Abstract
As success of reduced intensity conditioning (RIC) hematopoietic stem cell transplantation (HSCT) relies primarily on graft-versus-leukemia (GVL) activity, increased minor HLA disparity in unrelated compared to related donors could have a significant impact on transplant outcomes. To assess whether use of unrelated donors (URD) engenders more potent GVL in RIC HSCT compared to matched related donors (MRD), we retrospectively studied 433 consecutive T-replete 6/6 HLA matched URD (n= 246) and MRD (n=187) RIC HSCT for hematologic malignancies at our institution. Diseases included: AML(127), NHL(71), CLL (68), MDS (64), HD(40), CML (25), MM(23), MPD (12), ALL(7), other leukemia (1). All received uniform fludarabine and intravenous busulfan conditioning, and GVHD prophylaxis with tacrolimus/mini-MTX or tacrolimus/sirolimus +/− mini-MTX. Unrelated donors were younger compared to MRD (median age: 33 yrs vs. 52 yrs, p<0.0001), and provided larger CD34+ products (median CD34+ cells infused: 8.7 × 106/kg vs. 7.5 × 106/kg, p=0.002). Distribution of diseases, disease risk, prior transplant, and CMV status was similar in both cohorts. Cumulative incidence of grade II–IV acute GVHD (at day+180), 2 year-chronic GVHD, and 2-year non-relapse mortality (NRM) were 20% vs. 16%, 55% vs. 50%, and 8% vs. 6% in URD and MRD, respectively (p=NS). Cumulative incidence of relapse at 2 years was lower in URD, 52% vs. 65% (p=0.005). With median follow-up of 26.5 and 35.8 months, 2-yr progression free survival (PFS) was significantly better in unrelated donor transplants, 39.5% for URD and 29% for MRD (p= 0.01). Overall survival at 2 years were 56% for URD vs. 50% for MRD (p=0.53). In multivariable analysis, URD was associated with a lower risk of relapse (HR 0.67, p =0.002) and superior PFS (HR 0.69, p=0.002). These results suggest that URD is associated with greater GVL activity than MRD, and could have practice changing impact on future donor selection in RIC HSCT.
INTRODUCTION
Reduced intensity conditioning (RIC) regimens are increasingly used to facilitate hematopoietic stem cell transplantation (HSCT) in patients with advanced age or medical co-morbidity, primarily because RIC HSCT is well tolerated and associated with less toxicity. [1–3] Unlike myeloablative HSCT where dose intensity intrinsically reduces tumor burden, RIC HSCT depends largely on the graft-versus-leukemia (GVL) effect. As such, the extent of minor HLA disparity in unrelated compared to related donors could have a significant impact on transplant outcomes.
A number of studies in the myeloablative setting have shown that MRD is superior to URD, mainly because the latter is associated with greater GVHD and transplant related mortality (TRM). [4–8] With more refined HLA typing and improved supportive care, TRM after allogeneic HSCT has declined over the past 2 decades[9], and differences in outcomes between URD and MRD HSCT have become less apparent.[10–14]
The relative benefits and risks of MRD vs. URD in RIC HSCT remain to be elucidated. In RIC HSCT, early TRM is reduced, and the importance of GVL in preventing relapse is magnified. Recent reports have shown that URD RIC HSCT can be associated with equivalent,[15–17] or possibly superior disease control after RIC HSCT for conditions such as advanced AML, CLL and mantle cell lymphoma.[12, 18, 19] Moreover, there may be an advantage in the ability to use younger donors and to avoid female donors in male recipients.
To further address this question, we performed a retrospective cohort analysis comparing patients who underwent RIC HSCT from MRD and URD at our institution.
PATIENTS AND METHODS
Patients
All patients undergoing RIC HSCT with fludarabine/busulfan conditioning from HLA matched URD or MRD at the Dana-Farber/Brigham and Women’s Cancer Center between August 2002 and December 2008, and who received tacrolimus based GVHD prophylaxis without T cell depletion were included. All donor-recipient pairs were at minimum HLA allele matched at A, B, and DRB1 (6/6 match). High risk disease was defined as acute leukemia or CML beyond CR1/CP1, MDS other than de-novo RA/RARS, and CLL/lymphoma/myeloma without CR/PR at the time of transplant. All patients provided consent for use of protected health data for research as approved by Institutional Review Board of the Dana-Farber/Harvard Cancer Center.
