Abstract
Objective
To determine the precise timing of PR disappearing from the uterine luminal epithelium (LE) to help understand the significance of the dynamic PR expression in the LE during embryo implantation.
Design
Experimental rodent models.
Setting
University research laboratories.
Animal(s)
Mice and hamsters.
Intervention(s)
Pseudopregnancy and artificial decidaulization.
Main Outcome Measure(s)
Blue dye injection for detecting embryo attachment; immunohistochemistry, immunofluorescence, and in situ hybridization for detecting gene expression.
Result(s)
PR remains expressed in the LE up to 6 hours after the initial detection of blue dye reaction in mice (day three 22:00 hours). PR disappears first from LE cells at the implantation site and subsequently from the entire LE layer by day four 06:00 hours when uterine stromal decidualization has become obvious. PR remains highly expressed in the LE of day four 11:00 hours pseudopregnant mice, but it disappears from the entire LE layer by day four 06:00 hours of artificially decidualized pseudopregnant mice.
Conclusion(s)
1) PR disappears from the LE after implantation has initiated and before the histological decidualization is manifested, suggesting an active role of continued PR expression in the LE for the initial implantation process; and 2) the disappearance of PR expression in the LE is regulated by uterine factor(s) produced upon embryo attachment.
Keywords: progesterone receptor, uterine luminal epithelium, embryo implantation, embryo attachment
Introduction
Progesterone receptor (PR) has unique uterine expression patterns during early pregnancy, especially peri-implantation period, in mice: it increases significantly in the uterine luminal epithelium (LE) from gestation day 0.5 to day 1.5 (mating night as day 0); it is upregulated in both LE and stroma during preimplantation day 2.5 and day 3.5; it disappears from the LE but is strongly expressed in the primary decidual zone in the postimplantation day 4.5 uterus (1, 2).
PR-mediated progesterone (P) signaling is indispensable for embryo implantation in all mammals studied (3–5). There are two main PR isoforms, PR-A and PR-B. The ratio of PR-A/PR-B is 3:1 in the mouse uterus (6). Studies from PR knockout mice demonstrate that PR-A, but not PR-B, is critical for uterine function, including embryo implantation and decidualization (7–9).
Embryo implantation, which takes place between gestation day 3.5 and 4.5 in mice, is a multi-step process, including embryo apposition, attachment, and invasion (4, 10). LE is the first layer of cells that an embryo communicates with for implantation. PR disappears from the LE between gestation day 3.5 and day 4.5 in mice (1, 2). Multiple events happen during the hours between these two time points. When exactly does PR disappear from the LE? It is our objective to define the time of PR disappearance from the uterine LE during implantation. This will help us understand any potential role that PR plays in the LE during the implantation process and thus provide more insight into the molecular mechanism of embryo implantation.
Materials and Methods
Animals
Young virgin mice (C57BL6) and hamsters (golden) were purchased from the Jackson and Charles River Laboratories, respectively. They were housed in polypropylene cages with free access to regular food and water. The animal rooms were maintained on a 12-hour light/dark cycle (6:00 AM to 6:00 PM) at the University of Georgia and Vanderbilt University Medical Center. All methods used in this study were approved by the University of Georgia and Vanderbilt University Committees of Use and Care of animals and conform to National Institutes of Health guidelines and public law.
Treatments
In natural pregnancy, the females were mated with fertile males and checked for a vaginal plug in mice and the presence of sperms in vaginal smear in hamsters next morning (midnight of mating night as day 0 00:00). Implantation sites were detected by intravenous injection of blue dye as previously described (11). Mouse uterine tissues were collected on day three 11:00, 22:00, 23:00, 24:00 hours, and day four 01:00, 02:00, 04:00, 06:00, and 11:00 hours. Hamster uterine tissues were collected on day three 09:00, 12:00, and 15:00 hours. Pseudopregnancy was induced by mating female mice with vasectomized males and uteri were dissected on day three 11:00 hours and day four 11:00 hours. Artificial decidualization was induced by oil injection in one uterine horn and two pinches with a hemostat in the contralateral horn in pseudopregnant mice on day three 10:00 hours, and blue dye reaction was determined prior to uterine collection on day three 24:00 hours and day four 06:00 hours. At least three pregnant mice and hamsters were included in each time point in each study.
Immunohistochemistry
Frozen uterine sections (10 µm) from different groups in the same set of study were mounted on the same slide to keep consistent staining; fixed in 4% paraformaldehyde in PBS; washed in PBS; subjected to antigen retrieval in 0.01M sodium citrate buffer, pH 6.0, for 20 minutes. Endogenous peroxidase was inactivated with 3% H2O2 and non-specific staining was subsequently blocked using 10% goat serum. Sections were then incubated with primary rabbit-anti-human PR antibody (1:200, A0098, Dako, Denmark) at 4°C for overnight; washed in PBS and incubated with biotinylated goat anti-rabbit secondary antibody (1:200, Santa Cruz Biotechnology, Inc, Santa Cruz, CA) for 30 min at RT. PBS washed sections were next incubated with ABComplex/HRP (Santa Cruz Biotechnology, Inc); washed in PBS; incubated with 3, 3’-diaminobenzidine tetrahydrochloride (DAB, Bio Basic Inc. Ontario, Canada); counterstained with Hematoxylin; and mounted for imaging. Negative control was processed exactly the same except that the primary antibody was replaced with rabbit IgG. This DAKO anti-human PR antibody recognizes mouse PR-A and PR-B, both of which are expressed in the mouse uterus (12), with PR-A being the main PR isoform (6). It can also recognize hamster PR (Fig. 1O~1T).
