Abstract
Breast cancers with BRCA2 mutations exhibit DNA repair defects and are particularly sensitive to radiation. BRCA2 interacts with Rad51 in a complex manner involving internal BRC and C-terminal TR2 domains which play a key role in homologous recombination. BRCA2 expression also modulates Rad51 protein levels such that Rad51 protein is relatively decreased in BRCA2-defective cancer cells. This is mediated in part through BRCA2's capacity to protect Rad51 from caspase-3 proteolytic degradation. In order to distinguish between functional and expression related roles for BRCA2 we studied the results of Rad51 overexpression in mouse and human cells with inactivating BRCA2 mutations. The results show that overexpression of wild-type Rad51 partially rescues BRCA2 deficiency but that overexpression of a caspase-3 resistant Rad51 completely complements the BRCA2 defect in radiation responsiveness. These results indicate that Rad51 can compensate for some aspects of a BRCA2 gene defect and suggest that Rad51 expression levels may be an important modifier of the BRCA2 defective genotype.
Keywords: BRCA2, Rad51, breast cancer
Introduction
BRCA2 has been shown to interact with Rad51 through both a central BRC domain and a regulated C-terminal domain mediated by phosphorylation at serine 3291 (reviewed in Ref. [1]). One proposed model suggests that BRCA2 disrupts Rad51 oligomers, sequestering Rad51 in an inactive monomeric form. Dephosphorylation of BRCA2 supports the oligomerization of Rad51 on the nucleiprotein filament and then BRCA2 phosphorylation may terminate the process by inactivating Rad51. This model is supported by protein structural data [2,3] and studies of the C. elegans BRCA2 ortholog [4]. In addition to these functional protein interactions, BRCA2 also influences Rad51 protein levels since BRCA2 protein protects Rad51 from caspase-3 cleavage [5]. Rad51 is also thought to be transported to the nucleus by both BRCA2-dependent and BRCA2-independent pathways [6].
Mutations in the BRCA2 gene are responsible for 30% of early-onset, inherited forms of breast cancer [7]. Partial and complete knockout studies performed in mice have shown phenotypes ranging from the development of lymphomas to embryonic lethality [8–12]. Furthermore, BRCA2 knockout studies resulted in an accumulation of chromosomal aberrations [13,14]. Both observations substantiate that BRCA2 plays an important role in maintaining genomic integrity, which as revealed from these studies, is integral for embryonic development and regulation of cell growth. Functionally, BRCA2 has been defined as a tumor suppressor [15,16], due to the observations correlating BRCA2 mutations, particularly those that are C-terminal truncations, with tumor development.
In order to distinguish between functional and expression related roles for BRCA2 the present study evaluates results of Rad51 overexpression in mouse and human cells with inactivating BRCA2 mutations. The results show that Rad51 protein levels are increased in BRCA2 expressing cells and that Rad51 overexpression decreases the radiation sensitivity observed in BRCA2-defective cells. Caspase-3 resistant Rad51 (Rad51-D184, 187A) produces a greater decrease in radiation sensitivity than wild-type Rad51, presumably due to its greater stability in the setting of BRCA2-defective cells.
Materials and Methods
Cell Culture
Capan-l pancreatic cancer cells and BRCA2lexl/lex2 (brca2 −/−) mouse embryonic fibroblast (MEF) cells were maintained in Dulbecco's Modified Eagle Medium (DMEM) at 37°C, with 5% CO2.
Transfections and Generation of Stable Cell Lines
Full-length human BRCA2 cDNA (confirmed by DNA sequence) was cloned into pREP4 to produce an RSV-regulated expression vector. Transient and stable transfections were performed in Capan-l by lipofection using Targefect F-l (Targeting Systems) and in brca2−/− by a calcium phosphate-mediated protocol. Stable transfectants were pooled (at least 100 clones for each transfectant pool). BRCA2 expression in both cell types was confirmed by Western blot analysis using an antibody raised against the C-terminus of BRCA2 (BRCA2, amino acids 3245–3418, Pharmingen, San Diego, CA). Wild-type and non-cleavable GFP-tagged human RAD51 constructs were a generous gift from Yinyin Huang at theDana-Farber Cancer Institute [17].