Conditioning regimen and supportive care
All patients received fludarabine (30 mg/m2/d IV × 4) and intravenous busulfan (0.8 mg/kg q12h or q24h × 4 days). The twice daily dosing busulfan regimen was restricted to a few protocols in patients with advanced CLL and MDS/AML. A majority (97%) received G-CSF mobilized PBSC. During the first year, all patients received acyclovir as HSV/VZV prophylaxis, and sulfamethoxazole/trimethoprim or atovaquone as prophylaxis against Pneumocystis jirovecii. Patients were monitored for CMV reactivation during the first 100 days after transplantation, and pre-emptive therapy with valganciclovir was given if CMV reactivation occurred.
GVHD prophylaxis
GVHD prophylaxis consisted of either tacrolimus + mini-methotrexate (days +1,3,6,11), or tacrolimus and sirolimus ± mini-methotrexate (day +1,3,6) as previously described.[20, 21] Taper of immune suppression was initiated 2–4 months post transplant, with the goal to be off by approximately 6 months in the absence of GVHD. No pre-emptive or planned prophylactic donor lymphocyte infusions were given. Acute GVHD was graded by the consensus grading criteria.[22]
Chimerism Analysis
Total donor chimerism was assessed from unfractionated bone marrow aspirates and/or blood at approximately day +30 and +100. Genotyping was determined by short tandem repeat typing using the ABI Profiler Plus Kit (Applied Biosystems Inc.) and ABI 310 Genetic Analyzer. “Informative” alleles specific to donor or recipient were used for chimerism determination.
Statistical Methods
Descriptive statistics was used to summarize patient characteristics. The Wilcoxon rank-sum test, Chi-square test, or Fisher’s exact test was used for two sample comparisons. All tests were two-sided. Cumulative incidence curves for relapse and non-relapse mortality were constructed reflecting time to relapse and time to non-relapse death as competing risks. The difference between cumulative incidence curves in the presence of a competing risk was tested using the Gray method. [23] For patients who received more than one RIC HSCT, only the first transplant was considered in the analysis, except in the analysis of overall survival, which was measured from the time transplant to death from any cause. Overall survival (OS) and progression-free survival (PFS) were calculated using the Kaplan-Meier method. The Log-rank test was used for comparisons of Kaplan-Meier curves. Prognostic factors for OS and PFS were examined in Cox proportional hazard models. Prognostic factors for relapse and non-relapse death were examined in competing risks regression model.[24] Proportional hazards assumption was examined in each model. Prior to modeling, a variable clustering analysis on all covariates was performed using a hierarchical clustering with Hoeffding’s D statistic and squared Pearson correlation in R (v2.10.1) and VARCLUS in SAS 9.2 to assess collinearity and/or redundancy of covariates. Of covariates, donor age and donor type were found to be highly correlated. Thus, donor age was not included in the final model for all patients. Instead, donor age was examined separately in each donor type (URD, MRD).
RESULTS
Patient Characteristics
Characteristics of the 433 patients are shown on Table 1. The median patient age were 57 (range 18–73) and 56 years (range 19–71) for the URD and MRD groups, respectively. The two cohorts were balanced in gender, disease, disease risk, prior transplantation, CMV serology status, and stem cell source. The distribution of MRD and URD RIC SCT performed each year from 2002 through 2008 were also well balanced. As expected, donors for MRD were significantly older: median related donor age was 52 years (range, 12–73), compared to 33 years (range 18–60) for URD, p < 0.0001. Median number of CD34+ stem cells infused was higher in the URD cohort, 8.8 × 106 CD34+ cells/kg (range, 0.26–48) vs. 7.6 × 106 CD34+ cells/kg (range, 0.69–38), p=0.002. There were more female donor/male recipient gender mismatches in the MRD cohort (33% vs. 22%, p= 0.002). Among URD, 26(11%) had a single mismatch at HLA-C, compared to 2 (1%) in the MRD cohort, p <0.0001.
Table 1.
