Abstract
Hypothalamic-hypophysiotropic peptides are the proximate regulators of pituitary cells, but they cannot fully account for the complex functioning of these cells. Accordingly, awareness is growing that an array of peptides produced in the pituitary exert paracrine/autocrine functions. One such peptide, pituitary adenylate cyclase-activating polypeptide (PACAP), was originally identified as a hypothalamic activator of cAMP production in pituitary cells. Gonadotrophs and folliculostellate cells are the main source of pituitary PACAP, and each pituitary cell type expresses a PACAP receptor. PACAP increases alpha-subunit (Cga) and Lhb mRNAs, and it stimulates the transcription of follistatin (Fst) that, in turn, restrains activin signaling to repress Fshb and gonadotropin-releasing hormone-receptor (Gnrhr) expression as well as other activin-responsive genes. The PACAP (Adcyap1) promoter is activated by cAMP, and pituitary cells may communicate by a feed-forward, cAMP-dependent mechanism to maintain a high level of PACAP in the fetal pituitary. At birth, pituitary PACAP declines and pituitary follistatin levels decrease, which together with increased gonadotropin-releasing hormone secretion allow Gnrhr and Fshb to increase and facilitate activation of the newborn gonads. Changes in Adcyap1 expression levels in the adult pituitary may contribute to the selective rise in follicle-stimulating hormone (FSH) from age 20–30 days to the midcycle surge and to the secondary increase in FSH that occurs before estrus. These results provide further support for the notion that PACAP is a key player in reproduction through its actions as a pituitary autocrine/paracrine hormone.
Keywords: follicle-stimulating hormone (FSH/FSH receptor), follistatin, luteinizing hormone (LH/LH receptor), pituitary/pituitary hormones, pituitary adenylate cyclase-activating polypeptide
This minireview summarizes new evidence that PACAP is produced in the pituitary and regulates gonadotropin secretion and subunit gene expression.
INTRODUCTION
Pituitary adenylate cyclase-activating polypeptide (PACAP) was isolated in 1989 from sheep hypothalamic extracts based on its action to stimulate cAMP production by rat pituitary cell cultures [1]. PACAP is the most highly conserved member of the VIP (vasoactive intestinal peptide)-secretin-glucagon peptide superfamily, with expression in tunicates, fish, amphibians, rodents, and higher mammals, including humans; a related protein, amnesiac, is found in drosophila [2]. This high degree of conservation across species suggests essential functions. Two isoforms of PACAP have been found: a 38-amino-acid form and a C-terminally truncated, 27-amino-acid form, with PACAP-38 accounting for 90% of the protein in most tissues.
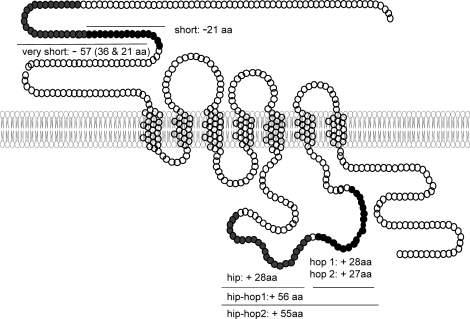
Three distinct receptors are activated by PACAP: the VPAC1 and VPAC2 receptors (official symbols VIPR1 and VIPR2, respectively), which have relatively similar affinity for VIP and PACAP, and the specific PAC1 receptor (PAC1-R; official symbol ADCYAP1R1) [3]. Multiple splice variants of the Adecyap1r1 (Fig. 1) result from the alternative splicing of two exons in the third intracellular loop (hip and hop) and were named null (neither hip nor hop), hip, hop1, hop2, hiphop1, and hiphop2 [4]. Adcyap1r1 variants that differ from the null receptor in the amino-terminal extracellular domain have also been identified [5]. VIPR1, VIPR2, and all ADCYAP1R1 variants bind PACAP-38 and PACAP-27 with high affinity and, like other members of the group B, G protein-coupled receptor family of proteins, couple with GNAS to stimulate adenylate cyclase (although in certain systems, PACAP-38 is more potent than PACAP-27). In some cell types, PACAP also stimulates inositol phosphate (IP) production and increases intracellular calcium concentrations. Evidence suggests that the hip cassette reduces the calcium response [6] and that the hop cassette elevates intracellular calcium [7]. Thus, the variable expression of the ADCYAP1R1 variants would be expected to influence PACAP signaling and produce different transcriptomes. The interested reader is referred to a recent comprehensive review [3].
FIG. 1.
Splice variants of the Acyap1r1 as compared to Adcyap1r1 null. For each cassette, the number of amino acids (aa) that are inserted (+) or deleted (−) is illustrated.
Consequent to the extensive distribution of PACAP and its receptors, PACAP exerts an array of functions on the nervous, immune, gastrointestinal, cardiac, and endocrine systems [8]. PACAP affects the exocrine and endocrine pancreas, hepatocytes, osteoblasts, adrenal medulla and cortex, testis and ovary, thyroid, pineal, neurohypophysis and pars tuberalis, as well as the anterior pituitary.
EVIDENCE PACAP REGULATES GONADOTROPHS
At least one of the PACAP receptors is present in each of the anterior pituitary endocrine cell types and in pituitary folliculostellate (FS) cells [9, 10]. The null and hop ADCYAP1R1 forms predominate in the rat pituitary [11] and are expressed in mouse αT3–1 gonadotroph cells [12]. PACAP stimulates cAMP production in these cells and increases intracellular calcium concentrations independent of cAMP activation by stimulating IP turnover through phospholipase C [13]. Stimulation of cAMP occurs at a lower concentration (median effective concentration [EC50] ∼ 3 nM) than the rise in IP production (EC50 ∼ 30 nM) [14], and PACAP-38 and PACAP-27 produce equipotent effects. Although the level of expression of ADCYAP1R1 is much lower in LβT-2 gonadotrophs than in αT3–1 cells [15], PACAP increases cAMP signaling in this more mature gonadotroph as well [16]. Information on signaling in normal gonadotrophs is limited; however, PACAP has been shown to increase intracellular calcium concentrations in rat gonadotrophs that were identified by reverse hemolytic plaque assay [17]. PACAP and gonadotropin-releasing hormone (GnRH) signaling interact in that PACAP-stimulated cAMP production is inhibited by GnRH through protein kinase C (PKC) in both αT3–1 [18] and LβT2 cells [19].
