Abstract
Purpose of review
Lipid accumulation in nonadipose tissues is increasingly recognized to contribute to organ injury through a process termed lipotoxicity, but whether this process occurs in the kidney is still uncertain. This article briefly summarizes the normal role of lipids in renal physiology and the current evidence linking excess lipids and lipotoxicity to renal dysfunction.
Recent findings
Evidence suggesting that renal lipid accumulation and lipotoxicity may lead to kidney dysfunction has mounted significantly over recent years. Abnormal renal lipid content has been described in a number of animal models and has been successfully manipulated using pharmacologic or genetic strategies. There is some heterogeneity among studies with regard to the mechanisms, consequences, and localization of lipid accumulation in the kidney, explainable at least in part by inherent differences between animal models. The relevance of these findings for human pathophysiology remains to be established.
Summary
Current knowledge on renal lipid physiology and pathophysiology is insufficient, but provides a strong foundation and incentive for further exploration. The future holds significant challenges in this area, especially with regard to applicability of research findings to the human kidney in vivo, but also the opportunity to transform our understanding of an array of kidney disorders.
Keywords: free nonesterified fatty acids, kidney disease, lipotoxicity, triglycerides
Introduction
Lipids are fundamental constituents of all living cells. They are the main structural components of biological membranes, are a source and reservoir of energy, interact with proteins to modulate their localization and function, and have key roles in intracellular and intercellular signaling. It is, therefore, not surprising that most cells are endowed with complex machineries to regulate the import, synthesis, storage, utilization, and export of lipids. Some eukaryotic cells have evolved specialized lipid handling functions, one notable example being the capacity of adipocytes to store and manage massive intracellular lipid droplets containing primarily triglycerides. Smaller lipid droplets exist in most other cell types and are increasingly recognized as highly dynamic cellular components with important functions that may extend beyond lipid storage [1•,2•]. However, lipid accumulation in cells that are not equipped with the molecular tools to handle large lipid loads has been associated with cellular injury and dysfunction [3•,4–7,8•]. Whether excess lipids are a consequence or a cause of cell injury has not been universally established, but a growing body of evidence suggests that, at least in some tissues and cell types, lipid overload per se can be deleterious [5–7,8•,9•,10]. This process has been termed lipotoxicity and has been linked to dysfunction in multiple organs, including the liver, heart, skeletal muscle, and endocrine pancreas [5–7,8•,9•,10,11•,12•]. Lipid accumulation has also been described in the kidney in a variety of pathological and physiological conditions, but whether renal lipids and lipotoxicity contribute to human kidney dysfunction has not been established.
Lipids in renal physiology
The total lipid content of the normal human kidney has been estimated at approximately 3% of wet weight [13,14]. Of this (by mass), more than half are phospholipids, the major constituent of cell membranes, approximately one-fifth are triglycerides, and about one-tenth are free nonesterified fatty acids (FFAs) [13]. For comparison, the total lipid content of normal nonsteatotic human liver is 4–5% of wet weight [15]. In both kidney and liver, triglyceride content exhibits the largest inter-individual variability [13–15].
The kidney can use a relatively wide variety of substrates as fuels, depending on their availability, but substrate utilization may vary by region [16–18]. The mitochondrial b-oxidation of FFA is a major source for renal ATP production, particularly in the proximal tubule, which has a high energy demand and relatively little glycolytic capacity [19–21]. In-vivo studies using radiolabeled FFA in dogs and measurement of substrate differences between arterial and renal venous blood in humans have shown that the kidney extracts FAs from the circulation and that FFA oxidation could account for more than half of renal oxygen consumption [22,23]. Importantly, FFA extraction by the human kidney in vivo was linearly dependent, over a wide range, on plasma FFA concentration (the higher the arterial FFA concentration, the higher the arteriovenous difference) [23].
The vast majority of plasma FFA is carried on albumin, with unbound FFA representing only a minor fraction (less than 0.01%) [24,25]. Tissue uptake of circulating FFA involves dissociation from albumin, mediated by specific membrane proteins such as FA translocase (CD36) and FA-binding protein (FABP) [26]. In addition, the renal proximal tubule retrieves albumin-bound FFA from the filtrate by receptor-mediated albumin endocytosis [27–30]. There is a significant and ongoing controversy with regard to the actual amount of albumin normally filtered (with values ranging from 0.5–3.5 g/day to upwards of 200 g/day) and with regard to its breakdown in the proximal tubule cell versus intact return to the circulation by transcytosis [31–37]. There is agreement, however, that the glomerular sieving coefficient for albumin is greater than zero, with some albumin normally escaping the filtration barrier and being retrieved by the proximal tubule [27–29,36]. The FFAs carried by this reclaimed albumin likely contribute to the overall energy balance of the proximal tubule.
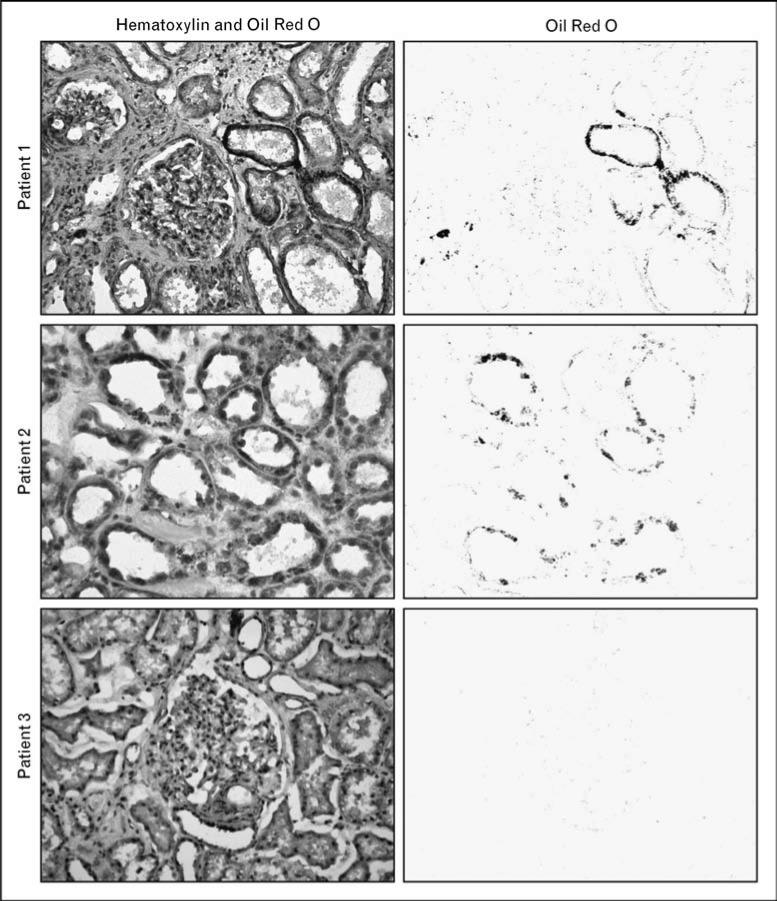
FFAs delivered to the kidney in excess of its energy needs can be esterified with glycerol and deposited as triglycerides in intracellular lipid droplets. These energy stores can be rapidly mobilized in periods of scarcity, as first suggested by ex-vivo experiments in canine kidney slices conducted by Weidemann and Krebs [38] more than 40 years ago. The physical size of individual lipid droplets can vary from submicrometer range (thus, undetectable by light microscopy) [1•] to larger lipid droplets detectable by Oil Red O staining in human kidney samples (Fig. 1).
Figure 1. Lipid accumulation in human kidney samples visualized by Oil Red O staining.