Patient Characteristics
| URD | MRD | p-value | |||
|---|---|---|---|---|---|
| N = 246 | % | N= 187 | % | ||
| Patient Age | 0.91 | ||||
| ≥ 50 | 181 | 74 | 136 | 73 | |
| < 50 | 65 | 26 | 51 | 27 | |
| median (range) | 57 (18, 73) | 56 (19, 71) | 0.12 | ||
| Donor Age, median (range) | 33 (18, 60) | 52 (12, 73) | <0.0001 | ||
| Sex | 0.42 | ||||
| M | 161 | 65 | 115 | 62 | |
| F | 85 | 35 | 72 | 39 | |
| Donor-Recipient Gender | 0.002 | ||||
| Female/Male | 53 | 22 | 62 | 33 | |
| MM | 108 | 44 | 53 | 28 | |
| FF | 37 | 15 | 39 | 21 | |
| MF | 48 | 20 | 33 | 18 | |
| Prior Myeloablative Transplant | 79 | 32 | 63 | 34 | 0.76 |
| Autologous | 73 | 30 | 50 | 27 | |
| Allogeneic | 5 | 2 | 12 | 6 | |
| Syngeneic | 1 | <1.0 | 1 | <1.0 | |
| Diagnosis | - | ||||
| ALL | 6 | 2 | 1 | < 1.0 | |
| AML | 68 | 28 | 59 | 32 | |
| CLL, SLL, PLL | 42 | 17 | 26 | 14 | |
| CML | 12 | 5 | 8 | 4 | |
| Hodgkin’s Disease | 27 | 11 | 13 | 7 | |
| MDS | 39 | 16 | 25 | 13 | |
| Multiple myeloma | 6 | 2 | 17 | 9 | |
| MPD | 7 | 3 | 5 | 3 | |
| NHL | 39 | 16 | 32 | 17 | |
| Mast Cell Leukemia | - | - | 1 | < 1.0 | |
| Risk Status | 0.12 | ||||
| High Risk* | 144 | 59 | 95 | 51 | |
| Low Risk | 102 | 41 | 92 | 49 | |
| Stem Cell Source | - | ||||
| Bone Marrow | 11 | 4 | 1 | <1.0 | |
| PBSC | 235 | 96 | 185 | 99 | |
| BM and PBSC | - | - | 1 | < 1.0 | |
| Conditioning Regimen | 0.2 | ||||
| Flu-BU1 (total 3.2mg/kg BU) | 200 | 81 | 161 | 86 | |
| Flu-BU2 (total 6.4mg/kg BU) | 46 | 19 | 26 | 14 | |
| GVHD Prophylaxis | 0.91 | ||||
| Tacrolimus/Sirolimus +/− mMTX | 192 | 78 | 145 | 78 | |
| Tacrolimus/mMTX | 54 | 22 | 42 | 22 | |
| HLA Matching | <0.0001 | ||||
| 8/8 HLA Match | 220 | 89 | 185 | 99 | |
| Single C Mismatch (7/8 match) | 26 | 11 | 2 | 1 | |
| D/R CMV Serology Status | 0.86 | ||||
| Negative/Negative | 79 | 32 | 64 | 34 | |
| Any Positive | 165 | 67 | 121 | 65 | |
| Missing | 2 | 0.8 | 2 | 1.1 | |
| Year of Transplant | 0.17 | ||||
| 2002 | 14 | 6 | 11 | 6 | |
| 2003 | 37 | 15 | 32 | 17 | |
| 2004 | 39 | 16 | 34 | 18 | |
| 2005 | 35 | 14 | 26 | 14 | |
| 2006 | 31 | 13 | 37 | 20 | |
| 2007 | 42 | 17 | 25 | 13 | |
| 2008 | 48 | 20 | 22 | 12 | |
| Median CD34+ cells (×106/kg) infused | 8.68 | 7.46 | 0.002 | ||
| Median Follow-up for survivors (mos) | 26.5 (6–80) | 35.8 (6.7–74) | 0.25 | ||
High risk defined as: Acute leukemia or CML beyond CR1/CP1, MDS other than de novo RA/RARS, or CLL, lymphoma, myeloma not in CR/PR at the time of transplant.
The preparative regimen was fludarabine and single daily dosing intravenous busulfan in 81% of the URD and 86% of the MRD patients (p= 0.20). Distribution of GVHD prophylaxis regimens and year of transplantation were similar. Median follow-up time among survivors was 26.5 months (range 6.0– 80.4 months) for URD and 35.8 months (range, 6.7–74.3 months) for MRD (p=0.25).