Immunofluorescence
The cells were seeded and allowed to grow onto cover slips in six well tissue culture plates. After the designated treatments, the cells were fixed in 2% paraformaldehyde, neutralized with 1 M glycine-tris, pH 7.4 and washed for 5 min in PBS. Next, the cells were probed with an affinity purified rabbit anti-human RAD51 antibody (Ab-l, Oncogene Research Products, La Jolla, CA) diluted 1:5000 in dilution buffer (5% normal donkey serum, 1% BSA-Fraction V and 0.1% Triton X-l00 in PBS), washed three times for 5 min in PBS, probed with Cy3-conjugated anti-rabbit secondary antibody (Jackson Laboratories, Bar Harbor, ME) diluted 1:8000 in dilution buffer, and washed three times for 5 min in PBS. Finally, the nuclei were DAPI (4,6-diamidino-2-phenylindole) stained for immunofluorescence analysis.
Cells transfected with GFP-Rad51 were washed and fixed in 2% paraformaldehyde and performed directly without use of antibodies or conjugates.
Western Blotting Analyses
We used affinity purified rabbit anti-human RAD51 (Ab-1, Oncogene Research Products) at a dilution of 1:2500 and affinity purified rabbit antihuman BRCA2 (anti-BRCA2, amino acids 3245–3418, Pharmingen) at a dilution of 1:1000 as primary antibodies. For detection of green fluorescent protein (GFP)-tagged RAD51 (both wild-type and the non-cleavable mutant), anti-rabbit GFP (Clontech, Mountain View, CA) was used. For Western blotting, cell lysates were subjected to polyacrylamide gel electrophoresis (12% gel) and transferred to PVDF filters (Millipore, Boston, MA, Irnmobilon-P) overnight at 30 V, To assess loading and transfer efficiency, the membrane was stained with Ponceau S prior to blocking. The membrane was blocked using 5% nonfat dry milk, 0.2% Tween-20 in Tris-buffered saline for 1 h at room temperature. After blocking and subsequent washing, the blot was exposed to the primary antibody. The blot was washed extensively with Tris-buffered saline, hour at room temperature, 0.2% Tween-20 before being exposed to a horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch) at a dilution of 1:7500 at room temperature for 1 h. The blot was again washed extensively. Bands were visualized using the Mosal western detection employing Luminol (3 aminophthalydrazide) and p-coumaric acid.
Cell Survival Assays
Colony forming assays to assess cell survival after irradiation were performed as described previously [18]. Briefly, cells were plated at 500 cells/dish and irradiated at a given dose. After irradiation, cells were returned to the 37°C incubator. They were fed fresh media every 2 d. After 14 d, the colonies were fixed in ethanol and stained with crystal violet. Colonies were counted manually. Relative survival was determined by comparing colony number of cells irradiated at a given dose to the colony number of unirradiated cells plated at the same density and cultured for the same amount of time.
Results and Discussion
BRCA2 Expression Increases Rad51 Protein Before and After Ionizing Radiation
We have previously reported that BRCA2 protein protects Rad51 from caspase-3 degradation, suggesting that BRCA2 protein modulates Rad51 protein stability. In order to directly test this hypothesis we performed Western blots of Rad51 protein in BRCA2-defective Capan-1 cells and in Capan-1 cells rescued with wild-type BRCA2. Capan-1 cells are a pancreatic carcinoma cell lines which we have previously demonstrated is hemizygous for the 6174delT Askenazi BRCA2 mutation [18]. Figure 1A shows that Rad51 protein levels are increased in BRCA2-transfected Capan-1 cells and that irradiation produces a further increase in Rad51 protein levels. Figure 1B shows quantitation of this protein expression standardized to gamma-actin. From the Western blot it appears that wild-type BRCA2 increases Rad51 protein levels. Our previous studies have demonstrated that BRCA2 stabilizes Rad51 protein in part by inhibiting caspase-3 cleavage of Rad51. However, since less than 20% of Rad51 is caspase-3 cleaved even in irradiated BRCA2-defective cells [5] it is difficult to attribute all Rad51 expression effects of BRCA2 to an inhibition of caspase-3 cleavage.
Figure 1.