Whereas PACAP has variable and species-specific effects on the secretion of adrenocorticotropin hormone, growth hormone, prolactin, and thyroid-stimulating hormone, evidence is growing that PACAP is an important regulator of gonadotropin secretion and subunit gene expression [20]. PACAP stimulates the release of luteinizing hormone (LH) and uncombined glycoprotein α-subunit from primary pituitary cell cultures [21]. The effect of PACAP on LH secretion is modest compared to that of GnRH, however, and PACAP-stimulated LH secretion desensitizes rapidly [22]. PACAP effects are abolished by the protein kinase A inhibitor H-89, by the PKC inhibitor bisindolylmaleimide, and by decreasing the extracellular calcium concentration with ethylene glycol tetraacetic acid [23]. In addition to stimulating LH secretion directly, PACAP augments the response to GnRH stimulation by shifting the GnRH-LH secretion dose-response curve to the left [24], although peak LH secretion appears to be unaffected [25]. When pituitary cells are perifused with pulses of GnRH, a more physiological model of the hypothalamic-pituitary unit, continuous treatment with PACAP markedly enhances GnRH-stimulated LH secretion [25].
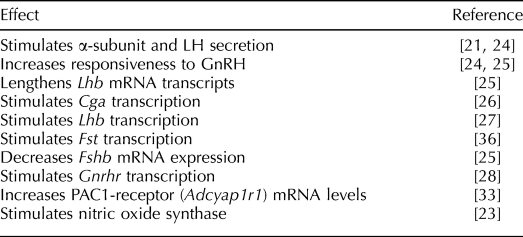
In addition, PACAP affects the expression of each gonadotropin subunit gene. PACAP increases Cga mRNA levels by stimulating transcription primarily through the protein kinase A (PKA)-cAMP signaling pathway [26]. PACAP lengthens Lhb mRNA transcripts in primary pituitary cultures [25] and activates the Lhb promoter in LβT2 cells, partly by increasing EGR1 [27]. PACAP stimulates transcription of the Gnrhr in LβT2 cells through CREB and NR5A1 (SF-1) [28], and Gnrhr mRNA in αT3–1 cells is increased by PKA activation. In other cell types, PACAP increases transcription of fos and c-jun. The ADCYAP1R1 influences cell growth and development through the extracellular signal-related kinase (ERK)-mitogen-activated protein kinase (MAPK) pathway in a variety of tissues [29, 30], and whereas PACAP stimulates ERK in αT3–1 gonadotrophs [31], to our knowledge no studies of PACAP effects on gonadotroph development or proliferation have been published. In contrast to its stimulation of the Cga, Lhb, and Gnrhr genes, PACAP reduces Fshb mRNA levels in primary rat pituitary cells [25], although not in LβT-2 pituitary cells [32, 33]. These observations imply that PACAP may enhance responsiveness to GnRH and regulate the differential secretion of LH and follicle-stimulating hormone (FSH). The reported effects of PACAP on gonadotrophs are summarized in Table 1.
TABLE 1.
Effects of PACAP on gonadotrophs.
FOLLISTATIN PLAYS A PIVOTAL ROLE IN THE PITUITARY ACTIONS OF PACAP
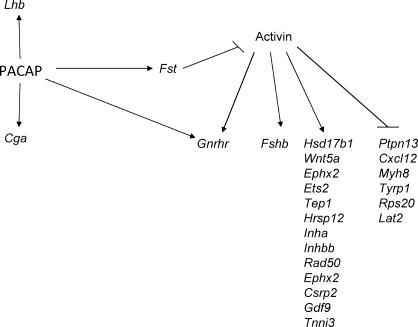
An elaborate mechanism selectively regulates Fshb gene expression and, thereby, FSH production, independently of LH, that involves activin and follistatin from the pituitary as well as inhibin from the gonads [34]. In this schema, follistatin binds activin with high affinity to form a biologically inactive complex that restrains activin signaling. Follistatin is highly regulated, and with its rapid half-life [35], the effect of follistatin can begin and terminate abruptly. PACAP increases follistatin (Fst) mRNA levels in primary pituitary cell cultures and activates the Fst promoter by stimulating cAMP-PKA signaling [16, 36]. Quantitative in situ hybridization coupled to immunostaining revealed that Fst expression in both gonadotrophs and FS cells is increased by PACAP [32]. Two alternatively spliced mRNAs are produced from the Fst gene, with follistatin-288 having no exon 6 sequence and the greater activin-neutralizing activity [37], and PACAP may have its greatest effect on the production of follistatin-288. Thus, PACAP induction of Fst expression is likely to explain, at least in part, its suppression of Fshb mRNA and may regulate many of the downstream targets of activin signaling. A diagram of the proposed role of follistatin in PACAP control of gonadotropin subunit gene expression is shown in Figure 2.
FIG. 2.
A schematic diagram to illustrate the direct effects of PACAP on gonadotropins and effects that may be mediated through regulation of follistatin. Expression of other activin-regulated genes is from studies with LβT2 gonadotroph cells by Mazhawidza et al. [85] and Zhang et al. [86].
Nitric oxide synthase type 1 (NOS1; neuronal NOS) is expressed in gonadotrophs and FS cells, and PACAP increases NOS in pituitary cells and potentiates the effect of GnRH to increase nitric oxide-dependent cGMP production [23, 38]. PACAP and NO/GMP enhance the action of GnRH to stimulate LH release in some systems. NOS1 has been linked to the control of gene expression by activin, as has GnRH that is administered continuously [39], and NO increases the expression of Fst in myoblasts [40]. Thus, NO might play a role in the actions of PACAP and in the regulation of follistatin and FSH by PACAP.