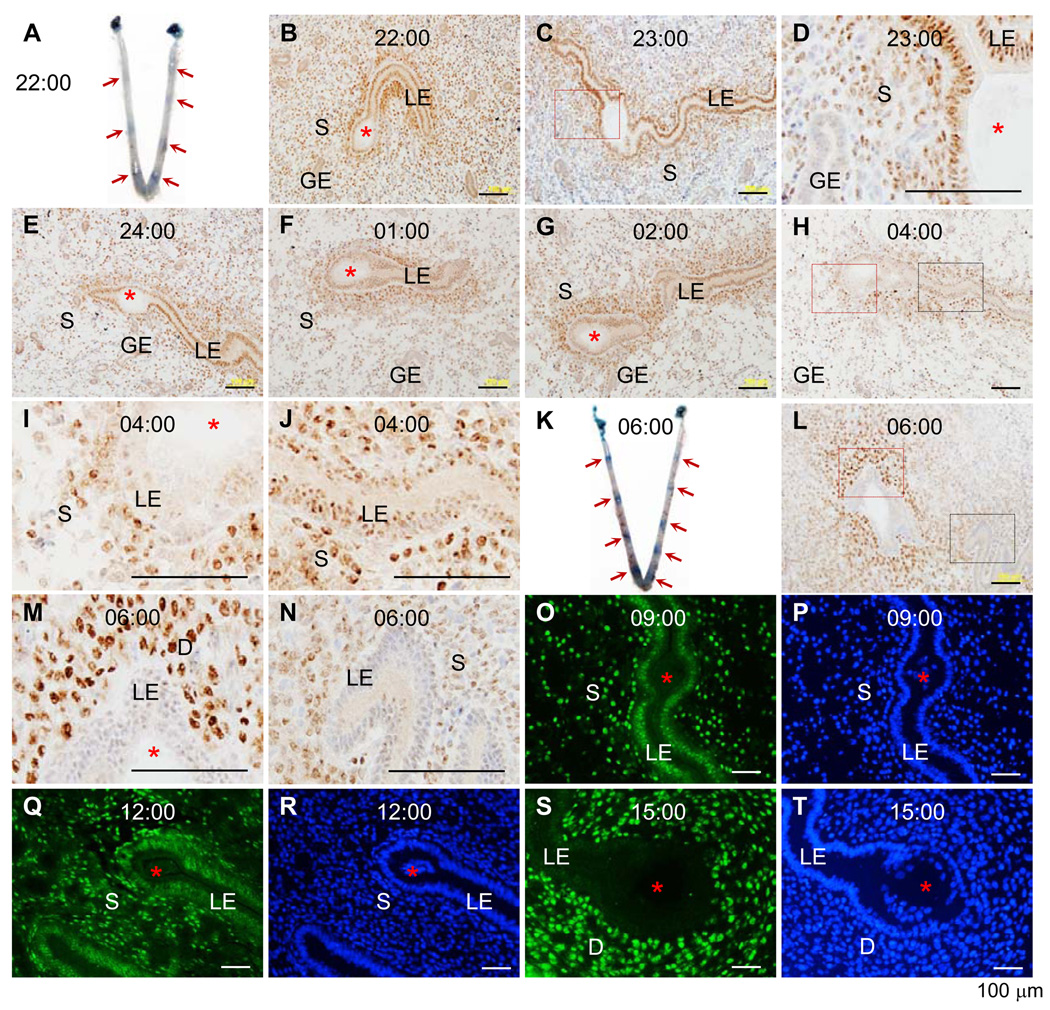
Figure 1.
Time-course expression of progesterone receptor (PR) during the early hours of embryo implantation in mouse (A~N) uterus detected by immunohistochemistry (IHC) and hamster (O~T) uterus detected by immunofluorescence (IF). Frozen sections (10 µm) through an embryo were used. A. A representative uterine image on day three 22:00 hours. B. PR IHC of an implantation site on A. C. PR IHC, day three 23:00 hours. D. An enlarged view of the red boxed area in C. E. PR IHC, day three 24:00 hours. F. PR IHC, day four 01:00 hour. G. PR IHC, day four 02:00 hours. H. PR IHC, day four 04:00 hours. I. An enlarged view of the red boxed area in H. J. An enlarged view of the black boxed area in H. K. A representative uterine image on day four 06:00 hours. L. PR IHC of an implantation site on K. M. An enlarged view of the red boxed area in L. N. An enlarged view of the black boxed area in L. O. PR IF, day three 09:00 hours. P. DAPI stain of the section on O. Q. PR IF, day three 12:00 hours. R. DAPI stain of the section on Q. S. PR IF, day three 15:00 hours. T. DAPI stain of the section on S. Red arrows indicate implantation sites detected by blue dye reaction (A & K). LE, luminal epithelium; S, stroma; GE, glandular epithelium; D, decidual zone; red asterisk, embryo; scale bar, 100 µm. N=3–4. No specific staining was detected in the negative control (data not shown).