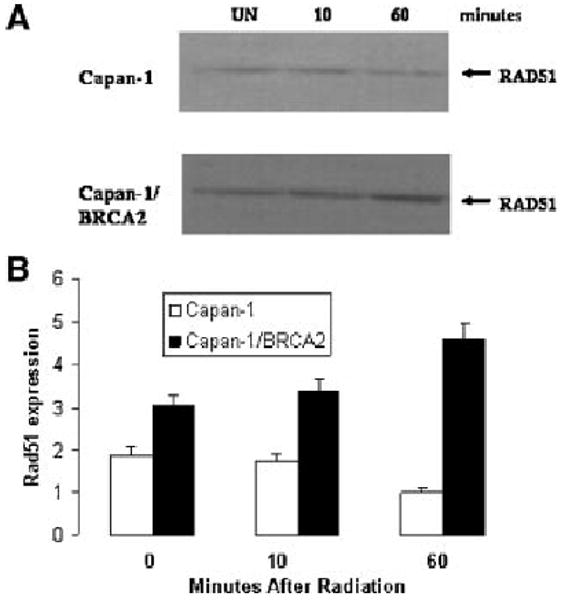
Expression of Rad51 protein in BRCA2-defective Capan-1 cells. (A) Western blot of Rad51 protein in untransfected Capan-1 (upper panel) and BRCA2-transfected Capan1 (lower panel) in unirradiated cells (UN) or cells either 10 min (10) or 60 min (60) after irradiation with 10 Gy. (B) Quantitation of Western blots of Rad51 protein standardized to tubulin protein levels using relative expression units. Four separate Western blots were quantitated and standard error is shown by error bars.
BRCA2 Expression Increases Rad51 Localization to Nuclear Foci
In addition to measuring levels of Rad51 in BRCA2-defective and transfected cells, we also performed immunocytochemistry to determine the localization of Rad51 in cells before and after 10 Gy ionizing radiation. Rad51 appears to be mixed nuclear and cytoplasmic and expressed at relatively low levels in Capan-1 cells before and after 10 Gy irradiation (Figure 2; first column). Rad51 is highly expressed and visualized in clearly apparent Rad51 foci in BRCA2 transfected Capan-1 cells 10 min following 10 Gy irradiation (Figure 2; second column). These results are consistent with prior studies showing a reduction of Rad51 foci in BRCA2-defective cells [6,11]. These data indicate that Rad51 is more highly expressed and correctly localized in BRCA2 transfected cells than in BRCA2-defective cells. Figure 2 shows that Rad51 localization is highly affected by the presence of BRCA2 which results in much stronger Rad51 foci formation following radiation. Immunofluorescence studies were performed, in which the cellular location of endogenous RAD51 in Capan-l cells with and without wild-type BRCA2 was observed before and 10 min after exposure to ionizing radiation. In cells that were rescued with wild-type BRCA2, there was an increase in nuclear concentrations of RAD51 as early as 10 min after irradiation. Some of this increase in Rad51 nuclear protein may occur because BRCA2 may physically transport Rad51 to the nucleus as has been previous reported [6].
Figure 2.

Cellular localization of RAD51. Endogenous Rad51 immunofluorescence shown in the first two columns: First column is Capan-1 and second column is BRCA2-transfected Capan-1. Composite overlay of endogenous RAD51 and DAPI-stained nuclei. Exogenous GFP-Rad51 fluorescence shown in the last two columns: 3rd column is GFP-Rad51 wild-type transfected Capan-l and 4th column is Rad51D-A transfected Capan-1. The upper row represents untreated cells and the lower row are cells that were fixed 10 min after 10 Gy ionizing radiation. The white arrow in first panel points to a cell in which RAD51 is primarily nuclear.
