Abstract
Mutations of GJB2 and GJB6 (connexin-26 and 30) at the DFNB1 locus are the most common cause of autosomal recessive, nonsyndromic deafness. Despite their widespread expression throughout the vestibular system, vestibular dysfunction has not been widely recognized as a commonly associated clinical feature. The observations of vertigo accompanying DFNB1 deafness in several large families prompted our hypothesis that vestibular dysfunction may be an integral, but often overlooked, component of DFNB1 deafness. Our aim was to define the prevalence of vestibular dysfunction in Cases of DFNB1 deafness and Controls with other forms of deafness. We developed and used a survey to assess symptoms of vestibular dysfunction, medical, and family history was distributed to Cases with deafness due to pathogenic GJB2 and/or GJB6 mutations and deaf Controls without DFNB1 deafness. Our results showed: Surveys were returned by 235/515 Cases (46%) with DFNB1 mutations and 121/ 321 Controls (38%) without these mutations. The mean age of Cases (41) was younger than Controls (51; p<0.001). Vestibular dysfunction was reported by 127 (54%) of Cases and was present at significantly higher rates in Cases than in deaf Controls without DFNB1 deafness (p< 0.03). Most (63%) had to lie down in order for vertigo to subside, and 48% reported that vertigo interfered with activities of daily living. Vertigo was reported by significantly more Cases with truncating than non-truncating mutations and was also associated with a family history of dizziness. We conclude that vestibular dysfunction appears to be more common in DFNB1 deafness than previously recognized and affects activities of daily living in many patients.
Keywords: Vertigo, Vestibular Dysfunction, Connexin, DFNB1, Deafness
INTRODUCTION
Hearing loss (HL) is a common neurosensory impairment affecting up to 3 of every 1,000 children at birth and nearly doubling in frequency by the age of 10 years [Morton and Nance, 2006]. At least 60% of deafness in infancy and childhood is genetically determined [Marazita et al., 1993; Morton and Nance, 2006], and more than 100 loci for either syndromic or nonsyndromic forms have been mapped or sequenced (http://webhost.ua.ac.be/hhh/). A remarkable feature of the genetic epidemiology of deafness was the discovery that recessive mutations at a single locus (DFNB1) account for 30–40% of all probands with deafness in many, but not all, populations [Denoyelle et al., 1999; Zelante et al., 1997]. DFNB1 is located at 13q11-q12 [Guilford et al., 1994] and includes the GJB2 and GJB6 genes, which encode the connexin 26 (Cx26) and connexin 30 (Cx30) proteins, respectively. More than 100 mutations have been discovered in the GJB2 gene, ranging from a very common mutation, 35delG [Cryns et al., 2004] to “private” mutations found only in single families. Digenic interactions with deletion mutations involving the GJB6 gene (Cx30) are a frequent explanation for the hearing loss in some individuals heterozygous for a single GJB2 mutation [del Castillo et al., 2003] while approximately 18 GJB2 mutations exhibit dominant transmission.
Cx26 and 30 are structurally related subunits of a gap junction protein, which aggregate to form homomeric or heteromeric connexons [Kumar and Gilula, 1996], which facilitate the passage of ions and small molecules between cells. Histologic studies have shown that the Cx26 and 30 proteins are both widely distributed in the functional compartments of the cochlea and the vestibular sensory epithelia [Forge et al., 2003; Kikuchi et al., 1994]. Cx26 is also the major gap junction protein expressed in the melanocytes of the human vestibular dark cell area, where it may play a pivotal role in the passive movement of ions between the endolymph and perilymph [Masuda et al., 2001]. Animal studies demonstrate that gap junction channels in both the auditory and vestibular regions of the cochlea are comprised mainly of connexin 26 and 30 subunits [Qu et al., 2007]. Additionally, there is extensive expression of connexin proteins in the ampulla, utricle and the nonsensory epithelia of the saccule accompanied by specific saccular hair cell loss in connexin 30 knockout mice [Qu et al., 2007; Forge et al., 2003].
The spectrum of phenotypic features seen in DFNB1 deafness has continued to expand and is influenced by several factors including the type of mutation, as well as other genetic and environmental modifiers. Originally, the DFNB1 phenotype was characterized as an isolated congenital, stable, bilateral, profound sensorineural hearing loss. Several studies now indicate that the hearing loss can vary from mild to profound, even within the same family [Murgia et al., 1999; Denoyelle et al., 1999; Loffler et al., 2001; Lerer et al., 2000; Mueller et al., 1999; Sobe et al., 2000; Azaiez et al., 2004; Cryns et al., 2004]. A few reports have documented the progression of hearing loss, as well as sudden hearing loss and losses confined to the high frequencies [Janecke et al., 2002; Wilcox et al., 2000]. Chain-termination mutations, which result in a truncated defective protein, are generally associated with greater degrees of hearing loss than non-truncating mutations, which lead to amino acid substitutions but no change in protein size [Snoeckx et al., 2005; Azaiez et al., 2004]. Finally, although DFNB1 deafness was originally considered to be invariably congenital in onset, it is now recognized that perhaps as many as 4%–8% of affected infants could have normal hearing at birth and then develop a later-onset hearing loss ([Norris et al., 2006; Orzan and Murgia, 2007; Schimmenti et al., 2008]). These infants would escape detection by newborn hearing screening programs and result in a later age of identification and treatment. These studies are consistent with animal studies that found a gradual postnatal deterioration in the outer hair cells in the cochlea [Cohen-Salmon et al., 2002] and suggest that newborn hearing screening does not identify all children with prelingual hearing loss.
Earlier reports on associated inner ear malformations based largely on visual inspection showed no conspicuous abnormalities of the temporal bones on CT imaging [Cohn et al., 1999; Denoyelle et al., 1999]. However, recent data suggests that anomalies are common in GJB2 deafness when the cochlear and vestibular architecture is precisely measured with electronic calipers [Propst et al., 2006]. In this study at least one temporal bone anomaly was found in 72% (38/53) of the participants (more than 50% of ears) with 47% (18/38) of them having bilateral anomalies. The detected abnormalities have included dilated endolymphatic fossa, hypoplastic modiolus, enlarged vestibular aqueduct (EVA), hypoplastic lateral semicircular canals, and hypoplastic cochlea [Propst et al., 2006]. It is of interest, and possible relevance, that dilation of the endolymphatic fossa, which has been reported in 1/3 of patients with GJB2 deafness, has also been associated with vertigo in patients with Meniere’s disease [Propst et al., 2006; Krombach et al., 2005]. In addition, Kenna et al [2007] found that 18% of children with biallelic GJB2 mutations had additional clinical manifestations apart from hearing loss, including structural ear abnormalities as well as vertigo [Kenna et al., 2007].