Immunofluorescence
Immunofluorescence was performed as previously described (13) with the exceptions that primary rabbit-anti-PR antibody (1:100, Dako) was used and the sections were counterstained with 0.01% 4’6-diamidino-2-phenylindole dihydrochloride (DAPI, Sigma-Aldrich Corporation, St. Louis, MO).
In situ hybridization
Results
PR expression in the LE during early hours of embryo implantation in mice
Embryo-uterine attachment can be visualized via blue dye reaction (16), which became detectable on day three 22:00 hours. Among all the 23 pregnant mice (with either blue dye reaction or healthy-looking blastocysts) examined from day three 22:00 hours to day four 06:00 hours, only one (dissected on day three 22:00 hours) didn’t show blue dye reaction. Although there were individual variations, the intensity of the blue bands (indicating implantation sites) roughly increased from almost undetectable faint bands on day three 22:00 hours (Fig. 1A) to defined blue bands on day four 06:00 hours (Fig. 1K). Immunohistochemistry (IHC) showed sustained PR expression in the LE by day four 2:00 hours, including the flattened LE cells at the implantation site (Fig. 1B~G). PR was detectable in the stroma, especially the subepithelial stromal area (Fig. 1B~J). From day three 24:00 hours to day four 04:00 hours, stromal cell density was decreased (Fig. 1E~H), indicating stromal edema. Disappearing PR expression was initially detected in the LE at the implantation site where decidualization was not obvious yet and PR was still detectable in the rest LE on day four 04:00 hours (Fig. 1H~J). On day four 06:00 hours, PR had disappeared from the entire LE layer of all four pregnant mice examined and it became highly expressed in the primary decidual cells (Fig. 1L~N). Low levels of PR seemed to remain in some, but not all, glandular epithelial cells (GE) of some, but not all, uterine samples by day three 24:00 hours (Fig. 1B, 1E). PR was undetectable in the GE from day four 01:00 hour and later (Fig. 1F~H).
Expression of histidine decarboxylase (Hdc), proline-rich acidic protein 1 (Prap1), and amiloride-binding protein 1 (Abp1) during early hours of embryo implantation in mice
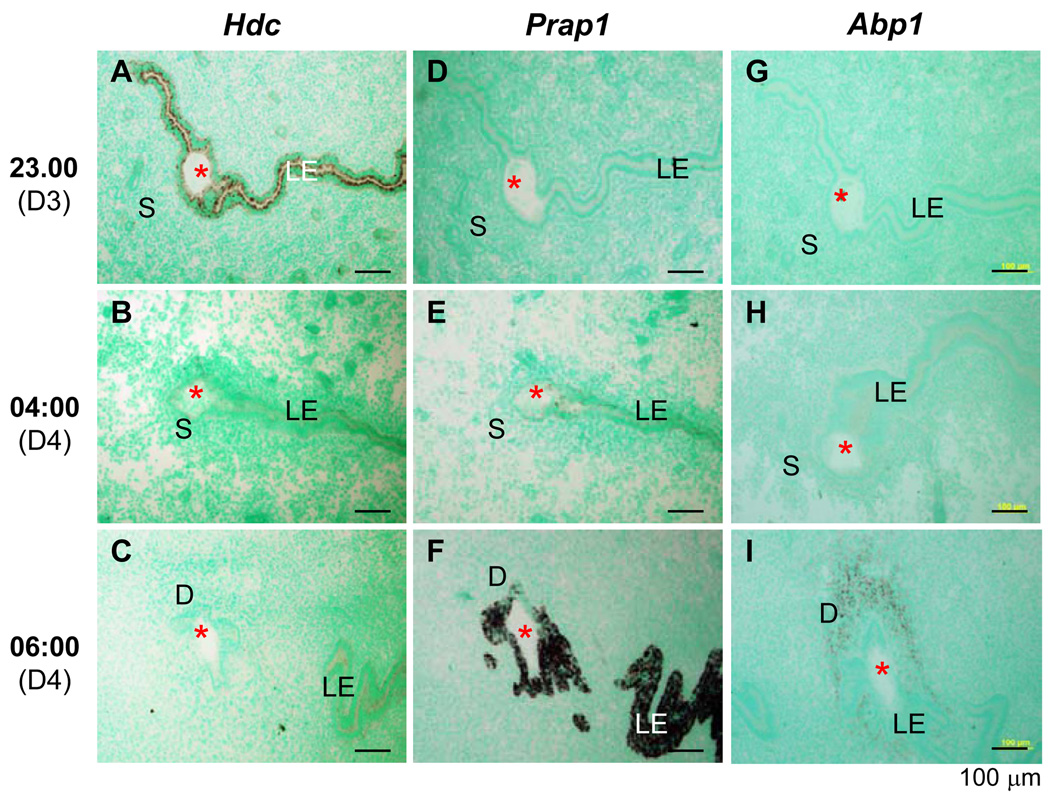
Hdc is an epithelial specific gene highly expressed in the preimplantation uterus and upregulated by P (17). Prap1 is an LE specific gene highly expressed in the postimplantation uterus and downregulated by P (14). They were selected as representative PR target genes to demonstrate correlating temporal expression patterns between PR and PR target genes. In situ hybridization on the serial mouse uterine sections next to those for PR IHC (Fig. 1C, 1H, 1L) indicated downregulation of Hdc in LE from day three 23:00 hours to day four 06:00 hours (Fig. 2A–C), paralleling to PR expression pattern (Fig. 1C, 1H, 1L); and upregulation of Prap1 at the same time period (Fig. 2D–F), opposite to PR expression pattern (Fig. 1C, 1H, 1L). Decidual cell-specific gene Abp1 (18) was only detectable on day four 06:00 hours (Fig. 2G–I), consistent with the decidualization status indicated by PR expression (Fig. 1C, 1H, 1L).
