Abstract
Using both a volume of interest (VOI) and whole brain voxel-wise approach, we compared rates of decline of 6-L-[18F]-fluorodopa (FDOPA) positron emission tomography (PET) uptake ipsilateral (IL) and contralateral (CL) to the initially symptomatic limbs over 4.5 years in 26 subjects with Parkinson’s disease (PD) and 11 controls. The VOI approach used six subregions: Head/body of caudate nucleus, whole putamen, and posterior putamen. The absolute rate of decline in PD was significantly greater than in controls in all subregions, but did not differ significantly by region. Ratios of uptake between regions did not change during the study with the exception of the IL putamen/caudate ratio. Both male gender and advancing age were associated with lower baseline FDOPA uptake in PD, but no difference in decline rates. In the PD group, decline rates were marginally greater during earlier time segments. Striatal FDOPA uptake was significantly correlated with disease duration and with progression of time during the study, but only moderately correlated with UPDRS scores. We conclude that FDOPA uptake in subregions of the striatum is strongly correlated with disease duration and age, and declines equally from symptom onset in PD. This implies that in idiopathic PD, relative preservation of uptake in the anterior striatum reflects a delay in pathologic involvement of nigrostriatal projections to these regions.
Keywords: Parkinson’s disease, Fluorodopa positron emission tomography, disease progression, aging, gender
Introduction
Rate of decline in side-to-side averaged striatal 6-L-[18F]-fluorodopa (FDOPA) uptake, imaged using positron emission tomography (PET), has been used as a surrogate for disease progression in Parkinson’s disease (PD) 1, 2. Therefore, understanding the evolution of changes in this biomarker is of utmost importance to interpreting intervention studies. The PET hallmarks of PD are abnormal cerebral hemispheric, caudal to rostral (putamen to caudate), and dorsal to ventral gradients in striatal FDOPA uptake 3, with most severely compromised uptake in the dorsal posterior putamen contralateral (CL) to the initially symptomatic limbs 4–7. The caudal-rostral gradient is attributed to predominant involvement of the ventrolateral portion of the substantia nigra (SN) that projects to the putamen, with relative preservation of the dorsomedial SN that projects to the caudate 8, 9. The annual rate of FDOPA uptake decline is greatest in early PD 10, 11, and has been quantified as 8.3%-12.5 of baseline for the putamen and 3.5–9.5% for the caudate 1, 10, 12, 13, in contrast to less than 1% in normal aging 12, 14. These observations suggest that the rate of decline should differ between striatal subregions, depending on length of disease involvement. The goal of this study was to compare the rate of change in FDOPA uptake between striatal subregions in PD, and to investigate the evolution of left-right and caudal-rostral gradients.
Methods
Subjects
Twenty-six subjects with idiopathic PD by UK Brain Bank criteria 15 (aged 56+/−11 years; 15 male, 11 female), and 11 controls (aged 61+/−12 years; 5 male, 6 female) completed a protocol approved by the local Institutional Review Board. At enrollment, 14 PD subjects were Hoehn and Yahr (HY) stage I, 11 HY II, and 1 HY III. The date and location of earliest motor symptoms were used to calculate disease duration (mean 3.2 years) and to designate onset laterality. At enrollment, 15 PD subjects were taking levodopa, 11 selegiline and/or pramipexole, and none catechol-o-methyltransferase (COMT) inhibitors. One additional subject began taking levodopa between study sessions 2 and 3. Mean Mini Mental State Examination (MMSE) scores were 28/30 for PD subjects and 29.7/30 for controls.
Procedures
Each study session included administration of the UPDRS by one of three movement disorders neurologists and 6-L-[18F]-fluorodopa (FDOPA) PET scanning. Over the 4-year span, 19 PD subjects and 11 controls completed 3 sessions, and the remaining 7 PD subjects completed two sessions. The mean interval between sessions was 1.8 years. Unified Parkinson’s Disease Rating Scale (UPDRS) scores for the maximally affected side were 15.7 +/− 8.4, 16.4 +/− 7.7, and 20.3 +/− 11.8 for the three sessions consecutively.