Engraftment
Approximately half of the patients (47% URD vs. 49% MRD) never developed absolute neutropenia, and 44% and 48%, respectively, did not have platelets less than < 20,000 cells/μL. Among patients whose neutrophil or platelet counts nadired, median neutrophil recovery time was 13 days for both cohorts (p=0.20), and time to platelet recovery was 20 days for URD, 21 days for MRD (p=0.79). Donor chimerism results in the first 4 months after transplantation were also similar. At approximately day +30, 67% and 61% of URD and MRD recipients, respectively, achieved ≥ 90% donor chimerism (p=0.24). At approximately day +100, 65% of the URD recipients retained > 90% donor chimerism, compared to 57% in the MRD (p= 0.17). Incidence of graft failure, defined as complete loss of donor chimerism in the absence of disease relapse, or decline in donor chimerism <50% in the absence of disease relapse that necessitated donor lymphocyte infusion or second HSCT, were 1.6% and 2.1% for URD and MRD, respectively (p=0.73). Two patients developed a post transplant B-cell lymphoproliferative disorder at 3 and 6 months after RIC HCT, both were URD and both cases were EBV negative by pathologic stains.
Graft-versus-Host Disease
As shown on Figure 1A, the cumulative incidence of grade II-IV acute GVHD at day +180 was 20% in URD, vs. 14% in MRD (p=0.18). The 180-day incidence of grade III–IV acute GVHD was 8% for URD and 5% and MRD (p=0.27). The cumulative incidence of chronic GVHD at 2 years was 55% among URD, and 51% among MRD (p=0.13), Figure 1B. The 2-year cumulative incidence of extensive chronic GVHD was 45% in URD and 36% in MRD (p = 0.03).
Figure 1. Cumulative Incidence of GVHD, with Death as a Competing Risk.
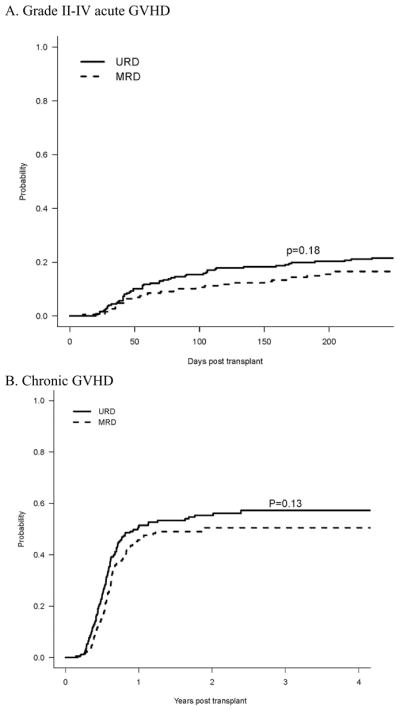
A. Grade II–IV Acute GVHD; B. Chronic GVHD
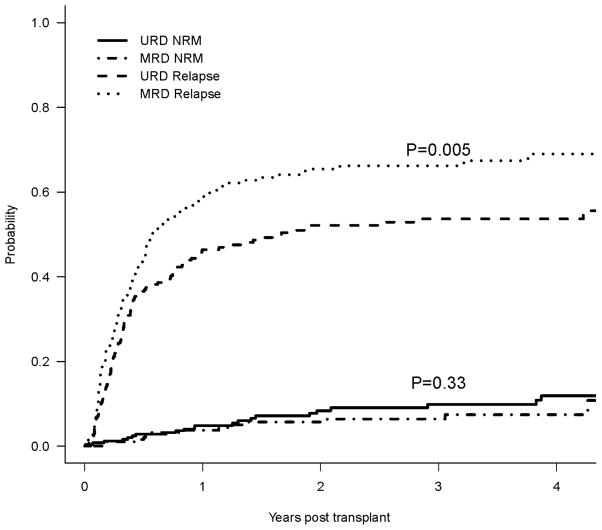