Figure 2.
Time-course expression of histidine decarboxylase (Hdc), proline-rich acidic protein 1 (Prap1), and amiloride-binding protein 1 (Abp1) during the early hours of embryo implantation in mouse uterus detected by in situ hybridization using antisense probes. Longitudinal frozen sections (10 µm) through an embryo were used. A. Hdc, day three 23:00 hours. B. Hdc, day four 04:00 hours. C. Hdc, day four 06:00 hours. D. Prap, day three 23:00 hours. E. Prap1, day four 04:00 hours. F. Prap1, day four 06:00 hours. G. Abp1, day three 23:00 hours. H. Abp1, day four 04:00 hours. I. Abp1, day four 06:00 hours. LE, luminal epithelium; S, stroma; D, decidual zone; red asterisk, embryo; scale bar, 100 µm. N=3. No specific staining was detected with sense probes (data not shown).
PR expression in the LE during early hours of embryo implantation in hamsters
Hamsters have an earlier implantation and a shorter gestation period than mice and do not require estrogen for implantation. Hamster blastocysts were already so tightly attached to the LE that they were difficult to be flushed on day three 09:00 hours when blue dye reaction was not obvious yet (19–21). PR was highly expressed in the LE on day three 09:00 hours (Fig. 1O, 1P) and remained expressed in the LE on day three 12:00 hours when blue dye reaction was detectable but decidualization was not obvious (Fig. 1Q, 1R). On day three 15:00 hours, decidualization was apparent and PR was highly expressed in the decidual cells but not the LE (Fig. 1S, 1T), indicating similar temporal expression pattern of PR in the LE between hamsters and mice (Fig. 1).
PR expression in the LE of pseudopregnant mice
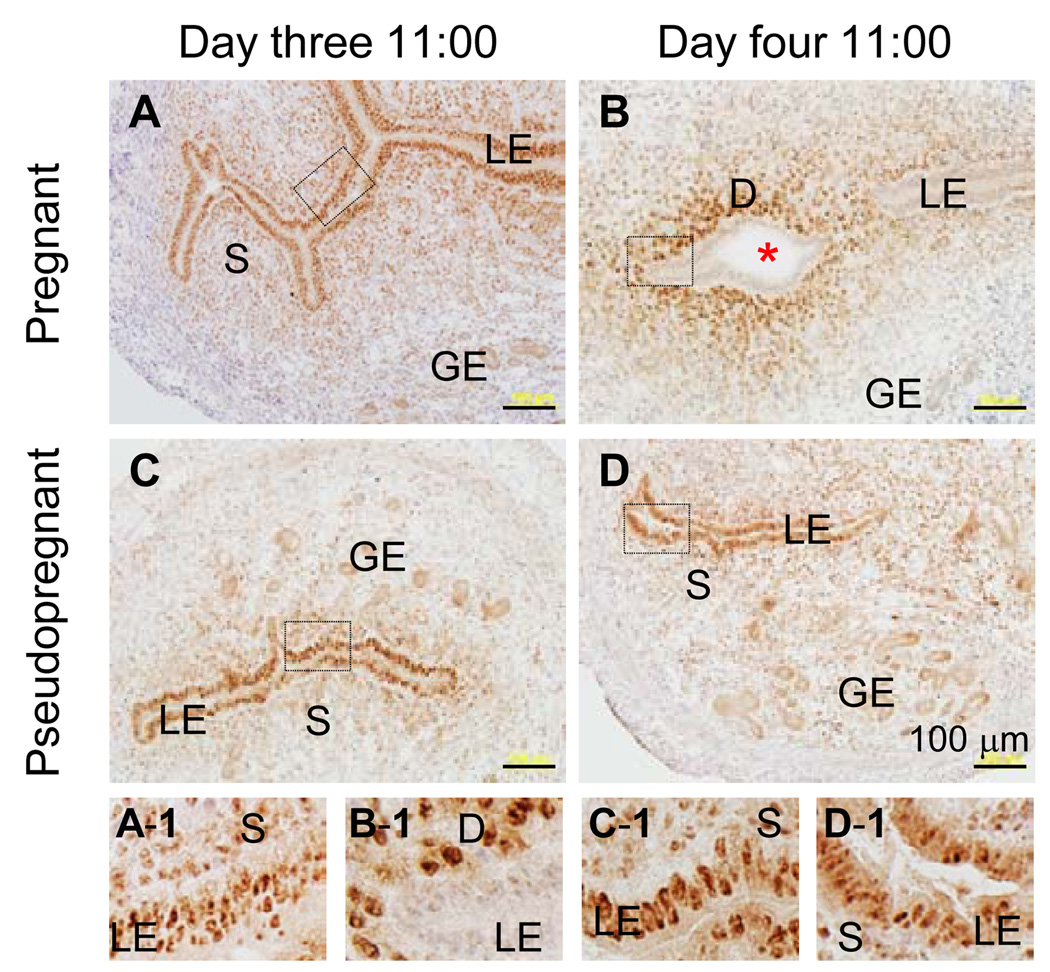
To differentiate hormonal factor(s) from local factor(s) in regulating PR expression in the LE, PR expression in the LE was compared between pregnant and pseudopregnant mouse uterus during peri-implantation. In pregnant uterus, PR was highly expressed in the LE on day three 11:00 hours (Fig. 3A, 3A1) and undetectable in the LE on day four 11:00 hours, but was highly expressed in the primary decidual zone (Fig. 3B, 3B-1). PR was highly expressed in the LE of pseudopregnant uterus on both day three 11:00 hours and day four 11:00 hours although the immunostaining in the former seemed stronger than in the latter (Fig. 3C, 3C-1, 3D, 3D-1). PR had disappeared from the GE on day four 11:00 hours in pregnant uterus (Fig. 3B) but not pseudopregnant uterus (Fig. 3D). These results indicate that local factor(s) produced in the implantation process is required to downregulate PR expression in the LE.