Ninety-minute 3D dynamic PET images were acquired on the same Advance scanner (General Electric Medical Systems, Waukesha, WI) after the IV administration of 204–284 MBq (5.5 to7.7 mCi) of FDOPA 16. Subjects were off anti-Parkinson medication for 18 hours prior to scanning, and ingested 100 mg of carbidopa 30 minutes before radiotracer injection. Brain MRI scans, obtained on a 1.5 Tesla scanner (General Electric Medical Systems, Waukesha, WI) that included a 124-section axial spoiled-gradient readout (SPGR) sequence (repetition time 29 ms, echo time 13 ms, flip angle=35 degrees, FOV = 220 mm; slice thickness = 1.2 mm), available for all but 4 subjects, were coregistered to PET for anatomic specificity.
Image analysis
PET and MRI images were realigned, within-subject, using FSL/FLIRT (http://fmrib.ox.ac.uk/fsl), then spatially normalized and resampled to 2mm cubic voxels. Using an in-house software package (http://brainimaging.waisman.wisc.edu/~oakes/spam), volumes of interest (VOIs) encompassing the head and body of caudate nucleus, putamen, posterior of putamen by volume, and a 900–1000 voxel occipital cortex reference region, were defined manually over each subject’s normalized MRI scan by one rater (CLG). The caudate and putamen were divided ventrally along the plane of internal capsular fibers visible on coronal views. This technique allowed for individual differences in the location of subcortical structures, and applied the same subject-specific VOI’s to repeat PET scans. For four subjects with missing MRI scan data, VOI’s were drawn on the spatially normalized PET sum image. Once drawn, all VOI volumes were reinspected and revised if inaccurate. Average radiotracer influx (Kocc) values for each VOI, and voxel-wise parametric maps, were computed from 30–90 minute PET frames using multiple-time graphical analysis method (MTGA) with occipital cortex (tissue) input function 17, 18. For the purpose of the voxel-wise analysis, the initially affected hemispheres of PD subjects were aligned on the left.
Statistical Analysis
To define regions of significant change between sessions, an exploratory analysis was conducted in Statistical Parametric Mapping 5 (SPM5; http://www.fil.ion.ucl.ac.uk/spm). Realigned Kocc images from first and last sessions were entered into a repeated measures analysis of variance (without global normalization), masked exclusively to basal ganglia, that included as factors subject, group (PD/control), and session. For analysis of the VOI data, Kocc values for the putamen, caudate nucleus, and posterior putamen were divided into pools according to their anatomic relationship as ipsilateral (IL) or contralateral (CL) to the initially symptomatic limbs. Data from PD subjects were compared with the corresponding side in control subjects. Caudal-rostral (putamen Kocc/caudate Kocc) 19 and hemispheric (CL putamen Kocc/IL putamen Kocc) ratios were generated. Several linear mixed effects models, in which a response variable (UPDRS score, Kocc value, or ratio) was regressed against explanatory variables of time (age, disease duration, or time from baseline session), gender, group, and time-by-group interaction, were used to model the main effects of these covariates, and the strength of interactions (http://www.math.mcgill.ca/keith/surfstat). Then, using the subgroup of 19 PD subjects who had completed three sessions, both annualized raw decline rates [(Kocc earlier− Kocc later) min−1/years between] and annualized percent decline rates for the session 1–2 and session 1–3 intervals were compared using paired samples t-tests in SPSS 16 20.
