Abstract
Biomaterials play pivotal roles in engineering tissue regeneration and repair. To regenerate irregular shaped defects, injectable cell carriers are desirable. Here, we report the development of self-assembled nanofibrous hollow microspheres from star-shaped biodegradable polymers as an injectable cell carrier for tissue regeneration. The nanofibrous hollow microspheres were shown to efficiently accommodate cells and enhance cartilage regeneration over control microspheres. The nanofibrous hollow microspheres also supported a significantly larger amount and higher quality cartilage regeneration over the chondrocytes alone group in an ectopic implantation model. In a critical-size rabbit osteochondral defect repair model, the nanofibrous hollow microspheres/chondrocytes group achieved substantially better cartilage repair and integration compared to the chondrocytes alone group that simulates the clinically available autologous chondrocyte implantation (ACI) procedure. These results indicate that the nanofibrous hollow microspheres are an excellent cell carrier for cartilage regeneration and are worthy of further investigation towards the aimed clinical application.
Biomaterials play pivotal roles in engineering tissue regeneration and repair1. To fabricate an entire organ or a large piece of tissue for transplantation, a predesigned scaffold with the patient-specific anatomy is required2–4. However, there are often irregular shaped defects and wounds that need to be filled and repaired in clinics. In such cases, injectable materials can be advantageous5 because they allow for easy manipulation or minimally invasive procedures by surgeons to reduce complications and to improve patient comfort and satisfaction. Hydrogels have been explored for such potential applications in research showing limitations, which are being tackled by various approaches6–10, and are not used clinically for cartilage repair. In this work, we synthesized star-shaped poly(L-lactic acid) (SS-PLLA) and developed technologies for such polymers to self-assemble into nanofibrous hollow microspheres. We also developed nanofibrous microspheres from linear poly(L-lactic acid) (PLLA). We hypothesized that the extracellular matrix (ECM)-mimicking nanofibrous architecture advantageously enhances cell-material interactions; channels/pores at multiple scales (between spheres, within spheres, and between nanofibres) promote cell migration, proliferation and mass transport conditions, facilitating tissue regeneration and integration with host. These microspheres were evaluated as injectable cell carriers for tissue regeneration using several experimental models.
We synthesized star-shaped poly(L-lactic acid) (SS-PLLA) by using poly(amidoamine) (PAMAM) dendrimers as initiators (Fig. 1A&B, and Supplementary Fig. S1). PAMAM dendrimers have been reported to be non-immunogenic and non-toxic at lower concentrations and lower generations (G ≤ 5)11, 12. We therefore chose low-generation PAMAM dendrimers (G2, G3, G4, G5) as initiators to synthesize SS-PLLA, and use the star-shaped polymers as building blocks to assemble nano and/or mesoscopic structures as well as to tune the degradation rate and possibly surface functionalities. The average molecular weights of PLLA branches and the whole SS-PLLA polymers were tailored by varying the PAMAM/L-lactide ratio and the number of generations of PAMAM (Supplementary Table S1). A SS-PLLA with a molecular weight of 69300 g/mol (branch molecular weight of 6600 g/mol) initiated by PAMAM (G2) was used for the rest of the study if not specifically indicated otherwise.
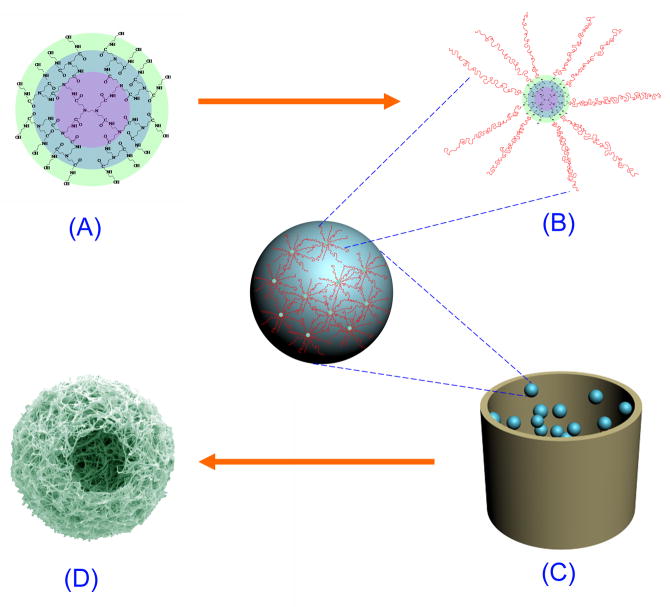
Figure 1. Schematic illustration of SS-PLLA synthesis and nanofibrous hollow microsphere fabrication.
(A) PAMAM (G2) as an initiator for the synthesis of SS-PLLA. The colours show the successive generations of the PAMAM. (B) The SS-PLLA synthesized. Pink coils represent the PLLA chains. Note that some hydroxyl groups on the PAMAM surface were not reacted with L-lactide. (C) Preparation of SS-PLLA microspheres using a surfactant-free emulsification process. (D) Nanofibrous hollow microspheres were obtained after phase separation, solvent extraction, and freeze-drying.
The ECM is a natural web of nanoscale structures and serves an important role in the maintenance of cell and tissue structure and function13–16. As an artificial ECM, a good scaffolding material should mimic the advantageous features of the natural ECM17. The nanofibres in the fabricated nanofibrous hollow microspheres (Fig. 1C&D) mimic the structural feature of collagen fibres (a main component of ECM). A representative nanofibrous hollow microsphere fabrication process is as follows: The SS-PLLA is dissolved in THF and emulsified into liquid microspheres in glycerol under rigorous stirring. The mixture is then quenched in liquid nitrogen to induce phase separation for nanofibre formation. After solvent extraction with distilled water and freeze-drying, the nanofibrous hollow microspheres are obtained without using any prefabricated template (Fig. 2A). The nanofibrous hollow microspheres are composed entirely of nanofibres with an average diameter of 160±67 nm (Fig. 2B&C), which is at the same scale as collagen fibres. In tissue engineering, a high porosity (often >90%) is desired for scaffolds to provide sufficient space for cell growth and ECM deposition18. The open and hollow structure (Fig. 2B,D,E) contributes to the high porosity (96.7±1.3%, which can be further increased by decreasing the SS-PLLA concentration) to facilitate cell seeding, proliferation and tissue regeneration. The average size of the nanofibrous hollow microspheres can be controlled from a few micrometers to a few hundred micrometers by varying the stirring speed and the SS-PLLA concentration. A higher stirring speed or/and a lower SS-PLLA concentration can decrease the average size of the nanofibrous hollow microspheres. Because no surfactant is added during the emulsification, the method has avoided potential complications associated with the surfactant removal.