Despite the widespread expression of the GJB2 and GJB6 genes throughout the inner ear, vestibular dysfunction has not been commonly associated with DFNB1 deafness in previous reports [Cohn and Kelley, 1999; Denoyelle et al., 1999] as it has been with some forms of autosomal dominant deafness, like DFNA9, caused by mutations of the cochlin protein within the inner ear [Robertson et al., 1998]. Epidemiologic studies of vestibular vertigo in the general population have shown a point prevalence of 1.5% and a lifetime incidence of 8% with female gender, self reported depression, tinnitus, hypertension, and dyslipidemia having independent effect on the incidence in multivariate analyses [Neuhauser et al., 2005]. Previous studies of dizziness suggest that the lifetime prevalence may be as high as 20 to 30% of the general population, but most studies suffer from a lack of standardization and difficulty quantitating the perceived severity [Neuhauser and Lempert, 2009; Kroenke and Price, 1993; Hannaford et al., 2005; Yardley et al., 1998].
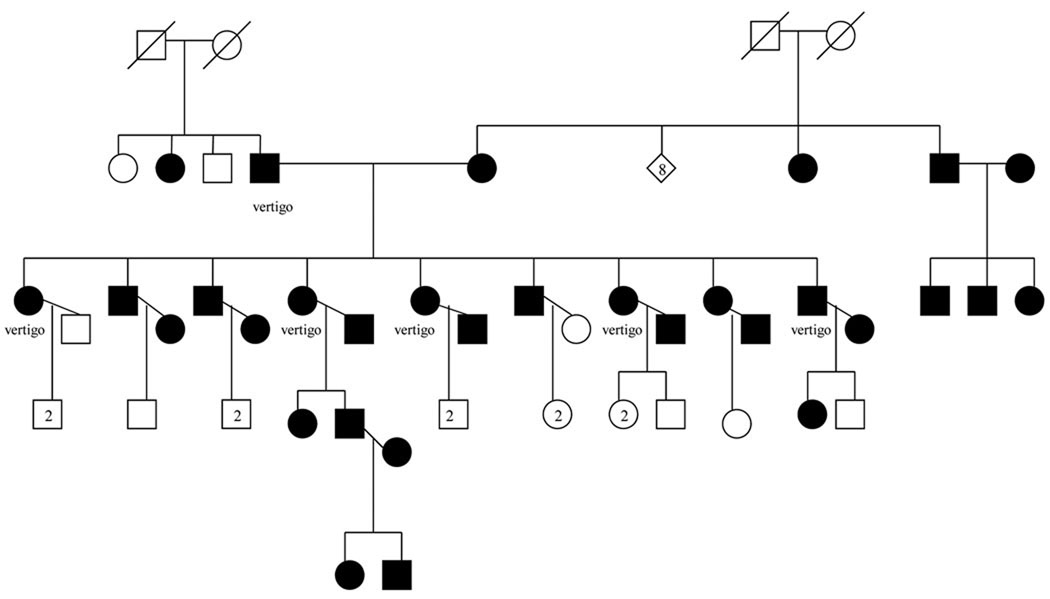
Anecdotal evidence of associated vestibular dysfunction in a large family where both parents, and all nine of their offspring were deaf was the impetus for this study. GJB2 testing in the parents and four of the nine children revealed that all were homozygous for the 35delG mutation (Fig 1). The father and six of the nine deaf offspring also reported symptoms of vertigo. While it was clearly possible that the hearing loss and vertigo were unrelated, this family prompted us to examine the prevalence of vertigo and in a large sample of deaf probands with GJB2 deafness in an effort to determine the frequency of this association.
Figure 1.
35delG/35delG DFNB1 family with vestibular dysfunction. Shading represents deafness.
METHODS
After obtaining appropriate IRB approval, we designed an internet-based survey instrument (Supplement 1) which was distributed to deaf subjects who had been tested for mutations in GJB2 and GJB6 through the North American Repository of deaf individuals [Pandya et al., 2003] or as part of a study of the alumni of Gallaudet University [Arnos et al., 2008]. The survey link was sent by electronic mail to those subjects aged 18 and above and for whom we had a valid address. In cases where a valid e-mail address was not available or had expired, a paper copy of the survey with a return envelope was mailed. A reminder notice was sent to all non-responders one to two months later.
The questionnaire sought information on the presence, characteristics, age of onset, duration, frequency, and length of episodes of vertigo and/or tinnitus, as well as the past medical and/or a family history of vertigo, migraine, and other medical conditions. The survey also sought specific information regarding severity of vertigo, including need for medications, emergency room visits, and interference with activities of daily living, requiring bedrest, and /or associated nausea and vomiting. We designed this survey instrument to establish the presence and severity of vertigo based upon published validated neurotologic data on the epidemiology of vestibular vertigo in the general population obtained through structured interviews [Neuhauser et al., 2005]. The responses were entered into an Excel database, and statistical analyses were performed using JMP Software version 6.0.0 (SAS Institute, Cary, NC). Summary statistics were calculated, and inferential tests, including contingency analyses with Fisher’s Exact Test and Chi-Square test and logistic regression, were performed.