PACAP IN VIVO
Less is known about the role of PACAP in vivo. PACAP and its receptors are widely expressed in rodents, and they are found in high concentration throughout the central nervous system, with the highest concentration in the hypothalamus [41, 42]. PACAP increases LH secretion when administered in vivo to rats [43], although not to ovariectomized ewes [44], and is thought to be a hypophysiotropic hormone because its concentration in rat hypothalamic portal blood exceeds that of peripheral blood [45]. PACAP protein levels in most regions of the rat brain increase from low levels at birth to peak levels by age 30–60 days and are maintained throughout adulthood [46]. Adcyap1 mRNA expression within the paraventricular nucleus (PVN) declines in the male rat between 20 and 30 days of age and then increases, with reciprocal changes in Fshb and Gnrhr mRNA levels [47]. Levels of Adcyap1 mRNA in the PVN vary during the rat estrous cycle, increasing 3 h before the proestrous LH/FSH surge and then declining [48]. Because the PVN projects axons to the median eminence, the increase in PVN PACAP may play a role in the LH surge, in the increase in anterior pituitary cAMP that occurs at the time of the surge [49], and in the developmental changes in Fshb and Gnrhr that occur in the rat between 20 and 30 days of age. Evidence also indicates that PACAP enhances progesterone-mediated female sexual behavior [50, 51].
In addition, PACAP may regulate GnRH secretion. PACAP injected s.c. on Day 1 of life delayed vaginal opening and reduced GnRH immunoreactivity in the preoptic region of 9- and 30-day-old rats [52]. On the other hand, PACAP-specific receptors are also expressed in GT1–7 GnRH neuronal cells, but PACAP stimulates, rather than inhibits, cAMP production and GnRH secretion by these cells [53].
Several groups have developed PACAP-deficient mice, but unfortunately, most of these pups die at birth or by the second week of life with wasting, ketosis, and dyslipidemia [54]. Survival improves if the environmental temperature is maintained at 25–28°C [55]. PACAP-deficient females are subfertile [56], and males are testosterone-deficient because of gonadotropin insufficiency [57]. Because reproduction is sensitive to environmental stress and nutritional deficiency, additional models of PACAP deficiency are needed before definitive conclusions about the effects of PACAP in these models are possible.
PITUITARY PRODUCTION OF PACAP
Although initially viewed as a hypothalamic-hypophysiotropic neuropeptide, evidence is increasing that PACAP is produced in the pituitary and has a paracrine/autocrine mechanism of action. Early immunoassays detected PACAP in adult rat pituitary, although at much lower levels than in hypothalamus, as well as in human pituitary tissue [41, 42]. Koves et al. [58] identified immunoreactive PACAP in rat gonadotrophs at proestrus, and Jin et al. [59] used laser-capture microdissection to demonstrate Adcyap1 mRNA in FS cells. PACAP secretion by pituitary cell cultures rises in late proestrus [60], and a transient increase in pituitary Adcyap1 expression occurs in female rats during the overnight hours between proestrus and estrus when FSH levels are elevated [48]. Accordingly, pituitary PACAP could contribute to the termination of the secondary FSH surge during the early hours of estrus. Pituitary PACAP levels are lower in males [61]; however, a significant decline in pituitary Adcyap1 mRNA in male rats between 17 and 21 days of age coincides with a pronounced rise in Fshb compared to Lhb mRNA [47].
PITUITARY PACAP IN THE FETUS
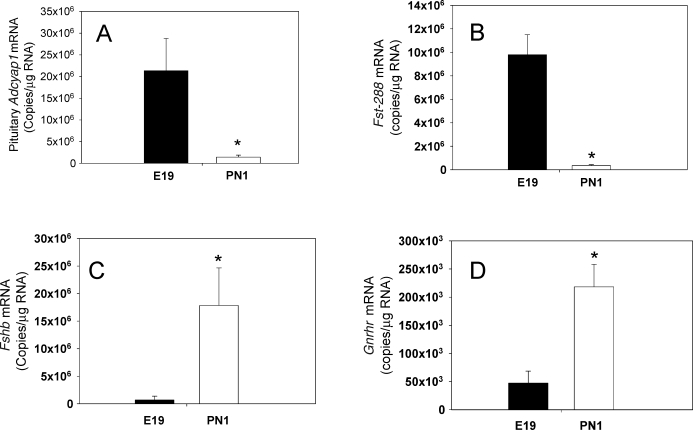
Moore et al. [62] reported recently that Adcyap1 mRNA and PACAP protein levels are high in the embryonic (Embryonic Day 19) rat pituitary, then decline strikingly and abruptly at birth (Fig. 3A). Photomicrographs in previous studies have suggested the expression of Adcyap1 mRNA within the embryonic rat pituitary [63], and Adcyap1 mRNA is expressed at higher levels in the fetal human pituitary (gestational age, 16–20 wk) than in the adult human pituitary [64].
FIG. 3.
Levels of Adcyap1 (A), Fst-288 (B), Fshb (C), and Gnrhr (D) mRNAs in the male rat pituitary on Embryonic Day 19 (E19) and Postnatal Day 1 (PN1). Results were determined by quantitative RT-PCR and represent the mean ± SEM (n = 6–8 animals/group). Pituitary Adcyap1 mRNA levels declined 94% from E19 to PN1 and were accompanied by a comparable decrease in Fst-288 mRNA. The fall in follistatin is thought to release activin signaling and allow expression of Fshb and Gnrhr as well as other genes to increase. *P < 0.05 vs. E19. (Adapted from Moore et al. [62], with permission from The Endocrine Society, Copyright 2009.)
The level of pituitary Fst mRNA falls sharply (Fig. 3B) at birth, in parallel with the decrease in PACAP; this is in keeping with the idea that PACAP is a major regulator of Fst expression. Interestingly, the magnitude of the decline in follistatin-288 exceeds the change in total pituitary Fst, suggesting that PACAP influences the splicing of this mRNA. Moreover, the fall in pituitary Fst-288 at birth is accompanied by a substantial increase in Fshb (Fig. 3C) and Gnrhr (Fig. 3D) mRNA levels, a change that could be explained by increased activin signaling because follistatin has decreased. These and previous results suggest that the high level of PACAP in the embryonic anterior pituitary facilitates the early appearance of gonadotropin Cga and delays the ontogeny of Fshb relative to Lhb by stimulating Fst transcription.