Results
Exploratory voxel-wise analysis
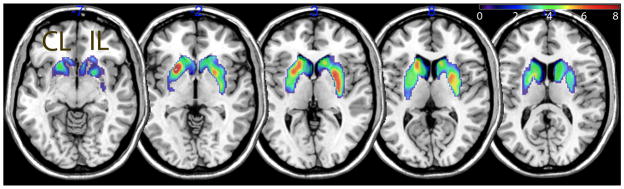
A contrast comparing the first and last PET scan sessions in all 26 PD subjects (corrected for false discovery rate [FDR] at a threshold of p<0.05) showed two significant clusters spanning the basal ganglia, with local maxima in the IL posterior putamen (MNI coordinates 30, −8, 2, voxel-level T=6.62) and CL anterior putamen (−24, 12, −2, T=7.63) (Figure 1). A similar contrast produced no significant clusters in the control group. Since this statistical model did not take into account variation in time interval between sessions, we proceeded to mixed effects analysis of the the manually-drawn VOIs.
Figure 1.
Group differences at baseline (Table 1)
Table 1.
Subregional baseline values and mean raw annual decline rates
| Subregion | Parkinson’s Group | Control Group | ||||
|---|---|---|---|---|---|---|
| Mean Kocc (min−1) | SD (min−1) | Annual decline (min−1) | Mean Kocc (min−1) | SD (min−1) | Annual decline (min−1) | |
| CL Caudate. | .0100 | .0015 | .0005 | .0117 | .0011 | .0001 |
| CL Putamen | .0072 | .0015 | .0005 | .0137 | .0014 | .0001 |
| CL Post. Putamen | .0052 | .0016 | .0004 | .0135 | .0010 | .0002 |
| IL Caudate | .0106 | .0017 | .0006 | .0122 | .0015 | .0002 |
| IL Putamen | .0083 | .0023 | .0006 | .0136 | .0011 | .0001 |
| IL Post. Putamen | .0065 | .0022 | .0006 | .0131 | .0018 | .0001 |
Baseline means, standard deviations, and linear regression decline rate estimatesfor Kocc values derived from striatal subregions: CL, contralateral to initially symptomatic limbs; IL, ipsilateral to initially symptomatic limbs; Kocc, uptake rate constant for FDOPA; Post., posterior; SD, Standard deviation.
As expected, an anterior-posterior gradient, with decreasing Kocc values in whole putamen compared with caudate nucleus, and in posterior putamen compared with whole putamen, was present in the PD group but not the control group. IL and CL putamen, caudate, and posterior putamen Kocc, and hemispheric putamen, and putamen/caudate ratios were significantly different between the PD and control groups (p<0.005). CL putamen and posterior putamen Kocc values did not overlap between groups. In the PD group, uptake in all structures, was higher in women (t[df],p =−2.2[26], 0.035), while mean disease duration was 3.2 years for both genders. This gender effect was most significant for the CL caudate nucleus (t[df],p =−3.7[26],0.001). Caudate nucleus (mean 420 voxels) and putamen (mean 675 voxels) VOI volumes did not differ by group or gender.
Within subject longitudinal data stability
For the control group, the average absolute scan-to-scan difference in VOI Kocc measurements was 0.0008 min−1 for the caudate and 0.0009 min−1 for the putamen, representing 7% of baseline for both structures.