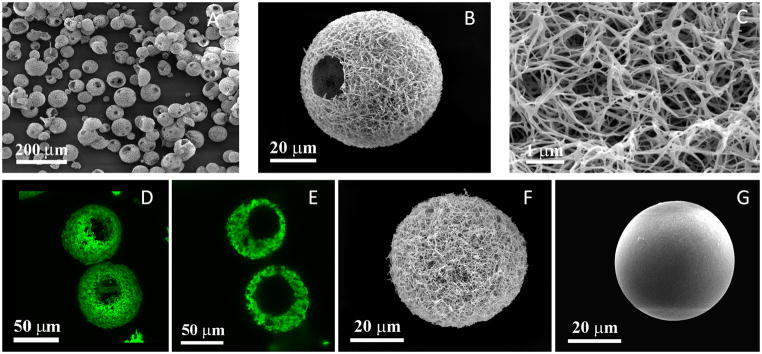
Figure 2. Characterization of nanofibrous hollow microspheres, nanofibrous microspheres, and solid interior microspheres.
(A) SEM image of nanofibrous hollow microspheres fabricated from SS-PLLA, noting that almost every microsphere had one or more open hole(s) on the shell. (B) SEM image of a representative nanofibrous hollow microspheres, showing the nanofibrous architecture and a hole of approximately 20 μm on the microsphere shell. (C) A high magnification image of (B) showing the nanofibres of the microsphere with an average diameter of about 160 nm. (D) A 3D reconstruction of nanofibrous hollow microspheres from confocal image stacks. (E) A 2D cross-section confocal image of the nanofibrous hollow microspheres, confirming the hollow structure of the microsphere. (F) SEM image of a representative nanofibrous microsphere, showing a nanofibrous architecture on the microsphere surface. (G) SEM image of a representative solid interior microspheres, showing a smooth surface of the microsphere. All the microspheres shown in this figure were fabricated from a 2% (wt/v) polymer solution. The nanofibrous hollow microspheres were fabricated from SS-PLLA. Both nanofibrous microspheres and solid interior microspheres were fabricated from a linear PLLA with an inherent viscosity of approximately 1.6 dl/g.
For comparison, two other types of microspheres were fabricated using linear PLLA (Fig. 2F&G). All of these microspheres were found not to be toxic to cells (Supplementary Fig. S2). Nanofibrous microspheres were prepared using the same procedure as that for preparing nanofibrous hollow microspheres when linear PLLA was used (Fig. 2F). The conventional solid PLLA microspheres, which were fabricated by using the solvent evaporation method, had a smooth surface (Fig. 2G). Significantly different from solid interior microspheres and nanofibrous microspheres, a hollow structure with an open hole (or multiple holes) on the shell of nanofibrous hollow microspheres was generated when SS-PLLA was used without the assistance of any template (Fig. 2D&E). This result indicates that the star-shaped architecture of the SS-PLLA is a unique building block to assemble the nanofibrous hollow microspheres. The diameter of most open pores ranged from 10 μm to 50 μm and was affected by the SS-PLLA concentration (Supplementary Fig. S3). Lower SS-PLLA concentration led to larger open holes. A lower stirring speed led to a larger diameter of the assembled nanofibrous hollow microspheres (Supplementary Fig. S4). Multiple open holes were observed on the nanofibrous hollow microspheres when the microsphere diameter was greater than 60 μm (Supplementary Fig. S5). Because of the nanofibrous and the hollow architecture, the nanofibrous hollow microspheres had an overall density of 0.043 g/cm3, which was less than 1/2 of the density of the nanofibrous microspheres and less than 1/30 of the density of the solid interior microspheres (Supplementary Table S2). The surface area of the nanofibrous hollow microspheres was 120.70±0.91 m2/g, which was similar to that of nanofibrous microspheres but was more than three orders of magnitude higher than that of the solid interior microspheres. When higher generations of PAMAM (G3, G4, and G5) were used to synthesize the SS-PLLA, the resulting SS-PLLA also formed nanofibrous hollow microspheres (Supplementary Fig. S6). One of the advantages of the nanofibrous hollow microspheres is that their degradation rate can be tailored by the molecular weight and molecular architecture of the SS-PLLA (Supplementary Fig. S7&S8).
Both the SS-PLLA polymers and the special emulsification process are required for the formation of the nanofibrous hollow microspheres. We have demonstrated that glycerol was encapsulated inside the SS-PLLA microspheres after the emulsification and phase separation procedures (Supplementary Fig. S9). The formation of open holes on the nanofibrous hollow microspheres shells was also attributed to the star-shaped architecture of the SS-PLLA. We demonstrated that linear PLLA could not form open hole structure by the same process of preparing nanofibrous hollow microspheres (Fig. 2F). When a small amount of linear PLLA was blended with the SS-PLLA, the obtained microspheres contained smaller open holes on the shells than those from the SS-PLLA alone (Supplementary Fig. S10). The open holes on the shells disappeared as the amount of linear PLLA in the blend was increased to 60% (wt/v). By analyzing the SS-PLLA molecular structure, it was found that there were un-reacted hydroxyl groups on the surface of PAMAM molecules likely resulted from steric hindrance. Based on the above results, the unique emulsification process and the remaining un-reacted hydroxyl groups (glycerol-philic) on SS-PLLA are believed to be critical to the formation of the hollow cores and open holes of the nanofibrous hollow microspheres (discussed in Supplementary information Part 4, Supplementary Fig. S11).
We examined the nanofibrous hollow microspheres as an injectable scaffold for cartilage regeneration using three experimental models: (I) In vitro cartilage formation; (II) Subcutaneous injection in nude mice for ectopic cartilage formation; and (III) Rabbit osteochondral defect repair.
