Abstract
Previous studies in MA-10 tumor Leydig cells demonstrated that disruption of the mitochondrial electron-transport chain (ETC), membrane potential (ΔΨm), or ATP synthesis independently inhibited steroidogenesis. In contrast, studies of primary Leydig cells indicated that the ETC, ΔΨm, and ATP synthesis cooperatively affected steroidogenesis. These results suggest significant differences between the two systems and call into question the extent to which results from tumor Leydig cells relate to primary cells. Thus, to further understand the similarities and differences between the two systems as well as the impact of ATP disruption on steroidogenesis, we performed comparative studies of MA-10 and primary Leydig cells under similar conditions of mitochondrial disruption. We show that mitochondrial ATP synthesis is critical for steroidogenesis in both primary and tumor Leydig cells. However, in striking contrast to primary cells, perturbation of ΔΨm in MA-10 cells did not substantially decrease cellular ATP content, a perplexing finding because ΔΨm powers the mitochondrial ATP synthase. Further studies revealed that a significant proportion of cellular ATP in MA-10 cells derives from glycolysis. In contrast, primary cells appear to be almost completely dependent on mitochondrial respiration for their energy provision. Inhibitor studies also suggested that the MA-10 ETC is impaired. This work underscores the importance of mitochondrial ATP for hormone-stimulated steroid production in both MA-10 and primary Leydig cells while indicating that caution must be exercised in extrapolating data from tumor cells to primary tissue.
Keywords: ATP, glycolysis, Leydig cells, mitochondria, respiration, steroid hormones/steroid hormone receptors, testosterone
Primary and tumor Leydig cells display significant differences in sources of ATP production, but mitochondrial ATP synthesis is important for steroidogenesis in both cell types.
INTRODUCTION
Synthesis of testosterone in mammalian males is performed almost exclusively by testicular Leydig cells. Acute synthesis of testosterone is stimulated by the binding of circulating luteinizing hormone (LH) to high-affinity receptors on the Leydig cell plasma membrane, which results in the formation of 3′,5′-cAMP [1]. LH, through cAMP, promotes the transfer of cholesterol to the inner mitochondrial membrane, where it is metabolized into pregnenolone (P5) via the P450 cholesterol side-chain cleavage enzyme (CYP11A1). This is the primary point of postreceptor control of steroidogenesis, because cholesterol does not freely diffuse across the mitochondrial intermembrane space. Several proteins, including the steroidogenic acute regulatory (STAR) protein [2] and translocator protein (TSPO) [3], are critical for this mitochondrial cholesterol transfer, operating as components of a larger “cholesterol transfer” complex recently named the transduceosome [4, 5]. Upon transfer to the mitochondrial matrix, cholesterol is metabolized to P5 by CYP11A1. Subsequently, P5 is metabolized by the 3β-hydroxysteroid dehydrogenase/isomerase (3βHSD) enzyme in the endoplasmic reticulum (ER) to progesterone (P4), which is converted to androstenedione by the ER cytochrome P450 17α-hydroxylase/17,20-lyase (CYP17). Androstenedione is finally metabolized in the ER by 17β-hydroxysteroid dehydrogenase (17βHSD) to yield the androgen testosterone [6].
In addition to its central role in cholesterol transport and metabolism in steroidogenic cells, the mitochondria are best known for their role in the synthesis of ATP. In many cells, mitochondrial ATP synthesis provides the bulk of cellular ATP through oxidative phosphorylation, a process in which electrons flow from electron donors (NADH and FADH2) generated by mitochondrial metabolic processes to the terminal electron acceptor, oxygen. Electron transfer occurs along a series of mitochondrial polypeptide complexes—complex I (NADH dehydrogenase), complex III (cytochrome c reductase), and complex IV (cytochrome c oxidase) [7, 8]—and is electrochemically coupled to the translocation of protons across the inner mitochondrial membrane, generating a proton-motive force composed of an electrical gradient (ΔΨm) and an H+ gradient (ΔpH). The mitochondrial membrane potential (ΔΨm) is utilized by the mitochondria for numerous processes, including the powering of mitochondrial ATP synthase [7, 8].
In addition to its production by oxidative phosphorylation, ATP is synthesized by cytosolic glycolysis [9]. Though glycolysis produces much less ATP per cycle than oxidative phosphorylation [9], it nonetheless plays an important role in some mammalian cells. For example, spermatozoa contain respiring mitochondria, but glycolytically derived ATP appears to be the primary energy source for sperm motility [10, 11]. Whether this also is true of the somatic cells in the testis remains an open question.
In previous studies using MA-10 mouse tumor Leydig cells, inhibition of mitochondrial electron transport with antimycin A and of mitochochondrial ATP synthesis with oligomycin suppressed cAMP-stimulated steroid (P4) synthesis [12]. These studies suggested that the energetic state of the mitochondria of the MA-10 cells is critically involved in the regulation of steroidogenesis. Building on these findings with tumor cells, we examined the effects of mitochondrial electron-transport chain (ETC) inhibition in primary Leydig cells with the ETC complex III inhibitor myxothiazol [13]. Myxothiazol inhibited cAMP and testosterone synthesis in response to LH as well as the activities of the downstream steroidogenic enzymes 3βHSD, CYP17, and 17βHSD [13]. Collectively, these studies demonstrated that mitochondrial disruption inhibits steroid biosynthesis at multiple steps in the steroidogenic pathway. These studies did not address the relative contributions of particular mitochondrial energetic functions—electron transport, ΔΨm, and ATP synthesis—to the control of steroidogenesis. Knowledge of these contributions is important for our mechanistic understanding of steroid synthesis and metabolism.
Many previous studies of cellular energetics in relationship to Leydig cell steroidogenesis have utilized hormone-responsive MA-10 mouse tumor Leydig cells as a model system [12, 14, 15]. The extent to which findings with these cells can be extrapolated to primary cells is uncertain, however, because fast-growing tumor cell types, such as MA-10 cells, typically display markedly modified energy metabolism in comparison to cells freshly isolated from their tissue of origin [9, 16, 17]. A major objective of the present study was to critically compare the relationship between mitochondrial metabolism and steroid synthesis in primary and tumor Leydig cells. To this end, relationships among ΔΨm, cellular ATP levels, sources of ATP synthesis, and steroidogenesis were analyzed in primary Leydig cells freshly isolated from rat testes in comparison to MA-10 tumor Leydig cells. We report that that primary Leydig cell ATP levels were highly sensitive to ΔΨm disruption, whereas MA-10 cells derived a significant proportion of their cellular ATP from glycolysis. Additionally, differences in mitochondrial ETC function were observed between the two cell types. However, both cell types were highly dependent on mitochondrial ATP for their steroidogenic function. The present results, taken together, extend our knowledge regarding energetic regulation of steroidogenesis and point to a central role of mitochondrial-derived ATP in steroidogenesis. The results also indicate, however, that given the important differences between MA-10 and primary cells, caution must be exercised before extrapolating data obtained with tumor to primary Leydig cells.
