Abstract
Since being proposed as a unique subtype of late-life depression (LLD), the vascular depression hypothesis has received considerable research attention. Although this effort has generated considerable empirical support for the validity of the subtype, fundamental questions remain including how the illness is defined, whether cerebrovascular disease and executive dysfunction (ED) define two separate entities or one underlying subtype, and whether ED is responsible for poor response to antidepressant treatment. In this guest editorial, we explore these and other issues (i.e., the role of personality and social support, psychosocial treatments targeting cognitive abilities frequently impaired in LLD) using a number of important papers that are either directly or indirectly related to the vascular depression hypothesis. In so doing we highlight a range of critical problems facing the vascular depression hypothesis and the effort to establish the illness as a unique diagnostic entity in late-life.
Keywords: vascular depression, late-onset depression, executive dysfunction, cerebrovascular disease, treatment outcome, geriatric depression
Since being proposed as a subtype of depression in late-life (1–4), the vascular depression hypothesis has received considerable research attention. The vascular depression hypothesis originated from the following findings: 1) patients with late-onset depression (LOD) had higher rates and greater severity of hyperintensities on T2-weighted brain MRI when compared with patients with early-onset depression (EOD) (3, 5–7), 2) patients with LOD and MRI hyperintesities demonstrated greater neuropsychological impairment than patients with EOD (1, 5, 8), and 3) greater severity and higher rates of MRI hyperintensities were associated with poor response to treatment (3). A coherent theory of vascular depression began to emerge in which cerebrovascular disease causes structural damage to corticostriatal circuits creating a vulnerability to antidepressant treatment resistant LOD that is characterized by the presence of deficits in executive functioning (1, 6, 9) (see Figure 1).
Figure 1.
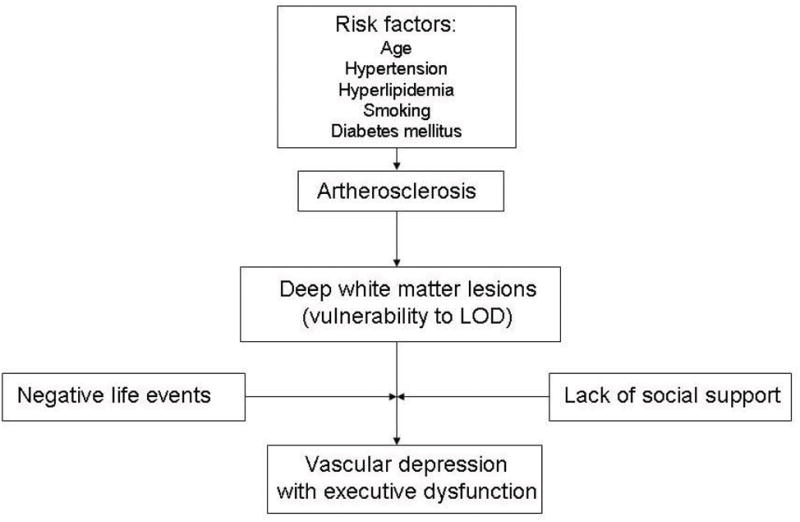
A schematic diagram depicting the vascular depression hypothesis [based on Krishnan and McDonald (4)].
Throughout the history of the vascular depression hypothesis, a number of researchers have proposed different diagnostic criteria (1, 2, 10–12). Alexopoulos et al (13) regarded clinical and or/laboratory evidence of vascular disease and late depression onset (after age 65) as cardinal features whereas Krishnan et al (6) considered clinical and/or neuroimaging evidence of cerebrovascular disease and NP impairment as the defining features of the illness. Since then, both researchers have further refined their notions of vascular depression. Krishnan and his colleagues (10) have proposed a subcortical ischemic depression (SID) in which only MRI evidence of cerebrovascular pathology is needed to define the disorder. Alexopoulos and colleagues proposed a depression executive dysfunction (DED) disorder of late-life that, while recognizing the role of cerebrovascular pathology in the etiology of the illness, only requires executive dysfunction (ED) to meet diagnostic criteria (11, 12).
Lack of consensus on criteria makes it difficult to interpret discrepant findings across studies (14). For example, Sheline et al (15) found that neuropsychological test performance and MRI hyperintensities predicted depression symptom severity over a 12-week course of treatment in depressed older adults. However, their definition of vascular depression did not correspond to any prior definition of the illness making it difficult to compare findings across studies (6, 10, 16). To address problems like these, Sneed et al (16) used data from two, large clinical samples of late-life depressed patients and showed that the vascular group, defined by a high probability of having MRI hyperintensities (deep white matter and periventricular), ED, and late age-at-onset, was most accurately identified by deep white matter lesions. Although deep white matter hyperintensities (DWMH) were the only indicator necessary to determine class membership, the use of ED as a secondary feature increased the accuracy of diagnostic classification suggesting that ED represents a critical secondary feature of the subtype.