The in vitro chondrocyte attachment on nanofibrous hollow microspheres was examined against nanofibrous microspheres and solid interior microspheres. Chondrocytes were readily attached to both the nanofibrous hollow microspheres and the nanofibrous microspheres. About 100% of the chondrocytes were attached to the nanofibrous hollow microspheres and the nanofibrous microspheres, while less than 60% of the chondrocytes were attached to the solid interior microspheres 24 hours after cell seeding (Fig. 3A). The high attachment efficiency of the cells on the nanofibrous hollow microspheres and the nanofibrous microspheres could be attributed to their nanofibrous architecture, which has high surface area and can adsorb cell adhesion proteins (such as fibronectin and vitronectin) at significantly higher levels than the smooth surface as previously found with non-injectable porous foam scaffolds19. The chondrocytes on both the nanofibrous hollow microspheres and the nanofibrous microspheres exhibited a more rounded morphology while they were flat and spread over significantly larger areas on the solid microspheres (Fig. 3B–D). Further, a significant number of cells was found to have migrated inside the nanofibrous hollow microspheres. It has been demonstrated that the rounded cell shape enhances the maintenance of chondrocyte phenotype, while a flat morphology promotes the dedifferentiation of chondrocytes20. Meanwhile, the chondrocytes seeded on both the nanofibrous hollow microspheres and the nanofibrous microspheres had significantly higher proliferation rates and produced higher amounts of glycosaminoglycans (GAG) than those on the solid interior microspheres (Supplementary Fig. S12A&B). After 3 weeks, the cartilage-specific genes (aggrecan and collagen type II) were down-regulated on the solid interior microspheres while the gene expression level of collagen type I was similar among the 3 types of microspheres (Supplementary Fig. S12C). In contrast, continuous expression of aggrecan and collagen type II genes at high levels was detected in cells on the nanofibrous hollow microspheres and nanofibrous microspheres, suggesting the capability of nanofibrous architecture to retain chondrocyte phenotype.
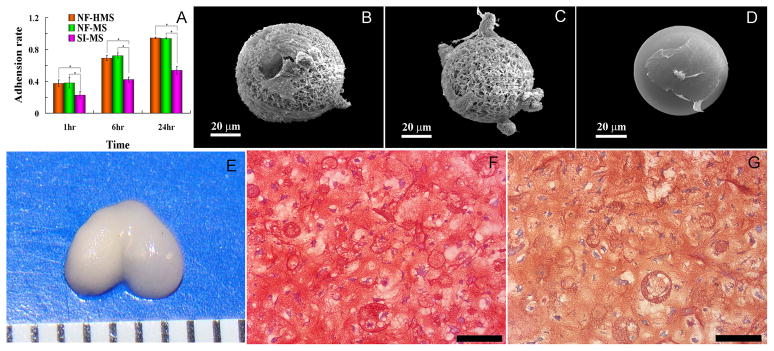
Figure 3. Assessment of chondrocyte adhesion and the in vitro cartilage tissue formation on different microspheres.
(A) Chondrocyte adhesion on nanofibrous hollow microspheres (NF-HMS), nanofibrous microspheres (NF-MS), and solid interior microspheres (SI-MS), by measuring the DNA content using Hoechst 33258 dye at various times. Error bars indicate standard deviation. *p < 0.05. (B) The 3D morphology of chondrocytes on nanofibrous hollow microspheres, noting that some chondrocytes migrated inside the holes. (C) The 3D morphology of chondrocytes on nanofibrous microspheres. (D) The morphology of chondrocytes on solid interior microspheres, noting that the chondrocytes were flat and spread over a large area. (E) De novo cartilage tissue formation with the anatomical shape of a rat femoral condyle after culture for 4 weeks, noting that the newly formed tissue had a white and glistening appearance. Safranin-O (F) and immunohistochemical (G) staining demonstrated GAG and collagen type II accumulation. Nanofibrous hollow microspheres were uniformly distributed inside the new tissue. Chondrocytes migrated into the hollow cores of some nanofibrous hollow microspheres. Note that microspheres may appear to have a range of sizes due to the difference in the plane of sectioning. The tick mark in E is 1 mm, and the scale bars in F and G represent 100 μm.
For clinical applications, injectable/moldable scaffolds are often needed to fill cartilage defects of irregular geometries. To test the capacity of the nanofibrous hollow microspheres as an injectable scaffold to fill cartilage defects, nanofibrous hollow microspheres were mixed with chondrocytes and injected into a mould with the shape of a rat femoral condyle. After 4 weeks of incubation in vitro, a piece of glistening new cartilage tissue with the identical shape to the rat femoral condyle was obtained (Fig. 3E). The histological results revealed a homogeneous distribution of nanofibrous hollow microspheres inside the tissue (Fig. 3F&G). The safranin-O and immunohistochemical staining indicated that the nanofibrous hollow microspheres were surrounded by abundant amounts of GAG and type II collagen (Fig. 3F&G). These in vitro results demonstrated the capability of the nanofibrous hollow microspheres as an injectable scaffold to fill a complex cartilage defect or to be moulded into a predesigned 3D tissue shape.