Linear mixed effects analysis
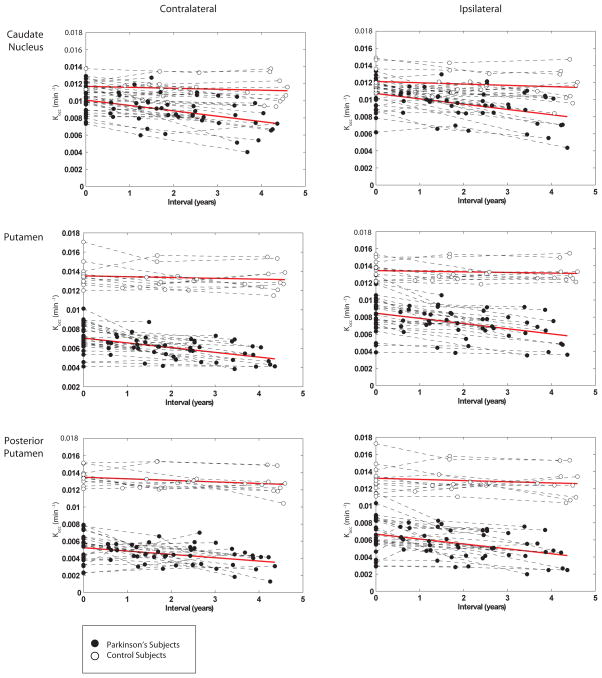
Models evaluating the main effects of group (PD versus control), gender, and age on FDOPA uptake identified group effects for all subregions, and for caudal-rostral, and hemispheric putamen ratios, age effects for all subregions except the CL posterior putamen, and gender effects for all structures except the CL caudate nucleus (Table 2). When the small control group was analyzed separately, the effect of age on putamen Kocc was marginal (p<0.06), and the decline rate estimated by linear regression (Table 1) as 0.0001–0.0002 min−1, or 0.7–1.5% of baseline uptake annually, did not reach significance. Models evaluating the rate of decline difference between groups, or time-by-group interactions, showed significant differences for all substructures and for the IL putamen/caudate ratio. Subtle differences in the slopes of linear regression lines fitted to the data from each substructure by group were not significant (Figure 2). Also, gender differences in the rate of decline were not significant. In the PD group, disease duration affected uptake in all substructures (Table 2). Intercept values for the disease duration models, which represent the projected Kocc at symptom onset, were 0.0114 (caudate nucleus), 0.0092 (putamen), 0.0072 (posterior putamen) for the IL brain and 0.0106 (caudate nucleus), 0.0075 (putamen), 0.0053 (posterior putamen) for the CL brain (compared with mean baseline control Kocc of 0.0136 for putamen and 0.0120 for the caudate nucleus). When similar models were applied to the PD group using total UPDRS scores as the outcome variable, age was a strong contributor (t[df],p=2.92[68], 0.002) and FDOPA uptake in all substructures a weak contributor ((t[df],p=1.5[68], 0.05) to clinical outcome. Total UPDRS scores were not significantly related to time or to disease duration. At session 1, HY I PD subjects had smaller mean hemispheric putamen ratio than HYII/III subjects (t[df],p=2.8[24], 0.01); however, this ratio did not change significantly as HYI subjects transitioned to HYII.
Figure 2.
Evaluation of annual rate of change between time intervals
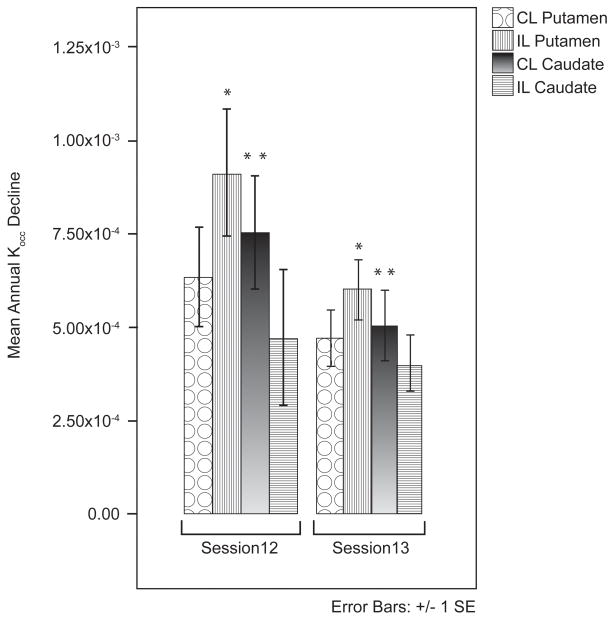
A within-subject comparison of time intervals for PD subjects who completed three sessions showed that decline rates were not uniform across sessions. For the session 1–2 interval, annual decline rates were 9.6 (IL putamen), 7.8 (CL putamen), 7.4 (CL caudate), and 3.8 (IL caudate) percent of baseline, and for the session 1–3 interval, 6.6 (IL putamen), 6.0 (CL putamen), 4.9 (CL caudate), and 3.8 (IL caudate) percent. Raw annual rates of decline were different between time intervals for the IL putamen (t[df],p=2.4[18], 0.03) and CL caudate (t[df],p=2.2[18], 0.04) (Figure 3).