Figure 3.
Immnuohistochemistry of progesterone receptor in the normal pregnant and pseudopregnant mouse uterus on day three 11:00 hours and day four 11:00 hours. An enlarged view of each of the boxed area in A–D is shown on the smaller panels A-1 to D-1, respectively. A & A-1. NP, day three 11:00 hours. B & B-1. NP, day four 11:00 hours. C & C-1. PP, day three 11:00 hours. D & D-1. PP, day four 11:00 hours. LE, luminal epithelium; S, stroma; GE, glandular epithelium; D, decidual zone; red asterisk, embryo; scale bar, 100 µm. N=3. No specific staining was detected in the negative control (data not shown).
PR expression in mouse uterus with artificial decidualization
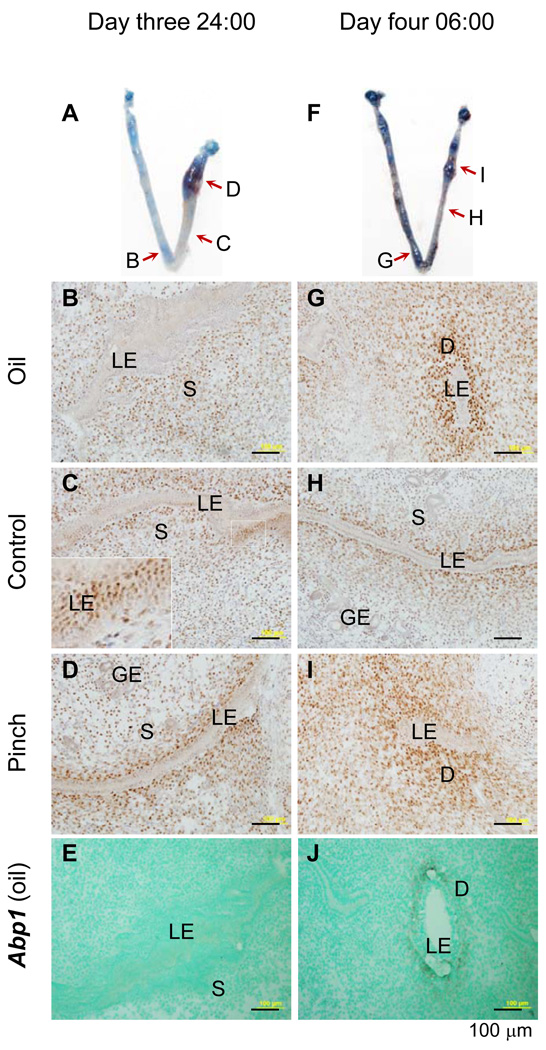
Implantation involves an embryo and a uterus (4, 10). Artificial decidualization was performed to differentiate embryonic from uterine factor(s) in regulating PR expression in the LE. Blue dye reaction was detectable on day three 24:00 hours and day four 06:00 hours (Fig. 4A, 4F). PR had already disappeared from the LE on day three 24:00 hours on sites with blue dye reaction by oil injection (Fig. 4B) or by a pinch (Fig. 4D), which didn’t introduce any exogenous substance, but it remained in the LE of some areas without blue dye reaction (Fig. 4C). PR had disappeared from the entire LE layer on day four 06:00 hours (Fig. 4G~I) when decidualization was apparent in the treated areas (Fig. 4G, 4I). Decidualization was confirmed by Abp1 expression (Fig. 4E, 4J). These data demonstrate that the downregulation of PR in the LE requires uterine but not embryonic factor(s) produced from the initial implantation process.
Figure 4.