A subcutaneous injection model in nude mice was used to test cartilage regeneration in vivo. We first compared the cartilage regeneration using the same mass of the three different microspheres. Chondrocytes (from New Zealand white rabbits, passage 2) were mixed with each type of microspheres and injected into the subcutaneous pockets of the nude mice. The injection of chondrocytes alone without microspheres was used as a control group. Samples were collected 8 weeks after the injection. While all regenerated cartilage tissues were white and glistening in gross appearance, the tissue sizes were significantly different. The average tissue mass formed from the nanofibrous hollow microspheres/chondrocytes group was 36.9% higher than that formed from the nanofibrous microspheres/chondrocytes group, 197.3% higher than that formed from the solid interior microspheres/chondrocytes group, and 235.0% higher than that formed from the chondrocytes alone group (Fig. 4A). Biochemical quantification showed that both the GAG/wet-weight (ww) and the GAG/DNA ratios of the nanofibrous hollow microspheres group were significantly higher than those of the nanofibrous microspheres, the solid interior microspheres, and the chondrocytes alone groups (Fig. 4B&C). Furthermore, the DNA/ww ratio of the nanofibrous hollow microspheres was significantly lower than those of the other groups (Fig. 4D). Among the four groups, the average DNA/ww ratio of the nanofibrous hollow microspheres group was the closest to that of the native cartilage. Histological examination showed that the tissue formed from the simple chondrocyte injection was hypercellular and consisted of larger areas of fibrous tissue, which was stained negative with safranin-O (Fig. 4E). Most of the solid interior microspheres aggregated and stained weakly by safranin-O, indicating poor cartilage tissue formation (Fig. 4F). In contrast, the nanofibrous hollow microspheres and the nanofibrous microspheres were uniformly distributed throughout the new tissues. The histological and immunohistochemical staining indicated that sulphate proteoglycan (Fig. 4G&H) and type II collagen (Fig. 4J) were evenly distributed throughout tissues from the nanofibrous hollow microspheres and the nanofibrous microspheres specimens. The in vivo regenerated cartilage from nanofibrous hollow microspheres (Fig. 4H) was closest to the native cartilage (Fig. 4I) in histological appearance. Results of similar trends in terms of histological and biochemical analyses were obtained when the same number of different microspheres was used in the ectopic cartilage regeneration model (Supplementary Fig. S13). The in vitro results (Supplementary Fig. S14) were consistent with the in vivo results when different microspheres were compared, although the quality of tissues regenerated in vitro was inferior to the quality of those regenerated in vivo. When different microspheres of the same mass were compared, the different 3D architectures (including surface architectures) were evaluated in terms of biological performance of the same amount of polymer (and eventually the same amount of degradation by-products), which is an important consideration for a tissue-engineering scaffold. When the same number of different microspheres was compared, the biological outcome in a short-term culture was mainly associated with the effect of the surface architecture. However, in the long-term, the large amounts of degradation by-products from the solid interior microspheres would be a major concern (a lesser degree of concern for nanofibrous microspheres, which would still generate significantly more degradation by-products than nanofibrous hollow microspheres though).
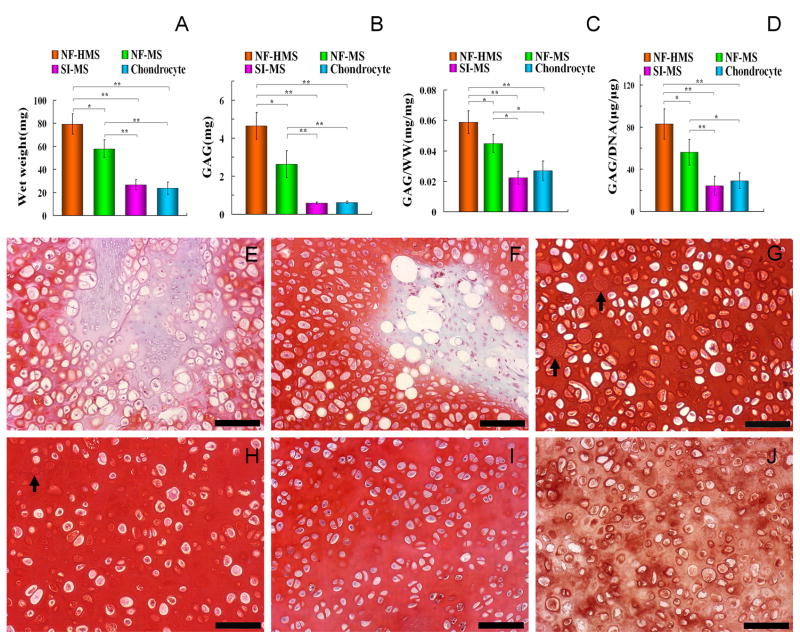
Figure 4. Comparison of various microspheres as cell carriers for ectopic cartilage regeneration in vivo.
Eight weeks after subcutaneous injection of the chondrocyte-microsphere suspension, the biochemical compositions and histological images of the ectopically engineered cartilage tissue from the same number of chondrocytes and the same mass of different types of microspheres were comparatively evaluated. The nanofibrous hollow microspheres (NF-HMS) group had significantly higher tissue mass (A), GAG content (B), GAG/wet-weight ratio (C), and GAG/DNA ratio (D) than the nanofibrous microspheres (NF-MS), the solid interior microspheres (SI-MS), and the chondrocytes alone groups. Error bars in panels A through D indicate standard deviation. * p < 0.05, ** p < 0.01. Tissue sections were stained with safranin-O for proteoglycans 8 weeks after subcutaneous injection: (E) Chondrocytes alone group, (F) solid interior microspheres/chondrocytes group, (G) nanofibrous microspheres/chondrocytes group (The arrow indicates a nanofibrous microsphere), (H) nanofibrous hollow microspheres/chondrocytes group (The arrow indicates a nanofibrous hollow microsphere). Native rabbit knee cartilage (I) was used as the positive control. Both the nanofibrous hollow microspheres and the nanofibrous microspheres were more uniformly distributed in the new cartilage tissue, where chondrocytes were embedded in the lacunae surrounded by the GAG-positive ECM. The cell density in the new tissue from the nanofibrous microspheres/chondrocytes appeared higher than that in the tissue from the nanofibrous hollow microspheres/chondrocytes group, which is the closest to that of the native cartilage. The solid interior microspheres clustered together and most of the tissue from the solid interior microspheres/chondrocytes group showed higher cellularity than that in native cartilage. The new tissue from chondrocytes alone group was hypercellular with fibrous tissue appearance in the centre. (J) Immunohistochemical staining demonstrated that there was abundant collagen type II in the new tissue from the nanofibrous hollow microspheres/chondrocytes group. Note that microspheres may appear to have a range of sizes due to the difference in the plane of sectioning. Scale bars in panels E through J represent 100 μm.
The outcome differences between nanofibrous carriers (both nanofibrous hollow microspheres and nanofibrous microspheres) and solid interior microspheres could be partially attributed to the nanofibrous architecture (which has been demonstrated to enhance in vitro cartilage differentiation using mesenchymal stem cells in a non-injectable nanofibrous scaffold21). The overall low material densities and high surface areas of the nanofibrous hollow microspheres and the nanofibrous microspheres likely enhance protein adsorption for cell-scaffold interactions and facilitate mass transfer for tissue regeneration. The faster degradation rate of the nanofibrous hollow microspheres (made of SS-PLLS) and their hollow structure likely provided additional space for the matrix accumulation, facilitating cartilage tissue formation. After 8 weeks, most of the nanofibrous hollow microspheres had degraded and abundant cartilage-specific matrix (GAG and type II collagen) had been deposited into the void spaces. In contrast, all the nanofibrous microspheres and the solid interior microspheres were still in the tissues with a round shape after 8 weeks of implantation. When compared with the solid interior microspheres and the nanofibrous microspheres based on the same polymer chemistry (PLLA), the nanofibrous hollow microspheres group had a lower cell density and a substantially larger amount of new tissue matrix per cell, similar to the native rabbit cartilage (Fig. 4F–I). These findings demonstrate the significant impact of the unique physical features of the nanofibrous hollow microspheres on cell behaviour and tissue regeneration. The importance of certain other physical features of biomaterials is also being recognized22.
