., Amlaiky N., Maroteaux L., Hen R., Borrelli E. Differential expression of the mouse D2 dopamine receptor isoforms. FEBS Lett. 1991 Jan 28;278(2):239–243. doi: 10.1016/0014-5793(91)80125-m. [DOI] [PubMed] [Google Scholar]
- Monyer H., Seeburg P. H., Wisden W. Glutamate-operated channels: developmentally early and mature forms arise by alternative splicing. Neuron. 1991 May;6(5):799–810. doi: 10.1016/0896-6273(91)90176-z. [DOI] [PubMed] [Google Scholar]
- Morgan B. A., Johnson W. A., Hirsh J. Regulated splicing produces different forms of dopa decarboxylase in the central nervous system and hypoderm of Drosophila melanogaster. EMBO J. 1986 Dec 1;5(12):3335–3342. doi: 10.1002/j.1460-2075.1986.tb04648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan G. J., Guo W., Wormsley S., Helfman D. M. Polypyrimidine tract binding protein interacts with sequences involved in alternative splicing of beta-tropomyosin pre-mRNA. J Biol Chem. 1992 Dec 15;267(35):25480–25487. [PubMed] [Google Scholar]
- Nakanishi N., Axel R., Shneider N. A. Alternative splicing generates functionally distinct N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8552–8556. doi: 10.1073/pnas.89.18.8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawa H., Kotani H., Nakanishi S. Tissue-specific generation of two preprotachykinin mRNAs from one gene by alternative RNA splicing. Nature. 1984 Dec 20;312(5996):729–734. doi: 10.1038/312729a0. [DOI] [PubMed] [Google Scholar]
- O'Malley K. L., Anhalt M. J., Martin B. M., Kelsoe J. R., Winfield S. L., Ginns E. I. Isolation and characterization of the human tyrosine hydroxylase gene: identification of 5' alternative splice sites responsible for multiple mRNAs. Biochemistry. 1987 Nov 3;26(22):6910–6914. doi: 10.1021/bi00396a007. [DOI] [PubMed] [Google Scholar]
- Oates E., Herbert E. 5' sequence of porcine and rat pro-opiomelanocortin mRNA. One porcine and two rat forms. J Biol Chem. 1984 Jun 25;259(12):7421–7425. [PubMed] [Google Scholar]
- Ono Y., Kikkawa U., Ogita K., Fujii T., Kurokawa T., Asaoka Y., Sekiguchi K., Ase K., Igarashi K., Nishizuka Y. Expression and properties of two types of protein kinase C: alternative splicing from a single gene. Science. 1987 May 29;236(4805):1116–1120. doi: 10.1126/science.3576226. [DOI] [PubMed] [Google Scholar]
- Owens G. C., Edelman G. M., Cunningham B. A. Organization of the neural cell adhesion molecule (N-CAM) gene: alternative exon usage as the basis for different membrane-associated domains. Proc Natl Acad Sci U S A. 1987 Jan;84(1):294–298. doi: 10.1073/pnas.84.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozçelik T., Leff S., Robinson W., Donlon T., Lalande M., Sanjines E., Schinzel A., Francke U. Small nuclear ribonucleoprotein polypeptide N (SNRPN), an expressed gene in the Prader-Willi syndrome critical region. Nat Genet. 1992 Dec;2(4):265–269. doi: 10.1038/ng1292-265. [DOI] [PubMed] [Google Scholar]
- Papandrikopoulou A., Doll T., Tucker R. P., Garner C. C., Matus A. Embryonic MAP2 lacks the cross-linking sidearm sequences and dendritic targeting signal of adult MAP2. Nature. 1989 Aug 24;340(6235):650–652. doi: 10.1038/340650a0. [DOI] [PubMed] [Google Scholar]
- Prior P., Schmitt B., Grenningloh G., Pribilla I., Multhaup G., Beyreuther K., Maulet Y., Werner P., Langosch D., Kirsch J. Primary structure and alternative splice variants of gephyrin, a putative glycine receptor-tubulin linker protein. Neuron. 1992 Jun;8(6):1161–1170. doi: 10.1016/0896-6273(92)90136-2. [DOI] [PubMed] [Google Scholar]
- Pyper J. M., Bolen J. B. Identification of a novel neuronal C-SRC exon expressed in human brain. Mol Cell Biol. 1990 May;10(5):2035–2040. doi: 10.1128/mcb.10.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyper J. M., Bolen J. B. Neuron-specific splicing of C-SRC RNA in human brain. J Neurosci Res. 1989 Sep;24(1):89–96. doi: 10.1002/jnr.490240113. [DOI] [PubMed] [Google Scholar]
- Raulf F., Robertson S. M., Schartl M. Evolution of the neuron-specific alternative splicing product of the c-src proto-oncogene. J Neurosci Res. 1989 Sep;24(1):81–88. doi: 10.1002/jnr.490240112. [DOI] [PubMed] [Google Scholar]