Figure 3.
The effect of pharmacotherapy
Annual decline rates for PD subjects taking pramipexole and/or selegiline were greater than those taking carbidopa/levodopa; however, use of carbidopa/levodopa was also correlated with disease duration (r=0.66, p<0.001).
Discussion
Studies that have used annual rates of change in striatal FDOPA uptake as a surrogate outcome measure have averaged side-to-side differences to improve scan-to-scan reproducibility 1. We addressed the question of whether rates of FDOPA uptake decline show regional differences within the striatum in PD. We first used an exploratory voxel-wise approach that suggested the regions of most significant change between sessions to be the CL anterior dorsal and IL posterior putamen. Previous voxel-based analyses have localized the region of greatest PD-related FDOPA decline to the anterior striatum 21, and to the IL posterior putamen,12. We then used a detailed VOI selection technique to comprehensively sample FDOPA uptake in striatal subregions. This technique yielded a favorable scan-rescan reproducibility in the control group 13, but also averaged uptake over fairly large volumes and was therefore expected to be less sensitive to small regional changes than the voxelwise approach. Commensurate with the voxel-wise findings, the VOI analysis showed a slight decline in the IL caudal-rostral gradient (putamen Kocc/caudate Kocc), and a slightly greater decline rate in the IL putamen (Figure 3) that might reach statistical significance in a larger sample. Although different in techniques of image sampling and radiopharmacology, a study using a dopamine transporter ligand did demonstrate significantly faster PD-related decline in the IL putamen22. Although the linear random effects analyses demonstrated significant differences in the rate of decline between PD and control groups (significant time-by-group interaction) for all subregions, we found no significant within-group differences in the decline rate between the selected VOIs. The roughly parallel regional decline trajectories was further supported by lack of change over the study interval in the hemispheric putamen ratio (CL/IL putamen Kocc) and CL caudal-rostral gradient, and the linear relationship of uptake in all subregions to disease duration. The hemispheric putamen ratio was smaller for subjects who entered the study at HYI than for subjects of HYII/III, but did not change at later sessions as HYI subjects transitioned clinically to HYII. These conclusions are commensurate with two previous studies that found no difference in the rate of decline between striatal subregions in 31 PD subjects over 2 years 23, and in 21 PD subjects over 5 years 12. The advantage of our study in confirming this observation is lack of potentially confounding medication changes, as only 1/26 of our subjects began taking levodopa during the study interval, whereas 27/31 and 10/21 began taking levodopa in the former studies. The finding of equivalent decline rates is at odds with previous studies using FDOPA 2, 10 that have described different rates of change for the caudate nucleus and putamen. Whereas the rate of change expressed as a percentage of baseline uptake may differ between striatal subregions, our analysis suggests the raw rate of change in Kocc is roughly equivalent when averaged over large subregions.
Age and gender effects
We found increasing age to be associated with reduced FDOPA uptake in the PD group, with a marginal effect in the control group, and a trivial decline rate 0.7–1.5% per annum in the control group, consistent with one previous investigation 12. Female gender also had a moderate effect on FDOPA uptake in all structures, especially the IL caudate nucleus. Higher striatal, especially caudate, FDOPA uptake in women has been described in both normal and PD groups controlled for disease duration 24. Since the incidence of PD is higher in men, it is possible that women are partially protected by higher baseline striatal dopaminergic function 25.