RESULTS
The response rate for the 515 Cases with GJB2/6 mutations was 46%, with 235 completed surveys. The mean age of the Cases was 41.7 years (range 18–84 years). Of these respondents, 127 (54%) reported vertigo, and 111 (47%) reported tinnitus while 73 (31%) reported both symptoms. The median age of onset of vertigo was 31, with a mean of 34.7 years. Sixty-two percent of respondents were female, and 86% reported being of Caucasian ancestry. Surveys were also sent to 321 deaf Controls from the same North American Deafness Repository who were also sequenced and did not carry GJB2/6 mutations, and responses were obtained from 121 (38% response rate). Of these, we analyzed responses from 61 subjects who had either autosomal recessive inheritance of deafness (based on a family history of an affected sibling) or an unknown cause of deafness. Responses from subjects who were documented or suspected to have autosomal dominant forms of deafness were not analyzed in order to decrease the confounding effect of autosomal dominant forms of hearing loss, which can be associated with vertigo. The mean age of Controls was older than the GJB2/6 Cases at 51 (range 22–84; p<0.001). Of the Controls, there were an equal number (62%) of female respondents compared with Cases, and 90% of Controls were of Caucasian ancestry. Significantly fewer Controls (25/61 or 41%) reported vertigo (p<0.03). The mean age of onset of vertigo in the Control group was 26.4 years. A comparable percentage of Controls (47%) reported baseline tinnitus, and 30% of Controls reported both tinnitus and vertigo.
Descriptive Data: Tables I and II summarize the reported clinical features of vertigo in both study groups. For Cases in the GJB2/6 group, of those reporting vertigo, symptoms were intermittent in 96% and had a positional component in 68%. Among the Controls, symptoms were intermittent in 92%; but much less likely to include a positional component (36%; p<0.0005). Of the GJB2/6 Cases, vertigo episodes were described as a spinning sensation (70%) a well as feelings of lightheadedness (74%) with (44%) noting disequilibrium. In the Control group, 60% reported spinning, 52% lightheadedness, and 52% imbalance. Thirty-six percent of the DFNB1 Cases reported that vertigo lasted only minutes, while 48% reported that the episodes lasted minutes to hours. For Controls, vertigo episodes were of shorter duration; 68% reported that episodes lasted less than one minute. In the Control group, the episodes of vertigo were of significantly shorter duration, lasting less than 1 minute in most subjects, while most of the Cases reported duration of minutes to hours or hours to days (Chi Square 10.544, 2df, p=0.005). Seventy-one percent in the GJB2/6 Cases and 78% of the Controls experienced more than three lifetime episodes of vertigo
Table I.
Comparison of Survey Results in Cases and Controls with Vertigo
| Vertigo Characteristics |
GJB2/6 Respondents (N=127) |
Control Respondents (N=25) |
|---|---|---|
| Presence of Vertigo | 127/235 (54%) | 25/61 (41%)*(P<0.03) |
| Characteristics | ||
| ➢ Spinning vertigo | 70% | 60% |
| ➢ Lightheadedness | 74% | 52% |
| ➢ Dysequilibrium | 44% | 52% |
| Positional Component | 68% | 36%*(P<0.0005) |
| Intermittent | 96% | 92% |
| Duration | ||
| ➢ Less than 1 minute | 36% | 68% |
| ➢ Minutes to Hours | 48% | 16% |
| ➢ Hours to Days | 16% | 16% |
| > 3 lifetime episodes | 71% | 78% |
Table II.
Summary of Survey Results in Cases and Controls with Vertigo
| Vestibular Dysfunction Characteristics |
GJB2/6 Respondents (N=127) |
Control Respondents (N=25) |
|---|---|---|
| Vertigo Requires Bedrest to Resolve |
63% | 46%*(P<0.05) |
| Vertigo Interferes with Activities of Daily Living |
48% | 28% *(P<0.03) |
| Associated Nausea/Vomiting |
38% | 36% |
| Vertigo Requires Chronic Medication Use |
27% | 25% |
| Baseline Tinnitus | 57% | 72% |
| Tinnitus Which Worsens During Vertigo Spell |
23% | 28% |
| Emergency Room Visits Due to Vertigo |
14% | 20% |
| Aural Fullness | 27% | 29% |
| Headaches | 29% | 24% |
Most (63%) Cases in the GJB2/6 group had to lie down in order for the vertigo to subside, and 48% reported that vertigo interfered with activities of daily living. In contrast, 46% of Controls had to lie down (p<0.05) and 28% reported interference with activities of daily living (p<0.03). Nausea and vomiting associated with vertigo were reported by 38% of GJB2/6 Cases and 36% of Controls. Baseline tinnitus was present in 57% of Cases with vertigo and 72% of Controls with vertigo. In those with tinnitus and vertigo, tinnitus was reported to worsen with vertiginous spells in 23% of the GJB2/6 Cases and in 28% of the Controls. Medications for vertigo, most commonly meclizine or diuretics, were used by 27% of the GJB2/6 Cases and 25% of the Controls. Emergency room visits due to vertigo were present in 14% of Cases and 20% of Controls.
Tables III and IV outlines relevant past medical and family history data in Cases and Controls with and without vertigo. Table III demonstrates that 40% of Cases with vertigo reported other medical conditions, including hypertension, type II diabetes, mitral valve prolapse, hypothyroidism and gastroesophageal reflux disease. This finding did not differ from the Cases without vertigo, in which 35% reported similar additional medical conditions. Similarly, in Table IV, 24% of Controls with vertigo as well as 50% of Controls without vertigo reported other chronic conditions. Thus, overall the total reporting of other medical conditions in Cases and Controls was 75% and 74%. Eighteen Cases (14%) with vertigo in the GJB2/6 group had undergone prior ear surgery, most frequently cochlear implantation (44% of this subgroup) or myringotomy with tube placement (22% of this subgroup). This was not significantly different from the Controls, in which 32% of those with vertigo reported prior ear surgery, most commonly cochlear implantation (38%) or tube placement (25%). In the GJB2/6 Cases without vertigo, 13 respondents (12%) had undergone prior ear surgery, again, most frequently cochlear implantation (54%) or tube placement (38%). Similarly, 11% of the Controls without vertigo had undergone prior ear surgery, most commonly cochlear implantation (50%).
Table III.