- Rettig J., Wunder F., Stocker M., Lichtinghagen R., Mastiaux F., Beckh S., Kues W., Pedarzani P., Schröter K. H., Ruppersberg J. P. Characterization of a Shaw-related potassium channel family in rat brain. EMBO J. 1992 Jul;11(7):2473–2486. doi: 10.1002/j.1460-2075.1992.tb05312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K., Good P. J., Dawid I. B. A developmentally regulated, nervous system-specific gene in Xenopus encodes a putative RNA-binding protein. New Biol. 1990 Jun;2(6):556–565. [PubMed] [Google Scholar]
- Robberson B. L., Cote G. J., Berget S. M. Exon definition may facilitate splice site selection in RNAs with multiple exons. Mol Cell Biol. 1990 Jan;10(1):84–94. doi: 10.1128/mcb.10.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesser J. R., Liittschwager K., Leff S. E. Regulation of tissue-specific splicing of the calcitonin/calcitonin gene-related peptide gene by RNA-binding proteins. J Biol Chem. 1993 Apr 15;268(11):8366–8375. [PubMed] [Google Scholar]
- Ruegg M. A., Tsim K. W., Horton S. E., Kröger S., Escher G., Gensch E. M., McMahan U. J. The agrin gene codes for a family of basal lamina proteins that differ in function and distribution. Neuron. 1992 Apr;8(4):691–699. doi: 10.1016/0896-6273(92)90090-z. [DOI] [PubMed] [Google Scholar]
- Santoni M. J., Barthels D., Vopper G., Boned A., Goridis C., Wille W. Differential exon usage involving an unusual splicing mechanism generates at least eight types of NCAM cDNA in mouse brain. EMBO J. 1989 Feb;8(2):385–392. doi: 10.1002/j.1460-2075.1989.tb03389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M., Picciotto M. R., Kreiner T., Kaldany R. R., Taussig R., Scheller R. H. Aplysia neurons express a gene encoding multiple FMRFamide neuropeptides. Cell. 1985 Jun;41(2):457–467. doi: 10.1016/s0092-8674(85)80019-2. [DOI] [PubMed] [Google Scholar]
- Schaller K. L., Krzemien D. M., McKenna N. M., Caldwell J. H. Alternatively spliced sodium channel transcripts in brain and muscle. J Neurosci. 1992 Apr;12(4):1370–1381. doi: 10.1523/JNEUROSCI.12-04-01370.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbie L. A., Hayes G., Shine J. The major dopamine D2 receptor: molecular analysis of the human D2A subtype. DNA. 1989 Nov;8(9):683–689. doi: 10.1089/dna.1.1989.8.683. [DOI] [PubMed] [Google Scholar]
- Small S. J., Akeson R. Expression of the unique NCAM VASE exon is independently regulated in distinct tissues during development. J Cell Biol. 1990 Nov;111(5 Pt 1):2089–2096. doi: 10.1083/jcb.111.5.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small S. J., Haines S. L., Akeson R. A. Polypeptide variation in an N-CAM extracellular immunoglobulin-like fold is developmentally regulated through alternative splicing. Neuron. 1988 Dec;1(10):1007–1017. doi: 10.1016/0896-6273(88)90158-4. [DOI] [PubMed] [Google Scholar]
- Snutch T. P., Tomlinson W. J., Leonard J. P., Gilbert M. M. Distinct calcium channels are generated by alternative splicing and are differentially expressed in the mammalian CNS. Neuron. 1991 Jul;7(1):45–57. doi: 10.1016/0896-6273(91)90073-9. [DOI] [PubMed] [Google Scholar]
- Sommer B., Keinänen K., Verdoorn T. A., Wisden W., Burnashev N., Herb A., Köhler M., Takagi T., Sakmann B., Seeburg P. H. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990 Sep 28;249(4976):1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- Sommer B., Köhler M., Sprengel R., Seeburg P. H. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991 Oct 4;67(1):11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Sontheimer E. J., Steitz J. A. The U5 and U6 small nuclear RNAs as active site components of the spliceosome. Science. 1993 Dec 24;262(5142):1989–1996. doi: 10.1126/science.8266094. [DOI] [PubMed] [Google Scholar]
- Stamm S., Casper D., Dinsmore J., Kaufmann C. A., Brosius J., Helfman D. M. Clathrin light chain B: gene structure and neuron-specific splicing. Nucleic Acids Res. 1992 Oct 11;20(19):5097–5103. doi: 10.1093/nar/20.19.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm S., Casper D., Lees-Miller J. P., Helfman D. M. Brain-specific tropomyosins TMBr-1 and TMBr-3 have distinct patterns of expression during development and in adult brain. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9857–9861. doi: 10.1073/pnas.90.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staugaitis S. M., Smith P. R., Colman D. R. Expression of myelin basic protein isoforms in nonglial cells. J Cell Biol. 1990 May;110(5):1719–1727. doi: 10.1083/jcb.110.5.