REGULATION OF PACAP (Adcyap1) GENE EXPRESSION
The Adcyap1 promoter contains sequences that are homologous to the CRE and is activated by both PACAP and forskolin [65, 66]. Furthermore, treatment of rats with PACAP-38 increased pituitary Adcyap1 mRNA levels in vivo [67], and PACAP increased ADCYAP1R1 expression in LβT2 gonadotroph cells [33]. Together, these experiments support the idea of a feed-forward mechanism through which PACAP increases cAMP production, which in turn increases pituitary Adcyap1 expression.
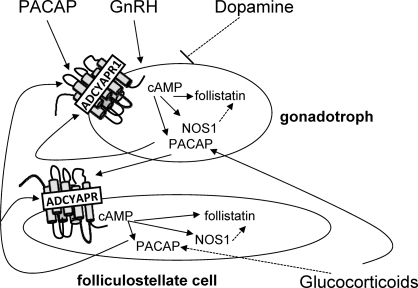
Gonadotropin-releasing hormone stimulates Adcyap1 expression via the PKA, PKC, and MAPK pathways in LβT2 cells through CREB, JUNB, and FOS [68] and increases the mRNA for Adcyap1r1 [33]. Thus, GnRH may play a role in PACAP activation. Receptors for dopamine are found in αT3–1 [69] and LβT2 gonadotroph cells [70], and when dopamine activates Gαi-coupled D2 receptors, adenylate cyclase 5 activity is blocked and cAMP levels decline [71]. Thus, an increase in dopamine signaling at birth [72] might suppress cAMP and, thereby, Adcyap1 mRNA levels. Adcyap1 mRNA in PC12 rat pheochromocytoma cells is increased by dexamethasone [73]. In addition, estrogens stimulate PACAP in the ventromedial nucleus and arcuate nucleus [50], whereas progesterone increases Adcyap1 and Adacyap1r1 mRNAs in the rat hypothalamus [74]. Thus, these steroid hormones may also regulate pituitary PACAP. Because gonadotrophs and FS cells are the major sources of pituitary PACAP [75, 76] and both cell types express PACAP receptors [10], it is interesting to propose that these cells communicate [77] to maintain the high level of PACAP in the fetal pituitary, as diagrammed in Figure 4. Experiments are underway to understand the factors that maintain PACAP in the fetal pituitary and to unravel the mechanism for the dramatic decline that occurs in Adacyap1 expression near the time of birth.
FIG. 4.
A proposed mechanism for the up-regulation of pituitary Adcyap1 expression through cAMP signaling in gonadotrophs and folliculostellate cells. PACAP stimulates ADCYAP1R1 to increase cAMP production, which induces Adcyap1 and Adcyap1r1 as well as Nos1 and Fst gene expression. PACAP secreted by gonadotrophs may activate folliculostellate cells, which likewise secrete PACAP that stimulates gonadotrophs. Dopamine, GnRH, central nervous system PACAP, and glucocorticoids may influence Adcyap1 expression. nNOS, neuronal NOS.
CONCLUSIONS
It has been more than 15 years since PACAP was hypothesized to be a key player in reproduction [78]. PACAP may be a hypophysotropic regulator with a role in the midcycle surge, and PACAP in the pituitary may control the differential expression of the gonadotropin subunit genes in the fetus and contribute to this control mechanism in adults. The decline in pituitary PACAP at birth may initiate a series of steps that activate gonadal function in the neonate and help explain the finding that mice deficient in PACAP [79, 80] or in ADACYAP1R1 [81] expression have reduced fertility.
It is important to emphasize that the relevance of these findings to reproduction in humans is not yet known. However, as in rodents, the level of FSH in human preterm infants is less than the level of LH [82], and PACAP increases Fst mRNA levels in primate FS cell-enriched pituitary cultures [83]. Thus, PACAP might regulate gonadotropin gene expression in the human fetal pituitary and might play a role in the differential secretion of LH and FSH that occurs in normal adolescence, in hypogonadotropic hypogonadism including hyperprolactinemia, during fasting, in women with polycystic ovary syndrome, and in some pituitary tumors in which functional, high-affinity PACAP-binding sites have been found [84]. Further research is needed to determine the role of PACAP in reproduction and to understand whether dysfunction of this peptide is important in disorders of the human pituitary.
Acknowledgment
The authors thank Mr. Dushan Ghoory for his many essential contributions to the conduct of our research.
Footnotes
Supported by NIH R01-HD050571 and by the Walter F. and Avis Jacobs Foundation.