MATERIALS AND METHODS
Materials
Rotenone, antimycin A, sodium cyanide, carbonyl cyanide m-chlorophenyl hydrazone (CCCP), oligomycin, 2-deoxyglucose (2-DG), dibutyryl cAMP (dbcAMP), and 22(R)-hydroxycholesterol (HC) were obtained from Sigma-Aldrich. P5, P4, androstenedione, and testosterone were purchased from Steraloids. Tetramethylrhodamine methyl ester (TMRM) and tetramethylrhodamine ethyl ester (TMRE) were purchased from Molecular Probes. Type IV collagenase was obtained from Worthington. Testosterone and P4 antibodies were obtained from MP Biomedicals. Bovine LH (USDA-bLH-B-6) was provided by the U.S. Department of Agriculture Animal Hormone Program.
Animals
Brown Norway rats (age, 4 mo) were obtained from Harlan Sprague Dawley through the National Institute on Aging and housed in the animal facilities of the Johns Hopkins Bloomberg School of Public Health (22°C, 14L:10D) with access to feed and water ad libitum. Animal handling and care were in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University.
Leydig Cell Isolation and Culture of Tumor Leydig Cells
Leydig cells were isolated from 4-mo-old Brown Norway rats as previously described [18]. In brief, the testicular artery was cannulated and perfused with type IV collagenase (1 mg/ml) in dissociation buffer (M199 medium with 2.2 g/L of Hepes, 0.1% bovine serum albumin, 25 mg/L of trypsin inhibitor, and 0.7 g/L of sodium bicarbonate [pH 7.4]). Testes were then decapsulated, and dissociation was continued at 34°C at a lower concentration (0.25 mg/ml) of collagenase, with low-speed shaking (90 cycles/min). Seminiferous tubules were removed by filtration through nylon mesh (pore size, 100 μm). The remaining fraction was centrifuged at 250 × g and the pellet resuspended in 55% isotonic Percoll. The Percoll suspension was centrifuged for 1 h at 27 000 × g, and Leydig cells with a density of 1.07 g/ml and greater were harvested for subsequent experimentation. Leydig cell purity, as determined by their staining for 3βHSD activity [19], was greater than 95% in all experiments. The viability of the cells, as assessed by trypan blue exclusion, was greater than 90%.
The MA-10 mouse tumor Leydig cells, derived from mouse Leydig tumors [20], were a gift from Dr. Mario Ascoli (Department of Pharmacology, Carver College of Medicine, The University of Iowa, Iowa City, IA). Cells were cultured in 75-cm2 cell culture flasks (Dow Corning Corp.) and were grown in Waymouth complete medium MB 752/1 (Invitrogen) containing 15% horse serum (Invitrogen) at 34°C in 5% CO2 in a humidified incubator.
Two-Photon Laser-Scanning Microscopy
The cationic potentiometric fluorescent dye TMRM was used to monitor changes in ΔΨm using two-photon laser-scanning fluorescence microscopy. The large potential gradient across the inner mitochondrial membrane results in the accumulation of TMRE within the matrix compartment according to its Nerst potential. Cells were loaded with 100 nM TMRM by adding dye to media and allowing uptake for 5 min at 34°C. Two-photon microscopy was performed as previously described [21]. Briefly, images were recorded using a two-photon laser-scanning microscope (Bio-Rad MRC-1024MP) with excitation at 740 nm (Tsunami Ti:Sa laser; Spectra Physics). Because of the overlap in the cross sections for two-photon excitation of the two fluorophores of interest (NADH and TMRM), this wavelength permitted simultaneous recording of the NAD(P)H:NAD(P)+ redox potential and ΔΨm. The red emission of TMRE was collected at 605 ± 25 nm, and NADH emission was collected as the total fluorescence at less than 490 nm. At 2-min intervals, 512- × 512-pixel, 8-bit, gray-scale images of the emission channels were collected simultaneously and stored. The average power from the Ti:Sa laser was 1000 mW, and the pulse bandwidth was approximately 12 nm, corresponding to a pulse duration of less than 60 fsec at a repetition rate of 80 MHz. This excitation was attenuated by the optical system and by a combination of neutral-density filters such that the average intensity at the focal plane was less than 10 mW. Images were analyzed and color added offline using ImageJ software (Wayne Rasband, National Institutes of Health; http://rsbweb.nih.gov/ij/).
Fluorescent Microplate Reading of ΔΨm
Fluorescent microplate readings of mitochondrial ΔΨm were performed as previously described [22] with modifications. Briefly, primary or MA-10 Leydig cells were plated in 96-well plates (50 000 cells/well) and allowed to adhere for 1 h before incubating the cells in phenol red M199 containing 100 nM of the potentiometric dye TMRE for 30 min at 34°C. Following loading, the cells were washed with PBS and exposed to various concentrations of the energy toxins used in the present study, suspended in phenol red-free M199, for 15 min at 34°C. Following this incubation period, medium was removed, and the cells were washed three times with PBS before leaving the cells suspended in PBS after the last wash. TMRE fluorescence was read immediately using a fluorescence microplate reader (Bio-Rad) with an excitation wavelength of 530 nm and an emission wavelength of 590 nm.
ATP Assay
Cellular ATP levels were assessed using the Promega luminescent cell viability assay (Promega G7570; Fisher Scientific). Purified primary or MA-10 tumor cells (3.3 × 104 cells/well) were plated in 96-well luminescence assay plates (Costar, Fisher Scientific). Treatment media were prepared in 100 μl of phenol red-free M199 medium (Invitrogen). Treatment groups were analyzed in replicates of three to four. After incubation at 34°C, cells were brought to room temperature, and an equal volume of Promega Cell Titer-Glo substrate (a mixture of Cell-Glo reagent and buffer) was added to the wells. Cells were incubated at room temperature on a shaker for 2 min, followed by an 8-min standing incubation at room temperature to enable cell lysis. Samples were analyzed for overall luminescence using a fluorescence microplate reader (Bio-Rad). Cellular ATP levels were determined according to standard curves of freshly prepared ATP solutions (Sigma-Aldrich).