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steingrimsdottir H., Rowley G., Dorado G., Cole J., Lehmann A. R. Mutations which alter splicing in the human hypoxanthine-guanine phosphoribosyltransferase gene. Nucleic Acids Res. 1992 Mar 25;20(6):1201–1208. doi: 10.1093/nar/20.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. M., Schneider T. D. Features of spliceosome evolution and function inferred from an analysis of the information at human splice sites. J Mol Biol. 1992 Dec 20;228(4):1124–1136. doi: 10.1016/0022-2836(92)90320-j. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. mRNA in the mammalian central nervous system. Annu Rev Neurosci. 1988;11:157–198. doi: 10.1146/annurev.ne.11.030188.001105. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Czernik A. J., Kao H. T., Takei K., Johnston P. A., Horiuchi A., Kanazir S. D., Wagner M. A., Perin M. S., De Camilli P. Synapsins: mosaics of shared and individual domains in a family of synaptic vesicle phosphoproteins. Science. 1989 Sep 29;245(4925):1474–1480. doi: 10.1126/science.2506642. [DOI] [PubMed] [Google Scholar]
- Südhof T. C. The structure of the human synapsin I gene and protein. J Biol Chem. 1990 May 15;265(14):7849–7852. [PubMed] [Google Scholar]
- Takahashi Y. Gene expression in cells of the central nervous system. Prog Neurobiol. 1992 Jun;38(6):523–569. doi: 10.1016/0301-0082(92)90041-c. [DOI] [PubMed] [Google Scholar]
- Tsukamoto T., Toyama R., Itoh H., Kozasa T., Matsuoka M., Kaziro Y. Structure of the human gene and two rat cDNAs encoding the alpha chain of GTP-binding regulatory protein Go: two different mRNAs are generated by alternative splicing. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):2974–2978. doi: 10.1073/pnas.88.8.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushkaryov Y. A., Petrenko A. G., Geppert M., Südhof T. C. Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science. 1992 Jul 3;257(5066):50–56. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- Ushkaryov Y. A., Südhof T. C. Neurexin III alpha: extensive alternative splicing generates membrane-bound and soluble forms. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6410–6414. doi: 10.1073/pnas.90.14.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. B., Burt D. R. Differential expression of two forms of GABAA receptor gamma 2-subunit in mice. Brain Res Bull. 1991 Nov;27(5):731–735. doi: 10.1016/0361-9230(91)90054-n. [DOI] [PubMed] [Google Scholar]
- Watakabe A., Tanaka K., Shimura Y. The role of exon sequences in splice site selection. Genes Dev. 1993 Mar;7(3):407–418. doi: 10.1101/gad.7.3.407. [DOI] [PubMed] [Google Scholar]
- Whiting P., McKernan R. M., Iversen L. L. Another mechanism for creating diversity in gamma-aminobutyrate type A receptors: RNA splicing directs expression of two forms of gamma 2 phosphorylation site. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9966–9970. doi: 10.1073/pnas.87.24.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth T., Priess A., Annweiler A., Zwilling S., Oeler B. Multiple Oct2 isoforms are generated by alternative splicing. Nucleic Acids Res. 1991 Jan 11;19(1):43–51. doi: 10.1093/nar/19.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R., Teng J., Cooper T. A. The cardiac troponin T alternative exon contains a novel purine-rich positive splicing element. Mol Cell Biol. 1993 Jun;13(6):3660–3674. doi: 10.1128/mcb.13.6.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeakley J. M., Hedjran F., Morfin J. P., Merillat N., Rosenfeld M. G., Emeson R. B. Control of calcitonin/calcitonin gene-related peptide pre-mRNA processing by constitutive intron and exon elements. Mol Cell Biol. 1993 Oct;13(10):5999–6011. doi: 10.1128/mcb.13.10.5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S., Imoto K., Kawamura T., Higashida H., Iwabe N., Miyata T., Numa S. Potassium channels from NG108-15 neuroblastoma-glioma hybrid cells. Primary structure and functional expression from cDNAs. FEBS Lett. 1989 Dec 18;259(1):37–42. doi: 10.1016/0014-5793(89)81488-7. [DOI] [PubMed] [Google Scholar]
- Zahler A. M., Neugebauer K. M., Lane W. S., Roth M. B. Distinct functions of SR proteins in alternative pre-mRNA splicing. Science. 1993 Apr 9;260(5105):219–222. doi: 10.1126/science.8385799. [DOI] [PubMed] [Google Scholar]
- Zhang M. Q., Marr T. G. Fission yeast gene structure and recognition. Nucleic Acids Res. 1994 May 11;22(9):1750–1759. doi: 10.1093/nar/22.9.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]