Deceleration of FDOPA uptake loss over time
This study provides a within-subject comparison of the slope of FDOPA uptake decline between sequential time intervals. The results support previous hypotheses, based on single time intervals, that FDOPA uptake loss is greater in earlier than later stages of disease 10, 11. However, the significance of our findings (p=0.03–0.05) would not withstand a rigorous correction for multiple comparisons, and greater statistical power might have been achieved had subjects representing a greater spectrum of disease stages been enrolled. A slower rate of decline in more depleted structures suggests that regions in which the disease is more advanced may be experiencing a slower rate of cell loss 21. However, the relationship between FDOPA uptake decline and cell loss is uncertain, 26 partially because factors that cannot be estimated from 90-minute dynamic FDOPA PET data, specifically dopamine turnover, vary with disease stage and influence the Kocc value27, 28. Although small doses of levodopa do not have short-term effects on quantification of striatal FDOPA uptake in PD29, measured rates of decline are influenced by treatment1. In this sample, the apparent slower progression rate in subjects treated with selegiline/pramipexole in comparison to those treated with levodopa may reflect greater disease duration in the levodopa-treated group rather than an effect of pharmacotherapy.
Conclusions
In this study, PET scan measurements were more consistently correlated with time and disease duration than were UPDRS scores. However, time may not be the most valuable surrogate for disease progression in a heterogeneous clinical population. A surrogate outcome measure should be “used as a substitute for a clinically meaningful end point that measures directly how the patient feels, functions, or survives.”30 An uncertain relationship between imaging measures and clinical outcomes has plagued many drug development trials 31. This study brings to light two issues related to using PET as a surrogate outcome measure in PD. First, age, disease duration, and gender influence FDOPA uptake and should continue to be matched between groups in studies that use PET as a surrogate outcome measure 1, 11. Second, the use of relative normalization methods in an effort to control for individual differences (in this case, normalizing annual rates of change to baseline FDOPA uptake), may contribute to uncertainty about how a biomarker reflects pathology 32. We found that striatal structures experience a largely parallel average rate of FDOPA loss in PD, such that rostrocaudal and hemispheric gradients do not change significantly over time. Our findings imply that all striatonigral projections are similarly affected from symptom onset, but that striatal subregions are involved sequentially with the disease process. Therefore, estimates of the “preclinical” period for these subregions will differ 10.
Acknowledgments
Figure preparation by Jennifer Broihahn, University of Wisconsin Department of Neurology Media Specialist.
Research support: This work was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Science Research and Development Service [grant to C.G]; National Institutes of Health [grant numbers 1R29NS31612 to D.B., and NIA T32 AG000213]; and University of Wisconsin Institute for Clinical and Translational Research, funded through a National Institutes of Health Clinical and Translational Science Award, [grant number 1UL1RR025011 to C.G.]. This work was supported with use of facilities at the William S. Middleton Memorial Veterans Hospital Geriatric Research Education and Clinical Center and the Waisman Laboratory for Brain Imaging and Behavior, Madison, WI, USA.
Footnotes
Authors roles:
- Research project: A. Conception, B. Organization, C. Execution;
- Statistical Analysis: A. Design, B. Execution, C. Review and Critique;
-
Manuscript: A. Writing of the first draft, B. Review and Critique;Gallagher: 1C, 2A, 2B, 2C, 3A, 3B. Oakes: 1C, 2A, 3B. Johnson: 1A, 2C, 3B. Chung: 2A, 2B, 2C. Holden: 1A, 3B. Bendlin: 2B, 3B. McLaren: 1C, 2A, 3B. Xu: 1C, 3B. Nickles: 1 C. Pyzalski: 1C. Dejesus: 1A. Brown: 1A, 1B, 1C, 3B.
Financial disclosures: The authors report no other financial disclosures.