Disease Relapse and Non-relapse Mortality
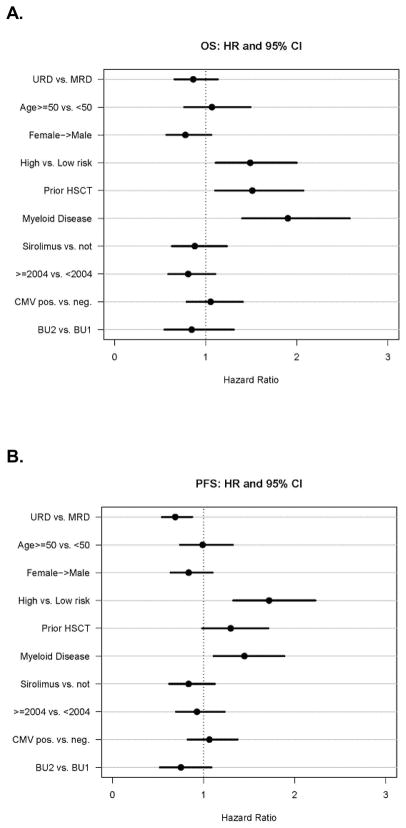
As shown on Table 2a and Figure 2, the 2-year cumulative incidence of relapse was 52% for URD vs. 65% for MRD (p=0.005). In a multivariable competing risks regression model, the hazard ratio for relapse remained much lower in patients with unrelated donors (HR 0.67; 95%CI 0.52–0.86; p= 0.002) (Table 2b). High-risk disease (HR 1.69, 95%CI 1.28–2.22; p=0.0002), myeloid disease (HR 1.41, 95%CI 1.06–1.86; p=0.02) were independently associated with increased relapse risk. Higher busulfan dose was associated with a lower relapse risk (HR 0.65, 95%CI 0.44–0.97; p=0.03) (Figure 4C).
Table 2a.
Results of univariable analysis for URD vs MRD
| URD | MRD | ||
|---|---|---|---|
| 2-year estimate (95% CI) | 2-year estimate (95% CI) | p-value | |
| Relapse* | 52% (46% – 59%) | 65% (58% – 72%) | 0.005 |
| NRM* | 8.4% (4.6% – 12.2%) | 5.7% (7.2% – 9.2%) | 0.33 |
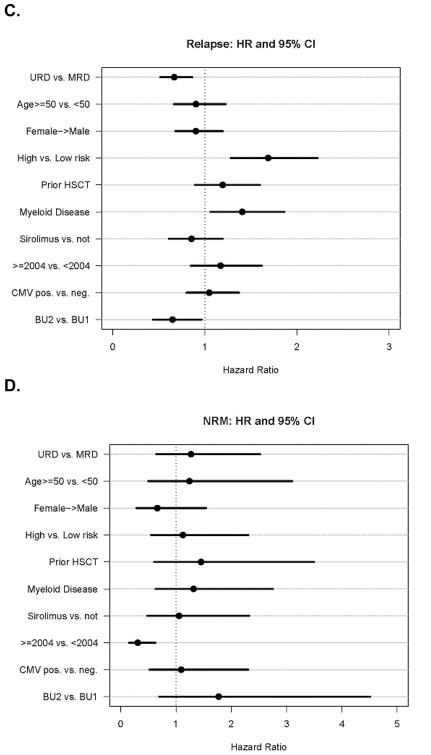
| PFS | 39.5% (33% – 46%) | 29% (22% – 36%) | 0.01 |
| OS | 56% (48% – 62%) | 50% (42% – 57%) | 0.53 |
cumulative incidence
Figure 2.
Cumulative Incidence of Relapse and Non-Relapse Mortality as Competing Risks
Table 2b.
Results of multivariable regression analysis* for URD vs MRD
| HR | 95% CI | p-value | ||
|---|---|---|---|---|
| Relapse | 0.67 | 0.52 | 0.86 | 0.002 |
| NRM | 1.27 | 0.64 | 2.52 | 0.49 |
| PFS | 0.69 | 0.54 | 0.88 | 0.002 |
| OS | 0.86 | 0.66 | 1.13 | 0.29 |
adjusting for age, prior ablative transplant, risk status, donor-recipient gender (F/M vs other), GVHD prophylaxis (Sirolimus vs. other), year of transplant, donor-recipient CMV status (any positive vs. neg/neg), conditioning regimen (FluBu2 vs FluBU1).
Figure 4. Multivariable Regression Modeling showing Hazard Ratios and 95% Confidence Intervals.