Testosterone and P4 Radioimmunoassay
To assess the time and dose responses of primary and tumor Leydig cells to CCCP, oligomycin, 2-DG, rotenone, and antimycin A, the purified primary or MA-10 tumor Leydig cells (1 × 105 cells/well) were incubated in 96-well Falcon culture plates with LH (100 ng/ml) or dbcAMP (1 mM) in the presence of increasing concentrations of CCCP (0–10 μM), oligomycin (0–10 μg/ml), 2-DG (0–100 mM), antimycin A (0–10 μM), or rotenone (0–10 μM) for 0–2 h. At the end of incubation, the media were collected for testosterone analysis (primary cell cultures) or P4 analysis (MA-10 cell cultures) by radioimmunoassay (RIA). Briefly, rabbit antitestosterone polyclonal antibodies were utilized as per the manufacturer's instructions (MP Biomedicals). The sensitivity of the antibody was 10 pg testosterone/tube, with inter- and intra-assay coefficients of variation of 14.0% and 13.7%, respectively. For analysis of P4, rabbit anti-P4 monoclonal antibodies were utilized as per the manufacturer's instructions (MP Biomedicals). The sensitivity of the antibody was 15 pg P4/tube, with inter- and intra-assay coefficients of variation of 8.6% and 7.4%, respectively. To assay LH signaling and the steroidogenic enzymes 3βHSD, CYP17, and 17βHSD in primary cells, the cells were incubated with LH (100 ng/ml), dbcAMP (1 mM), HC (20 μM), P5 (10 μM), P4 (10 μM), or androstenedione (5 μM) for 2 h. At the end of the incubation, media were collected for testosterone measurements by RIA.
Statistical Analysis
Experiments were performed in triplicate unless otherwise stated. Each experiment contained a minimum of three replicates. The data are reported as the mean ± SEM of three (or more) independent experiments. As appropriate, statistical analysis was performed by Student t-test (comparison of two data sets) or one-way ANOVA, with the latter followed by the Student-Newman-Keuls test of multiple data sets if P < 0.05 by ANOVA, using the Prism 4.02 software package from GraphPad, Inc.
RESULTS
Leydig Cell Response to Mitochondrial Depolarization
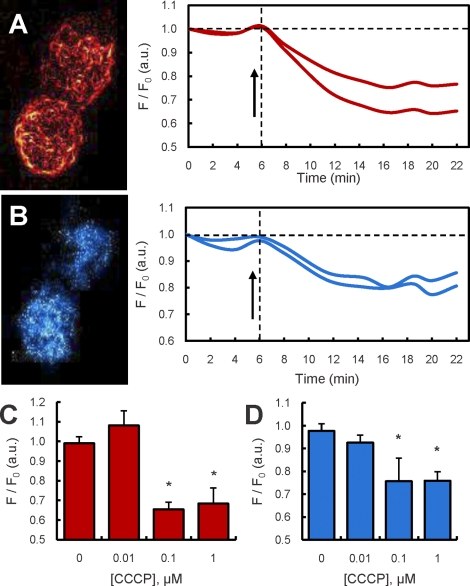
The effect of the mitochondrial protonophore and respiration uncoupler CCCP on ΔΨm was examined in primary rat Leydig cells in real time using two-photon microscopy. Cells were loaded with the potentiometric fluorescent dye TMRM, and the relative fluorescence intensity derived from images of single Leydig cells was quantified over time (Fig. 1, A, B, and associated time traces). The dose-dependent effect of CCCP exposure in this experiment was obtained by comparing relative fluorescence of cells before and after exposure to CCCP for 5 min, a time at which the fluorescence stabilized. As shown in Figure 1C, mitochondria maintained their ΔΨm at 0.01 μM CCCP, but a decline was observed in the mean intracellular fluorescence of TMRM at doses of 0.1 μM and greater, indicative of loss of the dye from the mitochondria because of depolarization. Studies in brain mitochondria have demonstrated that dissipation of the ΔΨm results in mitochondrial oxidation of NAD(P)H and decrease in autofluorescence [23]. As shown in Figure 1, B and D, in conjunction with loss of ΔΨm, cellular levels of NAD(P)H autofluorescence in the Leydig cells decreased correspondingly.
FIG. 1.
Two-photon microscopy (TPM) of mitochondrial ΔΨm and cellular NAD(P)H levels in LH-stimulated and in LH- and CCCP-stimulated primary Leydig cells. A) TPM image of TMRM fluorescence from two Leydig cells and corresponding time traces of TMRM fluorescence in the same cells exposed to LH (100 ng/ml) and to LH plus 1 μM CCCP (arrow). B) TPM image of NAD(P)H autofluorescence from the same Leydig cells as in A and corresponding time traces of NAD(P)H autofluorescence in the same cells exposed to LH (100 ng/ml) and to LH and 1 μM CCCP (arrow). C) TPM readings (mean ± SEM) of TMRM fluorescence of Leydig cells exposed to increasing concentrations of CCCP (0–1 μM) (summary of two experiments, n = 20 cells). D) TPM readings (mean ± SEM) of NAD(P)H autofluorescence of Leydig cells exposed to increasing concentrations of CCCP (0–1 μM) (summary of two experiments, n = 20 cells). *P < 0.001 vs. cells incubated without CCCP.
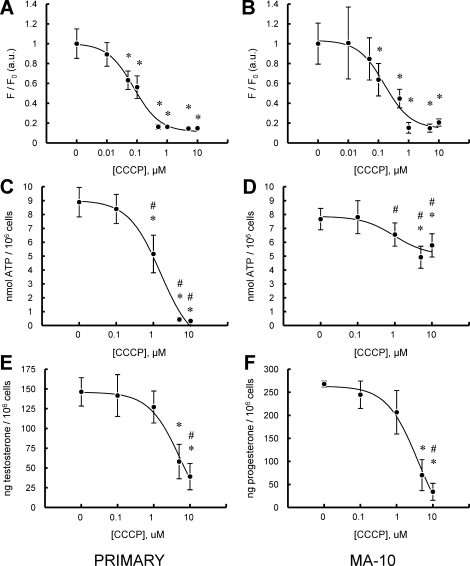
Plate-based assays were used to assess ΔΨm and ATP in relationship to the steroidogenic capacity of primary and tumor Leydig cells. Figure 2 shows the effect of treatment of MA-10 and primary Leydig cells with increasing concentrations of CCCP on ΔΨm (Fig. 2, A and B), intracellular ATP content (Fig. 2, C and D), and steroid production (Fig. 2, E and F). The ΔΨm in both MA-10 and primary Leydig cells, as measured by the fluorescent dye TMRE, was highly responsive to CCCP, with significant decreases seen at 0.05–10 μM CCCP and median inhibitory concentrations (IC50) of 0.08 ± 0.09 and 0.15 ± 0.16 μM CCCP for primary and MA-10 cells, respectively (Fig. 2, A and B). Mitochondrial depolarization was not accompanied by changes in trypan blue dye exclusion in either cell type, indicating that the effects seen were not a consequence of generalized toxicity. In the case of MA-10 cells, treatment with increasing concentrations of CCCP caused a significant, but modest (∼30%), reduction in ATP levels at concentrations above 1 μM (Fig. 2D). In striking contrast, a substantial decrease was observed in the cellular ATP content in primary Leydig cells exposed to increasing concentrations of CCCP, with inhibition of approximately 90% seen at greater than 1 μM (Fig. 2C). IC50 values for ATP inhibition of 1.56 ± 0.14 and 0.97 ± 0.54 μM were observed for primary and MA-10 cells, respectively (Fig. 2, C and D). Similar treatment of both MA-10 tumor and primary Leydig cells with increasing concentrations of CCCP resulted in significantly decreased steroidogenic capacity (P4 production in the case of MA-10 cells; testosterone production in the case of primary Leydig cells), with IC50 values of 5.20 ± 1.66 and 3.87 ± 1.51 μM for primary and MA-10 cells, respectively (Fig. 2, E and F). Notably, the CCCP concentration that elicited significant changes in ATP levels by MA-10 and primary Leydig cells (1 μM) was nearly an order of magnitude greater than the concentration found to alter ΔΨm (0.1 μM) (Fig. 2, A and B). Even higher CCCP concentrations were required to significantly inhibit steroid production (Fig. 2, E and F).