Overexpression of Wild-Type or Non-Cleavable RAD51 Decreases Radiation Sensitivity Similar to BRCA2-Rescue
The defective BRCA2 in Capan-l cells causes them to exhibit inefficient repair of double-strand DNA breaks and radiosensitivity-they are unable to recover from exposure to dosages of ionizing radiation that are not lethal to normal cells, subsequently leading to cell death [18]. However, after transfection of the Capan-l cells with wild-type BRCA2, a twofold decrease in radiation sensitivity was observed compared to parental Capan-l cells not rescued with wild-type BRCA2. This same study was performed in Capan-l cells transfected with either wild-type RAD51 or non-cleavable RAD51 (D-A RAD51), such that there was overexpression of either recombinant protein (Figure 3A). The Rad51 recombinant proteins were appropriately localized to the nucleus and showed Rad51 foci after 10 Gy radiation (Figure 2; 3rd and 4th columns). The Rad51 proteins were highly overexpressed and as a result there was only minimal caspase-3 cleavage of the wild-type Rad51 protein (Figure 3C, left panel) and no caspase-3 cleavage of the Rad51D-A protein. Interestingly, transfection of either Rad51 wild-type or Rad51 D-A resulted in changes in radiation sensitivity that were close to, or comparable to those seen in Capan-l cells transfected with wild-type BRCA2 (Figure 3B). Similar results were observed in transfected mouse brca2−/− cells (Figure 4).
Figure 3.

Capan-1 Cell survival after irradiation. (A) Western blot of GFP-RAD51 expression. Lane 1: Mock transfection. Lane 2: Transfection with construct expressing 62 kDa wild-type GFP-RAD51. Lane 3: Transfection with construct expressing 62 kDa noncleavable GFP-RAD51 (D-A RAD51). (B) Plates of Capan-l cells transfected with either wild-type BRCA2, wild-type RAD51 or non-cleavable RAD51 (D-A RAD51) were stained and counted for cell survival at 14 d after irradiation (N = 3). Note that the error bars of means near 0.01 are wide due to the use of a log scale. (C) Western blot of GFP-Rad51 transfected cells showing presence of caspase-3 cleavage product (arrow) compared with 62 kDa GFP-Rad51 (bar). Time after irradiation is shown in hours: Un represents unirradiated and 0.1 h represents cells frozen 6 min after 10 Gy radiation.
Figure 4.

Mouse brca2−/− Cell survival after Irradiation. Plates of mouse brca2−/− cells transfected with either wild-type BRCA2, wild-type RAD51 or non-cleavable RAD51 (D-A RAD51) were stained and counted for cell survival at 14 d after irradiation (N = 3).
These results suggest that Rad 51 and is caspase-3 resistant mutant are able to partially or fully complement the increased radiation sensitivity which is observed in BRCA2-defective mouse and human cells. However, it is unclear whether Rad51 overexpression can complement the tumor suppression function of BRCA2 or other possible functions unrelated to radiation sensitivity or DNA repair.
Individuals with a single inherited mutation in BRCA2 develop hereditary breast cancer, but individuals with both mutant BRCA2 alleles develop a hemolytic anemia called Fanconi's Anemia [19]. BRCA2 has been shown to be identical to FancD1 which is one of the components of the FANC pathway composed of other genes whose mutation leads to the disease. The FANC pathway is central to DNA damage detection in cells and leads to the coordination of repair/recombination foci of key proteins including Rad51 [20]. Understanding this pathway and the interaction of these proteins may lead to important knowledge about DNA repair and about hereditary cancer.
Acknowledgments
We thank Yinyin Huang for RAD51 and D184,187-A RAD51 expression vectors. This study was supported by the Public Health Service grants CA85269 1 (J.T.H.) and CA9694402 (E.T.B.).