A. Overall Survival; B. Progression Free Survival; C. Relapse D. Non-Relapse Mortality
Cumulative incidence of NRM at 2 years was 8% for URD vs. 6% for MRD recipients (p=0.33). There was a significant improvement in NRM for both cohorts correlated with year of transplantation. Two-year NRM incidence for URD and MRD transplants performed prior to 2004 were 16% and 14%, respectively, compared to 6% and 3% after 2004 (p = 0.17 for URD; p=0.03 for MRD).
In multivariable analysis, URD vs. MRD was not associated with a risk of NRM (HR 1.29; 95% CI 0.65–2.57; p = 0.47). Year of transplantation (< 2004 vs. ≥ 2004) was the only significant factor for the risk of NRM (HR 0.31, 95% CI 0.15–0.63, p=0.001) (Figure 4D).
Progression free and overall survival
With median follow-up of over 2 years for survivors in both cohorts, the 2-year PFS was 39.5% (95% CI, 33–46%) for URD, and 29% (95% CI, 22–36%) for MRD, p= 0.01 (Table 2a, Figure 3A). The 2-year estimate of OS was 56% for URD vs. 50% for MRD, p= 0.53. (Table 2a, Figure 3B)
Figure 3. Kaplan-Meier estimate of Survival after RIC HSCT.
A. Progression Free Survival; B. Overall Survival
In a multivariable Cox regression model for PFS (Table 2b, Figure 4B), URD was associated with superior PFS compared with MRD (HR 0.69; 95% CI 0.54–0.88; p=0.002). High risk disease (HR 1.72, 95% CI 1.33–2.23; p<0.0001), and myeloid malignancy (HR 1.45, 95% CI 1.11–1.89; p=0.007) were associated with worse PFS. Prior HSCT was borderline significantly associated with lower PFS (HR 1.30, 95% CI 0.98–1.71; p=0.065). Recipient age, F/M gender mismatch, sirolimus GVHD prophylaxis, CMV seropositivity, year of transplantation, and busulfan dose were not risk factors.
In a multivariable Cox regression model for OS using the same factors, there was no difference between URD and MRD (HR 0.86, 95% CI 0.66–1.13; p=0.29). Prior HSCT (HR 1.51, 95% CI 1.10–2.07; p=0.01), high risk disease (HR 1.49, 95% CI 1.11–2.00; p=0.008), and myeloid malignancy (HR 1.90, 95% CI 1.40–2.58; p<0.0001) were associated with inferior OS (Figure 4A).
Effect of donor type in disease subsets
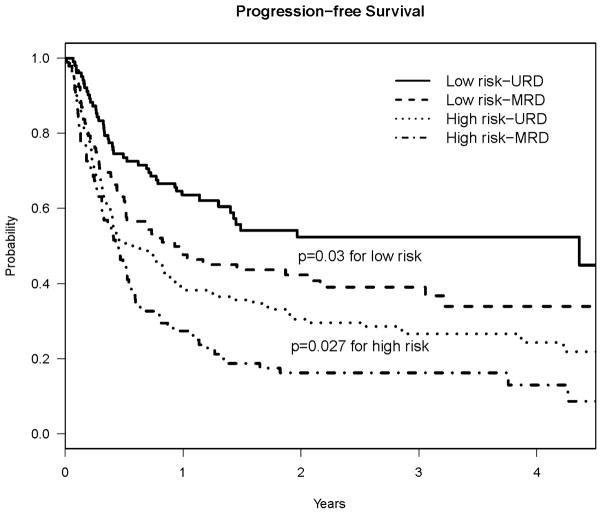
PFS and relapse benefit for URD was observed in both high and low risk diseases (Figure 5). For patients transplanted with high risk disease, 2-year PFS was 30% for URD, vs. 16% for MRD (p = 0.027), and 2-year cumulative incidence of relapse was 62% in URD vs. 75% in MRD (p=0.03). For patients transplanted with low-risk disease, 2 year PFS was 52% for URD, 42% for MRD (p= 0.03). The 2-year cumulative incidence of relapse was 38% in URD and 56% in MRD (p=0.006). In multivariable Cox models stratified by disease risk, URD is associated with improved PFS in both low risk (HR. 0.67, 95% CI 0.452–1.00, p=0.05) and high risk disease cohorts (HR 0.69, 95% CI 0.52–0.95, p= 0.02). There was a trend toward improved PFS for URD in both myeloid and lymphoid malignancies: 2-year PFS 34% vs. 24% in myeloid (p =0.09) and 45% vs. 35% in lymphoid (p= 0.06). There was no statistically significant difference in PFS outcomes for any single disease diagnosis.