FIG. 2.
Effect of CCCP on LH-stimulated primary and MA-10 tumor Leydig cell mitochondrial ΔΨm, intracellular ATP content, and steroid production. A and B) Fluorescence plate reader assay of ΔΨm in primary (A) and MA-10 tumor (B) Leydig cultures incubated with increasing doses of CCCP (0–10 μM) in the presence of maximally stimulating LH (100 ng/ml) for 15 min. C and D) ATP levels of primary (C) and MA-10 tumor (D) Leydig cultures incubated with increasing doses of CCCP (0–10 μM) in the presence of maximally stimulating LH (100 ng/ml) for 2 h. E and F) Steroid production by primary (E) and MA-10 tumor (F) Leydig cultures were incubated with increasing doses of CCCP (0–10 μM) in the presence of maximally stimulating LH (100 ng/ml) for 2 h. Points shown represent the mean ± SEM of three to four experiments, with three replicates per experiment. *P < 0.01 vs. cells incubated without CCCP, #P < 0.05 vs. corresponding primary or MA-10 cells.
ATP Synthesis and Steroidogenesis in Tumor Versus Primary Leydig Cells
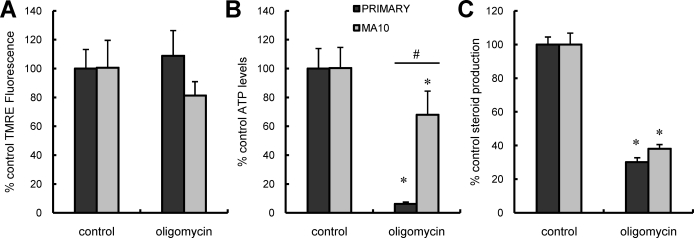
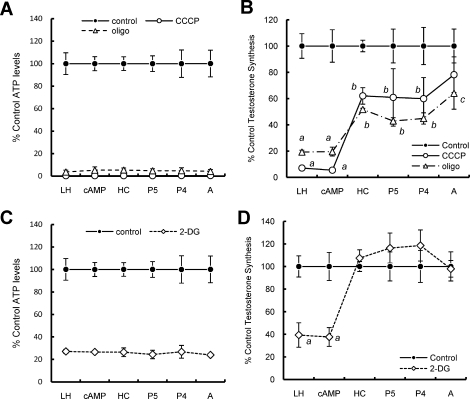
The observations that ΔΨm depolarizing concentrations of CCCP elicited only modest changes in MA-10 ATP levels cells but severely depleted levels in primary Leydig cells, whereas steroidogenesis was reduced in both cell types, suggested that the roles of ΔΨm depolarization and intracellular ATP levels on steroidogenesis might differ between the two cell types. We hypothesized that differences may exist in the importance of glycolysis versus mitochondrial ATP production for MA-10 and primary Leydig cells. To test this, the effects of culturing MA-10 versus primary Leydig cells with the ATP synthase inhibitor oligomycin on ATP levels and steroid production were assessed. Culturing the cells with oligomycin had no effect on ΔΨm in either cell type (Fig. 3A). As seen in Figure 3B, oligomycin treatment significantly decreased ATP levels in both MA-10 and primary Leydig cells. However, whereas primary cells only retained 6.4% ± 4.2% of their cellular ATP upon CCCP exposure, MA-10 cells retained 67.9% ± 11.9% (Fig. 3B). These results suggest that whereas the primary cells derive nearly all of their cellular ATP from mitochondrial respiration, the MA-10 cells derive a substantial proportion of their cellular ATP from an oligomycin-insensitive, nonmitochondrial pool. Steroid production in response to LH was affected similarly in the two cell types (Fig. 3C).
FIG. 3.
Effect of oligomycin on LH-stimulated mitochondrial ΔΨm, intracellular ATP content, and steroid production in primary and MA-10 tumor Leydig cells. Black bars correspond to controls; gray bars correspond to oligomycin treatment. Data are presented as a percentage of control values. A) ΔΨm in primary and MA-10 tumor Leydig cultures incubated with oligomycin (1 μg/ml) in the presence of maximally stimulating LH (100 ng/ml) for 15 min. B) ATP levels of primary and MA-10 tumor Leydig cultures incubated with oligomycin (1 μg/ml) in the presence of maximally stimulating LH (100 ng/ml) for 2 h. C) Steroid production by primary and MA-10 tumor Leydig cultures incubated with oligomycin (1 μg/ml) in the presence of maximally stimulating LH (100 ng/ml) for 2 h. Values shown represent the mean ± SEM of three experiments, with three replicates per experiment. *P < 0.001 vs. cells incubated without oligomycin, #P < 0.05 vs. corresponding primary or MA-10 cells.