REFERENCES
- Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun 1989; 164: 567 574 [DOI] [PubMed] [Google Scholar]
- Sherwood NM, Krueckl SL, McRory JE. The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr Rev 2000; 21: 619 670 [DOI] [PubMed] [Google Scholar]
- Dickson L, Finlayson K. VPAC and PAC receptors: from ligands to function. Pharmacol Ther 2009; 121: 294 316 [DOI] [PubMed] [Google Scholar]
- Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature 1993; 365: 170 175 [DOI] [PubMed] [Google Scholar]
- Pantaloni C, Brabet P, Bilanges B, Dumuis A, Houssami S, Spengler D, Bockaert J, Journot L. Alternative splicing in the N-terminal extracellular domain of the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor modulates receptor selectivity and relative potencies of PACAP-27 and PACAP-38 in phospholipase C activation. J Biol Chem 1996; 271: 22146 22151 [DOI] [PubMed] [Google Scholar]
- Journot L, Waeber C, Pantaloni C, Holsboer F, Seeburg PH, Bockaert J, Spengler D. Differential signal transduction by six splice variants of the pituitary adenylate cyclase-activating peptide (PACAP) receptor. Biochem Soc Trans 1995; 23: 133 137 [DOI] [PubMed] [Google Scholar]
- Mustafa T, Grimaldi M, Eiden LE. The hop cassette of the PAC1 receptor confers coupling to Ca2+ elevation required for pituitary adenylate cyclase-activating polypeptide-evoked neurosecretion. J Biol Chem 2007; 282: 8079 8091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 2009; 61: 283 357 [DOI] [PubMed] [Google Scholar]
- Vigh S, Arimura A, Gottschall PE, Kitada C, Somogyvari-Vigh A, Childs GV. Cytochemical characterization of anterior pituitary target cells for the neuropeptide, pituitary adenylate cyclase-activating polypeptide (PACAP), using biotinylated ligands. Peptides 1993; 14: 59 65 [DOI] [PubMed] [Google Scholar]
- Rawlings SR, Hezareh M. Pituitary adenylate cyclase-activating polypeptide (PACAP) and PACAP/vasoactive intestinal polypeptide receptors: actions on the anterior pituitary gland. Endocr Rev 1996; 17: 4 29 [DOI] [PubMed] [Google Scholar]
- Vertongen P, Velkeniers B, Hooghe-Peters E, Robberecht P. Differential alternative splicing of PACAP receptor in pituitary cell subpopulations. Mol Cell Endocrinol 1995; 113: 131 135 [DOI] [PubMed] [Google Scholar]
- Rawlings SR, Piuz I, Schlegel W, Bockaert J, Journot L. Differential expression of pituitary adenylate cyclase-activating polypeptide/vasoactive intestinal polypeptide receptor subtypes in clonal pituitary somatotrophs and gonadotrophs. Endocrinology 1995; 136: 2088 2098 [DOI] [PubMed] [Google Scholar]
- Rawlings SR, Canny BJ, Leong DA. Pituitary adenylate cyclase-activating polypeptide regulates cytosolic Ca2+ in rat gonadotropes and somatotropes through different intracellular mechanisms. Endocrinology 1993; 132: 1447 1452 [DOI] [PubMed] [Google Scholar]
- Schomerus E, Poch A, Bunting R, Mason WT, McArdle CA. Effects of pituitary adenylate cyclase-activating polypeptide in the pituitary: activation of two signal transduction pathways in the gonadotrope-derived alpha T3–1 cell line. Endocrinology 1994; 134: 315 323 [DOI] [PubMed] [Google Scholar]
- Fowkes RC, Sidhu KK, Sosabowski JK, King P, Burrin JM. Absence of pituitary adenylate cyclase-activating polypeptide-stimulated transcription of the human glycoprotein alpha-subunit gene in LbetaT2 gonadotrophs reveals disrupted cAMP-mediated gene transcription. J Mol Endocrinol 2003; 31: 263 278 [DOI] [PubMed] [Google Scholar]
- Winters SJ, Ghooray D, Fujii Y, Moore JP, Jr, Nevitt JR, Kakar SS. Transcriptional regulation of follistatin expression by GnRH in mouse gonadotroph cell lines: evidence for a role for cAMP signaling. Mol Cell Endocrinol 2007; 271: 45 54 [DOI] [PubMed] [Google Scholar]
- Canny BJ, Rawlings SR, Leong DA. Pituitary adenylate cyclase-activating polypeptide specifically increases cytosolic calcium ion concentration in rat gonadotropes and somatotropes. Endocrinology 1992; 130: 211 215 [DOI] [PubMed] [Google Scholar]
- McArdle CA, Poch A, Schomerus E, Kratzmeier M. Pituitary adenylate cyclase-activating polypeptide effects in pituitary cells: modulation by gonadotropin-releasing hormone in alpha T3–1 cells. Endocrinology 1994; 134: 2599 2605 [DOI] [PubMed] [Google Scholar]
- Lariviere S, Garrel-Lazayres G, Simon V, Shintani N, Baba A, Counis R, Cohen-Tannoudji J. Gonadotropin-releasing hormone inhibits pituitary adenylyl cyclase-activating polypeptide coupling to 3′,5′-cyclic adenosine-5′-monophosphate pathway in LbetaT2 gonadotrope cells through novel protein kinase C isoforms and phosphorylation of pituitary adenylyl cyclase-activating polypeptide type I receptor. Endocrinology 2008; 149: 6389 6398 [DOI] [PubMed] [Google Scholar]
- Counis R, Laverriere JN, Garrel-Lazayres G, Cohen-Tannoudji J, Lariviere S, Bleux C, Magre S. What is the role of PACAP in gonadotrope function? Peptides 2007; 28: 1797 1804 [DOI] [PubMed] [Google Scholar]
- Hart GR, Gowing H, Burrin JM. Effects of a novel hypothalamic peptide, pituitary adenylate cyclase-activating polypeptide, on pituitary hormone release in rats. J Endocrinol 1992; 134: 33 41 [DOI] [PubMed] [Google Scholar]
- Tsujii T, Winters SJ. Effects of pulsatile pituitary adenylate cyclase-activating polypeptide (PACAP) on gonadotropin secretion and subunit mRNA levels in perifused rat pituitary cells. Life Sci 1995; 56: 1103 1111 [DOI] [PubMed] [Google Scholar]