Immnuostaining of progesterone receptor (PR) and in situ hybridization (ISH) detection of decidualization marker amiloride-binding protein 1 (Abp1) in mouse uterus with artificial decidualization. Longitudinal frozen sections (10 µm) crossing luminal epithelium were used. A. A representative uterine image on day three 24:00 hours: left uterine horn with oil injection, right uterine horn with two pinches near the uterotubal junction, red arrows indicating the areas sectioned for PR immunohistochemistry (IHC) in B~D. B. PR IHC, day three 24:00 hours, oil-injected uterine horn. C. PR IHC, day three 24:00 hours, control uterine segment without stimulation; an enlarged view of the white boxed area shown in the insert on left bottom corner; the seemingly multiple luminal epithelial (LE) layers indicating a particular sectioning angle that cut through several LE cells on the single LE layer. D. PR IHC, day three 24:00 hours, a pinched area. E. Abp1, ISH, antisense probe, day three 24:00 hours, the same oil injected area as in B. Abp1 is undetectable at this hour. F. A representative uterine image on day four 06:00 hours: left uterine horn with oil injection, right uterine horn with two pinches near the uterotubal junction, red arrows indicating the areas sectioned for PR IHC in G~I. G. PR IHC, day four 06:00 hours, oil-injected uterine horn. H. PR IHC, day four 06:00 hours, control uterine segment without stimulation. I. PR IHC, day four 6:00 hours, a pinched area. J. Abp1, ISH, antisense probe, day four 06:00 hours, the same oil injected area as in G. Abp1 is detectable in the subepithelial stromal area at this hour. LE, luminal epithelium; S, stroma; GE, glandular epithelium; D, decidual zone; scale bar, 100 µm. N=3. No specific staining was detected in the negative control for PR IHC or using a sense probe for Abp1 ISH (data not shown).
Discussion
Embryo implantation is associated with dynamic changes in the endometrium. For example, during the early hours of embryo attachment, which can be detected by blue dye reaction (16), sequential changes were reported to take place in the mouse endometrium: blue dye reaction, local edema of the uterine stroma, and histological decidualization (22). These sequential changes were also observed in this study (Fig. 1).
PR mediates the effects of P that is critical for embryo implantation in all mammals, yet PR expression in the uterine epithelium during the expected “implantation window” is negatively associated with the establishment of uterine receptivity (3, 5, 23–26). PR is critical for embryo implantation (5) yet the loss of PR expression in the uterine epithelium is considered as a prerequisite for implantation (3). The precise temporal disappearing of PR in the uterine epithelium has not been reported, not to mention its correlation with the multi-step implantation process. The results from this study fill in the knowledge gap mentioned above.
This study demonstrates that PR disappears from the GE when the blue dye reaction becomes detectable, but it disappears from the LE a few hours after the blue dye reaction. The expression of PR sustains in the LE when local edema is present at the implantation site. It initially disappears from the LE at the implantation site when decidualization is not obvious and it is undetectable in the entire LE layer when histological decidualization becomes apparent (Fig. 1). These results demonstrate that PR disappears from the LE not before implantation but in the middle of implantation process.
The continued expression of PR in the LE during the initial implantation process suggests that PR expression in the LE may play an active role in the implantation initiation, such as embryo attachment and decidualization. PR knockout mice have implantation failure (7). Since PR is expressed in all major uterine compartments (1), potential roles of PR in the uterine epithelium cannot be differentiated from other uterine compartments in PR knockout mice. Fortunately, the implantation defects in PR knockout mice are recapitulated in the conditional knockout of indian hedgehog (Ihh). IHH is a P-PR target gene mainly expressed in the uterine epithelium (27, 28) and is an essential mediator of PR action in the uterus (29). Conditional knockout of Ihh in the uterine epithelium leads to embryo attachment failure and decidualization failure (29), demonstrating the critical role of P-PR-IHH signaling in the uterine epithelium in the initial implantation process. Since LE is the cell layer that an embryo initially attaches to during implantation and PR disappears from the LE later than PR disappearing from the GE, it is reasonable to speculate that P-PR-IHH signaling in the LE plays a critical role in the embryo attachment and decidualization. The role of PR in downregulating LE apical glycoproteins such as mucin-1, which is upregulated by PR antagonist RU486 and has to be downregulated prior to successful embryo attachment (30), may contribute to its role in embryo attachment.
The temporal expression of PR indicates that PR begins to disappear from the LE before histological decidualization is manifested (Fig. 1). How would PR expression in the LE still be important for decidualization? Decidualization does not occur naturally in the pseudopregnant mice although it can be induced in the pseudopregnant mice by deciduogenic stimuli when the uterus is receptive (31, 32). It suggests the importance of initial changes in the implantation process, such embryo attachment, in inducing decidualization in mice during natural pregnancy. This statement is supported by the observation in the conditional Ihh knockout mice that have both embryo attachment failure and decidualization failure (29). The presence of PR in the LE at the embryo attachment stage suggests its active role in embryo attachment, which is critical for the subsequent decidualization. Therefore, the loss of PR expression from uterine LE should not be considered as a prerequisite for implantation.