Comparison of Past Medical, Family History, and Associated Factors Between Cases with and without vestibular dysfunction
| Associated Factors |
GJB2/6 Respondents (Cases) with Vertigo (N=127) |
GJB2/6 Respondents (Cases) without Vertigo (N=108) |
|---|---|---|
| Baseline Tinnitus | 57% | 35% *(P<0.05) |
| Other Medical Conditions |
40% | 35% |
| Prior Ear Surgery | 14% | 12% |
| Cochlear Implantation |
6% | 6% |
| Female Gender | 70% | 53%*(P<0.05) |
| Biallelic Truncating Mutations |
95% | 86%*(P<0.05) |
| Personal Hx of Migraine |
38% | 23% *(P<0.05) |
| Family Hx of Dizziness |
40% | 20%*(P<0.05) |
| Family Hx of Migraine |
59% | 37% |
Table IV.
Comparison of Past Medical, Family History, and Associated Factors Between Controls with and without vestibular dysfunction
| Associated Factors |
Control Respondents with Vertigo (N=25) |
Control Respondents without Vertigo (N=36) |
|---|---|---|
| Baseline Tinnitus | 72% | 29% *(P<0.0009) |
| Other Medical Conditions |
24% | 50% |
| Prior Ear Surgery | 32% | 11% |
| Cochlear Implantation |
12% | 6% |
| Female Gender | 68% | 60% |
| Personal Hx of Migraine |
42% | 20% * (P<0.05) |
| Family Hx of Dizziness |
29% | 21% |
| Family Hx of Migraine |
46% | 34% |
A family history of vertigo was reported in 40% of GJB2/6 Cases with vertigo, but in only in 20% of Cases without vertigo (p<0.05). In contrast, a family history of vertigo was reported in 29% of Controls with vertigo and in 21% of Controls without vertigo. Overall, dizzy subjects were more likely to report a family history of dizziness (p<.005); but there was no significant difference between the Cases and Controls in this regard.
The phenotypic effects of truncating and nontruncating mutations on the presence of vertigo in Cases with GJB2/6 deafness were compared using a contingency analysis and chi square test. Table V presents the genotypes of the 127 Cases in the GJB2/6 group reporting vertigo in comparison with the Cases without vertigo. Ninety three percent of those with vertigo carried two truncating mutations in comparison to 86.1% among those without vertigo (p<0.03). A chi square analysis revealed a significant association between the presence of vertigo in the subject and several personal and family history variables as summarized in Table III. Perhaps somewhat surprisingly, there was no significant association between the presence or absence of vertigo and prior ear surgery.
Table V.
Frequency of GJB2 and GJB6 mutations in Study Participants with and without vertigo*
| With Vertigo | Without Vertigo | ||||
|---|---|---|---|---|---|
| Genotype | n | % | n | % | |
| GJB2 Truncating/Truncating (T/T): | |||||
| 35delG/31del38 | 1 | 0.79 | |||
| 35delG/35delG | 91 | 71.65 | 69 | 64.49 | |
| 35delG/167delT | 5 | 3.94 | 6 | 5.61 | |
| 35delG/235delC | 1 | 0.93 | |||
| 35delG/289insA | 1 | 0.93 | |||
| 35delG/310del14 | 4 | 3.15 | 1 | 0.93 | |
| 35delG/312del14 | 3 | 2.80 | |||
| 35delG/333-334delAA | 2 | 1.57 | |||
| 35delG/408insA | 1 | 0.79 | 1 | 0.93 | |
| 35delG/E47X | 3 | 2.36 | 2 | 1.87 | |
| 35delG/Q57X | 2 | 1.57 | |||
| 167delT/167delT | 3 | 2.36 | 4 | 3.74 | |
| GJB6 309 kbdel/-, GJB2 167delT/- | 5 | 3.94 | 1 | 0.93 | |
| GJB6 309 kbdel/-, GJB2 35delG/- | 4 | 3.15 | 2 | 1.87 | |
| GJB6 309 kbdel/-, GJB2 312del14/- | 1 | 0.93 | |||
| GJB6 309 kbdel/GJB6 309 kbdel | 1 | 0.93 | |||
| Total T/T Genotypes | 121 | 95.28 | 93 | 86.92 | |
| GJB2 Truncating/Nontruncating(T/NT): | |||||
| 35delG/delE120 | 1 | 0.93 | |||
| 35delG/G130V | 1 | 0.93 | |||
| 35delG/K15T | 1 | 0.93 | |||
| 35delG/L90P | 1 | 0.93 | |||
| 35delG/M34T | 1 | 0.93 | |||
| 35delG/N206S | 1 | 0.93 | |||
| 35delG/R143W | 1 | 0.79 | 1 | 0.93 | |
| 35delG/R184P | 2 | 1.87 | |||
| 35delG/S139N | 1 | 0.79 | 1 | 0.93 | |
| 35delG/V37I | 1 | 0.93 | |||
| 35delG/W77R | 1 | 0.93 | |||
| 167delT/R184P | 1 | 0.79 | |||
| 269insT/H100Y | 1 | 0.93 | |||
| 310del14/R32C | 1 | 0.79 | |||
| Total T/NT Genotypes | 4 | 3.15 | 13 | 12.15 | |
| TOTAL (with T/NT mutations vs total) | |||||
| GJB2 Nontruncating/Nontruncating (NT/NT): | |||||
| I81M/V95M | 1 | 0.93 | |||
| M34T/H100Y | 1 | 0.79 | |||
| R143W/E147K | 1 | 0.79 | |||
| Total NT/NT Genotypes | 2 | 1.57 | 1 | 0.93 | |
| TOTAL All Genotypes | 127 | 107 | |||
one individual heterozygous for a dominant GJB2 mutation (W44R) not included
DISCUSSION
The results of our study indicate that dizziness appears to be an integral feature of the connexin deafness phenotype as indicated by the frequency and duration of vertigo, the presence of associated symptoms, and the use of medications to mitigate the effects of vertigo on activities of daily living. The reported prevalence of dizziness (54%) in Cases with GJB2/6 deafness far exceeds the reported prevalence and lifetime incidence of vestibular vertigo in the general population [Neuhauser et al., 2005; Neuhauser and Lempert, 2009], and also exceeded the frequency observed in a Control group composed of subjects with other recessive or unknown causes of deafness.