- Garrel G, Lozach A, Bachir LK, Laverriere JN, Counis R. Pituitary adenylate cyclase-activating polypeptide stimulates nitric oxide synthase type I expression and potentiates the cGMP response to gonadotropin-releasing hormone of rat pituitary gonadotrophs. J Biol Chem 2002; 277: 46391 46401 [DOI] [PubMed] [Google Scholar]
- Culler MD, Paschall CS. Pituitary adenylate cyclase-activating polypeptide (PACAP) potentiates the gonadotropin-releasing activity of luteinizing hormone-releasing hormone. Endocrinology 1991; 129: 2260 2262 [DOI] [PubMed] [Google Scholar]
- Tsujii T, Ishizaka K, Winters SJ. Effects of pituitary adenylate cyclase-activating polypeptide on gonadotropin secretion and subunit messenger ribonucleic acids in perifused rat pituitary cells. Endocrinology 1994; 135: 826 833 [DOI] [PubMed] [Google Scholar]
- Attardi B, Winters SJ. Transcriptional regulation of the glycoprotein hormone alpha-subunit gene by pituitary adenylate cyclase-activating polypeptide (PACAP) in alphaT3–1 cells. Mol Cell Endocrinol 1998; 137: 97 107 [DOI] [PubMed] [Google Scholar]
- Horton CD, Halvorson LM. The cAMP signaling system regulates LHbeta gene expression: roles of early growth response protein-1, SP1 and steroidogenic factor-1. J Mol Endocrinol 2004; 32: 291 306 [DOI] [PubMed] [Google Scholar]
- Pincas H, Laverriere JN, Counis R. Pituitary adenylate cyclase-activating polypeptide and cyclic adenosine 3′,5′-monophosphate stimulate the promoter activity of the rat gonadotropin-releasing hormone receptor gene via a bipartite response element in gonadotrope-derived cells. J Biol Chem 2001; 276: 23562 23571 [DOI] [PubMed] [Google Scholar]
- Sakai Y, Hashimoto H, Shintani N, Katoh H, Negishi M, Kawaguchi C, Kasai A, Baba A. PACAP activates Rac1 and synergizes with NGF to activate ERK1/2, thereby inducing neurite outgrowth in PC12 cells. Brain Res Mol Brain Res 2004; 123: 18 26 [DOI] [PubMed] [Google Scholar]
- Ohtaki H, Nakamachi T, Dohi K, Aizawa Y, Takaki A, Hodoyama K, Yofu S, Hashimoto H, Shintani N, Baba A, Kopf M, Iwakura Y, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) decreases ischemic neuronal cell death in association with IL-6. Proc Natl Acad Sci U S A 2006; 103: 7488 7493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowkes RC, Burch J, Burrin JM. Stimulation of extracellular signal-regulated kinase by pituitary adenylate cyclase-activating polypeptide in alpha T3–1 gonadotrophs. J Endocrinol 2001; 171: R5 R10 [DOI] [PubMed] [Google Scholar]
- Fujii Y, Okada Y, Moore JP, Jr, Dalkin AC, Winters SJ. Evidence that PACAP and GnRH down-regulate follicle-stimulating hormone-beta mRNA levels by stimulating follistatin gene expression: effects on folliculostellate cells, gonadotrophs and LbetaT2 gonadotroph cells. Mol Cell Endocrinol 2002; 192: 55 64 [DOI] [PubMed] [Google Scholar]
- Kanasaki H, Mutiara S, Oride A, Purwana IN, Miyazaki K. Pulse frequency-dependent gonadotropin gene expression by adenylate cyclase-activating polypeptide 1 in perifused mouse pituitary gonadotroph LbetaT2 cells. Biol Reprod 2009; 81: 465 472 [DOI] [PubMed] [Google Scholar]
- Harrison CA, Gray PC, Vale WW, Robertson DM. Antagonists of activin signaling: mechanisms and potential biological applications. Trends Endocrinol Metab 2005; 16: 73 78 [DOI] [PubMed] [Google Scholar]
- Kogure K, Zhang YQ, Kanzaki M, Omata W, Mine T, Kojima I. Intravenous administration of follistatin: delivery to the liver and effect on liver regeneration after partial hepatectomy. Hepatology 1996; 24: 361 366 [DOI] [PubMed] [Google Scholar]
- Winters SJ, Dalkin AC, Tsujii T. Evidence that pituitary adenylate cyclase-activating polypeptide suppresses follicle-stimulating hormone-beta messenger ribonucleic acid levels by stimulating follistatin gene transcription. Endocrinology 1997; 138: 4324 4329 [DOI] [PubMed] [Google Scholar]
- Sidis Y, Mukherjee A, Keutmann H, Delbaere A, Sadatsuki M, Schneyer A. Biological activity of follistatin isoforms and follistatin-like-3 is dependent on differential cell surface binding and specificity for activin, myostatin, and bone morphogenetic proteins. Endocrinology 2006; 147: 3586 3597 [DOI] [PubMed] [Google Scholar]
- Garrel G, Lerrant Y, Siriostis C, Berault A, Magre S, Bouchaud C, Counis R. Evidence that gonadotropin-releasing hormone stimulates gene expression and levels of active nitric oxide synthase type I in pituitary gonadotrophs, a process altered by desensitization and, indirectly, by gonadal steroids. Endocrinology 1998; 139: 2163 2170 [DOI] [PubMed] [Google Scholar]
- Shafiee-Kermani F, Han SO, Miller WL. Chronic gonadotropin-releasing hormone inhibits activin induction of the ovine follicle-stimulating hormone beta-subunit: involvement of 3′,5′-cyclic adenosine monophosphate response element binding protein and nitric oxide synthase type I. Endocrinology 2007; 148: 3346 3355 [DOI] [PubMed] [Google Scholar]
- Pisconti A, Brunelli S, Di Padova M, De Palma C, Deponti D, Baesso S, Sartorelli V, Cossu G, Clementi E. Follistatin induction by nitric oxide through cyclic GMP: a tightly regulated signaling pathway that controls myoblast fusion. J Cell Biol 2006; 172: 233 244 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology 1991; 129: 2787 2789 [DOI] [PubMed] [Google Scholar]
- Ghatei MA, Takahashi K, Suzuki Y, Gardiner J, Jones PM, Bloom SR. Distribution, molecular characterization of pituitary adenylate cyclase-activating polypeptide and its precursor encoding messenger RNA in human and rat tissues. J Endocrinol 1993; 136: 159 166 [DOI] [PubMed] [Google Scholar]
