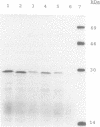
Abstract
Pokeweed antiviral protein (PAP) has N-glycosidase activity towards both eukaryotic and prokaryotic ribosomes. This is in marked contrast with the A chains of type 2 ribosome inactivating proteins (RIPs) such as ricin and abrin, which inactivate only eukaryotic ribosomes. A recent report described spontaneous mutations in PAP that implicated specific amino acids to be involved in determining the activity of PAP towards prokaryotic ribosomes. As part of an ongoing study into RIP--ribosome interactions these mutations were specifically recreated in a PAP clone encoding the mature 262 amino acid PAP sequence. Mutants were tested for their N-glycosidase activity by analysing the integrity of eukaryotic and prokaryotic ribosomes after mutant protein expression. Mutations of F196Y and K211R, either individually or within the same clone, were active toward both classes of ribosome, indicating that these amino acid positions are not involved in differentiating ribosomal substrates. Mutation R68G led to a protein that appeared to be inactive towards prokaryotic ribosomes, but also very poorly active towards eukaryotic ribosomes. This mutation is currently under further investigation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Straus J. W., Dudock B. S. Preparation of a cell-free protein-synthesizing system from wheat germ. Methods Enzymol. 1983;101:635–644. doi: 10.1016/0076-6879(83)01044-7. [DOI] [PubMed] [Google Scholar]
- Chaddock J. A., Roberts L. M. Mutagenesis and kinetic analysis of the active site Glu177 of ricin A-chain. Protein Eng. 1993 Jun;6(4):425–431. doi: 10.1093/protein/6.4.425. [DOI] [PubMed] [Google Scholar]
- Dore J. M., Gras E., Depierre F., Wijdenes J. Mutations dissociating the inhibitory activity of the pokeweed antiviral protein on eukaryote translation and Escherichia coli growth. Nucleic Acids Res. 1993 Sep 11;21(18):4200–4205. doi: 10.1093/nar/21.18.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y., Tsurugi K. The RNA N-glycosidase activity of ricin A-chain. The characteristics of the enzymatic activity of ricin A-chain with ribosomes and with rRNA. J Biol Chem. 1988 Jun 25;263(18):8735–8739. [PubMed] [Google Scholar]
- Habuka N., Murakami Y., Noma M., Kudo T., Horikoshi K. Amino acid sequence of Mirabilis antiviral protein, total synthesis of its gene and expression in Escherichia coli. J Biol Chem. 1989 Apr 25;264(12):6629–6637. [PubMed] [Google Scholar]
- Hartley M. R., Legname G., Osborn R., Chen Z., Lord J. M. Single-chain ribosome inactivating proteins from plants depurinate Escherichia coli 23S ribosomal RNA. FEBS Lett. 1991 Sep 23;290(1-2):65–68. doi: 10.1016/0014-5793(91)81227-y. [DOI] [PubMed] [Google Scholar]
- Kataoka J., Ago H., Habuka N., Furuno M., Masuta C., Miyano M., Koiwai A. Expression of a pokeweed antiviral protein in Escherichia coli and its characterization. FEBS Lett. 1993 Mar 29;320(1):31–34. doi: 10.1016/0014-5793(93)81651-f. [DOI] [PubMed] [Google Scholar]
- Kataoka J., Habuka N., Masuta C., Miyano M., Koiwai A. Isolation and analysis of a genomic clone encoding a pokeweed antiviral protein. Plant Mol Biol. 1992 Dec;20(5):879–886. doi: 10.1007/BF00027159. [DOI] [PubMed] [Google Scholar]
- Kim Y., Robertus J. D. Analysis of several key active site residues of ricin A chain by mutagenesis and X-ray crystallography. Protein Eng. 1992 Dec;5(8):775–779. doi: 10.1093/protein/5.8.775. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin Q., Chen Z. C., Antoniw J. F., White R. F. Isolation and characterization of a cDNA clone encoding the anti-viral protein from Phytolacca americana. Plant Mol Biol. 1991 Oct;17(4):609–614. doi: 10.1007/BF00037047. [DOI] [PubMed] [Google Scholar]
- Lord J. M., Hartley M. R., Roberts L. M. Ribosome inactivating proteins of plants. Semin Cell Biol. 1991 Feb;2(1):15–22. [PubMed] [Google Scholar]
- May M. J., Hartley M. R., Roberts L. M., Krieg P. A., Osborn R. W., Lord J. M. Ribosome inactivation by ricin A chain: a sensitive method to assess the activity of wild-type and mutant polypeptides. EMBO J. 1989 Jan;8(1):301–308. doi: 10.1002/j.1460-2075.1989.tb03377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzingo A. F., Collins E. J., Ernst S. R., Irvin J. D., Robertus J. D. The 2.5 A structure of pokeweed antiviral protein. J Mol Biol. 1993 Oct 20;233(4):705–715. doi: 10.1006/jmbi.1993.1547. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Vandeyar M. A., Weiner M. P., Hutton C. J., Batt C. A. A simple and rapid method for the selection of oligodeoxynucleotide-directed mutants. Gene. 1988 May 15;65(1):129–133. doi: 10.1016/0378-1119(88)90425-8. [DOI] [PubMed] [Google Scholar]