In what follows, we highlight a number of critical questions facing the vascular depression hypothesis. Interestingly, none of the articles selected for this editorial contains the term “vascular depression” in its title. A number of papers, however, contain the phrase “depression executive dysfunction syndrome” or “executive function.” This raises the first and perhaps most important question: What is the relationship between vascular depression and ED? In this issue, Kim et al (17) examine the role of vascular risk factors in the development of the proposed DED syndrome. The authors found a significant association between a previous cerebrovascular attack and DED whereas depression without ED was unrelated to a previous cerebrovascular attack. These findings raise the possibility that the different criteria used to define vascular depression and DED ultimately define that same patient population.
Diagnostic overlap is highlighted in studies of antidepressant treatment response. Some studies have shown that being classified as vascular depressed predicts poor antidepressant treatment response (3, 18–22) whereas other studies have shown a role for ED in predicting poor antidepressant treatment response (23–26). In one study examining the independent roles of hyperintensity burden, ED, and late age-at-onset in predicting antidepressant treatment response, Sneed et al (26) found that only ED (as measured by the Stroop Color Word Test) predicted poor response. In contrast to the predictive utility of hyperintensity burden, studies examining the predictive utility of ED rely on different tests that tap different domains of this construct. Therefore, it is unclear which aspect of this broad and multifaceted construct is responsible for poor antidepressant response. In this issue, Morimoto and colleagues (27) examine the role of semantic strategy in explaining the relationship between deficits on the initiation/perseveration subscale of the Mattis Dementia Rating Scale and remission. The authors found that only the verbal fluency task of this subscale predicted remission and that executive impairment in verbal strategy explained performance on the verbal fluency task. This suggests that verbal strategy is responsible for lack of remission.
The role of personality (e.g., personality traits like neuroticism, extraversion, openness to experience, conscientiousness, and agreeableness) in LOD in general, and vascular depression in particular, has been largely ignored. The theoretical justification for this stems from the early finding that patients with LOD had higher rates of DWMH compared to patients with EOD (3, 5–7) indicating that the etiology of LOD was vascular and not related to a long-standing predisposition to depression. This notion was supported by data showing that neuroticism levels are lower in depressed women with LOD compared to depressed women with EOD (28). However, this idea is based on the faulty assumption that someone with EOD could not have a vascular depression later in life (16, 29). Moreover, because there is an established relationship between psychosocial stress and the development of vascular risk factors (30, 31), a role for personality seems natural and compelling. Although not directly related to the vascular depression hypothesis, Oddone and colleagues (32) in this issue examine the relationship between social support, personality, and depression among older adults with and without depression. They found that depressed older adults reported higher neuroticism and lower extraversion, openness, and conscientiousness and had more impairment in instrumental social support, social interaction and subjective social support than non-depressed adults. Furthermore, subjective social support modified the association between personality and depressive symptoms. These findings are important not only because they support the role of personality in late-life depression (LLD) but also because they support the important role of social support, which was implicated in early models of the vascular depression hypothesis (4).
The vascular depression hypothesis is specific regarding the location of DWMH. In particular, the functional deficits of the subtype have been hypothesized to reflect lesions within frontostriatal circuits that are integral to emotion regulation and executive functioning (13). Studies have documented neuroimaging evidence of cerebrovascular irregularities in the form of DWMH and/or reduced volume in frontal and subcortical regions (33–37). Previous reports of morphometric studies in LLD have documented neuronal abnormalities within the prefrontal cortex (38, 39). In this issue, Khundakar and colleagues (40) extend existing findings by conducting the first morphometric investigation of the caudate nucleus in LLD. They found a significant reduction in neuronal density in both the dorsolateral and ventromedial areas of the caudate nucleus, although there was no evidence of changes in glial density or in neuronal volume. These findings suggest that neuronal abnormalities in some LLD are present not only within the frontal region but also within the striatal region, which is consistent with the vascular depression hypothesis.
Cognitive impairment is common in LLD, particularly in memory (41), visuospatial functioning (42), information processing speed (8), and executive functioning (43). In this issue, Yen and colleagues (44) examined the impact of depressive symptoms on memory, reasoning, speed of processing abilities and everyday problem-solving. The relationship between LLD and functional disability (i.e., everyday problem-solving difficulties) was mediated by cognitive abilities such as learning, memory, and reasoning (a component of executive functioning). Given the relationship between ED and poor antidepressant treatment response, problem-solving therapy has been proposed as an effective alternative to antidepressant medication in the treatment of LLD (45, 46). However, the findings of Yen et al (44) in this issue suggest that successful interventions may also need to target other cognitive abilities (i.e., learning and memory) that are impaired in LLD.