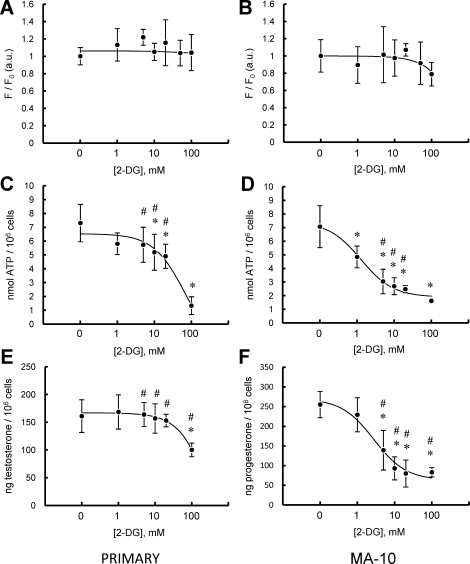
To further compare ATP production in MA-10 and primary Leydig cells, the respective abilities of these cell types to respond to acute inhibition of glycolysis was tested by culturing the cells with increasing concentrations of 2-DG (0–100 mM). Used this way, 2-DG can serve as a competitive inhibitor of glycolysis [24]. 2-DG did not affect ΔΨm in either cell type (Fig. 4, A and B). However, as seen in Figure 4, C and D, 2-DG inhibited both MA-10 and primary Leydig cell ATP levels, with the levels in MA-10 cells inhibited at far lower concentrations (IC50: primary cells, 69.94 ± 2.60 mM; MA-10 cells, 1.27 ± 1.33 mM). Though 2-DG inhibited hormone-driven testosterone synthesis in primary cells, it did so only at very high concentration, with an IC50 of 47.63 ± 7.26 mM. In contrast, MA-10 cell P4 production was far more sensitive to 2-DG, with an IC50 of 2.76 ± 1.50 mM (Fig. 4, E and F).
FIG. 4.
Effect of 2-DG on LH-stimulated primary and MA-10 tumor Leydig cell mitochondrial ΔΨm, intracellular ATP content, and steroid production. A and B) ΔΨm in primary (A) and MA-10 tumor (B) Leydig cultures incubated with increasing doses of 2-DG (0–100 mM) in the presence of maximally stimulating LH (100 ng/ml) for 15 min. C and D) ATP levels in primary (C) and MA-10 tumor (D) Leydig cultures incubated with increasing doses of 2-DG (0–100 mM) in the presence of maximally stimulating LH (100 ng/ml) for 2 h. E and F) Steroid production by primary (E) and MA-10 tumor (F) Leydig cultures incubated with increasing doses of 2-DG (0–100 mM) in the presence of maximally stimulating LH (100 ng/ml) for 2 h. Points shown represent the mean ± SEM of three experiments, with three replicates per experiment. *P < 0.01 vs. cells incubated with CCCP, #P < 0.05 vs. corresponding primary or MA-10 cells.
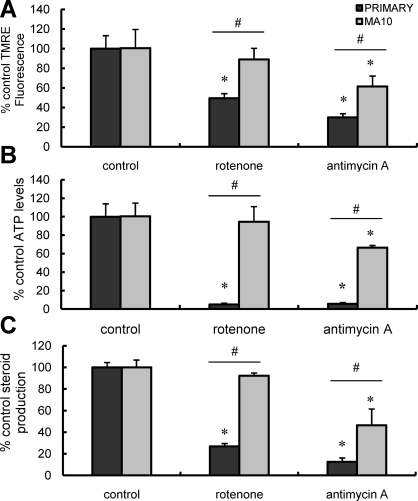
Altered ETC Dynamics and Steroidogenesis in MA-10 Versus Primary Leydig Cells
To compare the effect of inhibition of electron transport on MA-10 and primary cells, the cells were cultured in the presence of rotenone, a complex I electron-transport inhibitor, or antimycin A, a complex III electron-transport inhibitor. Steroid production, intracellular ATP content, and ΔΨm were measured. As seen in Figure 5, probing cellular response with the complex I inhibitor rotenone significantly reduced ΔΨm, ATP levels, and testosterone production in primary Leydig cells but not in MA-10 cells. The primary cells were exquisitely sensitive to complex I inhibition, with maximal inhibition of mitochondrial parameters and testosterone synthesis seen at low nanomolar concentrations of rotenone (data not shown). Treatment with the complex III inhibitor antimycin A resulted in reduced ΔΨm (Fig. 5A) and ATP content (Fig. 5B) in both primary and MA-10 cells. Consistent with the critical role played by mitochondrial electron transport in steroid biosynthesis [12, 13], antimycin A also significantly inhibited steroid production by both cell types (Fig. 5C). Cellular ATP and hormone-mediated steroid synthesis were more severely affected in primary cells than in MA-10 cells (Fig. 2, B and C), which is consistent with the hypotheses that mitochondrial energetics play a critical role in steroidogenesis and that primary cells are more dependent on mitochondrial ATP synthesis for cellular ATP compared with MA-10 cells.
FIG. 5.
Effect of ETC inhibition on LH-stimulated mitochondrial ΔΨm, intracellular ATP content, and steroid production in primary and MA-10 tumor Leydig cells. Black bars correspond to controls; gray bars correspond to rotenone or antimycin A treatment. Data are presented as a percentage of control values. A) ΔΨm in primary and MA-10 tumor Leydig cultures incubated with rotenone (0.1 μM) or antimycin A (1 μM) in the presence of maximally stimulating LH (100 ng/ml) for 15 min. B) ATP levels in primary and MA-10 tumor Leydig cultures incubated with rotenone (0.1 μM) or antimycin A (1 μM) in the presence of maximally stimulating LH (100 ng/ml) for 2 h. C) Steroid production by primary and MA-10 tumor Leydig cells incubated with rotenone (0.1 μM) or antimycin A (1 μM) in the presence of maximally stimulating LH (100 ng/ml) for 2 h. Points shown represent the mean ± SEM of three experiments, with three replicates per experiment. *P < 0.001 vs. cells incubated without ETC inhibitors, #P < 0.05 vs. corresponding primary or MA-10 cells.
Energetic Control of Steroidogenic Pathway
Though MA-10 and primary cells are both hormone-responsive, steroidogenic Leydig cells, the two cell types produce different steroids as their final product. Thus, whereas primary cells produce testosterone, MA-10 cells synthesize P4 as their final product, as a consequence of reduced CYP17 expression. This renders MA-10 cells an inappropriate model to study the later steps of testosterone synthesis. Therefore, to examine the hypothesis that cellular ATP is involved in the postmitochondrial steps of steroid production, primary Leydig cells were cultured in the presence of maximally inhibiting concentrations of CCCP, a mitochondrial uncoupler, or oligomycin, an ATP synthase inhibitor (Fig. 6A), or with 2-DG, an inhibitor of glycolysis, to deplete cellular ATP by two different methods (Fig. 6C). These cells also were cultured with stimulators of steroidogenesis (LH or dbcAMP) or substrates of the steroidogenic enzymes (HC for CYP11A1, P5 for 3βHSD, P4 for CYP17, or androstenedione for 17βHSD), and the ability of cells to synthesize testosterone was measured (Fig. 6, B and D). Inhibition of mitochondrial ATP synthesis reduced activity at all steps of steroidogenesis, with no difference in trypan blue dye exclusion. As seen in Figure 6B, the steps before mitochondrial cholesterol transport were affected more than were those after cholesterol transport. A more modest decrease in ATP with 2-DG (Fig. 6A) only affected mitochondrial cholesterol transport, leaving downstream activities unaffected (Fig. 6D).