Kidney surgical specimens were obtained from patients undergoing radical nephrectomy for renal cell carcinoma. Normal kidney cortex samples were dissected by an experienced pathologist, away from the tumor. The samples were frozen, sectioned, and stained with hematoxylin and Oil Red O to visualize the distribution of lipids within renal structures. Left panels are representative images from three different patients (original color images are shown here in gray scale). For each image, a computer-based color deconvolution algorithm was used to separately visualize Oil Red O staining in the red channel (right panels). In these examples, lipid deposits are localized mostly within tubular epithelial cells in patients 1 and 2, but are not detectable in patient 3.
Whether significant lipid synthesis from nonlipid substrates occurs in the normal kidney is not clear. Similarly, very little is known about lipid export from the kidney. Human kidney specimens ex vivo produce apolipoprotein E (Apo-E) [39], and Apo-B has been recently proposed to be critical for renal lipid export in mice [40•]. Arteriovenous FFA concentration difference experiments have shown that the kidney takes up FFA in fasted rats, but adds FFA to the circulation in fed rats [41], suggesting that the transport of FFA between blood and renal cells may be bidirectional. It is conceivable that lipid export could protect the kidney from excessive lipid accumulation in conditions of FFA oversupply. It is also theoretically possible that the proximal tubule retrieves important lipophilic compounds (e.g. vitamins) from the primary urine and returns them to the circulation through lipoprotein export [30].
Lipids in renal pathophysiology
Both plasma and intrarenal lipid disturbances may play a role in renal pathophysiology, as concisely reviewed below.
Role of dyslipidemia
Moorhead et al. [42] first outlined the lipid nephrotoxicity hypothesis in 1982, proposing that dyslipidemia may contribute to the progression of renal disease. Dyslipidemia in this context could be triggered by urinary albumin loss leading to a compensatory increase in hepatic lipoprotein synthesis and could in effect be part of a positive feedback loop causing further renal injury [42]. This hypothesis has since garnered significant experimental support and has been recently revised and expanded in a comprehensive review by the same group [43•]. Dyslipidemia may affect the kidney directly by causing deleterious renal lipid disturbances (renal lipotoxicity), as well as indirectly through systemic inflammation and oxidative stress, vascular injury, and changes in hormones and other signaling molecules with renal action [7,43•,44].
Dyslipidemia per se is not sufficient to lead to renal injury, but likely contributes to renal injury as part of a multihit mechanism, in combination with other systemic and/or local factors [43•]. Theoretically, the heterogeneity and variable involvement of these factors may explain why the preventive value of statins in human chronic kidney disease is still uncertain, in spite of promising data from small renal outcome trials [45,46] and from meta-analyses [47–50] and subgroup analyses [51–53] of nonrenal trials. Larger ongoing renal outcome trials, including LORD (Lipid lowering and Onset of Renal Disease) [54] and SHARP (Study of Heart and Renal Protection) [55], are expected to dissipate some of this uncertainty. It is, however, important to stress that such trials evaluate renal outcomes with a drug class that affects multiple tissues and organs, in addition to having potential pleio-tropic effects independent of lipid lowering [56]. Although the results of these trials will certainly be very important from a clinical practice standpoint, they cannot and should not be interpreted as a yes-or-no verdict confirming or dismissing the direct role of renal lipid disturbances in kidney pathophysiology.
Renal lipid accumulation
The link between lipid accumulation and kidney disease was first suggested more than 150 years ago by Rudolf Virchow [57] in his lectures at the Pathological Institute of Berlin, when referring to ‘fatty degeneration of the renal epithelium as a stage of Bright's disease’ (a historical designation for glomerulonephritis, described earlier in the 19th century by Richard Bright, one of the ‘founding fathers’ of nephrology) [58].
We now have an abundance of animal data showing an association between kidney dysfunction and renal lipid accumulation, including in models of metabolic disease (obesity, metabolic syndrome, and diabetes mellitus), chronic kidney disease, acute renal injury of several etiologies, as well as aging (Table 1) [40•,59,60,61•,62–67,68•,69–73,74•,75,76•,77–83]. Glomeruli and renal tubules (proximal tubules in particular) seem to be most susceptible to lipid accumulation, but the localization of excess lipids varies between animal models. There may also be species or strain-specific differences in the propensity to accumulate lipids in the kidney, as exemplified by the relative resistance to renal lipid accumulation in Sprague–Dawley rats fed a high-fat diet [67] compared with similarly fed mice [59,60,61•]. Whether these models adequately reflect lipid involvement in human kidney disease is up for debate, including in the case of diabetic nephropathy, for which an accurate animal model is not available as of the date of this review [84•].
Table 1.
Renal triglyceride accumulation in animal modelsa
| Condition | Animal model | Renal triglyceride accumulation | Ref(s) |
|---|---|---|---|
| Obesity and metabolic syndrome resulting from high-fat diet | C57BL/6J wild-type and SREBP1c–/– mice on HFD (60% kcal from fat) | Glomerular and tubulointerstitial Correlated with upregulation of renal SREBPs Prevented in the kidneys of SREBP1c –/– mice |
[59] |
| C57BL/6J wild-type and PPARγ+/– mice on HFD (45% kcal from fat) | Associated with upregulation of renal lipogenic genes Attenuated in PPARγ+/– mice (these mice also have less severe metabolic syndrome features) |
[60] | |
| DBA/2J mice on HFD (42% kcal from fat) | Prevented by treatment with a FXR agonist acting to downregulate lipogenic genes and upregulate genes involved in lipid oxidation | [61•] | |
| Obesity and metabolic syndrome resulting from leptin or leptin receptor deficiency | db/db mice in several genetic backgrounds | In glomeruli and proximal tubule cells Accompanied by differential expression of multiple lipid metabolism and transport genes Decreased by antidiabetic treatment with a GLP-1 agonist |
[62–65] |
| ob/ob mice | In glomeruli and proximal tubule cells | [66] | |
| Zucker diabetic fatty (ZDF) rats | Primarily in proximal tubule cells Reduced by treatment with PPARγ agonists |
[67,68•] | |
| F1 hybrid ZDF/spontaneous hypertensive heart failure (ZS) rats | Primarily in renal tubular cells Not affected by systemic immunosuppressive therapy Reduced by treatment with an antibody against LOX-1 |
[69,70] | |
| Type 1 diabetes | Streptozotocin-treated SD rats | Glomerular and tubular Accompanied by upregulation of SREBPs in the absence of plasma lipid abnormalities |
[71,72] |
| C57BL/6-Ins2Akita (Akita) mice | Mitigated by treatment with insulin Primarily in glomeruli Accompanied by upregulation of lipogenic genes and downregulation of genes involved in fatty acid oxidation |
[73] | |
| Other transgenic/knockout models | SREBP-1a overexpression | Within glomerular and tubular cells in C57BL/6J × SJL mice overexpressing human SREBP-1a controlled by the PEPCK promoter | [72] |
| Xantine oxidoreductase (XOR) deficiency | In the lumen of renal tubules of C57BL/6J XOR–/– mice Associated with upregulation of genes involved in lipogenesis |
[74•] | |
| Angiotensin II (Ang II)-induced hypertension | Infusion of Ang II by minipump for 7 days in SD rats | Primarily in renal tubular cells in hypertensive rats induced by Ang II but not by norepinephrine Partly suppressed by iron chelation with deferoxamine |
[75] |
| Models of chronic renal disease by renal mass reduction | Uninephrectomy in SD rats followed for 3–10 months | Redistribution of fat to nonadipose tissues, including liver, pancreas, adrenals, and kidney Prevented by ACE inhibition Accompanied by upregulation of lipogenic genes, downregulation of genes involved in fatty acid oxidation, and increased expression of the endocytic receptors megalin and cubilin |
[76•,77] |
| 5/6 nephrectomy in SD rats and Nagase analbuminemic rats | Marked glomerular lipid deposition after 2 months in Nagase rats, much less in SD rats Prevented by ACE inhibition |
[78] | |
| Models of acute renal injury | Unilateral ureteral ligation in SD rats | In the cortex and medulla of the obstructed kidney, and to a lesser extent in the contralateral kidney | [79] |
| Endotoxin nephrotoxicity (LPS injection in CD-1 mice) | In the cortex, including in proximal tubules LPS dose-dependent |
[80] | |
| Glycerol-induced rhabdomyolysis in CD-1 mice | In the cortex, including in proximal tubules Correlated with the severity of azotemia Inversely correlated with plasma triglyceride levels |
[80] | |
| Cisplatin injection in Sv129 mice | Primarily in proximal tubules Prevented by pretreatment with a PPARα agonist |
[81] | |
| Ischemia–reperfusion in uninephrectomized CD-1 mice | In the cortex, including in proximal tubules In mice subjected to 15 min ischemia followed by 18 h of reperfusion, but not in mice subjected to uninephrectomy alone |
[80] | |
| Normal animals | Aging in C57BL/6J mice | Age-related, in glomeruli and proximal tubule cells Correlated with age-related increase in renal expression of SREBPs Mitigated in part by caloric restriction |
[82,83] |
| Fasting in C57BL/6 mice | Likely due to mobilization of extrarenal lipid stores Exacerbated by in-vivo antisense LNA-mediated knockdown of ApoB |
[40•] |
ACE, angiotensin-converting enzyme; ApoB, apolipoprotein B; FXR, farnesoid X receptor; GLP-1, glucagon-like peptide-1; HFD, high-fat diet; LOX-1, oxidized low-density lipoprotein receptor 1; LPS, Escherichia coli lipopolysaccharide; PEPCK, phosphoenolpyruvate carboxylkinase; PPAR, peroxisome proliferator-activated receptor; SD, Sprague–Dawley; SREBP(s), sterol regulatory element-binding protein(s).