Our findings are consistent with recent radiologic evidence documenting the frequent presence of subtle cochlear and vestibular malformations on fine cut temporal bone CT scans in patients with DFNB1 deafness [Propst et al., 2006], and suggest that these small changes may have clinical relevance. Our data differs from most older studies of connexin deafness that have not documented a substantial frequency of symptoms attributable to vestibular dysfunction on rotary chair and caloric testing [Cohn and Kelley, 1999; Denoyelle et al., 1999]. This may have been due in part to the compensatory mechanisms which mitigate insult to the vestibular system, which makes abnormal responses intermittent.
However, newer studies incorporating Vestibular Evoked Myogenic Potentials (VEMP), indicate that a more substantial percentage of those with deafness, and specifically connexin deafness, may have vestibular abnormalities than previously recognized [Kasai et al., 2010; Tribukait et al., 2004; Zagolski 2007; Shinjo et al., 2007; Zhou et al., 2009]. The VEMP is a “short latency electromyogram that is evoked by high-level acoustic stimuli (either click or tone burst) and recorded from surface electrodes over the tonically contracted sternocleidomastoid muscle” and examines the saccule and inferior vestibular nerve, whereas traditional vestibular testing examines the lateral semicircular canal and superior vestibular nerve [Akinet al., 2003; Colebatch et al., 1994; Welgampola et al., 2003; Murofushi et al., 1996; Murofushi et al., 1995]. The VEMP response can be obtained on people with any degree of sensorineural hearing loss (SNHL) [Colebatch et al., 1994; Murofushi et al., 1998; Robertson and Ireland, 1995; Ackley, 2004] as long as there is no conductive component, making it applicable to the deaf population.
Our data were originally described in Dodson et al. [2006] and are in agreement with recent investigations of vestibular dysfunction in subjects with connexin deafness [Kasai et al., 2010]. This study demonstrated that the percentage of vestibular dysfunction, as measured by VEMPs and caloric testing, was statistically higher in GJB2 deafness than in patients with congenital deafness not caused by GJB2 mutations or in healthy controls. In 2007, Zagolski reported on vestibular studies in which VEMP and calorics were performed on 40 normal hearing children, and 18 children with nonsyndromic hereditary hearing loss, of which five had biallelic GJB2 mutations. Of these children with biallelic GJB2 mutations, three had no response to either VEMPs or caloric testing, while the remaining two had normal studies [Zagolski, 2007].
In our survey, GJB2/6 Cases who reported vertigo were significantly (p<0.03) more likely to have two truncating mutations. This is consistent with a previous study which found that the presence of two truncating mutations is associated with more severe audiologic phenotypes [Snoeckx et al., 2005]. We also found significant associations between the presence of vertigo and tinnitus, aural fullness, and a family history of dizziness in our Cases with DFNB1 deafness. In agreement with another study of vertigo [Neuhauser et al., 2005], female gender and a history of migraine were also significantly associated with vertigo. Future studies may well identify other genetic modifiers that interact with DFNB1 mutations to increase the risk of these symptoms.
The strengths of the current study include the investigation of vestibular complaints in deaf Cases who were all homozygous for recessive GJB2/6 mutations, compared to deaf Controls who did not have DFNB1. We found that self reported dizziness and vertigo was more frequent in the Cases than Controls and more severe in those Cases who were homozygous for chain terminating mutations. As with any survey in which the response rate is less than 100%, the potential responder bias must be considered. However, even if every non-respondent in the GJB2/6 group had not experienced vertigo, the observed prevalence in our sample would have been more than twice the reported prevalence of vestibular vertigo in the general population. In addition, responder bias cannot explain the effects we observed of truncating mutations on the severity of vestibular symptoms since the details of the molecular structure of the mutations were unknown to the participants. Detailed clinical, vestibular and radiologic studies of symptomatic and non-symptomatic GJB2/6 patients who are homozygous for truncating and non-truncating mutations respectively could provide objective support for our findings.
As data emerges regarding the vestibular dysfunction in individuals with GJB2 and GJB6 mutations, it will permit a greater appreciation by physicians and patients of the full range of symptoms that may be associated with connexin deafness. This in turn should improve the clinical management of a patient population that has often been underserved in the past [Harmer, 1999].
CONCLUSION
Symptoms of vestibular dysfunction are more frequent in patients with DFNB1 deafness than previously recognized. Vertigo appears to be a common feature of this form of genetic deafness, especially cases with truncating mutations, and in those with tinnitus, aural fullness, and a family history of dizziness. Further investigation into the genetics and pathophysiology of vertigo in DFNB1 utilizing VEMPs, is clearly warranted, and should lead to the increased recognition and treatment of these sometimes disabling symptoms.
Supplementary Material
ACKNOWLEDGMENTS
We wish to acknowledge Sarah Burton, M.S. and Amanda Dove, B.S. for coordination of survey distribution and data entry and Kevin Cole from the Gallaudet Research Institute for the implementation of the online survey instrument. We also wish to thank the survey respondents for their participation in this project. This work was supported by NIH grant 0R01DC006707 awarded to KSA.