FIG. 6.
Effect of mitochondrial disruption and glycolytic inhibition on metabolic flux through steroidogenic pathway in primary Leydig cells. A) ATP levels in cells incubated with 1 μg/ml of oligomycin or 5 μM CCCP in the presence of either LH (100 ng/ml), dbcAMP (1 mM), HC (20 μM), P5 (10 μM), P4 (10 μM), or androstenedione (5 μM) for 2 h. B) Testosterone production in cells treated as in A. After 2 h, medium was collected and testosterone levels assessed by RIA. Values are presented as the percentage of testosterone production without inhibitor. C) ATP levels in cells incubated with 100 mM 2-DG in the presence of either LH (100 ng/ml), dbcAMP (1 mM), HC (20 μM), P5 (10 μM), P4 (10 μM), or androstenedione (5 μM) for 2 h. D) Testosterone production in cells treated as in C. After 2 h, medium was collected and testosterone levels assessed by RIA. Values are presented as the percent of testosterone production without inhibitor. Lowercase letters designate groups that are statistically significant from each other (P < 0.05).
DISCUSSION
The mitochondrial ΔΨm is a central component of mitochondrial metabolism, providing the driving force for oxidative phosphorylation and the import of proteins and metabolites [7, 8, 25, 26]. Previous investigations of the regulation of steroidogenesis have demonstrated agents that disrupt ΔΨm, such as the protonophore CCCP, inhibit steroid formation by MA-10 tumor Leydig cells, the mitochondria from those cells, adrenal tumor cells, and nonsteroidogenic COS-1 cells transfected with CYP11A1 and STAR constructs [12, 14, 27]. Studies of the interplay between ΔΨm and mitochondrial steroid metabolism have indicated that in addition to ΔΨm, electron flux through the mitochondrial ETC [12] and the presence of ATP [28] are also critical for steroid formation in MA-10 cells.
Similar results have been obtained using primary Leydig cells. We recently showed that perturbation of the mitochondrial ETC with myxothiazol resulted in suppression of human chorionic gonadotropin/LH-stimulated testosterone formation and reduced intracellular ATP [13]. We also noted reductions in cAMP production and in the activities of 3βHSD, CYP17, and 17βHSD [13]. Because ATP is required for several steps in steroidogenesis, the results of these studies and of those on MA-10 cells suggest that myxothiazol might exert its effects through reduced ATP production and, thus, point to the importance of both mitochondrial ETC fidelity and ATP for steroidogenesis. However, results obtained on the interrelationships among ATP synthesis, ΔΨm, and steroidogenesis have not all been consistent. For example, it has been reported that dissipating ΔΨm in MA-10 cells with CCCP inhibited steroid formation but had no significant effect on cellular ATP levels [12]. Such results call into question whether ΔΨm plays a role in steroidogenesis beyond mitochondrial ATP synthesis, especially in light of findings that cholesterol transport in yeast is not affected by ΔΨm disruption [29].
Studies of mitochondrial energetics in relationship to steroid formation have been conducted using both primary and tumor Leydig cells. The extent to which results obtained from the tumor cells can be extrapolated to primary cells has not been tested critically, however, so it seemed possible to us that some of the apparently conflicting data in the literature might be a consequence of differences in the cell types used. We addressed this herein by conducting comparative studies on Leydig cell mitochondrial energetics and steroid synthesis using both primary and tumor Leydig cells. As had been shown by others in MA-10 cells [12, 14, 27], we found that CCCP depolarized mitochondria in primary rat as well as mouse tumor Leydig cells. The IC50 values of ΔΨm depolarization were not significantly different between the cell types. Surprisingly, the concentrations of CCCP necessary to depolarize the mitochondria, (IC50, ∼0.1 μM in both cell types) were significantly less than those required to affect LH-stimulated testosterone synthesis. Moreover, exposure of primary Leydig cells to CCCP and elicitation of ΔΨm depolarization was accompanied by a substantial reduction in cellular ATP levels, suggesting that primary Leydig cell ATP is derived almost entirely from mitochondrial oxidative phosphorylation. When cellular ATP responses to CCCP exposure were assessed, however, a striking difference was observed between the two cell types. In contrast to results with primary cells and in keeping with previous findings [12], CCCP treatment of MA-10 cells resulted in ΔΨm depolarization but not in a substantial decrease of cellular ATP levels in MA-10 Leydig cells.
The large difference between the cell types regarding cellular ATP levels raised the possibility that the tumor cells derive a majority of their ATP from glycolysis rather than oxidative phosphorylation. To investigate this possibility, the effect of the ATP synthase inhibitor oligomycin on Leydig cell parameters was assessed. As expected, oligomycin did not significantly affect ΔΨm [30]; rather, it potently inhibited testosterone and P4 synthesis in both primary and tumor Leydig cells. Oligomycin significantly inhibited cellular ATP levels in both cell types, but the degree of inhibition was markedly different. Inhibition of mitochondrial ATP synthesis resulted in almost complete depletion of cellular ATP levels in primary but not in tumor Leydig cells, as has been reported by others [12, 15]. Indeed, inhibition of mitochondrial ATP synthesis in MA-10 cells resulted in only 40%–50% reductions in cellular ATP levels. The existence of a large, oligomycin-insensitive pool supports the hypothesis that tumor Leydig cells derive ATP from sources other than, or in addition to, mitochondrial respiration.
To assess the glucose dependence of tumor and primary Leydig cells directly, cells were cultured with the nonhydrolyzable glucose analog 2-DG, which serves as a competitive inhibitor of the glycolytic pathway [24]. We noted dose-dependent inhibition of glucose metabolism and steroid formation in both primary and MA-10 tumor cells. However, steroid production was more significantly affected in the tumor Leydig cells than in the primary cells, with maximal inhibition at lower glucose concentrations. Importantly, MA-10 cell ATP levels were significantly more sensitive to glycolytic inhibition than were primary cell ATP levels.