Most articles cited in this table used quantitative biochemical methods to measure tissue triglyceride content and/or staining of kidney sections with Oil Red O or other Sudan dyes. These dyes stain neutral lipids, primarily triglycerides, but also some lipoproteins.
The available evidence in humans is much scarcer and less amenable to systematic study. Renal lipid accumulation has been described in several contexts, including hypertensive nephrosclerosis [85], focal segmental glomerulosclerosis [86], minimal change disease [87], hepatorenal syndrome [88], diabetic coma [89], severe whole-body hypothermia [90], as well as in rare genetic disorders, including Fabry's disease [91], familial dysbetalipoproteinemia [92], lecithin–cholesterol acyltransferase deficiency [93], arteriohepatic dysplasia (Alagille's syndrome) [94], and lipoprotein glomerulopathy [95].
The range of conditions that have excess renal lipids and kidney dysfunction as common elements implies that the two may be causally related, but whether renal lipid accumulation is a result or a mediator of renal injury in humans is not known. Animal experiments suggest that causality may be bidirectional and may be highly dependent on pathophysiological context.
Lipid accumulation as a consequence of injury
Renal ischemia–reperfusion in uninephrectomized mice causes triglyceride accumulation in the cortex, particularly in proximal tubules [80]. As mitochondrial aerobic respiration is necessary for ATP production from FFA, it is conceivable that ischemia leads to decreased FFA oxidation and redirects FFA to triglyceride pools. However, this view may be overly simplistic, as triglyceride synthesis does not depend only on FFA availability, but also on the activity of a series of enzymes and on the degree of ATP depletion (severe depletion may in fact inhibit triglyceride synthesis and raise FFA concentration, as the conversion of FFA to fatty acyl-CoA requires ATP). The mechanism of renal triglyceride accumulation may be highly dependent on the type of injury and may involve multiple injury-specific changes in lipid metabolic pathways [96].
Of course, the fact that lipid accumulation may in some cases result from renal injury does not preclude the possibility that accumulated lipids, in turn, lead to further damage. For example, lipid accumulation in the renal proximal tubule during ischemia–reperfusion may cause sustained energy deficit by FFA-mediated mitochondrial injury [97,98].
Lipid accumulation as a cause of injury
There is substantial evidence in animals that excess renal lipids can cause injury. The general approach for such experiments has been to show correlation between renal lipid content and markers of kidney dysfunction in one set of animals, while at the same time using genetic, pharmacologic, or dietary maneuvers to prevent or reduce renal lipid accumulation in another set of animals (Table 1). The concomitant reduction of renal lipids and improvement of histological or functional markers was interpreted to support the causal relationship. Although confounding factors could potentially plague individual experiments using this approach (if, for example, a drug reduces lipid accumulation because it improves kidney function rather than vice versa), this body of data taken as a whole strongly supports the hypothesis that excess lipids may be toxic for the kidney (renal lipotoxicity). This hypothesis is also supported by experiments in which lipid accumulation was triggered in renal epithelial cells by incubation with FFA and then reduced by starvation, in the absence of whole-animal confounding factors [67,68•].
The general mechanisms of lipotoxicity have been reviewed in detail elsewhere [5–7,8•,9•,10,11•,12•]. The principal determinant of lipotoxicity seems to be excessive intracellular FFA content, leading to accumulation of potentially toxic metabolites such as fatty acyl-CoA, diacylglycerol, and ceramides. Lipotoxic cellular dysfunction and injury occurs through several mechanisms, including the generation of reactive oxygen species, multiple organellar damage, disruption of intra-cellular signaling pathways, release of proinflammatory and profibrotic factors, and lipid-induced apoptosis (lipoapoptosis). Triglycerides are not considered toxic per se (and may in fact be protective), but are an active reservoir of FFA and an easily measurable indicator of tissue lipid overload [5,7,99,100]. It is important to note that not all FFAs have the same lipotoxic potential and that the cellular mechanisms of lipotoxicity are highly dependent on cell type [101•]. Consequently, not all studies of lipotoxicity in nonrenal cells or organs may be applicable to the kidney.
Finally, renal lipid accumulation has been linked to changes in gene expression, including the SREBPs (sterol regulatory element-binding proteins), ‘master’ regulators of lipid metabolism [59,61•,71,72,82,83], suggesting that the lipid-loaded kidney is not simply a passive victim of lipid oversupply. Intrarenal lipid metabolic disturbances may be important contributors to renal dysfunction, but the role of these disturbances in human kidney disease remains to be established.
Role of albumin reabsorption
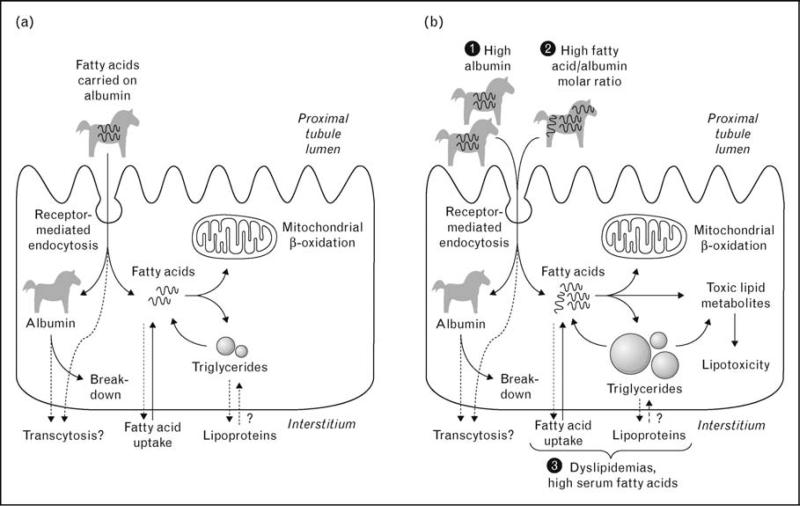
As discussed earlier, FFAs enter the proximal tubule from both the basolateral and the apical (luminal) sides. It has been proposed that increased filtration of FFA-bearing albumin and/or an increased FFA/albumin ratio may contribute to proximal tubule FFA overload and lipotoxicity [102–104] (Fig. 2). Filtered albumin would act in this setting as a ‘Trojan horse’ [105], by delivering a passive but deleterious load of FFA to the proximal tubule performing its physiologic task of albumin reclamation.