At this point, the critical question facing the field with regard to the vascular depression hypothesis is how to define the patient population (14, 16). Researchers have gravitated towards defining the population in two different but certainly related ways: One in terms of functional outcomes (ED and treatment outcome) and the other in terms of underlying pathophysiology (DWMH burden). To examine the relative value and contribution of these factors in defining the subtype, studies need to include comprehensive neuropsychological and neuroimaging evaluations. One of the critical issues facing such an endeavor is the definition and measurement of executive functioning as it consists of a number of different components (47, 48), and tests of executive functioning often measure multiple cognitive abilities making it difficult to know what aspect is operating (49). Consequently, it is also difficult to determine whether the underlying deficit is attributed to higher order functions or lower order cognitive processes (i.e., processing speed) (50–52). Finally, in order to make real progress regarding the validity of this proposed subtype, its longitudinal course needs to be characterized (16). By differentiating the course of illness between diagnostic groups much of the definitional issues that exist within the subtype may be resolved.
Acknowledgments
This research was supported by National Institute of Mental Health grants K23 MH075006 (JRS) and R21 MH087774 (JRS).
Contributor Information
Joel R. Sneed, Queens College, CUNY, Columbia University and the New York State Psychiatric Institute
Michelle E. Culang-Reinlieb, The Graduate Center, City University of New York
References
- 1.Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. [see comment] Archives of General Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 2.Steffens DC, Krishnan KR. Structural neuroimaging and mood disorders: recent findings, implications for classification, and future directions. Biological Psychiatry. 1998;43:705–712. doi: 10.1016/s0006-3223(98)00084-5. [DOI] [PubMed] [Google Scholar]
- 3.Hickie I, Scott E, Mitchell P, et al. Subcortical hyperintensities on magnetic resonance imaging: Clinical correlates and prognostic significance in patients with severe depression. Biological Psychiatry. 1995;37:151–160. doi: 10.1016/0006-3223(94)00174-2. [DOI] [PubMed] [Google Scholar]
- 4.Krishnan KR, McDonald WM. Arteriosclerotic Depression. Medical Hypotheses. 1995;44:111–115. doi: 10.1016/0306-9877(95)90081-0. [DOI] [PubMed] [Google Scholar]
- 5.Salloway S, Malloy P, Kohn R, et al. MRI and neuropsychological differences in early- and late-life-onset geriatric depression. Neurology. 1996;46:1567–1574. doi: 10.1212/wnl.46.6.1567. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. American Journal of Psychiatry. 1997;154:497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- 7.Figiel GS, Krishnan KR, Doraiswamy PM, et al. Subcortical hyperintensities on brain magnetic resonance imaging: a comparison between late age onset and early onset elderly depressed subjects. Neurobiol Aging. 1991;12:245–247. doi: 10.1016/0197-4580(91)90104-r. [DOI] [PubMed] [Google Scholar]
- 8.Lesser I, Boone K, Mehringer C, et al. Cognition and white matter hyperintensities in older depressed patients. American Journal of Psychiatry. 1996;153:1280–1287. doi: 10.1176/ajp.153.10.1280. [DOI] [PubMed] [Google Scholar]
- 9.Alexopoulos GS, Meyers BS, Young RC, et al. Clinically defined vascular depression. American Journal of Psychiatry. 1997;154:562–565. doi: 10.1176/ajp.154.4.562. [DOI] [PubMed] [Google Scholar]
- 10.Krishnan KR, Taylor WD, McQuoid DR, et al. Clinical characteristics of magnetic resonance imaging-defined subcortical ischemic depression. Biological Psychiatry. 2004;55:390–397. doi: 10.1016/j.biopsych.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Alexopoulos GS. “The depression-executive dysfunction syndrome of late life”: a specific target for D3 agonists? American Journal of Geriatric Psychiatry. 2001;9:22–29. [PubMed] [Google Scholar]
- 12.Alexopoulos GS, Kiosses DN, Klimstra S, et al. Clinical presentation of the “depression-executive dysfunction syndrome” of late life. American Journal of Geriatric Psychiatry. 2002;10:98–106. [PubMed] [Google Scholar]