The energetic differences between primary and tumor Leydig cells were not limited to glucose metabolism and ATP production. Though primary Leydig cells were highly sensitive to the complex I inhibitor rotenone, tumor Leydig cells were surprisingly refractory to ETC inhibition by rotenone. Because both cell types were strongly affected by inhibition of complex III by antimycin A and complex IV by NaCN (data not shown), these findings suggest that the tumor cells possess an impaired mitochondrial ETC at the level of complex I. Mutational and functional changes in complex I have been observed in other transformed and tumor cells [31, 32], though the precise alterations are heterogeneous. This may be an important consideration for reproductive toxicology studies, because rotenone and other complex I inhibitors are currently utilized in pesticides [33, 34] and have been used to examine complex I failure in models of Parkinson disease [35]. Importantly, exposure of rodent models has been demonstrated to decrease circulating testosterone levels [35]. Toxicological studies of complex I inhibitors would return false negatives if performed in assays using MA-10 tumor Leydig cells.
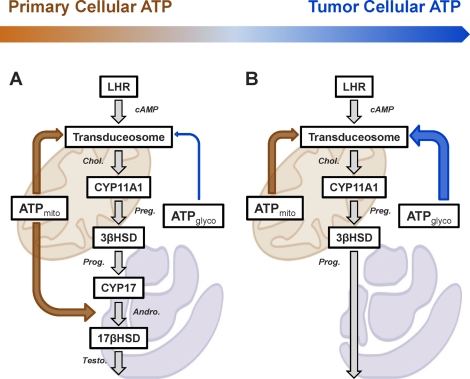
The data presented herein make it apparent that the seemingly inconsistent findings in the literature regarding the contribution of mitochondrial energetics to steroidogenesis likely are a consequence of significant, intrinsic differences between primary and tumor Leydig cells, as summarized in Figure 7. Both primary and MA-10 Leydig cells utilize mitochondrial ATP for mitochondrial cholesterol transport. However, the tumorigenic transition in MA-10 cells has resulted in a larger glycolytic contribution to cellular ATP. As a consequence, MA-10 cells utilize a greater proportion of glycolytic ATP for cholesterol transport than do primary cells, though mitochondria-derived ATP remains absolutely critical in these cells. Primary cells also utilize mitochondria-derived ATP for enzymatic reactions taking place in the ER, as previously demonstrated in other primary cell studies [36]. These reactions are missing in tumor Leydig cells. The glycolytic changes and dysfunctional complex I activity in MA-10 cells provide uncertainty regarding the extent to which studies of mitochondrial energetics and regulation of steroidogenesis in these transformed cells relate to the regulation of steroidogenesis in primary Leydig cells. Regardless, these findings demonstrate that steroidogenesis, especially mitochondrial cholesterol import, is exquisitely sensitive to ATP supply and demand, not to ΔΨm or ETC per se. The relative importance of this ATP demand, whether for phosphorylation of key steroidogenic components such as STAR [37–39], the mitochondrial transport of fatty acids [15], or unknown functions related to assembly of the transduceosome [4, 5], remains an area of active investigation.
FIG. 7.
Model of Leydig cell ATP utilization. Hormone-responsive primary (A) and tumor (B) Leydig cells critically utilize mitochondrial ATP for mitochondrial cholesterol transport (upper orange arrow). However, the tumorigenic transition has resulted in a larger glycolytic contribution to cellular ATP (gradient arrow above). Consequently, tumor Leydig cells utilize a greater proportion of glycolytic ATP (B; blue arrow) for cholesterol transport than do primary cells (A; blue arrow). Primary cells also utilize mitochondria-derived ATP for enzymatic reactions taking place in the ER (A; lower orange arrow), which are missing in tumor Leydig cells (B).
Acknowledgment
We thank Dr. M. Ascoli (University of Iowa, Iowa City, IA) for providing the MA-10 Leydig cells.
Footnotes
Supported by NIA grant R37-AG21092 (B.R.Z.), NIEHS Training Grant ES07141 (A.S.M.), and NIH grants RO1-HL091923-01 (M.A.A.) and R01-ES03495 (V.P.).
REFERENCES
- Puett D, Li Y, DeMars G, Angelova K, Fanelli F. A functional transmembrane complex: the luteinizing hormone receptor with bound ligand and G protein. Mol Cell Endocrinol 2007; 260–262: 126 136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WL. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochim Biophys Acta 2007; 1771: 663 676 [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci 2006; 27: 402 409 [DOI] [PubMed] [Google Scholar]
- Rone MB, Fan J, Papadopoulos V. Cholesterol transport in steroid biosynthesis: role of protein-protein interactions and implications in disease states. Biochim Biophys Acta 2009; 1791: 646 658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Rone MB, Papadopoulos V. Protein-protein interactions mediate mitochondrial cholesterol transport and steroid biosynthesis. J Biol Chem 2006; 281: 38879 38893 [DOI] [PubMed] [Google Scholar]
- Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev 2004; 25: 947 970 [DOI] [PubMed] [Google Scholar]
- Kadenbach B, Ramzan R, Wen L, Vogt S. New extension of the Mitchell Theory for oxidative phosphorylation in mitochondria of living organisms. Biochim Biophys Acta 2010; 1800: 205 212 [DOI] [PubMed] [Google Scholar]
- Wittig I, Schägger H. Supramolecular organization of ATP synthase and respiratory chain in mitochondrial membranes. Biochim Biophys Acta 2009; 1787: 672 680 [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg Effect: the metabolic requirements of cell proliferation. Science 2009; 324: 1029 1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento JM, Shi LZ, Tam J, Chandsawangbhuwana C, Durrant B, Botvinick EL, Berns MW. Comparison of glycolysis and oxidative phosphorylation as energy sources for mammalian sperm motility, using the combination of fluorescence imaging, laser tweezers, and real-time automated tracking and trapping. J Cell Physiol 2008; 217: 745 751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Hung PH, VandeVoort CA, Miller MG. 1H NMR to investigate metabolism and energy supply in rhesus macaque sperm. Reprod Toxicol 2009; 28: 75 80 [DOI] [PubMed] [Google Scholar]