Figure 2. Entry of free nonesterified fatty acids into proximal tubule cells and the role of albumin as ‘Trojan horse’.
(a) Under normal conditions, fatty acids enter the proximal tubule cell from the basolateral side as well as from the apical (luminal) side, carried on albumin. Albumin is degraded in lysosomes, but transcytosis has also been proposed. Depending on cellular energy needs, intracellular fatty acids are directed to mitochondrial b-oxidation or to triglyceride stores. (b) Several conditions can theoretically lead to increased fatty acid intake into the proximal tubule cell, including high albumin filtration, high fatty acid to albumin molar ratio, and circulating lipid disturbances. These conditions, alone or in combination, may cause increased intracellular concentration of fatty acids, exceeding the b-oxidative capacity of mitochondria. This leads to intracellular accumulation of triglycerides and to the generation of lipid metabolites with potential toxic effect.
Knowing what proportion of the total influx of FFA in the proximal tubule normally occurs via reabsorptive endocytosis of filtered albumin would be helpful to ascertain the relative importance of this pathway for renal lipotoxicity. As no direct experimental data are available to answer this question, a brief quantitative exercise is in order. Assuming that albumin filtration in humans is between 0.5 and 3.5 g/day, of which normally less than 0.15 g/day is excreted, the daily reabsorption of albumin by the proximal tubule (after conversion and rounding) is about 5–50 μmol. As the FFA to albumin molar ratio is normally around 1, it can be inferred that 5–50 μmol of FFA enter the proximal tubule daily with albumin from the lumen. At the upper extreme of the albumin filtration controversy, the same calculation would yield 2–4 mmol of FFA delivered to the proximal tubule from the apical side.
Nieth and Schollmeyer [23] measured a difference of FFA concentration between the brachial artery and the renal vein of approximately 35 μmol/l in humans. Assuming a renal blood flow of 2160 l/day (1.5 l/min), the calculated renal extraction of FFA in 24 h amounts to approximately 75 mmol (but may be less or more if FFA extraction varies circadially or with feeding). Based on these estimates, the amount of FFA taken up from the proximal tubule lumen is most likely three orders of magnitude less than the amount taken up from the circulation. Even if the normal kidney filters and then reabsorbs massive amounts of albumin, as proposed by Comper and coworkers [36,37,106], and even if all FFAs carried by albumin are retained in proximal tubule cells (although most of the albumin would in this scenario undergo transcytosis), apical uptake would still account for less than 5% of the total proximal tubule FFA uptake.
Does this mean that apical uptake of FFA-bound albumin could not be an important source of proximal tubule FFA overload and lipotoxicity in vivo? Not necessarily. A sustained increase in apical FFA uptake due to increased albumin filtration or increased FFA/albumin molar ratio (Fig. 2b) may still throw the system off balance by cumulative effect. In addition, FFA taken up from the apical and basolateral sides may have distinct intracellular fates, such as targeting of albumin-bound FFA to lysosomes, possibly leading to FFA-induced lysosomal injury [107,108]. These possibilities require further investigation.
Nonlipotoxic effects
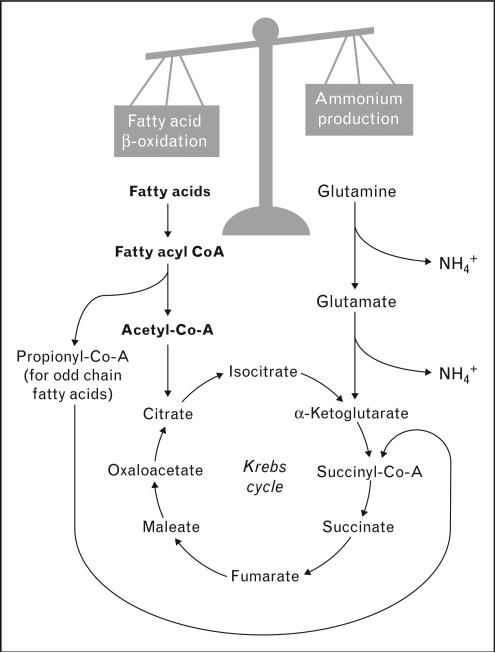
Lipid overload may also affect renal function through mechanisms that cannot be classified as lipotoxic. For example, excess FFA in the proximal tubule may compete with glutamine as oxidizable substrates in mitochondria, thus decreasing the production of ammonium [109,110] (Fig. 3). This is compatible with findings in Zucker diabetic fatty (ZDF) rats and in Sprague–Dawley rats fed a high-fat diet, in which plasma FFA levels (and renal lipid accumulation in the case of ZDF rats) were inversely correlated with the urinary excretion of ammonium [67,68•].
Figure 3. Fatty acids may affect proximal tubule ammonium production by mitochondrial substrate competition.
Ammonium (NH4+) is produced in the proximal tubule by the metabolism of glutamine to α-ketoglutarate, which then continues in the Krebs cycle. The products of fatty acid β-oxidation also enter the Krebs cycle. Increased intracellular concentration of fatty acids may compete with glutamine as mitochondrial substrate, decreasing its utilization and reducing ammonium production.
Moving beyond glomerulocentricity
Compared with other organs in which lipid accumulation and lipotoxicity may occur (such as the liver, heart, and skeletal muscle), the kidney has a high degree of histo-logical complexity, with individual structures having distinct contributions to the fundamental functional triad of filtration, reabsorption, and secretion, as well as to renal metabolic and endocrine functions. As discussed above, renal lipid accumulation has been described in both glomeruli and tubules, particularly in the proximal segment, and is likely to have different mechanisms and consequences within these different structures. However, most studies of lipids and the kidney to date, from clinical correlations to basic science experiments, have taken a predominantly glomerulocentric stance.
The general term ‘kidney dysfunction’ has been used deliberately in this article instead of ‘kidney disease’, because there is more to normal kidney function than a normal glomerular filtration rate (GFR) and absence of proteinuria. For example, humans with features of the metabolic syndrome have an increased risk of uric acid nephrolithiasis, caused at least in part by reduced renal ammonium excretion resulting in an overly acidic urinary pH, which in turn favors uric acid crystallization [111–115]. This ammonium excretion defect has been attributed to proximal tubular lipid accumulation in a rodent model of metabolic syndrome and in a cell culture model of proximal tubule lipotoxi-city [67,68•].
Although the potential links between renal lipid accumulation and kidney disease, as manifested by declining GFR and proteinuria, are undoubtedly of paramount importance, more subtle manifestations of lipid-induced renal tubular dysfunction (such as the one exemplified above) are no less worthy of further investigation.
Conclusion
The study of lipids, lipid accumulation, and lipotoxicity in other organs has gained considerable momentum over the past decade, but the role of lipids in kidney physiology and pathophysiology remains a relatively under-investigated area. Laboratory experiments have provided substantial evidence that intrarenal lipid metabolism disturbances may contribute to renal injury and dysfunction, but the applicability of these findings to human kidney disease remains to be established. Deciphering the role of lipids in the kidney requires further detailed studies in animal and cell culture models, but would also immensely benefit from potential noninvasive urinary ‘-omic’ assays reflective of intrarenal lipid metabolism in humans.
Acknowledgements
I.A.B.'s research is supported by the American Society of Nephrology Carl W. Gottschalk Research Scholar Award, by the Haberecht Wild-Hare Idea Research Program, and by the National Institutes of Health (coinvestigator on grant no. R01-DK081423 to K. Sakhaee).
Footnotes
The author declares no conflict of interest.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 411–412).