- 13.Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 14.Sneed JR, Roose SP, Sackeim HA. Vascular depression: A distinct diagnostic entity? Biological Psychiatry. 2006;60:1295–1298. doi: 10.1016/j.biopsych.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Sheline YI, Pieper CF, Barch DM, et al. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67:277–285. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sneed JR, Rindskopf D, Steffens DC, et al. The vascular depression subtype: Evidence of internal validity. Biological Psychiatry. 2008;64:491–497. doi: 10.1016/j.biopsych.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim B, Lee D, Lee D, et al. The role of vascular risk factors in the development of Depression with Executive Dysfunction(DED) syndrome among an elderly community sample. American Journal of Geriatric Psychiatry. doi: 10.1097/JGP.0b013e31820119b6. In press. [DOI] [PubMed] [Google Scholar]
- 18.Alexopoulos GS, Kiosses DN, Choi SJ, et al. Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. American Journal of Psychiatry. 2002;159:1929–1932. doi: 10.1176/appi.ajp.159.11.1929. [DOI] [PubMed] [Google Scholar]
- 19.Taylor WD, Steffens DC, MacFall JR, et al. White matter hyperintensity progression and late-life depression outcomes. Archives of General Psychiatry. 2003;60:1090–1096. doi: 10.1001/archpsyc.60.11.1090. [DOI] [PubMed] [Google Scholar]
- 20.Simpson S, Baldwin RC, Jackson A, et al. Is subcortical disease associated with a poor response to antidepressants? Neurological, neuropsychological, and neuroradiological findings in late-life depression. Psychological Medicine. 1998;28:1015–1026. doi: 10.1017/s003329179800693x. [DOI] [PubMed] [Google Scholar]
- 21.Navarro V, Gasto C, Lomena F, et al. Prognostic value of frontal functional neuroimaging in late-onset severe major depression. Br J Psychiatry. 2004;184:306–311. doi: 10.1192/bjp.184.4.306. [DOI] [PubMed] [Google Scholar]
- 22.Alexopoulos GS, Murphy CF, Gunning-Dixon FM, et al. Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry. 2008;165:238–244. doi: 10.1176/appi.ajp.2007.07050744. [DOI] [PubMed] [Google Scholar]
- 23.Dunkin JJ, Leuchter AF, Cook IA, et al. Executive dysfunction predicts nonresponse to fluoxetine in major depression. Journal of Affect Disorders. 2000;60:12–23. doi: 10.1016/s0165-0327(99)00157-3. [DOI] [PubMed] [Google Scholar]
- 24.Sneed JR, Culang ME, Keilp JG, et al. Antidepressant medication and executive dysfunction: a deleterious interaction in late-life depression. American Journal of Geriatric Psychiatry. 2010;18:128–135. doi: 10.1097/JGP.0b013e3181c796d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexopoulos G, Kiosses DN, MH, et al. Executive dysfunction and the course of geriatric depression. Biological Psychiatry. 2005;58:204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 26.Sneed JR, Roose SP, Keilp JG, et al. Response inhibition predicts poor antidepressant treatment response in very old depressed patients. American Journal of Geriatric Psychiatry. 2007;15:553–563. doi: 10.1097/JGP.0b013e3180302513. [DOI] [PubMed] [Google Scholar]
- 27.Morimoto S, Gunning FM, Murphy CF, et al. Executive function and short-term remission of geriatric depression: The role of semantic strategy. American Journal of Geriatric Psychiatry. doi: 10.1097/JGP.0b013e3181e751c4. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sneed JR, Kasen S, Cohen P. Early-life risk factors for late-onset depression. International Journal of Geriatric Psychiatry. 2007;22:663–667. doi: 10.1002/gps.1727. [DOI] [PubMed] [Google Scholar]
- 29.Alexopoulos GS. The vascular depression hypothesis: 10 years later. Biological Psychiatry. 2006;60:1304–1305. doi: 10.1016/j.biopsych.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Pickering TG. Stress, inflammation, and hypertension. J Clin Hypertens. 2007;9:567–571. doi: 10.1111/j.1524-6175.2007.06301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. J Psychosom Res. 2002;52:1–23. doi: 10.1016/s0022-3999(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 32.Oddone C, Hybels CF, McQuoid DR, et al. Social support modifies the relationship between personality and depressive symptoms in older adults. American Journal of Geriatric Psychiatry. doi: 10.1097/jgp.0b013e3181f7d89a. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Firbank MJ, Lloyd AJ, Ferrier N, et al. A volumetric study of MRI signal hyperintensities in late-life depression. American Journal of Geriatric Psychiatry. 2004;12:606–612. doi: 10.1176/appi.ajgp.12.6.606. [DOI] [PubMed] [Google Scholar]