How is PR downregulated in the LE during implantation? Tissue recombination demonstrates that stromal ERα mediates E2-induced downregulation of PR in the LE in ovariectomized mice (33). Since PR expression in the LE can be downregualted by artificial decidualization (Fig. 4), it suggests that embryonic factor is not critical for PR downregulation in the LE during implantation. Based on our observations that PR remains expressed in the LE of pseudopregnant mice on day four 11:00 hours (Fig. 3) and artificial decidualization can downregulate PR expression in the LE by day four 06:00 hours (Fig. 4), as well as the established uterine epithelial-stromal cross-talk (34), it is most likely that some uterine factors, which remain to be identified, produced during the initial implantation process are involved in downregualting PR expression in the LE. It is expected that the dynamic changes of PR expression in the LE will lead to the differential expression of genes that are controlled by P-PR signaling in the LE, such as Hdc and Prap1 (Fig. 2).
PR disappears from the LE after embryo attachment in both mice and hamsters (Fig. 1). In human, PR is highly expressed in the uterine epithelium in the proliferative phase and early secretory phase but decreases in the mid and late secretory phase (35), this reduction may be related to the decidualization process in human uterus regardless of the pregnancy status. The precise temporal expression of PR in the LE during human implantation process has not been reported. In sheep the pregnancy is noninvasive, yet PR also disappears from the LE during early pregnancy (36). The molecular mechanism and significance of the downregulation of PR in the LE during early pregnancy await further investigation.
Acknowledgements
The authors thank Dr. Zhen Fu at the College of Veterinary Medicine, University of Georgia for the access to the imaging system, and Ms. Heidi Y. Nguyen at Vanderbilt University Medical Center for technical support. This work was supported by funding from the Office of the Vice President for Research, Department of Physiology & Pharmacology, and the Interdisciplinary Toxicology Program at the University of Georgia; and grants from the National Institute of Health (R15HD066301 to X.Y., R01HD044741 to B.C.P.).
Funding: Office of the Vice President for Research at University of Georgia and National Institute of Health R15HD066301 (to X.Y.) and R01HD044741 (to B.C.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors, H.D., B.C.P., S.X., and X.Y., have nothing to disclose.
References
- 1.Tan J, Paria BC, Dey SK, Das SK. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology. 1999;140:5310–5321. doi: 10.1210/endo.140.11.7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheon YP, Li Q, Xu X, DeMayo FJ, Bagchi IC, Bagchi MK. A genomic approach to identify novel progesterone receptor regulated pathways in the uterus during implantation. Mol Endocrinol. 2002;16:2853–2871. doi: 10.1210/me.2002-0270. [DOI] [PubMed] [Google Scholar]
- 3.Bazer FW, Slayden OD. Progesterone-induced gene expression in uterine epithelia: a myth perpetuated by conventional wisdom. Biol Reprod. 2008;79:1008–1009. doi: 10.1095/biolreprod.108.072702. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- 5.Mulac-Jericevic B, Conneely OM. Reproductive tissue selective actions of progesterone receptors. Reproduction. 2004;128:139–146. doi: 10.1530/rep.1.00189. [DOI] [PubMed] [Google Scholar]
- 6.Schneider W, Ramachandran C, Satyaswaroop PG, Shyamala G. Murine progesterone receptor exists predominantly as the 83-kilodalton 'A' form. J Steroid Biochem Mol Biol. 1991;38:285–291. doi: 10.1016/0960-0760(91)90099-q. [DOI] [PubMed] [Google Scholar]
- 7.Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 8.Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- 9.Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A. 2003;100:9744–9749. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paria BC, Reese J, Das SK, Dey SK. Deciphering the cross-talk of implantation: advances and challenges. Science. 2002;296:2185–2188. doi: 10.1126/science.1071601. [DOI] [PubMed] [Google Scholar]
- 11.Ye X, Hama K, Contos JJ, Anliker B, Inoue A, Skinner MK, et al. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 2005;435:104–108. doi: 10.1038/nature03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mote PA, Arnett-Mansfield RL, Gava N, deFazio A, Mulac-Jericevic B, Conneely OM, et al. Overlapping and distinct expression of progesterone receptors A and B in mouse uterus and mammary gland during the estrous cycle. Endocrinology. 2006;147:5503–5512. doi: 10.1210/en.2006-0040. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Su Y, Deb K, Raposo M, Morrow JD, Reese J, et al. Prostaglandin E2 is a product of induced prostaglandin-endoperoxide synthase 2 (Ptgs2) and microsomal-type prostaglandin E synthase (mPtges) at the implantation site of the hamster. J Biol Chem. 2004 doi: 10.1074/jbc.M400573200. [DOI] [PubMed] [Google Scholar]
- 14.Diao H, Xiao S, Zhao F, Ye X. Uterine luminal epithelium-specific proline-rich acidic protein 1 (PRAP1) as a marker for successful embryo implantation. Fertil Steril. 2010;94:2808–2811. doi: 10.1016/j.fertnstert.2010.06.034. e1. [DOI] [PubMed] [Google Scholar]