REFERENCES
- Ackley RS, Tamaki C, Oliszewski C, Inverso D. Vestibular Evoked Myogenic Potentials in Deaf and Hard of Hearing Subjects. Insights in Practice, Clinical Practice in Otoneurology. 2004:1–8. September. Insights in Practice, Clinical Practice in Otoneurology September. [Google Scholar]
- Akin FW, Murnane OD, Proffitt TM. The effects of click and tone-burst stimulus parameters on the vestibular evoked myogenic potential (VEMP) J.Am.Acad.Audiol. 2003;14:500–509. doi: 10.3766/jaaa.14.9.5. [DOI] [PubMed] [Google Scholar]
- Arnos KS, Welch KO, Tekin M, Norris VW, Blanton SH, Pandya A, Nance WE. A comparative analysis of the genetic epidemiology of deafness in the United States in two sets of pedigrees collected more than a century apart. Am.J.Hum.Genet. 2008;83:200–207. doi: 10.1016/j.ajhg.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azaiez H, Chamberlin GP, Fischer SM, Welp CL, Prasad SD, Taggart RT, del CI, Van Camp G, Smith RJ. GJB2: the spectrum of deafness-causing allele variants and their phenotype. Hum.Mutat. 2004;24:305–311. doi: 10.1002/humu.20084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Salmon M, Ott T, Michel V, Hardelin JP, Perfettini I, Eybalin M, Wu T, Marcus DC, Wangemann P, Willecke K, Petit C. Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impairment and cell death. Curr.Biol. 2002;12:1106–1111. doi: 10.1016/s0960-9822(02)00904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn ES, Kelley PM. Clinical phenotype and mutations in connexin 26 (DFNB1/GJB2), the most common cause of childhood hearing loss. Am.J.Med.Genet. 1999;89(3):130–136. [PubMed] [Google Scholar]
- Cohn ES, Kelley PM, Fowler TW, Gorga MP, Lefkowitz DM, Kuehn HJ, Schaefer GB, Gobar LS, Hahn FJ, Harris DJ, Kimberling WJ. Clinical studies of families with hearing loss attributable to mutations in the connexin 26 gene (GJB2/DFNB1) Pediatrics. 1999;103:546–550. doi: 10.1542/peds.103.3.546. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J.Neurol.Neurosurg.Psychiatry. 1994;57:190–197. doi: 10.1136/jnnp.57.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryns K, Orzan E, Murgia A, Huygen PL, Moreno F, del CI, Chamberlin GP, Azaiez H, Prasad S, Cucci RA, Leonardi E, Snoeckx RL, Govaerts PJ, Van de Heyning PH, Van de Heyning CM, Smith RJ, Van Camp G. A genotype-phenotype correlation for GJB2 (connexin 26) deafness. J.Med.Genet. 2004;41:147–154. doi: 10.1136/jmg.2003.013896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waele C, Huy PT, Diard JP, Freyss G, Vidal PP. Saccular dysfunction in Meniere's disease. Am.J.Otol. 1999;20:223–232. [PubMed] [Google Scholar]
- del Castillo I, Moreno-Pelayo MA, del Castillo FJ, Brownstein Z, Marlin S, Adina Q, Cockburn DJ, Pandya A, Siemering KR, Chamberlin GP, Ballana E, Wuyts W, Maciel-Guerra AT, Alvarez A, Villamar M, Shohat M, Abeliovich D, Dahl HH, Estivill X, Gasparini P, Hutchin T, Nance WE, Sartorato EL, Smith RJ, Van Camp G, Avraham KB, Petit C, Moreno F. Prevalence and evolutionary origins of the del(GJB6-D13S1830) mutation in the DFNB1 locus in hearing-impaired subjects: a multicenter study. Am.J.Hum.Genet. 2003;73:1452–1458. doi: 10.1086/380205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denoyelle F, Marlin S, Weil D, Moatti L, Chauvin P, Garabedian EN, Petit C. Clinical features of the prevalent form of childhood deafness, DFNB1, due to a connexin-26 gene defect: implications for genetic counselling. Lancet. 1999;353(9161):1298–1303. doi: 10.1016/S0140-6736(98)11071-1. [DOI] [PubMed] [Google Scholar]
- Dodson KM, Arnos K, Burton S, Marin R, Welch K, Norris V, Ackley S, Nance W, Pandya A. Prevalence of Vertigo in GJB2 Deafness. Abstract 695 American Society of Human Genetics. 2006 [Google Scholar]
- Forge A, Becker D, Casalotti S, Edwards J, Marziano N, Nevill G. Gap junctions in the inner ear: comparison of distribution patterns in different vertebrates and assessement of connexin composition in mammals. J.Comp Neurol. 2003;467:207–231. doi: 10.1002/cne.10916. [DOI] [PubMed] [Google Scholar]
- Guilford P, Ben Arab S, Blanchard S, Levilliers J, Weissenbach J, Belkahia A, Petit C. A non-syndrome form of neurosensory, recessive deafness maps to the pericentromeric region of chromosome 13q. Nat.Genet. 1994;6:24–28. doi: 10.1038/ng0194-24. [DOI] [PubMed] [Google Scholar]
- Hannaford PC, Simpson JA, Bisset AF, Davis A, McKerrow W, Mills R. The prevalence of ear, nose and throat problems in the community: results from a national cross-sectional postal survey in Scotland. Fam.Pract. 2005;22:227–233. doi: 10.1093/fampra/cmi004. [DOI] [PubMed] [Google Scholar]
- Harmer L. Health care delivery and deaf people: practice, problems, and recommendations for change. J.Deaf Stud.Deaf Educ. 1999;4:73–110. doi: 10.1093/deafed/4.2.73. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Takai Y, Ito K, Murofushi T. Abnormal vestibular evoked myogenic potentials in the presence of normal caloric responses. Otol.Neurotol. 2005a;26:1196–1199. doi: 10.1097/01.mao.0000194890.44023.e6. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Takai Y, Ozeki H, Ito K, Karino S, Murofushi T. Extent of lesions in idiopathic sudden hearing loss with vertigo: study using click and galvanic vestibular evoked myogenic potentials. Arch.Otolaryngol.Head Neck Surg. 2005b;13:857–862. doi: 10.1001/archotol.131.10.857. [DOI] [PubMed] [Google Scholar]