- 1•.Olofsson SO, Bostrom P, Andersson L, et al. Lipid droplets as dynamic organelles connecting storage and efflux of lipids. Biochim Biophys Acta. 2009;1791:448–458. doi: 10.1016/j.bbalip.2008.08.001. [An overview of the novel and fascinating field of lipid droplet biology, including a discussion of the potential role of lipid droplets in insulin resistance and atherosclerosis.] [DOI] [PubMed] [Google Scholar]
- 2•.Walther TC, Farese RV., Jr The life of lipid droplets. Biochim Biophys Acta. 2009;1791:459–466. doi: 10.1016/j.bbalip.2008.10.009. [A detailed look at the biology of lipid droplets, referred to by the authors as ‘the least characterized of cellular organelles’.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Meex RC, Schrauwen P, Hesselink MK. Modulation of myocellular fat stores: lipid droplet dynamics in health and disease. Am J Physiol Regul Integr Comp Physiol. 2009;297:R913–R924. doi: 10.1152/ajpregu.91053.2008. [An excellent review of lipid droplet dynamics and their potential role in skeletal muscle insulin sensitivity.] [DOI] [PubMed] [Google Scholar]
- 4.Lee Y, Hirose H, Ohneda M, et al. Beta-cell lipotoxicity in the pathogenesis of noninsulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc Natl Acad Sci U S A. 1994;91:10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14:281–287. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Unger RH. Lipotoxic diseases. Annu Rev Med. 2002;53:319–336. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- 7.Weinberg JM. Lipotoxicity. Kidney Int. 2006;70:1560–1566. doi: 10.1038/sj.ki.5001834. [DOI] [PubMed] [Google Scholar]
- 8•.Unger RH, Clark GO, Scherer PE, et al. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;1801:209–214. doi: 10.1016/j.bbalip.2009.10.006. [An excellent overview of the lipotoxicity hypothesis in the context of whole-organism lipid partitioning. This is the opening article in a special journal issue dedicated to lipotoxicity.] [DOI] [PubMed] [Google Scholar]
- 9•.Muoio DM. Intramuscular triacylglycerol and insulin resistance: guilty as charged or wrongly accused? Biochim Biophys Acta. 2010;1801:281–288. doi: 10.1016/j.bbalip.2009.11.007. [An in-depth discussion of the mechanisms linking lipid excess, lipotoxicity, and insulin resistance in the skeletal muscle.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unger RH, Orci L. Diseases of liporegulation: new perspective on obesity and related disorders. FASEB J. 2001;15:312–321. doi: 10.1096/fj.00-0590. [DOI] [PubMed] [Google Scholar]
- 11•.Wende AR, Abel ED. Lipotoxicity in the heart. Biochim Biophys Acta. 2010;1801:311–319. doi: 10.1016/j.bbalip.2009.09.023. [Lipotoxicity is highly dependent on cell type. Specific mechanisms by which lipotoxicity alters cardiac structure and function are discussed in this article.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.Poitout V, Amyot J, Semache M, et al. Glucolipotoxicity of the pancreatic beta cell. Biochim Biophys Acta. 2010;1801:289–298. doi: 10.1016/j.bbalip.2009.08.006. [An overview of the potential role of lipotoxicity in the specific setting of the pancreatic beta cell.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Druilhet RE, Overturf ML, Kirkendall WM. Structure of neutral glycerides and phosphoglycerides of human kidney. Int J Biochem. 1975;6:893–901. [Google Scholar]
- 14.Rouser G, Simon G, Kritchevsky G. Species variations in phospholipid class distribution of organs. I. Kidney, liver and spleen. Lipids. 1969;4:599–606. doi: 10.1007/BF02531047. [DOI] [PubMed] [Google Scholar]
- 15.Kwiterovich PO, Jr, Sloan HR, Fredrickson DS. Glycolipids and other lipid constituents of normal human liver. J Lipid Res. 1970;11:322–330. [PubMed] [Google Scholar]
- 16.Klein KL, Wang MS, Torikai S, et al. Substrate oxidation by isolated single nephron segments of the rat. Kidney Int. 1981;20:29–35. doi: 10.1038/ki.1981.100. [DOI] [PubMed] [Google Scholar]
- 17.Guder WG, Wagner S, Wirthensohn G. Metabolic fuels along the nephron: pathways and intracellular mechanisms of interaction. Kidney Int. 1986;29:41–45. doi: 10.1038/ki.1986.6. [DOI] [PubMed] [Google Scholar]
- 18.Elhamri M, Martin M, Ferrier B, et al. Substrate uptake and utilization by the kidney of fed and starved rats in vivo. Ren Physiol Biochem. 1993;16:311–324. doi: 10.1159/000173777. [DOI] [PubMed] [Google Scholar]
- 19.Balaban RS, Mandel LJ. Metabolic substrate utilization by rabbit proximal tubule. An NADH fluorescence study. Am J Physiol. 1988;254:F407–F416. doi: 10.1152/ajprenal.1988.254.3.F407. [DOI] [PubMed] [Google Scholar]
- 20.Gullans SR, Brazy PC, Mandel LJ, et al. Stimulation of phosphate transport in the proximal tubule by metabolic substrates. Am J Physiol. 1984;247:F582–F587. doi: 10.1152/ajprenal.1984.247.4.F582. [DOI] [PubMed] [Google Scholar]
- 21.Uchida S, Endou H. Substrate specificity to maintain cellular ATP along the mouse nephron. Am J Physiol. 1988;255:F977–F983. doi: 10.1152/ajprenal.1988.255.5.F977. [DOI] [PubMed] [Google Scholar]
- 22.Gold M, Spitzer JJ. Metabolism of free fatty acids by myocardium and kidney. Am J Physiol. 1964;206:153–158. doi: 10.1152/ajplegacy.1964.206.1.153. [DOI] [PubMed] [Google Scholar]
- 23.Nieth H, Schollmeyer P. Substrate-utilization of the human kidney. Nature. 1966;209:1244–1245. doi: 10.1038/2091244a0. [DOI] [PubMed] [Google Scholar]
- 24.Richieri GV, Kleinfeld AM. Unbound free fatty acid levels in human serum. J Lipid Res. 1995;36:229–240. [PubMed] [Google Scholar]
- 25.Frayn KN, Summers LK, Fielding BA. Regulation of the plasma nonesterified fatty acid concentration in the postprandial state. Proc Nutr Soc. 1997;56:713–721. doi: 10.1079/pns19970071. [DOI] [PubMed] [Google Scholar]
- 26.Stremmel W, Pohl L, Ring A, et al. A new concept of cellular uptake and intracellular trafficking of long-chain fatty acids. Lipids. 2001;36:981–989. doi: 10.1007/s11745-001-0809-2. [DOI] [PubMed] [Google Scholar]
- 27.Birn H, Christensen EI. Renal albumin absorption in physiology and pathology. Kidney Int. 2006;69:440–449. doi: 10.1038/sj.ki.5000141. [DOI] [PubMed] [Google Scholar]
- 28.Gekle M. Renal tubule albumin transport. Annu Rev Physiol. 2005;67:573–594. doi: 10.1146/annurev.physiol.67.031103.154845. [DOI] [PubMed] [Google Scholar]
- 29.Pollock CA, Poronnik P. Albumin transport and processing by the proximal tubule: physiology and pathophysiology. Curr Opin Nephrol Hypertens. 2007;16:359–364. doi: 10.1097/MNH.0b013e3281eb9059. [DOI] [PubMed] [Google Scholar]
- 30.Moestrup SK, Nielsen LB. The role of the kidney in lipid metabolism. Curr Opin Lipidol. 2005;16:301–306. doi: 10.1097/01.mol.0000169350.45944.d4. [DOI] [PubMed] [Google Scholar]
- 31.Christensen EI, Birn H, Rippe B, et al. Controversies in nephrology: renal albumin handling, facts, and artifacts! Kidney Int. 2007;72:1192–1194. doi: 10.1038/sj.ki.5002526. [DOI] [PubMed] [Google Scholar]