References
- 1.Whone AL, Watts RL, Stoessl AJ, et al. Slower progression of Parkinson’s disease with ropinirole versus levodopa: The REAL-PET study. Ann Neurol. 2003;54(1):93–101. doi: 10.1002/ana.10609. [DOI] [PubMed] [Google Scholar]
- 2.Hilker R, Portman AT, Voges J, et al. Disease progression continues in patients with advanced Parkinson’s disease and effective subthalamic nucleus stimulation. J Neurol Neurosurg Psychiatry. 2005;76(9):1217–1221. doi: 10.1136/jnnp.2004.057893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrish PK, Sawle GV, Brooks DJ. Regional changes in [18F]dopa metabolism in the striatum in Parkinson’s disease. Brain. 1996;119 ( Pt 6):2097–2103. doi: 10.1093/brain/119.6.2097. [DOI] [PubMed] [Google Scholar]
- 4.Garnett ES, Nahmias C, Firnau G. Central dopaminergic pathways in hemiparkinsonism examined by positron emission tomography. Can J Neurol Sci. 1984;11(1 Suppl):174–179. doi: 10.1017/s0317167100046369. [DOI] [PubMed] [Google Scholar]
- 5.Marek KL, Seibyl JP, Zoghbi SS, et al. [123I] beta-CIT/SPECT imaging demonstrates bilateral loss of dopamine transporters in hemi-Parkinson’s disease. Neurology. 1996;46(1):231–237. doi: 10.1212/wnl.46.1.231. [DOI] [PubMed] [Google Scholar]
- 6.Rinne OJ, Nurmi E, Ruottinen HM, Bergman J, Eskola O, Solin O. [(18)F]FDOPA and [(18)F]CFT are both sensitive PET markers to detect presynaptic dopaminergic hypofunction in early Parkinson’s disease. Synapse. 2001;40(3):193–200. doi: 10.1002/syn.1042. [DOI] [PubMed] [Google Scholar]
- 7.Kaasinen V, Nurmi E, Bruck A, et al. Increased frontal [(18)F]fluorodopa uptake in early Parkinson’s disease: sex differences in the prefrontal cortex. Brain. 2001;124(Pt 6):1125–1130. doi: 10.1093/brain/124.6.1125. [DOI] [PubMed] [Google Scholar]
- 8.Goto S, Hirano A, Matsumoto S. Subdivisional involvement of nigrostriatal loop in idiopathic Parkinson’s disease and striatonigral degeneration. Ann Neurol. 1989;26(6):766–770. doi: 10.1002/ana.410260613. [DOI] [PubMed] [Google Scholar]
- 9.Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20(4):415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- 10.Morrish PK, Rakshi JS, Bailey DL, Sawle GV, Brooks DJ. Measuring the rate of progression and estimating the preclinical period of Parkinson’s disease with [18F]dopa PET. J Neurol Neurosurg Psychiatry. 1998;64(3):314–319. doi: 10.1136/jnnp.64.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hilker R, Schweitzer K, Coburger S, et al. Nonlinear progression of Parkinson disease as determined by serial positron emission tomographic imaging of striatal fluorodopa F 18 activity. Arch Neurol. 2005;62(3):378–382. doi: 10.1001/archneur.62.3.378. [DOI] [PubMed] [Google Scholar]
- 12.Nurmi E, Ruottinen HM, Bergman J, et al. Rate of progression in Parkinson’s disease: a 6-[18F]fluoro-L-dopa PET study. Mov Disord. 2001;16(4):608–615. doi: 10.1002/mds.1139. [DOI] [PubMed] [Google Scholar]
- 13.Morrish PK, Sawle GV, Brooks DJ. An [18F]dopa-PET and clinical study of the rate of progression in Parkinson’s disease. Brain. 1996;119 ( Pt 2):585–591. doi: 10.1093/brain/119.2.585. [DOI] [PubMed] [Google Scholar]
- 14.Bhatt MH, Snow BJ, Martin WR, Pate BD, Ruth TJ, Calne DB. Positron emission tomography suggests that the rate of progression of idiopathic parkinsonism is slow. Ann Neurol. 1991;29(6):673–677. doi: 10.1002/ana.410290617. [DOI] [PubMed] [Google Scholar]
- 15.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51(6):745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown WD, Taylor MD, Roberts AD, et al. FluoroDOPA PET shows the nondopaminergic as well as dopaminergic destinations of levodopa. Neurology. 1999;53(6):1212–1218. doi: 10.1212/wnl.53.6.1212. [DOI] [PubMed] [Google Scholar]