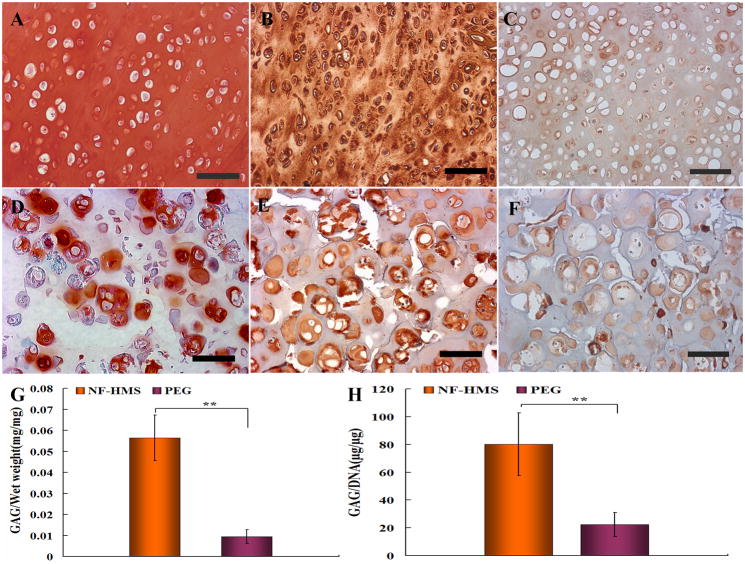
Given that the above results had shown the superiority of the nanofibrous hollow microspheres over other types of microspheres in regenerating cartilage, the nanofibrous hollow microspheres were selected as the injectable carrier for chondrocytes to further test their ability to regenerate hyaline cartilage. Poly(ethylene glycol) (PEG), as one of the most widely studied experimental hydrogels for cartilage regeneration, was selected for comparison. Eight weeks after subcutaneous injection of 2.0 ml either nanofibrous hollow microspheres/chondrocytes suspension or PEG/chondrocytes suspension (4×106 chondrocytes each) in a nude mouse, the regenerated tissue constructs were harvested. Histological examination using safranin-O staining demonstrated that neo-cartilage tissue formed from the nanofibrous hollow microspheres/chondrocytes group contained uniform GAG-rich extracellular matrix throughout the construct (Fig. 5A), while the neo-cartilage tissue formed from the PEG/chondrocytes group contained GAG-positive matrix only in the pericellular regions of the chondrocytes (Fig. 5D). Consistently, immunohistochemical staining revealed collagen type II throughout the neo-cartilage from the nanofibrous hollow microspheres/chondrocytes group (Fig. 5B), while collagen type II was only stained positively in the pericellular regions of the chondrocytes in the PEG/chondrocytes group (Fig. 5E). While the collagen type II was significantly more abundant in the nanofibrous hollow microspheres/chondrocytes groupthan in the PEG/chondrocytes group, the collagen type I staining was similarly weak in overall intensity between the two groups (Fig. 5C and F).Biochemical analysis (n=6) revealed that the neo-cartilage tissue from the nanofibrous hollow microspheres/chondrocytes group had significantly higher GAG/Wet weight (Fig. 5G) and GAG/DNA (Fig. 5H) ratios. Consistently, the biomechanical properties of the engineered cartilage from the nanofibrous hollow microspheres were similar to those of native cartilage and superior to those from the PEG gel (Supplementary Fig. S15).
Figure 5. Comparison between neo cartilage tissues regenerated from nanofibrous hollow microspheres/chondrocytes and PEG/chondrocytes constructs after 8 weeks of subcutaneous implantation in nude mice.
Histological examination showed that neo-cartilage tissue regenerated from the nanofibrous hollow microspheres/chondrocytes group contained uniform GAG-rich ECM as evidenced by safranin-O staining (A), and abundant collagen type II as shown by immunohistochemical staining (B). In contrast, only pericellular regions of the chondrocytes were stained positively for GAG (D) and collagen type II (E) in the PEG/chondrocytes group. While the collagen type II was significantly more abundant in the nanofibrous hollow microspheres/chondrocytes group than in the PEG/chondrocytes group, the collagen type I staining was similarly weak in overall intensity between the two groups (C and F). Biochemical analysis (n=6) revealed that cartilage tissue formed from the nanofibrous hollow microspheres (NF-HMS)/chondrocytes constructs had significantly higher GAG/wet weight ratio (G) and GAG/DNA ratio (H) than those from the PEG/chondrocytes constructs. Error bars in panels G & H indicate standard deviation. ** p<0.01.