- 32.Russo LM, Sandoval RM, Brown D, et al. Controversies in nephrology: response to ’renal albumin handling, facts, and artifacts’. Kidney Int. 2007;72:1195–1197. doi: 10.1038/sj.ki.5002528. [DOI] [PubMed] [Google Scholar]
- 33.Gekle M. Renal albumin handling: a look at the dark side of the filter. Kidney Int. 2007;71:479–481. doi: 10.1038/sj.ki.5002123. [DOI] [PubMed] [Google Scholar]
- 34.Remuzzi A, Sangalli F, Fassi A, et al. Albumin concentration in the Bowman's capsule: multiphoton microscopy vs micropuncture technique. Kidney Int. 2007;72:1410–1411. doi: 10.1038/sj.ki.5002501. [DOI] [PubMed] [Google Scholar]
- 35.de Borst MH. On the origin of albuminuria. Kidney Int. 2007;72:1409. doi: 10.1038/sj.ki.5002499. [DOI] [PubMed] [Google Scholar]
- 36.Comper WD, Haraldsson B, Deen WM. Resolved: normal glomeruli filter nephrotic levels of albumin. J Am Soc Nephrol. 2008;19:427–432. doi: 10.1681/ASN.2007090997. [DOI] [PubMed] [Google Scholar]
- 37.Comper WD, Russo LM. The glomerular filter: an imperfect barrier is required for perfect renal function. Curr Opin Nephrol Hypertens. 2009;18:336–342. doi: 10.1097/MNH.0b013e32832cb96a. [DOI] [PubMed] [Google Scholar]
- 38.Weidemann MJ, Krebs HA. The fuel of respiration of rat kidney cortex. Biochem J. 1969;112:149–166. doi: 10.1042/bj1120149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blue ML, Williams DL, Zucker S, et al. Apolipoprotein E synthesis in human kidney, adrenal gland, and liver. Proc Natl Acad Sci U S A. 1983;80:283–287. doi: 10.1073/pnas.80.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Krzystanek MB, Pedersen TX, Bartels ED, et al. Expression of apolipoprotein B in the kidney attenuates renal lipid accumulation. J Biol Chem. 2010;285:10583–10590. doi: 10.1074/jbc.M109.078006. [Renal lipoprotein export, as shown in this article, may imply that the kidney participates in whole-body lipid metabolism to a greater extent than previously appreciated. This article is also interesting because of its use of in-vivo antisense locked nucleic acid (LNA) oligonucleotide-mediated gene knockdown.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hohenegger M, Schuh H. Uptake and fatty acid synthesis by the rat kidney. Int J Biochem. 1980;12:169–172. doi: 10.1016/0020-711x(80)90062-2. [DOI] [PubMed] [Google Scholar]
- 42.Moorhead JF, Chan MK, El-Nahas M, et al. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet. 1982;2:1309–1311. doi: 10.1016/s0140-6736(82)91513-6. [DOI] [PubMed] [Google Scholar]
- 43•.Ruan XZ, Varghese Z, Moorhead JF. An update on the lipid nephrotoxicity hypothesis. Nat Rev Nephrol. 2009;5:713–721. doi: 10.1038/nrneph.2009.184. [An excellent review, expansion, and update of the lipid nephrotoxicity hypothesis, originally proposed in 1982 (reference above).] [DOI] [PubMed] [Google Scholar]
- 44.Wahba IM, Mak RH. Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:550–562. doi: 10.2215/CJN.04071206. [DOI] [PubMed] [Google Scholar]
- 45.Bianchi S, Bigazzi R, Caiazza A, et al. A controlled, prospective study of the effects of atorvastatin on proteinuria and progression of kidney disease. Am J Kidney Dis. 2003;41:565–570. doi: 10.1053/ajkd.2003.50140. [DOI] [PubMed] [Google Scholar]
- 46.Lee TM, Su SF, Tsai CH. Effect of pravastatin on proteinuria in patients with well controlled hypertension. Hypertension. 2002;40:67–73. doi: 10.1161/01.hyp.0000022805.11288.7f. [DOI] [PubMed] [Google Scholar]
- 47.Fried LF, Orchard TJ, Kasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 2001;59:260–269. doi: 10.1046/j.1523-1755.2001.00487.x. [DOI] [PubMed] [Google Scholar]
- 48.Strippoli GF, Navaneethan SD, Johnson DW, et al. Effects of statins in patients with chronic kidney disease: meta-analysis and meta-regression of randomised controlled trials. BMJ. 2008;336:645–651. doi: 10.1136/bmj.39472.580984.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Douglas K, O'Malley PG, Jackson JL. Meta-analysis: the effect of statins on albuminuria. Ann Intern Med. 2006;145:117–124. doi: 10.7326/0003-4819-145-2-200607180-00009. [DOI] [PubMed] [Google Scholar]
- 50.Sandhu S, Wiebe N, Fried LF, et al. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol. 2006;17:2006–2016. doi: 10.1681/ASN.2006010012. [DOI] [PubMed] [Google Scholar]
- 51.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 52.Tonelli M, Moye L, Sacks FM, et al. Effect of pravastatin on loss of renal function in people with moderate chronic renal insufficiency and cardiovascular disease. J Am Soc Nephrol. 2003;14:1605–1613. doi: 10.1097/01.asn.0000068461.45784.2f. [DOI] [PubMed] [Google Scholar]
- 53.Chonchol M, Cook T, Kjekshus J, et al. Simvastatin for secondary prevention of all-cause mortality and major coronary events in patients with mild chronic renal insufficiency. Am J Kidney Dis. 2007;49:373–382. doi: 10.1053/j.ajkd.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 54.Fassett RG, Ball MJ, Robertson IK, et al. The Lipid lowering and Onset of Renal Disease (LORD) trial: a randomized double blind placebo controlled trial assessing the effect of atorvastatin on the progression of kidney disease. BMC Nephrol. 2008;9:4. doi: 10.1186/1471-2369-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baigent C, Landry M. Study of Heart and Renal Protection (SHARP). Kidney Int Suppl. 2003;63:S207–S210. doi: 10.1046/j.1523-1755.63.s84.4.x. [DOI] [PubMed] [Google Scholar]
- 56.Shaw SM, Fildes JE, Yonan N, et al. Pleiotropic effects and cholesterol-lowering therapy. Cardiology. 2009;112:4–12. doi: 10.1159/000137692. [DOI] [PubMed] [Google Scholar]
- 57.Virchow R, Chance Frank. Cellular pathology, as based on physiological and pathological histology. J. Churchill; London: 1860. A more precise account of fatty metamorphosis. p. 351. (full text available electronically via Google Books, http://books.google.com/) [Google Scholar]
- 58.Jay V. Richard Bright: physician extraordinaire. Arch Pathol Lab Med. 2000;124:1262–1263. doi: 10.5858/2000-124-1262-RBPE. [DOI] [PubMed] [Google Scholar]
- 59.Jiang T, Wang Z, Proctor G, et al. Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem. 2005;280:32317–32325. doi: 10.1074/jbc.M500801200. [DOI] [PubMed] [Google Scholar]
- 60.Kume S, Uzu T, Araki S, et al. Role of altered renal lipid metabolism in the development of renal injury induced by a high-fat diet. J Am Soc Nephrol. 2007;18:2715–2723. doi: 10.1681/ASN.2007010089. [DOI] [PubMed] [Google Scholar]
- 61•.Wang XX, Jiang T, Shen Y, et al. The farnesoid X receptor modulates renal lipid metabolism and diet-induced renal inflammation, fibrosis, and proteinuria. Am J Physiol Renal Physiol. 2009;297:F1587–F1596. doi: 10.1152/ajprenal.00404.2009. [The pathways involved in renal lipid accumulation and lipid-induced renal injury are highly complex. This article demonstrates that the farnesoid X receptor (FXR) modulates the activity of renal SREBP-1, glomerular lesions, and proteinuria.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Z, Jiang T, Li J, et al. Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes. 2005;54:2328–2335. doi: 10.2337/diabetes.54.8.2328. [DOI] [PubMed] [Google Scholar]