- 17.Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations J Cereb Blood Flow Metab. 1985;5(4):584–590. doi: 10.1038/jcbfm.1985.87. [DOI] [PubMed] [Google Scholar]
- 18.Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3(1):1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- 19.Ilgin N, Zubieta J, Reich SG, Dannals RF, Ravert HT, Frost JJ. PET imaging of the dopamine transporter in progressive supranuclear palsy and Parkinson’s disease. Neurology. 1999;52(6):1221–1226. doi: 10.1212/wnl.52.6.1221. [DOI] [PubMed] [Google Scholar]
- 20.SPSS, Inc. 16. Chicago: 2007. [Google Scholar]
- 21.Nurmi E, Bergman J, Eskola O, et al. Progression of dopaminergic hypofunction in striatal subregions in Parkinson’s disease using [18F]CFT PET. Synapse. 2003;48(3):109–115. doi: 10.1002/syn.10192. [DOI] [PubMed] [Google Scholar]
- 22.Tang CC, Poston KL, Dhawan V, Eidelberg D. Abnormalities in metabolic network activity precede the onset of motor symptoms in Parkinson’s disease. J Neurosci. 30(3):1049–1056. doi: 10.1523/JNEUROSCI.4188-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruck A, Aalto S, Nurmi E, Vahlberg T, Bergman J, Rinne JO. Striatal subregional 6-[18F]fluoro-L-dopa uptake in early Parkinson’s disease: a two-year follow-up study. Mov Disord. 2006;21(7):958–963. doi: 10.1002/mds.20855. [DOI] [PubMed] [Google Scholar]
- 24.Haaxma CA, Bloem BR, Borm GF, et al. Gender differences in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78(8):819–824. doi: 10.1136/jnnp.2006.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alves G, Muller B, Herlofson K, et al. Incidence of Parkinson’s disease in Norway: the Norwegian ParkWest study. J Neurol Neurosurg Psychiatry. 2009;80(8):851–857. doi: 10.1136/jnnp.2008.168211. [DOI] [PubMed] [Google Scholar]
- 26.Borghammer P, Kumakura Y, Cumming P. Fluorodopa F 18 positron emission tomography and the progression of Parkinson disease. Arch Neurol. 2005;62(9):1480. doi: 10.1001/archneur.62.9.1480-a. author reply 1480–1481. [DOI] [PubMed] [Google Scholar]
- 27.Sossi V, de La Fuente-Fernandez R, Holden JE, et al. Increase in dopamine turnover occurs early in Parkinson’s disease: evidence from a new modeling approach to PET 18 F-fluorodopa data. J Cereb Blood Flow Metab. 2002;22(2):232–239. doi: 10.1097/00004647-200202000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Sossi V, de la Fuente-Fernandez R, Holden JE, Schulzer M, Ruth TJ, Stoessl J. Changes of dopamine turnover in the progression of Parkinson’s disease as measured by positron emission tomography: their relation to disease-compensatory mechanisms. J Cereb Blood Flow Metab. 2004;24(8):869–876. doi: 10.1097/01.WCB.0000126563.85360.75. [DOI] [PubMed] [Google Scholar]
- 29.Kumakura Y, Danielsen EH, Reilhac A, Gjedde A, Cumming P. Levodopa effect on [18F]fluorodopa influx to brain: normal volunteers and patients with Parkinson’s disease. Acta Neurol Scand. 2004;110(3):188–195. doi: 10.1111/j.1600-0404.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 30.Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled? Ann Intern Med. 1996;125(7):605–613. doi: 10.7326/0003-4819-125-7-199610010-00011. [DOI] [PubMed] [Google Scholar]
- 31.Daumer M, Neuhaus A, Morrissey S, Hintzen R, Ebers GC. MRI as an outcome in multiple sclerosis clinical trials. Neurology. 2009;72(8):705–711. doi: 10.1212/01.wnl.0000336916.38629.43. [DOI] [PubMed] [Google Scholar]
- 32.Grunder G. “Absolute” or “relative”: choosing the right outcome measure in neuroimaging. Neuroimage. 2009;45(2):258–259. doi: 10.1016/j.neuroimage.2008.10.042. [DOI] [PubMed] [Google Scholar]