- Allen JA, Shankara T, Janus P, Buck S, Diemer T, Hales KH, Hales DB. Energized, polarized, and actively respiring mitochondria are required for acute Leydig cell steroidogenesis. Endocrinology 2006; 147: 3924 3935 [DOI] [PubMed] [Google Scholar]
- Midzak AS, Liu J, Zirkin BR, Chen H. Effect of myxothiazol on Leydig cell steroidogenesis: inhibition of luteinizing hormone-mediated testosterone synthesis but stimulation of basal steroidogenesis. Endocrinology 2007; 148: 2583 2590 [DOI] [PubMed] [Google Scholar]
- King SR, Liu Z, Soh J, Eimerl S, Orly J, Stocco DM. Effects of disruption of the mitochondrial electrochemical gradient on steroidogenesis and the steroidogenic acute regulatory (StAR) protein. J Steroid Biochem Mol Biol 1999; 69: 143 154 [DOI] [PubMed] [Google Scholar]
- Duarte A, Castillo AF, Castilla R, Maloberti P, Paz C, Podestá EJ, Cornejo Maciel F. An arachidonic acid generation/export system involved in the regulation of cholesterol transport in mitochondria of steroidogenic cells. FEBS Lett 2007; 581: 4023 4028 [DOI] [PubMed] [Google Scholar]
- Moreno-Sánchez R, Rodríguez-Enríquez S, Saavedra E, Marín-Hernández A, Gallardo-Pérez JC. The bioenergetics of cancer: is glycolysis the main ATP supplier in all tumor cells? Biofactors 2009; 35: 209 225 [DOI] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science 1956; 123: 309 314 [DOI] [PubMed] [Google Scholar]
- Klinefelter GR, Hall PF, Ewing LL. Effect of luteinizing hormone deprivation in situ on steroidogenesis of rat Leydig cells purified by a multistep procedure. Biol Reprod 1987; 36: 769 783 [DOI] [PubMed] [Google Scholar]
- Payne AH, Downing JR, Wong KL. Luteinizing hormone receptors and testosterone synthesis in two distinct populations of Leydig cells. Endocrinology 1980; 106: 1424 1429 [DOI] [PubMed] [Google Scholar]
- Ascoli M. Characterization of several clonal lines of cultured Leydig tumor cells: gonadotropin receptors and steroidogenic responses. Endocrinology 1981; 108: 88 95 [DOI] [PubMed] [Google Scholar]
- Aon MA, Cortassa S, Marbán E, O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem 2003; 278: 44735 44744 [DOI] [PubMed] [Google Scholar]
- Huang SG. Development of a high-throughput screening assay for mitochondrial membrane potential in living cells. J Biomol Screen 2002; 7: 383 389 [DOI] [PubMed] [Google Scholar]
- Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem 2003; 86: 1101 1107 [DOI] [PubMed] [Google Scholar]
- Zhu Z, Jiang W, McGinley JN, Thompson HJ. 2-Deoxyglucose as an energy restriction mimetic agent: effects on mammary carcinogenesis and on mammary tumor cell growth in vitro. Cancer Res 2005; 65: 7023 7030 [DOI] [PubMed] [Google Scholar]
- Daum G, Vance JE. Import of lipids into mitochondria. Prog Lipid Res 1997; 36: 103 130 [DOI] [PubMed] [Google Scholar]
- Mokranjac D, Neupert W. Energetics of protein translocation into mitochondria. Biochim Biophys Acta 2008; 1777: 758 762 [DOI] [PubMed] [Google Scholar]
- Artemenko IP, Zhao D, Hales DB, Hales KH, Jefcoate CR. Mitochondrial processing of newly synthesized steroidogenic acute regulatory protein (StAR), but not total StAR, mediates cholesterol transfer to cytochrome P450 side chain cleavage enzyme in adrenal cells. J Biol Chem 2001; 276: 46583 46596 [DOI] [PubMed] [Google Scholar]
- King SR, Stocco DM. ATP and a mitochondrial electrochemical gradient are required for functional activity of the steroidogenic acute regulatory (StAR) protein in isolated mitochondria. Endocr Res 1996; 22: 505 514 [DOI] [PubMed] [Google Scholar]
- Tuller G, Daum G. Import of sterols into mitochondria of the yeast Saccharomyces cerevisiae. FEBS Lett 1995; 372: 29 32 [DOI] [PubMed] [Google Scholar]
- Weber J, Senior AE. ATP synthesis driven by proton transport in F1F0-ATP synthase. FEBS Lett 2003; 545: 61 70 [DOI] [PubMed] [Google Scholar]
- Gasparre G, Porcelli AM, Bonora E, Pennisi LF, Toller M, Iommarini L, Ghelli A, Moretti M, Betts CM, Martinelli GN, Ceroni AR, Curcio F, et al. Disruptive mitochondrial DNA mutations in complex I subunits are markers of oncocytic phenotype in thyroid tumors. Proc Natl Acad Sci U S A 2007; 104: 9001 9006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baracca A, Chiaradonna F, Sgarbi G, Solaini G, Alberghina L, Lenaz G. Mitochondrial complex I decrease is responsible for bioenergetic dysfunction in K-ras transformed cells. Biochim Biophys Acta 2010; 1797: 314 323 [DOI] [PubMed] [Google Scholar]
- Lümmen P. Complex I inhibitors as insecticides and acaricides. Biochim Biophys Acta 1998; 1364: 287 296 [DOI] [PubMed] [Google Scholar]
- Cicchetti F, Drouin-Ouellet J, Gross RE. Environmental toxins and Parkinson's disease: what have we learned from pesticide-induced animal models? Trends Pharmacol Sci 2009, 30: 475 483 [DOI] [PubMed] [Google Scholar]
- Alam M, Schmidt WJ. Mitochondrial complex I inhibition depletes plasma testosterone in the rotenone model of Parkinson's disease. Physiol Behav 2004; 83: 395 400 [DOI] [PubMed] [Google Scholar]
- Khanum A, Buczko E, Dufau ML. Essential role of adenosine triphosphate in activation of 17beta-hydroxysteroid dehydrogenase in rat Leydig cells. Endocrinology 1997; 138: 1612 1620 [DOI] [PubMed] [Google Scholar]
- Arakane F, King SR, Du Y, Kallen CB, Walsh LP, Watari H, Stocco DM, Strauss JF., III Phosphorylation of steroidogenic acute regulatory protein (StAR) modulates its steroidogenic activity. J Biol Chem 1997; 272: 32656 32662 [DOI] [PubMed] [Google Scholar]
- Jo Y, King SR, Khan SA, Stocco DM. Involvement of protein kinase C and cyclic adenosine 3′,5′-monophosphate-dependent kinase in steroidogenic acute regulatory protein expression and steroid biosynthesis in Leydig cells. Biol Reprod 2005; 73: 244 255 [DOI] [PubMed] [Google Scholar]
- Poderoso C, Converso DP, Maloberti P, Duarte A, Neuman I, Galli S, Maciel FC, Paz C, Carreras MC, Poderoso JJ, Podestá EJ. A mitochondrial kinase complex is essential to mediate an ERK1/2-dependent phosphorylation of a key regulatory protein in steroid biosynthesis. PLoS ONE 2008; 3: e1443 [DOI] [PMC free article] [PubMed] [Google Scholar]