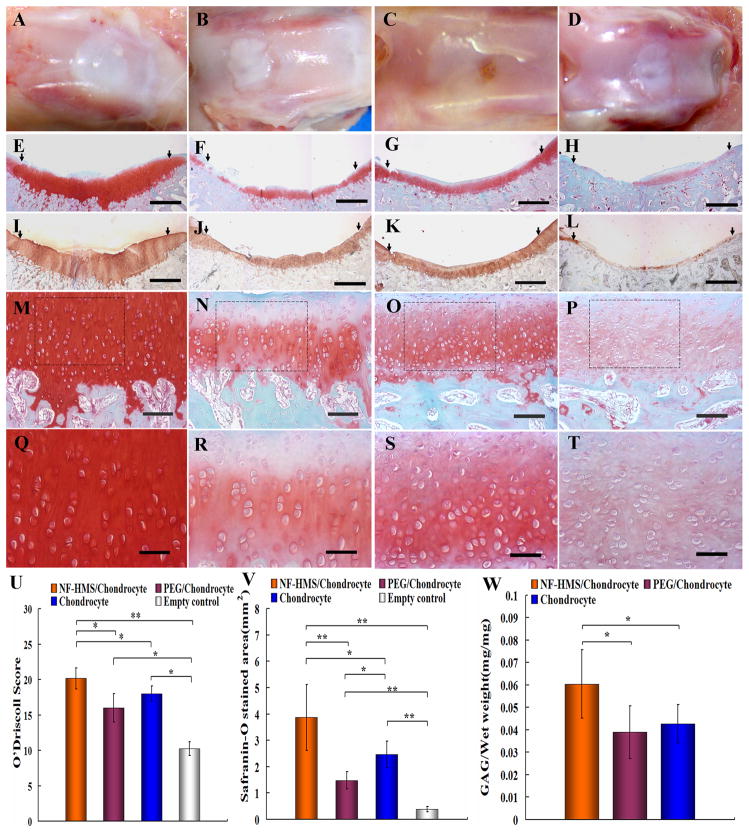
The nanofibrous hollow microspheres/chondrocytes group was also compared with the PEG/chondrocytes group and a chondrocytes alone group (simulating a clinically available autologous chondrocyte implantation (ACI) procedure) to test the ability of nanofibrous hollow microspheres as a chondrocyte carrier to regenerate hyaline cartilage in a rabbit osteochondral defect repair model. A full-thickness rabbit osteochondral defect was created in each knee of the rabbits. The cartilage defects were injected with the nanofibrous hollow microspheres/chondrocytes suspension, PEG/chondrocytes suspension, chondrocytes suspension (in DMEM), or DMEM (sham control). No obvious immunological or infectious complications were observed throughout the experiments. The regenerated tissue constructs were harvested and evaluated 8 weeks after injection. In the nanofibrous hollow microspheres/chondrocytes group, the regenerated tissue fully filled the defect, smoothly integrated with the host cartilage, and exhibited white glistening appearance (Fig. 6A). In the PEG/chondrocytes group, the regenerated tissue was white but there was clearly a gap between the regenerated tissue and the host tissue (Fig. 6B). In the chondrocytes alone group, the regenerated tissue was similarly thin but integrated more smoothly than that of the PEG/chondrocytes group with the host tissue (Fig. 6C). In the sham control group, the regenerated tissue was peripherally white but depressed in the centre (Fig. 6D). It is known that implanted tissues23–25 and the PEG hydrogels8 do not integrate well with the native cartilage. The smooth integration between the regenerated tissue from the nanofibrous hollow microspheres/chondrocytes group and the host tissue is an important advantage. In the nanofibrous hollow microspheres/chondrocytes group, the regenerated tissue was predominantly hyaline cartilage matrix evidenced by strong safranin-O staining for GAG (Fig. 6E, M, Q), strong immunohistochemical staining for collagen type II and good integration with host cartilage (Fig. 6I). In the PEG/chondrocytes group, the regenerated tissue was thin, uneven, weakly stained with safrinin-O (Fig. 6F, N, R) and collagen type II immunohistochemically (Fig. 6J). In the chondrocytes alone group, the regenerated cartilage tissue was significantly thinner, and was stained weaker by safranin-O (Fig. 6G, O, S) and collagen type II antibody (Fig 6K). In addition, the regenerated tissue from the chondrocytes alone group was hypercullular (Fig. 6O&S) compared to native cartilage (Fig. 4I). In the sham control, only small areas of the defect were stained positive with safranin-O (Fig. 6H, P, T), and minimal type II collagen was detected by immunohistochemical staining (Fig. 6L). At a higher magnification, superior bone-cartilage interface was revealed for the nanofibrous hollow microspheres/chondrocytes group (Fig. 6M) compared to the PEG/chondrocytes (Fig. 6N), chondrocytes alone (Fig. 6O), and the sham control (Fig. 6P) groups. The mean O’Driscoll histological score26 for the nanofibrous hollow microspheres/chondrocytes group (20.2±1.5) was significantly higher than those for the PEG/chondrocytes (16.0±2.0), chondrocytes alone (18.0±1.1), and sham control (10.3±1.0) groups (Fig. 6U). Histomorphometric analysis confirmed more GAG in the nanofibrous hollow microspheres/chondrocytes groupthan in PEG/chondrocytes, chondrocytes alone, or sham control groups (Fig. 6V). As one of the markers for the quality of cartilage, the GAG/wet weight ratio of the regenerated tissue from the nanofibrous hollow microspheres/chondrocytes group was significantly higher than those from the PEG/chondrocytes and chondrocytes alone groups (Fig. 6W), while the value of the sham control group was negligible. The regenerated cartilage in the rabbit knees from all groups were stained very weak for collagen type I, similar to the native cartilage (Supplementary Fig. S16). More positive staining for collagen type I was identified in the subchondral bone in all groups and fibrous tissue in the sham control group.
Figure 6. Evaluation of critical-size rabbit osteochondral defect repair 8 weeks after injection.
(A) Gross appearance of the nanofibrous hollow microspheres/chondrocytes group; (B) gross appearance of PEG/chondrocytes group; (C) gross appearance of the chondrocytes alone group; (D) gross appearance of the sham control group. There was predominantly hyaline cartilage formation in the nanofibrous hollow microspheres/chondrocytes group evidenced by strong safranin-O staining (E, M, Q) and immunohistochemical staining for collagen type II (I) with thick cartilage matrix and smooth integration with the host cartilage; there was thinner and uneven repair tissue in the PEG/chondrocytes group with poor safranin-O (F, N, R) and weaker immunohistochemical staining for collagen type II (J); there was similarly thinner repair tissue in the chondrocytes alone group with similarly poor safranin-O staining (G, O, S) and weaker immunohistochemical staining for collagen type II (K); there was minimal repair tissue in the sham control group evidenced by sparse safranin-O (H, P, T) and minimal type II collagen staining (L). Figures M, N, O, and P are higher magnification images of E, F, G and H, respectively, at the bone-cartilage interface. Figures Q, R, S, and T are higher magnification images selected from the dash lined boxes of Figures M, N, O, and P, respectively. Significant higher O’Driscoll score was achieved in the nanofibrous hollow microspheres (NF-HMS)/chondrocytes group compared with the PEG/chondrocytes group, the chondrocytes alone group and the sham control group (U). Histomorphometric analysis (areas of positive safranin-O staining) revealed significantly better repair with the nanofibrous hollow microspheres (NF-HMS)/chondrocytes group than with the PEG/chondrocytes group, chondrocyte alone group, or sham control group (V). The GAG/wet-weight ratio of the nanofibrous hollow microspheres (NF-HMS)/chondrocytes group was significantly higher than that of the PEG/chondrocytes group or the chondrocytes alone group (W). The tissue amount regenerated from the sham control group was too small and scattered to measure the GAG/wet-weight ratio. The scale bars represent 1 mm in E through L, 200 μm in M through P, and 100 μm in Q through T. The arrows in E through L point to the interface areas between the repair tissues and host cartilage. The error bars in panels U through W indicate standard deviation. * p < 0.05, ** p<0.01.