- 63.Jiang T, Wang XX, Scherzer P, et al. Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes. 2007;56:2485–2493. doi: 10.2337/db06-1642. [DOI] [PubMed] [Google Scholar]
- 64.Park CW, Kim HW, Ko SH, et al. Long-term treatment of glucagon-like peptide-1 analog exendin-4 ameliorates diabetic nephropathy through improving metabolic anomalies in db/db mice. J Am Soc Nephrol. 2007;18:1227–1238. doi: 10.1681/ASN.2006070778. [DOI] [PubMed] [Google Scholar]
- 65.Mishra R, Emancipator SN, Miller C, et al. Adipose differentiation-related protein and regulators of lipid homeostasis identified by gene expression profiling in the murine db/db diabetic kidney. Am J Physiol Renal Physiol. 2004;286:F913–F921. doi: 10.1152/ajprenal.00323.2003. [DOI] [PubMed] [Google Scholar]
- 66.Brandon E, do Carmo JM, da Silva AA, et al. Renal lipid accumulation and mitochondrial function in leptin deficient and melanocortin-4 receptor deficient obese mice (meeting abstract). FASEB J. 2008;22:947.3.. [Google Scholar]
- 67.Bobulescu IA, Dubree M, Zhang J, et al. Effect of renal lipid accumulation on proximal tubule Na+/H+ exchange and ammonium secretion. Am J Physiol Renal Physiol. 2008;294:F1315–F1322. doi: 10.1152/ajprenal.00550.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68•.Bobulescu IA, Dubree M, Zhang J, et al. Reduction of renal triglyceride accumulation: effects on proximal tubule Na+/H+ exchange and urinary acidification. Am J Physiol Renal Physiol. 2009;297:F1419–F1426. doi: 10.1152/ajprenal.00177.2009. [Lipid accumulation was studied in both rodent kidneys and proximal tubule cells and was found to cause a reversible decrease in renal ammonium production and transport. This could theoretically explain why humans with obesity and metabolic syndrome have an increased risk for idiopathic uric acid nephrolithiasis, a disease characterized by low urinary pH.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dominguez J, Wu P, Packer CS, et al. Lipotoxic and inflammatory phenotypes in rats with uncontrolled metabolic syndrome and nephropathy. Am J Physiol Renal Physiol. 2007;293:F670–F679. doi: 10.1152/ajprenal.00021.2007. [DOI] [PubMed] [Google Scholar]
- 70.Dominguez JH, Mehta JL, Li D, et al. Anti-LOX-1 therapy in rats with diabetes and dyslipidemia: ablation of renal vascular and epithelial manifestations. Am J Physiol Renal Physiol. 2008;294:F110–F119. doi: 10.1152/ajprenal.00013.2007. [DOI] [PubMed] [Google Scholar]
- 71.Lemieux G, Moulin B, Davignon J, et al. The lipid content of the diabetic kidney of the rat. Can J Physiol Pharmacol. 1984;62:1274–1278. doi: 10.1139/y84-213. [DOI] [PubMed] [Google Scholar]
- 72.Sun L, Halaihel N, Zhang W, et al. Role of sterol regulatory element-binding protein 1 in regulation of renal lipid metabolism and glomerulosclerosis in diabetes mellitus. J Biol Chem. 2002;277:18919–18927. doi: 10.1074/jbc.M110650200. [DOI] [PubMed] [Google Scholar]
- 73.Proctor G, Jiang T, Iwahashi M, et al. Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes. 2006;55:2502–2509. doi: 10.2337/db05-0603. [DOI] [PubMed] [Google Scholar]
- 74•.Ohtsubo T, Matsumura K, Sakagami K, et al. Xanthine oxidoreductase depletion induces renal interstitial fibrosis through aberrant lipid and purine accumulation in renal tubules. Hypertension. 2009;54:868–876. doi: 10.1161/HYPERTENSIONAHA.109.135152. [Xanthine oxidoreductase deficiency in mice leads to an unusual pattern of renal lipid accumulation, including triglycerides in the lumen of the nephron. The explanation for this phenomenon is not clear, but this study may have important implications for our understanding of oxidative stress and uric acid metabolism.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saito K, Ishizaka N, Hara M, et al. Lipid accumulation and transforming growth factor-beta upregulation in the kidneys of rats administered angiotensin II. Hypertension. 2005;46:1180–1185. doi: 10.1161/01.HYP.0000184653.75036.d5. [DOI] [PubMed] [Google Scholar]
- 76•.Kim HJ, Moradi H, Yuan J, et al. Renal mass reduction results in accumulation of lipids and dysregulation of lipid regulatory proteins in the remnant kidney. Am J Physiol Renal Physiol. 2009;296:F1297–F1306. doi: 10.1152/ajprenal.90761.2008. [A comprehensive study of the mechanisms of renal lipid accumulation in a rodent model of chronic kidney disease.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao HL, Sui Y, Guan J, et al. Fat redistribution and adipocyte transformation in uninephrectomized rats. Kidney Int. 2008;74:467–477. doi: 10.1038/ki.2008.195. [DOI] [PubMed] [Google Scholar]
- 78.Fujihara CK, Limongi DM, Falzone R, et al. Pathogenesis of glomerular sclerosis in subtotally nephrectomized analbuminemic rats. Am J Physiol. 1991;261:F256–F264. doi: 10.1152/ajprenal.1991.261.2.F256. [DOI] [PubMed] [Google Scholar]
- 79.Tannenbaum J, Purkerson ML, Klahr S. Effect of unilateral ureteral obstruction on metabolism of renal lipids in the rat. Am J Physiol. 1983;245:F254–F262. doi: 10.1152/ajprenal.1983.245.2.F254. [DOI] [PubMed] [Google Scholar]
- 80.Zager RA, Johnson AC, Hanson SY. Renal tubular triglyercide accumulation following endotoxic, toxic, and ischemic injury. Kidney Int. 2005;67:111–121. doi: 10.1111/j.1523-1755.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- 81.Portilla D, Li S, Nagothu KK, et al. Metabolomic study of cisplatin-induced nephrotoxicity. Kidney Int. 2006;69:2194–2204. doi: 10.1038/sj.ki.5000433. [DOI] [PubMed] [Google Scholar]
- 82.Jiang T, Liebman SE, Lucia MS, et al. Role of altered renal lipid metabolism and the sterol regulatory element binding proteins in the pathogenesis of age-related renal disease. Kidney Int. 2005;68:2608–2620. doi: 10.1111/j.1523-1755.2005.00733.x. [DOI] [PubMed] [Google Scholar]
- 83.Jiang T, Liebman SE, Lucia MS, et al. Calorie restriction modulates renal expression of sterol regulatory element binding proteins, lipid accumulation, and age-related renal disease. J Am Soc Nephrol. 2005;16:2385–2394. doi: 10.1681/ASN.2004080701. [DOI] [PubMed] [Google Scholar]
- 84•.Brosius FC, 3rd, Alpers CE, Bottinger EP, et al. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2009;20:2503–2512. doi: 10.1681/ASN.2009070721. [To date, there is no completely accurate rodent model of diabetic nephropathy. This article explains why, and presents a set of criteria for validating future mouse models of progressive diabetic nephropathy.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Druilhet RE, Overturf ML, Kirkendall WM. Cortical and medullary lipids of normal and nephrosclerotic human kidney. Int J Biochem. 1978;9:729–734. doi: 10.1016/0020-711x(78)90040-x. [DOI] [PubMed] [Google Scholar]