Figure 5.
Progression Free Survival of URD and MRD Cohorts, stratified by Disease Risk.
Additional analyses
To assess whether the inclusion of HLA-C mismatches affected the results, we performed a subset analysis on patients who were 8/8 HLA match (n = 405). The 2-year cumulative incidence of disease relapse was 52% in the 8/8 matched URD cohort, and 66% in the MRD (p=0.008). PFS at 2 years was 40% for 8/8 matched URD and 29% for MRD (p=0.01). These results suggest that the imbalance in HLA-C mismatches in the URD cohort did not account for the benefit in relapse and PFS we observed.
To assess the effect of CD34+ dose, we performed additional multivariable modeling including CD34+ dose as a covariate. CD34+ dose was not associated with a difference in PFS or OS (p= 0.10 and 0.56, respectively), and inclusion of CD34+ dose in the model did not change the hazard ratio showing benefit for URD over MRD.
Because donor age and donor type were highly correlated (clustering analysis R-squared correlation =0.81), we assessed the effect of donor age on PFS in the MRD and URD cohorts separately. In a Cox regression model, increasing donor age had no effect on PFS in either the MRD or the URD cohorts. (HR 1.01, p =0.31 for MRD; HR 0.99, p= 0.26 for URD).
To investigate why the improvements in relapse and PFS in the URD cohort did not translate to an OS benefit, we performed additional analyses considering salvage therapies after relapse. Of the 244 patients who relapsed after RIC HSCT, 123/246 (50%) in URD and 121/187 (65%), 63 underwent subsequent “salvage” allogeneic HSCT. There was more patients in the MRD cohort who received salvage HSCT: 31 (17%) in MRD vs. 32 (13%) in URD, p= 0.34. In fact, 12/31 (39%) patients who relapsed after MRD actually received ≥ 2 salvage allo-HSCT, compared to 7/32 (22%) in the URD group (p=0.13). There were also more patients who received DLI for relapse after transplant in the MRD vs. URD: 20% vs. 10% (p=0.006).
To assess whether subsequent HSCT might have obscured our ability to observe a OS benefit, we performed a subset analysis excluding pts (n=63) who received salvage HSCT. When salvage HSCT patients were excluded, the 2-year OS advantage for URD over MRD increased from 6% (56% vs. 50%) to 10% (57% vs. 47%). Although this 10% overall survival difference did not reach statistical significance (p=0.23), perhaps due to reduced sample size, this trend is consistent with the improvement in PFS. In a multivariable Cox model, there was also a trend toward improved overall survival in favor of URD, (HR=0.77 p=0.08). These results suggest that salvage HSCT after relapses may have, at least in part, negated differences in OS.
DISCUSSION
This study represents a large single-institution series of patients receiving a uniform Flu/Bu RIC HSCT. We observed a significant improvement in disease relapse and PFS for patients transplanted using 6/6 or 8/8 HLA matched URD compared with MRD without increased GVHD, graft failure, or NRM. We did not observe a difference in OS, but this could reflect, in part, the fact that many patients, especially in the MRD group, proceeded to a second or subsequent allogeneic transplantation, and that patients may survive longer despite relapse due to improved supportive and palliative therapies in recent years.
Investigators from Seattle have reported their non-myeloablative HSCT experience including 221 MRD and 184 URD patients after 2Gy TBI +/− fludarabine conditioning.[16] They also did not observe any increase in GVHD or non-relapse mortality in the URD cohort. However, they did not detect any significant difference in relapse based on related or unrelated donor type. The discordance with our findings may reflect differences in the conditioning regimens, GVHD prophylaxis, and distribution of diseases. In the Seattle series, all URD patients received Flu/TBI as conditioning, while among MRD patients, 60% received Flu/TBI, and 40% received low dose TBI alone. More recently, the Seattle group reported on 274 AML patients who received non-myeloablative HSCT with 2 Gy TBI ± fludarabine, along with a calcineurin inhibitor and mycophenolate mofetil.[17] They found equivalent incidence of relapse and survival for recipients of matched related and unrelated donors.