The above presented experimental data demonstrate that the molecular architecture of a biodegradable polymer (such as SS-PLLA) not only can be used to tailor degradation behaviour but also can be used to modulate the self-assembly of structural features of micro carriers at the nanometer and micrometer scales. The micro and nano-sized architectural features of cell carriers can regulate cellular function and tissue regeneration. The injectable nanofibrous hollow microspheres self-assembled from SS-PLLA are an excellent micro carrier for chondrocytes to facilitate high-quality hyaline cartilage regeneration. The nanofibrous hollow microspheres have been used to successfully repair a critical-size osteochondral defect in a widely-used rabbit model, and have been shown to be advantageous over the chondrocytes alone group that simulates the clinical available autologous chondrocyte implantation (ACI) procedure. Thus the injectable nanofibrous hollow microspheres have been demonstrated to be an excellent carrier for chondrocytes and worthy of further investigation towards the aimed clinical application. The nanofibrous hollow microspheres will also be evaluated as carriers for other cells to regenerate other tissues in the future.
Methods
Synthesis of Star-shaped PLLA (SS-PLLA)
The PAMAM-OH dendrimers (G2, G4, and G5) were injected into a dry glass ampoule and evaporated under vacuum at 45°C for 48 h to remove the solvent. L-lactide and Sn(Oct)2 were added, and the ampoule was purged six times with dry nitrogen and sealed under vacuum. Under rigorous magnetic stirring, the polymerization was carried out at 130–140°C for 24 h. After polymerization, the crude product was dissolved in chloroform. The polymer was purified by repeated precipitations from chloroform into methanol.
Fabrication of nanofibrous hollow microspheres
SS-PLLA was dissolved in THF at 50°C with a concentration of 2.0% (wt/v). Under rigorous mechanical stirring (speed 7, MAXIMA, Fisher Scientific Inc.), glycerol (50°C) with three times the volume of the SS-PLLA solution was gradually added into the SS-PLLA solution, and the stirring continued for 5 minutes afterwards. The mixture was then quickly poured into liquid nitrogen. After 10 min, water-ice mixture (1000 mL) was added for solvent exchange for 24 hours. The spheres were sieved and washed with excessive distilled water for 6 times to remove glycerol residue. The spheres were then lyophilized for 3 days.
Fabrication of nanofibrous microspheres
When a linear PLLA was used in the above process, nanofibrous microspheres without open holes were generated.
Subcutaneous injection of chondrocytes and microspheres
All animal procedures were carried out under the guidelines of the Institutional Animal Care and Use Committee of the University of Michigan. Nude mice (6–8 weeks old, NU/NU, Charles River Laboratories Inc, USA) were anesthetized with 2.5% isoflurane in balanced oxygen. The microspheres/chondrocytes suspension was injected into the subcutaneous pockets on both sides lateral to the dorsal midline using a 25-gauge needle. Each mouse received two injections with each injection containing 4×106 cells (0.2 mL) mixed with microspheres. Two separate experiments were performed: 1) using the same mass (0.3 mg: 2×105 nanofibrous hollow microspheres, 0.86×105 nanofibrous microspheres, and 0.075×105 solid interior microspheres) of microspheres (20–60 μm in diameter); 2) using the same number of microspheres (2×105: 0.3 mg nanofibrous hollow microspheres, 0.7 mg nanofibrous microspheres, and 8.0 mg solid interior microspheres). In each experiment, injections were randomly arranged and 8 nude mice were used, which allowed for four specimens in each group for the 4 groups (including the chondrocytes alone group). The new cartilage tissues were harvested after 8 weeks and the fibrous capsules were removed. The samples were weighted and used for biochemical, histological and immunohistochemical examinations.
Rabbit osteochondral defect repair
Twelve female three-month-old New Zealand white rabbits (Harlan Sprague Dawley Inc, USA) were used in this study. The rabbits were anesthetized with an intramuscular injection of a mixture of 150 mg ketamine hydrochloride (Ketaset III, Fort Dodge, USA), 35 mg xylazine hydrochloride (LLOYD laboratories, USA) and 5 mg acepromazine (Boehringer Ingelheim Vetmedica inc. USA). A medial parapatellar incision was made so that the knee joint was exposed. The patella was dislocated laterally and the anterior articular surface of the distal femur was exposed. A 5-mm diameter full-thickness cylindrical osteochondral defect (2–3 mm deep) was using an electrical trephine in the articular surface of the femoral patellar groove. After irrigating the joint with sterile isotonic saline, a 6×6 mm flap was removed from the overlying the quadriceps muscle and sutured to the peripheral rim of the artificial defect with 6-0 gut suture (Gut chromic, Hu-Friedy, USA). 2×107 cultured chondrocytes (passage 2) were mixed with 1.4 mg nanofibrous hollow microspheres into 1 ml DMEM medium. For each defect, 0.08 ml nanofibrous hollow microspheres/chondrocyte suspension (1.6×106 cells) was delivered (n=6). For the PEG comparison group (n=6), 2×107 chondrocytes (passage 2) were mixed into 1 ml PEG solution. 0.08 ml PEG/chondrocytes suspension (1.6×106 cells) was injected into each defect and irradiated with a UV light (365 nm) at an intensity of 10 mW/cm2 for 5 min under a UV lamp (Cole-Parmer, High intensity, long-wave UV lamps, Illinois, USA) as reported5, 7. The gel was also covered with a flap as described above. For the chondrocytes alone group (n=6), 2×107 chondrocytes (passage 2, in 0.08 ml DMEM medium) were injected into each defect. The defect was also covered with a flap. Similarly, the negative control defect was covered with a flap and received 0.08 mL DMEM medium (n=6). The wound was closed by suturing the knee joint capsule and the skin layer by layer. Rabbits were allowed to move freely within the individual cages. The regenerated tissue constructs were harvested 8 weeks later. Each construct was cut into two halves. One half was used for histological and immunohistochemical staining, and the other half was used for biochemical assays.