Abbreviation
- GFP
green fluorescent protein
References
- 1.Lord CJ, Ashworth A. Rad51, BRCA2 and DNA repair: A partial resolution. Nat Struct Mol Biol. 2007;14:461–462. doi: 10.1038/nsmb0607-461. [DOI] [PubMed] [Google Scholar]
- 2.Esashi F, Galkin V, Yu X, Egelman EH, West S. Stabilization of RAD51 nucleoprotein filaments by the C-terminal region of BRCA2. Nat Struct Mol Biol. 2007;14:468–474. doi: 10.1038/nsmb1245. [DOI] [PubMed] [Google Scholar]
- 3.Davies OR, Pellegrini L. Interaction with the BRCA2 C terminus protects RAD51-DNA filaments from disassembly by BRC repeats. Nat Struct Mol Biol. 2007;14:475–483. doi: 10.1038/nsmb1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petalcorin MI, Galkin VE, Yu X, Egelman EH, Boulton SJ. Stabilization of RAD-51-DNA filaments via an interaction domain in Caenorhabditis elegans BRCA2. Proc Natl Acad Sci USA. 2007;104:8299–8304. doi: 10.1073/pnas.0702805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown ET, Robinson-Benion C, Holt JT. Radiation enhances Caspase 3 cleavage of Rad51 in BRCA2-defective cells. Radiat Res. 2008;169:595–601. doi: 10.1667/RR1129.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumann P, West SC. Role of the human RAD51 protein in homologous recombination and double stranded break repair. TBIS. 1998;23:247–251. doi: 10.1016/s0968-0004(98)01232-8. [DOI] [PubMed] [Google Scholar]
- 7.Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCAI and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim DS, Hasty P. A mutation in mouse RAD51 result in an early embryonic lethal that is suppressed by a mutation in p53. Mol Cell Biol. 1996;16:7133–7143. doi: 10.1128/mcb.16.12.7133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuzuki T, Fuji Y, Sajuma K, et al. Targetted disruption of the RAD51 gene leads to lethality in embryonic mice. Proc Natl Acad Sci USA. 1996;93:6236–6240. doi: 10.1073/pnas.93.13.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludwig T, Chapman DL, Papioannou VE, Efstratiadis A. Targeted mutations of breast cancer susceptibility gene homologs in mice: Lethal phenotypes of BRCAl, BRCA2, BRCAl/BRCA2, BRCAl/p53, and BRCA2/p53 nullizygous embryos. Genes Dev. 1997;11:1226–1241. doi: 10.1101/gad.11.10.1226. [DOI] [PubMed] [Google Scholar]
- 11.Sharan SK, Morimatsu M, Albrecht U, et al. Embryonic lethality and radiation hypersensitivity mediated by RAD51 in mice lacking brca2. Nature. 1997;386:804–810. doi: 10.1038/386804a0. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki A, Delapompa JL, Hakem R, et al. Brca2 is required for embryonic cellular proliferation in the mouse. Genes Dev. 1997;11:1242–1252. doi: 10.1101/gad.11.10.1242. [DOI] [PubMed] [Google Scholar]
- 13.Patel KJ, Vo VP, Lee H, et al. Involvement of Brca2 in DNA repair. Mol Cell. 1998;1:347–357. doi: 10.1016/s1097-2765(00)80035-0. [DOI] [PubMed] [Google Scholar]
- 14.Yu VP, Koehler M, Steinlein C, et al. Gross chromosomal rearrangements and genetic exchange between nonhomologous chromosomes following BRCA2 inactivation. Genes Dev. 2000;14:1400–1406. [PMC free article] [PubMed] [Google Scholar]
- 15.Connor F, Bertwistel D, Mee PJ, et al. Tumorigenesis and a DNA repair defect in mice with a truncating brca2 mutation. Nat Genet. 1997;17:423–430. doi: 10.1038/ng1297-423. [DOI] [PubMed] [Google Scholar]
- 16.Friedman LS, Thistlethwaite FC, Patel KH, Yu VP, Lee H. Thymic lymphomas in mice with a truncating mutation in Brca2. Cancer Res. 1998;58:1338–1343. [PubMed] [Google Scholar]
- 17.Huang Y, Nakada S, Ishiko T, et al. Role for caspase-mediated cleavage of Rad51 in induction of apoptosis by DNA damage. Mol Cell Biol. 1999;19:2986–2997. doi: 10.1128/mcb.19.4.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbott DW, Freeman ML, Holt JT. Double-strand break repair deficiency and radiation sensitivity in BRCA2 mutant cancer cells. J Natl Cancer Inst. 1998;90:978–985. doi: 10.1093/jnci/90.13.978. [DOI] [PubMed] [Google Scholar]
- 19.D'Andrea AD. The Fanconi road to cancer. Genes Dev. 2003;17:1933–1936. doi: 10.1101/gad.1128303. [DOI] [PubMed] [Google Scholar]
- 20.Jacquemont C, Taniguchi T. The Fanconi anemia pathway and ubiquitin. BMC Biochem. 2007;8:S10. doi: 10.1186/1471-2091-8-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]