- 86.Magil AB, Cohen AH. Monocytes and focal glomerulosclerosis. Lab Invest. 1989;61:404–409. [PubMed] [Google Scholar]
- 87.Jennette JC, Falk RJ. Adult minimal change glomerulopathy with acute renal failure. Am J Kidney Dis. 1990;16:432–437. doi: 10.1016/s0272-6386(12)80055-2. [DOI] [PubMed] [Google Scholar]
- 88.Hovig T, Blomhoff JP, Holme R, et al. Plasma lipoprotein alterations and morphologic changes with lipid deposition in the kidney of patients with hepatorenal syndrome. Lab Invest. 1978;38:540–549. [PubMed] [Google Scholar]
- 89.Nielsen H, Thomsen JL, Kristensen IB, et al. Accumulation of triglycerides in the proximal tubule of the kidney in diabetic coma. Pathology. 2003;35:305–310. doi: 10.1080/0031302031000150551. [DOI] [PubMed] [Google Scholar]
- 90.Preuss J, Dettmeyer R, Lignitz E, et al. Fatty degeneration in renal tubule epithelium in accidental hypothermia victims. Forensic Sci Int. 2004;141:131–135. doi: 10.1016/j.forsciint.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 91.Gubler MC, Lenoir G, Grunfeld JP, et al. Early renal changes in hemizygous and heterozygous patients with Fabry's disease. Kidney Int. 1978;13:223–235. doi: 10.1038/ki.1978.32. [DOI] [PubMed] [Google Scholar]
- 92.Balson KR, Niall JF, Best JD. Glomerular lipid deposition and proteinuria in a patient with familial dysbetalipoproteinaemia. J Intern Med. 1996;240:157–159. doi: 10.1046/j.1365-2796.1996.501855000.x. [DOI] [PubMed] [Google Scholar]
- 93.Gjone E. Familial lecithin:cholesterol acyltransferase deficiency – a new metabolic disease with renal involvement. Adv Nephrol Necker Hosp. 1981;10:167–185. [PubMed] [Google Scholar]
- 94.Chung-Park M, Petrelli M, Tavill AS, et al. Renal lipidosis associated with arteriohepatic dysplasia (Alagille's syndrome). Clin Nephrol. 1982;18:314–320. [PubMed] [Google Scholar]
- 95.Sam R, Wu H, Yue L, et al. Lipoprotein glomerulopathy: a new apolipoprotein E mutation with enhanced glomerular binding. Am J Kidney Dis. 2006;47:539–548. doi: 10.1053/j.ajkd.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 96.Johnson AC, Stahl A, Zager RA. Triglyceride accumulation in injured renal tubular cells: alterations in both synthetic and catabolic pathways. Kidney Int. 2005;67:2196–2209. doi: 10.1111/j.1523-1755.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 97.Feldkamp T, Weinberg JM, Horbelt M, et al. Evidence for involvement of nonesterified fatty acid-induced protonophoric uncoupling during mitochondrial dysfunction caused by hypoxia and reoxygenation. Nephrol Dial Transplant. 2009;24:43–51. doi: 10.1093/ndt/gfn436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feldkamp T, Kribben A, Roeser NF, et al. Accumulation of nonesterified fatty acids causes the sustained energetic deficit in kidney proximal tubules after hypoxia-reoxygenation. Am J Physiol Renal Physiol. 2006;290:F465–F477. doi: 10.1152/ajprenal.00305.2005. [DOI] [PubMed] [Google Scholar]
- 99.Koyama K, Chen G, Lee Y, et al. Tissue triglycerides, insulin resistance, and insulin production: implications for hyperinsulinemia of obesity. Am J Physiol. 1997;273:E708–E713. doi: 10.1152/ajpendo.1997.273.4.E708. [DOI] [PubMed] [Google Scholar]
- 100.Listenberger LL, Han X, Lewis SE, et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101•.Garbarino J, Sturley SL. Saturated with fat: new perspectives on lipotoxicity. Curr Opin Clin Nutr Metab Care. 2009;12:110–116. doi: 10.1097/MCO.0b013e32832182ee. [This article reviews the mechanisms of lipotoxicity and discusses the dependence of these mechanisms on cell type and FA type.] [DOI] [PubMed] [Google Scholar]
- 102.Thomas ME, Schreiner GF. Contribution of proteinuria to progressive renal injury: consequences of tubular uptake of fatty acid bearing albumin. Am J Nephrol. 1993;13:385–398. doi: 10.1159/000168653. [DOI] [PubMed] [Google Scholar]
- 103.Thomas ME, Harris KP, Walls J, et al. Fatty acids exacerbate tubulointerstitial injury in protein-overload proteinuria. Am J Physiol Renal Physiol. 2002;283:F640–F647. doi: 10.1152/ajprenal.00001.2002. [DOI] [PubMed] [Google Scholar]
- 104.Arici M, Brown J, Williams M, et al. Fatty acids carried on albumin modulate proximal tubular cell fibronectin production: a role for protein kinase C. Nephrol Dial Transplant. 2002;17:1751–1757. doi: 10.1093/ndt/17.10.1751. [DOI] [PubMed] [Google Scholar]
- 105.Iglesias J, Levine JS. Albuminuria and renal injury: beware of proteins bearing gifts. Nephrol Dial Transplant. 2001;16:215–218. doi: 10.1093/ndt/16.2.215. [DOI] [PubMed] [Google Scholar]
- 106.Russo LM, Sandoval RM, McKee M, et al. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int. 2007;71:504–513. doi: 10.1038/sj.ki.5002041. [DOI] [PubMed] [Google Scholar]
- 107.Acosta D, Wenzel DG. Injury produced by free fatty acids to lysosomes and mitochondria in cultured heart muscle and endothelial cells. Atherosclerosis. 1974;20:417–426. doi: 10.1016/0021-9150(74)90023-9. [DOI] [PubMed] [Google Scholar]
- 108.Feldstein AE, Werneburg NW, Li Z, et al. Bax inhibition protects against free fatty acid-induced lysosomal permeabilization. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1339–G1346. doi: 10.1152/ajpgi.00509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bagnasco SM, Gaydos DS, Risquez A, et al. The regulation of renal ammoniagenesis in the rat by extracellular factors. III. Effects of various fuels on in vitro ammoniagenesis. Metabolism. 1983;32:900–905. doi: 10.1016/0026-0495(83)90204-4. [DOI] [PubMed] [Google Scholar]
- 110.Vinay P, Lemieux G, Cartier P, et al. Effect of fatty acids on renal ammonia-genesis in in vivo and in vitro studies. Am J Physiol. 1976;231:880–887. doi: 10.1152/ajplegacy.1976.231.3.880. [DOI] [PubMed] [Google Scholar]
- 111.Sakhaee K, Adams-Huet B, Moe OW, et al. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int. 2002;62:971–979. doi: 10.1046/j.1523-1755.2002.00508.x. [DOI] [PubMed] [Google Scholar]
- 112.Cameron MA, Maalouf NM, Adams-Huet B, et al. Urine composition in type 2 diabetes: predisposition to uric acid nephrolithiasis. J Am Soc Nephrol. 2006;17:1422–1428. doi: 10.1681/ASN.2005121246. [DOI] [PubMed] [Google Scholar]
- 113.Daudon M, Traxer O, Conort P, et al. Type 2 diabetes increases the risk for uric acid stones. J Am Soc Nephrol. 2006;17:2026–2033. doi: 10.1681/ASN.2006030262. [DOI] [PubMed] [Google Scholar]
- 114.Maalouf NM, Cameron MA, Moe OW, et al. Low urine pH: a novel feature of the metabolic syndrome. Clin J Am Soc Nephrol. 2007;2:883–888. doi: 10.2215/CJN.00670207. [DOI] [PubMed] [Google Scholar]
- 115.Sakhaee K, Maalouf NM. Metabolic syndrome and uric acid nephrolithiasis. Semin Nephrol. 2008;28:174–180. doi: 10.1016/j.semnephrol.2008.01.010. [DOI] [PubMed] [Google Scholar]