- 34.Greenwald BS, Kramer-Ginsberg E, Krishnan KR, et al. Neuroanatomic localization of magnetic resonance imaging signal hyperintensities in geriatric depression. Stroke. 1998;29:613–617. doi: 10.1161/01.str.29.3.613. [DOI] [PubMed] [Google Scholar]
- 35.Taylor WD, MacFall JR, Payne ME, et al. Orbitofrontal cortex volume in late life depression: influence of hyperintense lesions and genetic polymorphisms. Psychological Medicine. 2007;37:1763–1773. doi: 10.1017/S0033291707000128. [DOI] [PubMed] [Google Scholar]
- 36.Artero S, Tiemeier H, Prins ND, et al. Neuroanatomical localization and clinical correlates of white matter lesions in the elderly. J neurol Neurosurg. 2004;75:1304–1308. doi: 10.1136/jnnp.2003.023713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hannestad J, Taylor WD, McQuoid DR, et al. White matter lesion volumes and caudate volumes in late-life depression. International Journal of Geriatric Psychiatry. 2006;21:1193–1198. doi: 10.1002/gps.1640. [DOI] [PubMed] [Google Scholar]
- 38.Khundakar A, Morris C, Oakley A, et al. Morphometric analysis of neuronal and glial cell pathology in the dorsolateral prefrontal cortex in late-life depression. Br J Psychiatry. 2009;195:163–169. doi: 10.1192/bjp.bp.108.052688. [DOI] [PubMed] [Google Scholar]
- 39.Rajkowska G, Miguel-Hidalgo JJ, Dubey P, et al. Prominent reduction in pyramidal neurons density in the orbitofrontal cortex of elderly depressed patients. Biological Psychiatry. 2005;58:297–306. doi: 10.1016/j.biopsych.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khundakar A, Morris C, Oakley A, et al. Morphometric analysis of neuronal and glial cell pathology in the caudate nucleus in late-life depression. American Journal of Geriatric Psychiatry. doi: 10.1097/JGP.0b013e3181df4642. In press. [DOI] [PubMed] [Google Scholar]
- 41.Salloway S, Malloy P, Kohn R, et al. MRI and neuropsychological differences in early- and late-lifeonset geriatric depression. Neurology. 1996;46:1567–1574. doi: 10.1212/wnl.46.6.1567. [DOI] [PubMed] [Google Scholar]
- 42.Kramer-Ginsberg E, Greenwald BS, Krishnan KRR, et al. Neuropsychological Functioning and MRI Signal Hyperintensities in Geriatric Depression. Am J Psychiatry. 1999;156:438–444. doi: 10.1176/ajp.156.3.438. [DOI] [PubMed] [Google Scholar]
- 43.Lockwood KA, SAG, van Gorp WG. Executive Dysfunction in Geriatric Depression. Am J Psychiatry. 2002;159:1119–1126. doi: 10.1176/appi.ajp.159.7.1119. [DOI] [PubMed] [Google Scholar]
- 44.Yen Y, Rebok G, Gallo J, et al. Depressive symptoms impair everyday problem-solving ability through cognitive abilities in late life. American Journal of Geriatric Psychiatry. doi: 10.1097/JGP.0b013e3181e89894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiosses DN, Teri L, Velligan DI, et al. A home-delivered intervention for depressed, cognitively impaired, disabled elders. International Journal of Geriatric Psychiatry. 2010 doi: 10.1002/gps.2521. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alexopoulos GS, Raue P, Arean P. Problem-solving therapy versus supportive therapy in geriatric major depression with executive dysfunction. American Journal of Geriatric Psychiatry. 2003;11:46–52. [PubMed] [Google Scholar]
- 47.Miyake A, Freidman NP, Emerson MJ, et al. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 48.Podell K, Lovell MR. Neuropsychological assessment. In: Coffey CE, Cummings JL, editors. Textbook of Geriatric Neuropsychiatry. Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- 49.Lezak M, Howieson DB, Loring DW. Neuropsychological Assessment. 4. New York: Oxford; 2004. [Google Scholar]
- 50.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Reveiw. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 51.Fristoe NM, Salthouse TA, Woodard JL. Examination of age-related deficits on the Wisconsin Card Sorting Test. Neuropsychology. 1997;11:428–436. doi: 10.1037//0894-4105.11.3.428. [DOI] [PubMed] [Google Scholar]
- 52.Kail R, Salthouse TA. Processing speed as a mental capacity. Acta Psychologica. 1994;86:199–225. doi: 10.1016/0001-6918(94)90003-5. [DOI] [PubMed] [Google Scholar]