- Osuga Y, Mitsuhashi N, Mizuno M. In vivo effect of pituitary adenylate cyclase-activating polypeptide 38 (PACAP 38) on the secretion of luteinizing hormone (LH) in male rats. Endocrinol Jpn 1992; 39: 153 156 [DOI] [PubMed] [Google Scholar]
- Anderson ST, Sawangjaroen K, Curlewis JD. Pituitary adenylate cyclase-activating polypeptide acts within the medial basal hypothalamus to inhibit prolactin and luteinizing hormone secretion. Endocrinology 1996; 137: 3424 3429 [DOI] [PubMed] [Google Scholar]
- Dow RC, Bennie J, Fink G. Pituitary adenylate cyclase-activating peptide-38 (PACAP)-38 is released into hypophysial portal blood in the normal male and female rat. J Endocrinol 1994; 142: R1 R4 [DOI] [PubMed] [Google Scholar]
- Masuo Y, Tokito F, Matsumoto Y, Shimamoto N, Fujino M. Ontogeny of pituitary adenylate cyclase-activating polypeptide (PACAP) and its binding sites in the rat brain. Neurosci Lett 1994; 170: 43 46 [DOI] [PubMed] [Google Scholar]
- Moore JP, Jr, Wilson L, Dalkin AC, Winters SJ. Differential expression of the pituitary gonadotropin subunit genes during male rat sexual maturation: reciprocal relationship between hypothalamic pituitary adenylate cyclase-activating polypeptide and follicle-stimulating hormone beta expression. Biol Reprod 2003; 69: 234 241 [DOI] [PubMed] [Google Scholar]
- Moore JP, Jr, Burger LL, Dalkin AC, Winters SJ. Pituitary adenylate cyclase-activating polypeptide messenger RNA in the paraventricular nucleus and anterior pituitary during the rat estrous cycle. Biol Reprod 2005; 73: 491 499 [DOI] [PubMed] [Google Scholar]
- Kimura F, Kawakami M, Nakano H, McCann SM. Changes in adenosine 3′,5′-monophosphate and guanosine 3′,5′-monophosphate concentrations in the anterior pituitary and hypothalamus during the rat estrous cycle and effects of administration of sodium pentobarbital in proestrus. Endocrinology 1980; 106: 631 635 [DOI] [PubMed] [Google Scholar]
- Apostolakis EM, Lanz R, O'Malley BW. Pituitary adenylate cyclase-activating peptide: a pivotal modulator of steroid-induced reproductive behavior in female rodents. Mol Endocrinol 2004; 18: 173 183 [DOI] [PubMed] [Google Scholar]
- Apostolakis EM, Riherd DN, O'Malley BW. PAC1 receptors mediate pituitary adenylate cyclase-activating polypeptide- and progesterone-facilitated receptivity in female rats. Mol Endocrinol 2005; 19: 2798 2811 [DOI] [PubMed] [Google Scholar]
- Szabo E, Nemeskeri A, Heinzlmann A, Suzuki N, Arimura A, Koves K. Cell immunoblot assay study demonstrating the release of PACAP from individual anterior pituitary cells of rats and the effect of PACAP on LH release. Regul Pept 2002; 109: 75 81 [DOI] [PubMed] [Google Scholar]
- Olcese J, Middendorff R, Munker M, Schmidt C, McArdle CA. Natriuretic peptides stimulate cyclic GMP production in an immortalized LHRH neuronal cell line. J Neuroendocrinol 1994; 6: 127 130 [DOI] [PubMed] [Google Scholar]
- Gray SL, Cummings KJ, Jirik FR, Sherwood NM. Targeted disruption of the pituitary adenylate cyclase-activating polypeptide gene results in early postnatal death associated with dysfunction of lipid and carbohydrate metabolism. Mol Endocrinol 2001; 15: 1739 1747 [DOI] [PubMed] [Google Scholar]
- Gray SL, Yamaguchi N, Vencova P, Sherwood NM. Temperature-sensitive phenotype in mice lacking pituitary adenylate cyclase-activating polypeptide. Endocrinology 2002; 143: 3946 3954 [DOI] [PubMed] [Google Scholar]
- Isaac ER, Sherwood NM. Pituitary adenylate cyclase-activating polypeptide (PACAP) is important for embryo implantation in mice. Mol Cell Endocrinol 2008; 280: 13 19 [DOI] [PubMed] [Google Scholar]
- Lacombe A, Lelievre V, Roselli CE, Salameh W, Lue YH, Lawson G, Muller JM, Waschek JA, Vilain E. Delayed testicular aging in pituitary adenylate cyclase-activating peptide (PACAP) null mice. Proc Natl Acad Sci U S A 2006; 103: 3793 3798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koves K, Kantor O, Scammell JG, Arimura A. PACAP colocalizes with luteinizing and follicle-stimulating hormone immunoreactivities in the anterior lobe of the pituitary gland. Peptides 1998; 19: 1069 1072 [DOI] [PubMed] [Google Scholar]
- Jin L, Tsumanuma I, Ruebel KH, Bayliss JM, Lloyd RV. Analysis of homogeneous populations of anterior pituitary folliculostellate cells by laser capture microdissection and reverse transcription-polymerase chain reaction. Endocrinology 2001; 142: 1703 1709 [DOI] [PubMed] [Google Scholar]
- Koves K, Kantor O, Molnar J, Heinzlmann A, Szabo E, Szabo F, Nemeskeri A, Horvath J, Arimura A. The role of PACAP in gonadotropic hormone secretion at hypothalamic and pituitary levels. J Mol Neurosci 2003; 20: 141 152 [DOI] [PubMed] [Google Scholar]
- Heinzlmann A, Kirilly E, Meltzer K, Szabo E, Baba A, Hashimoto H, Koves K. PACAP is transiently expressed in anterior pituitary gland of rats: in situ hybridization and cell immunoblot assay studies. Peptides 2008; 29: 571 577 [DOI] [PubMed] [Google Scholar]
- Moore JP, Jr, Villafuerte BC, Unick CA, Winters SJ. Developmental changes in pituitary PACAP expression during the perinatal period: possible role in fetal gonadotroph regulation. Endocrinology 2009; 150: 4802 4809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski DM, Proctor MD. Developmental regulation of pituitary adenylate cyclase-activating polypeptide and PAC(1) receptor mRNA expression in the rat central nervous system. Brain Res Dev Brain Res 2000; 120: 27 39 [DOI] [PubMed] [Google Scholar]