- 15.Diao H, Xiao S, Cui J, Chun J, Xu Y, Ye X. Progesterone receptor-mediated up-regulation of transthyretin in preimplantation mouse uterus. Fertil Steril. 2010;93:2750–2753. doi: 10.1016/j.fertnstert.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Psychoyos A. [New contribution to the study of the nidation of the egg in the rat.] C R Hebd Seances Acad Sci. 1960;25:3073–3075. [PubMed] [Google Scholar]
- 17.Paria BC, Das N, Das SK, Zhao X, Dileepan KN, Dey SK. Histidine decarboxylase gene in the mouse uterus is regulated by progesterone and correlates with uterine differentiation for blastocyst implantation. Endocrinology. 1998;139:3958–3966. doi: 10.1210/endo.139.9.6173. [DOI] [PubMed] [Google Scholar]
- 18.Liang XH, Zhao ZA, Deng WB, Tian Z, Lei W, Xu X, et al. Estrogen regulates amiloride-binding protein 1 through CCAAT/enhancer-binding protein-beta in mouse uterus during embryo implantation and decidualization. Endocrinology. 2010;151:5007–5016. doi: 10.1210/en.2010-0170. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q, Paria BC. Importance of uterine cell death, renewal, and their hormonal regulation in hamsters that show progesterone-dependent implantation. Endocrinology. 2006;147:2215–2227. doi: 10.1210/en.2005-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenwald GS. Endocrinology of the pregnant hamster. In: Siegel HI, editor. The Hamster. New York: Plenum Press; 1985. pp. 53–72. [Google Scholar]
- 21.Reese J, Wang H, Ding T, Paria BC. The hamster as a model for embryo implantation: insights into a multifaceted process. Semin Cell Dev Biol. 2008;19:194–203. doi: 10.1016/j.semcdb.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finn CA, McLaren A. A study of the early stages of implantation in mice. J Reprod Fertil. 1967;13:259–267. doi: 10.1530/jrf.0.0130259. [DOI] [PubMed] [Google Scholar]
- 23.Palomino WA, Fuentes A, Gonzalez RR, Gabler F, Boric MA, Vega M, et al. Differential expression of endometrial integrins and progesterone receptor during the window of implantation in normo-ovulatory women treated with clomiphene citrate. Fertil Steril. 2005;83:587–593. doi: 10.1016/j.fertnstert.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KS., Jr Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab. 1988;67:334–340. doi: 10.1210/jcem-67-2-334. [DOI] [PubMed] [Google Scholar]
- 25.Wakitani S, Hondo E, Phichitraslip T, Stewart CL, Kiso Y. Upregulation of Indian hedgehog gene in the uterine epithelium by leukemia inhibitory factor during mouse implantation. J Reprod Dev. 2008;54:113–116. doi: 10.1262/jrd.19120. [DOI] [PubMed] [Google Scholar]
- 26.Bazer FW, Wu G, Spencer TE, Johnson GA, Burghardt RC, Bayless K. Novel pathways for implantation and establishment and maintenance of pregnancy in mammals. Mol Hum Reprod. 2010;16:135–152. doi: 10.1093/molehr/gap095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takamoto N, Zhao B, Tsai SY, DeMayo FJ. Identification of Indian hedgehog as a progesterone-responsive gene in the murine uterus. Mol Endocrinol. 2002;16:2338–2348. doi: 10.1210/me.2001-0154. [DOI] [PubMed] [Google Scholar]
- 28.Jeong JW, Lee KY, Kwak I, White LD, Hilsenbeck SG, Lydon JP, et al. Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Endocrinology. 2005;146:3490–3505. doi: 10.1210/en.2005-0016. [DOI] [PubMed] [Google Scholar]
- 29.Lee K, Jeong J, Kwak I, Yu CT, Lanske B, Soegiarto DW, et al. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet. 2006;38:1204–1209. doi: 10.1038/ng1874. [DOI] [PubMed] [Google Scholar]
- 30.Carson DD, DeSouza MM, Kardon R, Zhou X, Lagow E, Julian J. Mucin expression and function in the female reproductive tract. Hum Reprod Update. 1998;4:459–464. doi: 10.1093/humupd/4.5.459. [DOI] [PubMed] [Google Scholar]
- 31.Loeb L. The production of deciduomata and the relation between the ovaries and the formation of the decidua. J A M A. 1908;50:1897–1901. [Google Scholar]
- 32.De Feo VJ. Decidualization. In: Wynn RM, editor. Cellular Biology of the Uterus. New York, United States: Appleton-Century-Crofts, Inc; 1967. pp. 191–290. [Google Scholar]
- 33.Kurita T, Lee KJ, Cooke PS, Taylor JA, Lubahn DB, Cunha GR. Paracrine regulation of epithelial progesterone receptor by estradiol in the mouse female reproductive tract. Biol Reprod. 2000;62:821–830. doi: 10.1093/biolreprod/62.4.821. [DOI] [PubMed] [Google Scholar]
- 34.Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, et al. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007;3:e102. doi: 10.1371/journal.pgen.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Press MF, Udove JA, Greene GL. Progesterone receptor distribution in the human endometrium. Analysis using monoclonal antibodies to the human progesterone receptor. Am J Pathol. 1988;131:112–124. [PMC free article] [PubMed] [Google Scholar]
- 36.Spencer TE, Bazer FW. Temporal and spatial alterations in uterine estrogen receptor and progesterone receptor gene expression during the estrous cycle and early pregnancy in the ewe. Biol Reprod. 1995;53:1527–1543. doi: 10.1095/biolreprod53.6.1527. [DOI] [PubMed] [Google Scholar]