- Janecke AR, Hirst-Stadlmann A, Gunther B, Utermann B, Muller T, Loffler J, Utermann G, Nekahm-Heis D. Progressive hearing loss, and recurrent sudden sensorineural hearing loss associated with GJB2 mutations--phenotypic spectrum and frequencies of GJB2 mutations in Austria. Hum.Genet. 2002;111:145–153. doi: 10.1007/s00439-002-0762-y. [DOI] [PubMed] [Google Scholar]
- Kasai M, Hayashi C, Iizuka T, Inoshita A, Kamiya K, Okada H, Nakajima Y, Kaga K, Ikeda K. Vestibular function of patients with profound deafness related to GJB2 mutation. Acta Otolaryngol. 2010;130:990–995. doi: 10.3109/00016481003596508. [DOI] [PubMed] [Google Scholar]
- Kenna MA, Rehm HL, Robson CD, Frangulov A, McCallum J, Yaeger D, Krantz ID. Additional clinical manifestations in children with sensorineural hearing loss and biallelic GJB2 mutations: who should be offered GJB2 testing? Am.J.Med.Genet.A. 2007;143A(14):1560–1566. doi: 10.1002/ajmg.a.31706. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Adams JC, Paul DL, Kimura RS. Gap junction systems in the rat vestibular labyrinth: immunohistochemical and ultrastructural analysis. Acta Otolaryngol. 1994;114:520–528. doi: 10.3109/00016489409126097. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Price RK. Symptoms in the community. Prevalence, classification, and psychiatric comorbidity. Arch.Intern.Med. 1993;153:2474–2480. [PubMed] [Google Scholar]
- Krombach GA, van den BM, Di Martino E, Schmitz-Rode T, Westhofen M, Prescher A, Gunther RW, Wildberger JE. Computed tomography of the inner ear: size of anatomical structures in the normal temporal bone and in the temporal bone of patients with Meniere's disease. Eur.Radiol. 2005;15:1505–1513. doi: 10.1007/s00330-005-2750-9. [DOI] [PubMed] [Google Scholar]
- Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- Lerer I, Sagi M, Malamud E, Levi H, Raas-Rothschild A, Abeliovich D. Contribution of connexin 26 mutations to nonsyndromic deafness in Ashkenazi patients and the variable phenotypic effect of the mutation 167delT. Am.J.Med.Genet. 2000;95(1):53–56. doi: 10.1002/1096-8628(20001106)95:1<53::aid-ajmg11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Loffler J, Nekahm D, Hirst-Stadlmann A, Gunther B, Menzel HJ, Utermann G, Janecke AR. Sensorineural hearing loss and the incidence of Cx26 mutations in Austria. Eur.J.Hum.Genet. 2001;9:226–230. doi: 10.1038/sj.ejhg.5200607. [DOI] [PubMed] [Google Scholar]
- Marazita ML, Ploughman LM, Rawlings B, Remington E, Arnos KS, Nance WE. Genetic epidemiological studies of early-onset deafness in the U.S. school-age population. Am.J.Med.Genet. 1993;46:486–491. doi: 10.1002/ajmg.1320460504. [DOI] [PubMed] [Google Scholar]
- Masuda M, Usami S, Yamazaki K, Takumi Y, Shinkawa H, Kurashima K, Kunihiro T, Kanzaki J. Connexin 26 distribution in gap junctions between melanocytes in the human vestibular dark cell area. Anat.Rec. 2001;262:137–146. doi: 10.1002/1097-0185(20010201)262:2<137::AID-AR1018>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Morton CC, Nance WE. Newborn hearing screening--a silent revolution. N.Engl.J.Med. 2006;354:2151–2164. doi: 10.1056/NEJMra050700. [DOI] [PubMed] [Google Scholar]
- Mueller RF, Nehammer A, Middleton A, Houseman M, Taylor GR, Bitner-Glindzciz M, Van Camp G, Parker M, Young ID, Davis A, Newton VE, Lench NJ. Congenital non-syndromal sensorineural hearing impairment due to connexin 26 gene mutations--molecular and audiological findings. Int.J.Pediatr.Otorhinolaryngol. 1999;50:3–13. doi: 10.1016/s0165-5876(99)00242-6. [DOI] [PubMed] [Google Scholar]
- Murgia A, Orzan E, Polli R, Martella M, Vinanzi C, Leonardi E, Arslan E, Zacchello F. Cx26 deafness: mutation analysis and clinical variability. J.Med.Genet. 1999;36:829–832. [PMC free article] [PubMed] [Google Scholar]
- Murofushi T, Curthoys IS, Gilchrist DP. Response of guinea pig vestibular nucleus neurons to clicks. Exp.Brain Res. 1996;111:149–152. doi: 10.1007/BF00229565. [DOI] [PubMed] [Google Scholar]
- Murofushi T, Curthoys IS, Topple AN, Colebatch JG, Halmagyi GM. Responses of guinea pig primary vestibular neurons to clicks. Exp.Brain Res. 1995;103:174–178. doi: 10.1007/BF00241975. [DOI] [PubMed] [Google Scholar]
- Murofushi T, Matsuzaki M, Mizuno M. Vestibular evoked myogenic potentials in patients with acoustic neuromas. Arch.Otolaryngol.Head Neck Surg. 1998;124(5):509–512. doi: 10.1001/archotol.124.5.509. [DOI] [PubMed] [Google Scholar]
- Murofushi T, Takeuchi N, Ozeki H, Mizuno M. Acute vestibular dysfunction associated with interferon-alpha therapy. Eur.Arch.Otorhinolaryngol. 1998;255:77–78. doi: 10.1007/s004050050023. [DOI] [PubMed] [Google Scholar]
- Neuhauser HK, Lempert T. Vertigo: epidemiologic aspects. Semin.Neurol. 2009;29:473–481. doi: 10.1055/s-0029-1241043. [DOI] [PubMed] [Google Scholar]
- Neuhauser HK, von Brevern M, Radtke A, Lezius F, Feldmann M, Ziese T, Lempert T. Epidemiology of vestibular vertigo: a neurotologic survey of the general population. Neurology. 2005;65:898–904. doi: 10.1212/01.wnl.0000175987.59991.3d. [DOI] [PubMed] [Google Scholar]
- Norris VW, Arnos KS, Hanks WD, Xia X, Nance WE, Pandya A. Does universal newborn hearing screening identify all children with GJB2 (Connexin 26) deafness? Penetrance of GJB2 deafness. Ear Hear. 2006;27:732–741. doi: 10.1097/01.aud.0000240492.78561.d3. [DOI] [PubMed] [Google Scholar]