- Ma YY, Qi XF, Song SJ, Zhao ZY, Zhu ZD, Qi J, Zhang X, Xiao HS, Teng Y, Han ZG. cDNA microarray reveals signaling pathways involved in hormones expression of human pituitary. Gen Comp Endocrinol 2005; 143: 184 192 [DOI] [PubMed] [Google Scholar]
- Suzuki N, Harada M, Hosoya M, Fujino M. Enhanced production of pituitary adenylate-cyclase-activating polypeptide by 1,N6-dibutyryladenosine 3′,5′-monophosphate, phorbol 12-myristate 13-acetate and by the polypeptide itself in human neuroblastoma cells, IMR-32. Eur J Biochem 1994; 223: 147 153 [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Hashimoto H, Hagihara N, Nishino A, Fujita T, Matsuda T, Baba A. Cloning and characterization of the mouse pituitary adenylate cyclase-activating polypeptide (PACAP) gene. Gene 1998; 211: 63 69 [DOI] [PubMed] [Google Scholar]
- Radleff-Schlimme A, Leonhardt S, Wuttke W, Jarry H. Evidence for PACAP to be an autocrine factor on gonadotrope cells. Ann N Y Acad Sci 1998; 865: 486 491 [DOI] [PubMed] [Google Scholar]
- Grafer CM, Thomas R, Lambrakos L, Montoya I, White S, Halvorson LM. GnRH stimulates expression of PACAP in the pituitary gonadotropes via both the PKA and PKC signaling systems. Mol Endocrinol 2009; 23: 1022 1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanasaki H, Yonehara T, Yamada Y, Takahashi K, Hata K, Fujiwaki R, Yamamoto H, Takeuchi Y, Fukunaga K, Miyamoto E, Miyazaki K. Regulation of gonadotropin alpha subunit gene expression by dopamine D(2) receptor agonist in clonal mouse gonadotroph alphaT3–1 cells. Biol Reprod 2002; 67: 1218 1224 [DOI] [PubMed] [Google Scholar]
- Mutiara S, Kanasaki H, Harada T, Miyazaki K. Dopamine D(2) receptor expression and regulation of gonadotropin alpha-subunit gene in clonal gonadotroph LbetaT2 cells. Mol Cell Endocrinol 2006; 259: 22 29 [DOI] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res 2004; 24: 165 205 [DOI] [PubMed] [Google Scholar]
- Coulon JF, Biguet NF, Cavoy A, Delacour J, Mallet J, David JC. Gene expression of tyrosine hydroxylase in the developing fetal brain. J Neurochem 1990; 55: 1412 1417 [DOI] [PubMed] [Google Scholar]
- Yang TT, Tsao CW, Li JS, Wu HT, Hsu CT, Cheng JT. Changes of dopamine content and cell proliferation by dexamethsone via pituitary adenylate cyclase-activating polypeptide in PC12 cell. Neurosci Lett 2007; 426: 45 48 [DOI] [PubMed] [Google Scholar]
- Ha CM, Kang JH, Choi EJ, Kim MS, Park JW, Kim Y, Choi WS, Chun SY, Kwon HB, Lee BJ. Progesterone increases mRNA levels of pituitary adenylate cyclase-activating polypeptide (PACAP) and type I PACAP receptor (PAC(1)) in the rat hypothalamus. Brain Res Mol Brain Res 2000; 78: 59 68 [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Lee BL, Carroll RS, Unabia G, Chin WW, Childs GV. Follistatin gene expression in the pituitary: localization in gonadotropes and folliculostellate cells in diestrous rats. Endocrinology 1992; 130: 3048 3056 [DOI] [PubMed] [Google Scholar]
- Bilezikjian LM, Leal AM, Blount AL, Corrigan AZ, Turnbull AV, Vale WW. Rat anterior pituitary folliculostellate cells are targets of interleukin-1beta and a major source of intrapituitary follistatin. Endocrinology 2003; 144: 732 740 [DOI] [PubMed] [Google Scholar]
- Katayama T, Nakashima M, Kyan H, Murakami N, Kuroda H. A role of pituitary adenylate cyclase-activating polypeptide (PACAP) as a regulator of paracrine interactions between folliculo-stellate cells and gonadotropes through the control of activin-follistatin interactions. J Vet Med Sci 2000; 62: 731 736 [DOI] [PubMed] [Google Scholar]
- McArdle CA. Pituitary adenylate cyclase-activating polypeptide: a key player in reproduction? Endocrinology 1994; 135: 815 817 [DOI] [PubMed] [Google Scholar]
- Shintani N, Mori W, Hashimoto H, Imai M, Tanaka K, Tomimoto S, Hirose M, Kawaguchi C, Baba A. Defects in reproductive functions in PACAP-deficient female mice. Regul Pept 2002; 109: 45 48 [DOI] [PubMed] [Google Scholar]
- Sherwood NM, Adams BA, Isaac ER, Wu S, Fradinger EA. Knocked down and out: PACAP in development, reproduction and feeding. Peptides 2007; 28: 1680 1687 [DOI] [PubMed] [Google Scholar]
- Jamen F, Rodriguez-Henche N, Pralong F, Jegou B, Gaillard R, Bockaert J, Brabet P. PAC1 null females display decreased fertility. Ann N Y Acad Sci 2000; 921: 400 404 [DOI] [PubMed] [Google Scholar]
- Massa G, de Zegher F, Vanderschueren-Lodeweyckx M. Serum levels of immunoreactive inhibin, FSH, and LH in human infants at preterm and term birth. Biol Neonate 1992; 61: 150 155 [DOI] [PubMed] [Google Scholar]
- Kawakami S, Fujii Y, Okada Y, Winters SJ. Paracrine regulation of FSH by follistatin in folliculostellate cell-enriched primate pituitary cell cultures. Endocrinology 2002; 143: 2250 2258 [DOI] [PubMed] [Google Scholar]
- Vertongen P, d'Haens J, Michotte A, Velkeniers B, van Rampelbergh J, Svoboda M, Robberecht P. Expression of pituitary adenylate cyclase-activating polypeptide and receptors in human brain tumors. Peptides 1995; 16: 713 719 [DOI] [PubMed] [Google Scholar]
- Mazhawidza W, Winters SJ, Kaiser UB, Kakar SS. Identification of gene networks modulated by activin in LbetaT2 cells using DNA microarray analysis. Histol Histopathol 2006; 21: 167 178 [DOI] [PubMed] [Google Scholar]
- Zhang H, Bailey JS, Coss D, Lin B, Tsutsumi R, Lawson MA, Mellon PL, Webster NJ. Activin modulates the transcriptional response of LbetaT2 cells to gonadotropin-releasing hormone and alters cellular proliferation. Mol Endocrinol 2006; 20: 2909 2930 [DOI] [PMC free article] [PubMed] [Google Scholar]