Statistical analysis
All data were presented as means ± standard deviations (SD). In order to test the significance of observed differences between the study groups, an unpaired Student’s t-test was applied. A value of p < 0.05 was considered to be statistically significant.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the financial support from the National Institutes of Health (Research Grants DE015384 and DE017689: PXM). The authors would also like to acknowledge the assistance from Dr. Jiang Hu in the animal experiments.
Footnotes
Author contributions
XL and XJ contributed overall equally to the experimental work. XL carried out the polymer synthesis, fabrication of microspheres and structural characterization. XJ carried out the cell culture, animal studies and tissue analyses. PXM was responsible for the overall project design and manuscript organization. All authors contributed to the scientific planning, data analysis and interpretation.
The authors declare no competing financial interests.
Supplementary information accompanies this paper on www.nature.com/naturematerials
Supplementary information is provided on the characterizations of polymers and microspheres, solid interior microsphere fabrication, cytotoxicity and degradation of various microspheres, the isolation and culture of chondrocytes, in vitro cartilage regeneration, constructing femoral condyle shaped cartilage, subcutaneous PEG/chondrocytes injection, the detection of GAG and DNA, the real time PCR analysis, biomechanical properties of engineered cartilage, the sample preparation for SEM observation, and the histological, immunohistochemical, and histomorphometrical analyses of the engineered cartilage.
References
- 1.Langer R, Vacanti JP. Tissue Engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Oberpenning F, Meng J, Yoo JJ, Atala A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat Biotechnol. 1999;17:149–155. doi: 10.1038/6146. [DOI] [PubMed] [Google Scholar]
- 3.Wang P, Hu J, Ma PX. The engineering of patient-specific, anatomically shaped, digits. Biomaterials. 2009;30:2735–2740. doi: 10.1016/j.biomaterials.2009.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen VJ, Smith LA, Ma PX. Bone regeneration on computer-designed nano-fibrous scaffolds. Biomaterials. 2006;27:3973–3979. doi: 10.1016/j.biomaterials.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 5.Elisseeff J, Anseth K, Sims D, McIntosh W, Randolph M, Langer R. Transdermal photopolymerization for minimally invasive implantation. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:3104–3107. doi: 10.1073/pnas.96.6.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rice MA, Waters KR, Anseth KS. Ultrasound monitoring of cartilaginous matrix evolution in degradable PEG hydrogels. Acta Biomater. 2009;5:152–161. doi: 10.1016/j.actbio.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang DA, Varghese S, Sharma B, Strehin I, Fermanian S, Gorham J, Fairbrother DH, Cascio B, Elisseeff JH. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat Mater. 2007;6:385–392. doi: 10.1038/nmat1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benoit DS, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat Mater. 2008;7:816–823. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strehin I, Nahas Z, Arora K, Nguyen T, Elisseeff J. A versatile pH sensitive chondroitin sulfate-PEG tissue adhesive and hydrogel. Biomaterials. 2010;31:2788–2797. doi: 10.1016/j.biomaterials.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esfand R, Tomalia DA. Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discovery Today. 2001;6:427–436. doi: 10.1016/s1359-6446(01)01757-3. [DOI] [PubMed] [Google Scholar]
- 12.Roberts JC, Bhalgat MK, Zera RT. Preliminary biological evaluation of polyamidoamine (PAMAM) Starburst(TM) dendrimers. Journal of Biomedical Materials Research. 1996;30:53–65. doi: 10.1002/(SICI)1097-4636(199601)30:1<53::AID-JBM8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 13.Stevens MM, George JH. Exploring and engineering the cell surface interface. Science. 2005;310:1135–1138. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 14.Meredith JE, Fazeli B, Schwartz MA. The Extracellular-Matrix as a Cell-Survival Factor. Molecular Biology of the Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gullberg D, Ekblom P. Extracellular matrix and its receptors during development. International Journal of Developmental Biology. 1995;39:845–854. [PubMed] [Google Scholar]
- 16.Rosso F, Giordano A, Barbarisi M, Barbarisi A. From cell-ECM interactions to tissue engineering. Journal of Cellular Physiology. 2004;199:174–180. doi: 10.1002/jcp.10471. [DOI] [PubMed] [Google Scholar]
- 17.Ma PX. Biomimetic materials for tissue engineering. Advanced Drug Delivery Reviews. 2008;60:184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu XH, Ma PX. Polymeric scaffolds for bone tissue engineering. Annals of Biomedical Engineering. 2004;32:477–486. doi: 10.1023/b:abme.0000017544.36001.8e. [DOI] [PubMed] [Google Scholar]
- 19.Woo KM, Chen VJ, Ma PX. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. Journal of Biomedical Materials Research Part A. 2003;67A:531–537. doi: 10.1002/jbm.a.10098. [DOI] [PubMed] [Google Scholar]
- 20.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 21.Hu J, Feng K, Liu X, Ma PX. Chondrogenic and osteogenic differentiations of human bone marrow-derived mesenchymal stem cells on a nanofibrous scaffold with designed pore network. Biomaterials. 2009;30:5061–5067. doi: 10.1016/j.biomaterials.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitragotri S, Lahann J. Physical approaches to biomateiral design. Nature Materials. 2009;8:15–23. doi: 10.1038/nmat2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Z, McCaffery JM, Spencer RG, Francomano CA. Growth and integration of neocartilage with native cartilage in vitro. J Orthop Res. 2005;23:433–439. doi: 10.1016/j.orthres.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 24.Ahsan T, Sah RL. Biomechanics of integrative cartilage repair. Osteoarthritis Cartilage. 1999;7:29–40. doi: 10.1053/joca.1998.0160. [DOI] [PubMed] [Google Scholar]
- 25.Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 26.O'Driscoll SW, Keeley FW, Salter RB. The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1986;68:1017–1035. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.