- Orzan E, Murgia A. Connexin 26 deafness is not always congenital. Int.J.Pediatr.Otorhinolaryngol. 2007;71:501–507. doi: 10.1016/j.ijporl.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Pandya A, Arnos KS, Xia XJ, Welch KO, Blanton SH, Friedman TB, Garcia SG, Liu MDX, Morell R, Nance WE. Frequency and distribution of GJB2 (connexin 26) and GJB6 (connexin 30) mutations in a large North American repository of deaf probands. Genet.Med. 2003;5:295–303. doi: 10.1097/01.GIM.0000078026.01140.68. [DOI] [PubMed] [Google Scholar]
- Propst EJ, Blaser S, Stockley TL, Harrison RV, Gordon KA, Papsin BC. Temporal bone imaging in GJB2 deafness. Laryngoscope. 2006;116:2178–2186. doi: 10.1097/01.mlg.0000244389.68568.a7. [DOI] [PubMed] [Google Scholar]
- Qu Y, Tang W, Dahlke I, Ding D, Salvi R, Sohl G, Willecke K, Chen P, Lin X. Analysis of connexin subunits required for the survival of vestibular hair cells. J.Comp Neurol. 2007;504:499–507. doi: 10.1002/cne.21459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DD, Ireland DJ. Vestibular evoked myogenic potentials. J.Otolaryngol. 1995;24:3–8. [PubMed] [Google Scholar]
- Robertson NG, Lu L, Heller S, Merchant SN, Eavey RD, McKenna M, Nadol JB, Jr, Miyamoto RT, Linthicum FH, Jr, Lubianca Neto JF, Hudspeth AJ, Seidman CE, Morton CC, Seidman JG. Mutations in a novel cochlear gene cause DFNA9, a human nonsyndromic deafness with vestibular dysfunction. Nat.Genet. 1998;20:299–303. doi: 10.1038/3118. [DOI] [PubMed] [Google Scholar]
- Schimmenti LA, Martinez A, Telatar M, Lai CH, Shapiro N, Fox M, Warman B, McCarra M, Crandall B, Sininger Y, Grody WW, Palmer CG. Infant hearing loss and connexin testing in a diverse population. Genet. Med. 2008;10:517–524. doi: 10.1097/gim.0b013e31817708fa. [DOI] [PubMed] [Google Scholar]
- Shinjo Y, Jin Y, Kaga K. Assessment of vestibular function of infants and children with congenital and acquired deafness using the ice-water caloric test, rotational chair test and vestibular-evoked myogenic potential recording. Acta Otolaryngol. 2007;127:736–747. doi: 10.1080/00016480601002039. [DOI] [PubMed] [Google Scholar]
- Snoeckx RL, Huygen PL, Feldmann D, Marlin S, Denoyelle F, Waligora J, Mueller-Malesinska M, Pollak A, Ploski R, Murgia A, Orzan E, Castorina P, Ambrosetti U, Nowakowska-Szyrwinska E, Bal J, Wiszniewski W, Janecke AR, Nekahm-Heis D, Seeman P, Bendova O, Kenna MA, Frangulov A, Rehm HL, Tekin M, Incesulu A, Dahl HH, du SD, Jenkins L, Lucas D, Bitner-Glindzicz M, Avraham KB, Brownstein Z, del CI, Moreno F, Blin N, Pfister M, Sziklai I, Toth T, Kelley PM, Cohn ES, Van Maldergem L, Hilbert P, Roux AF, Mondain M, Hoefsloot LH, Cremers CW, Lopponen T, Lopponen H, Parving A, Gronskov K, Schrijver I, Roberson J, Gualandi F, Martini A, Lina-Granade G, Pallares-Ruiz N, Correia C, Fialho G, Cryns K, Hilgert N, Van de HP, Nishimura CJ, Smith RJ, Van Camp G. GJB2 mutations and degree of hearing loss: a multicenter study. Am.J.Hum.Genet. 2005;77:945–957. doi: 10.1086/497996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobe T, Vreugde S, Shahin H, Berlin M, Davis N, Kanaan M, Yaron Y, Orr-Urtreger A, Frydman M, Shohat M, Avraham KB. The prevalence and expression of inherited connexin 26 mutations associated with nonsyndromic hearing loss in the Israeli population. Hum.Genet. 2000;106:50–57. doi: 10.1007/s004390051009. [DOI] [PubMed] [Google Scholar]
- Todt I, Hennies HC, Basta D, a Ernst A. Vestibular dysfunction of patients with mutations of Connexin 26. Neuroreport. 2005;16:1179–1181. doi: 10.1097/00001756-200508010-00009. [DOI] [PubMed] [Google Scholar]
- Tribukait A, Brantberg K, Bergenius J. Function of semicircular canals, utricles and saccules in deaf children. Acta Otolaryngol. 2004;124:41–48. doi: 10.1080/00016480310002113. [DOI] [PubMed] [Google Scholar]
- Welgampola MS, Rosengren SM, Halmagyi GM, Colebatch JG. Vestibular activation by bone conducted sound. J.Neurol.Neurosurg.Psychiatry. 2003;74:771–778. doi: 10.1136/jnnp.74.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox SA, Saunders K, Osborn AH, Arnold A, Wunderlich J, Kelly T, Collins V, Wilcox LJ, McKinlay Gardner RJ, Kamarinos M, Cone-Wesson B, Williamson R, Dahl HH. High frequency hearing loss correlated with mutations in the GJB2 gene. Hum.Genet. 2000;106:399–405. doi: 10.1007/s004390000273. [DOI] [PubMed] [Google Scholar]
- Yardley L, Owen N, Nazareth I, Luxon L. Prevalence and presentation of dizziness in a general practice community sample of working age people. Br.J.Gen.Pract. 1998;48:1131–1135. [PMC free article] [PubMed] [Google Scholar]
- Zagolski O. Vestibular system in infants with hereditary nonsyndromic deafness. Otol.Neurotol. 2007;28:1053–1055. doi: 10.1097/MAO.0b013e31815145e9. [DOI] [PubMed] [Google Scholar]
- Zelante L, Gasparini P, Estivill X, Melchionda S, D'Agruma L, Govea N, Mila M, Monica MD, Lutfi J, Shohat M, Mansfield E, Delgrosso K, Rappaport E, Surrey S, Fortina P. Connexin26 mutations associated with the most common form of non-syndromic neurosensory autosomal recessive deafness (DFNB1) in Mediterraneans. Hum.Mol.Genet. 1997;6:1605–1609. doi: 10.1093/hmg/6.9.1605. [DOI] [PubMed] [Google Scholar]
- Zhou G, Kenna MA, Stevens K, Licameli G. Assessment of saccular function in children with sensorineural hearing loss. Arch.Otolaryngol.Head Neck Surg. 2009;135:40–44. doi: 10.1001/archoto.2008.508. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.