The German Cooperative Transplant Study Group has also reported on the effect of donor type on RIC HSCT outcomes in elderly patients with AML.[12] In a retrospective cohort analysis of 368 patients, Schetelig and colleagues found no association between donor type and AML relapse. However, in subset analysis of patients with high risk cytogenetics and advanced disease, there was an improvement in event-free survival (29% vs. 6%, p=0.04) for URD. In this study, a majority of URD patients also received ATG, compared to a minority in the MRD cohort, which could have confounded relapse results. Another publication by Hegenbart and colleagues reported on the outcomes of low dose TBI based non-myeloablative transplantation for AML across US and European centers.[15] There was again no significant difference in GVHD or TRM, but a trend toward a lower relapse incidence at 2 years for URD compared with MRD (33% vs. 47%, p=0.12). Taken together, these results suggest that in RIC and non-myeloablative HSCT, matched URD are at least comparable to MRD, and that URD might be superior in certain disease subsets.
Aside from minor HLA antigen differences, URD transplantation may be more likely to engender anti-leukemic activity mediated by NK cells on the basis of KIR mismatches, especially in patients with myeloid malignancies. [25] As such, further studies to assess for potential effect of KIR mismatches may be warranted. In our study, we are unable to assess the impact of HLA-C mismatching because the numbers of patients with this mismatch were small (n= 28).
Our analysis is fortified by its large sample size and the fact that both MRD and URD cohorts had extended follow-up, and were well balanced in disease characteristics, disease risk, prior HSCT, and GVHD prophylaxis regimens. Importantly, all patients received similar Flu/Bu conditioning without ATG or ex vivo T cell depletion, thereby eliminating conditioning regimen heterogeneity as a potential confounding factor. These considerations aside, our study is subject to inherent limitations due to its retrospective nature, and there may be uncontrolled factors that could have biased results. For example, one potential confounding factor is the longer time needed to identify unrelated donors compared to a donor in the family. As a result, some patients with aggressive malignancies could relapse before a volunteer donor is found, potentially confounding results in favor of URD. Conversely, there may also be selection bias in favor of MRD because patients with less aggressive malignancies are more likely to be offered transplantation if there is a family donor available.
It is also conceivable that sirolimus, with its potential anti-neoplastic properties, could have affected relapse outcomes after RIC HSCT, at least in certain disease subsets.[26] The fact that both the MRD and URD cohorts were identical with respect to percentage receiving sirolimus GVHD prophylaxis, and that sirolimus was not a significant factor for PFS or relapse in our multivariable models, appear to mitigate this issue. Nonetheless, by minimizing differences in GVHD and TRM between MRD and URD, use of sirolimus could have allowed the reduction in relapse in the URD cohort to result in a more discernable improvement in PFS.[27] It is also possible that the high relapse rates we observed, especially in MRD patients, reflect the fact that by minimizing acute GVHD, sirolimus also blunted the GVL effect. In this context, our findings of benefit for URD over MRD may only be applicable to Flu/Bu RIC with tacrolimus/sirolimus based GVHD prophylaxis, and caution should be exercised when extending these results to HSCT employing other conditioning or GVHD prophylaxis regimens.
An important consideration in the discussion of related versus unrelated donor choice in RIC HSCT is the issue of donor age. Since RIC HSCT is primarily offered to older patients, siblings are likely to be older than volunteer unrelated donors. Previous studies have shown that increased donor age is associated with inferior PBSC mobilization,[28] delayed immune recovery,[29] greater risk of GVHD, and worse survival.[30, 31] Given these considerations, one may question the desirability of older sibling donors when healthier, younger matched unrelated donors are available. In our analysis, we found that the median age of related donors was indeed much older than that of unrelated donors. The CD34 dose infused in the URD cohort was also greater than that in MRD. However, neither variable was independently associated with relapse or PFS in multivariable analyses.
In summary, our results demonstrate that matched URD RIC HSCT with Flu/Bu conditioning and primarily tacrolimus/sirolimus based GVHD prophylaxis is associated with an improvement in disease relapse and PFS for URD, possibly reflecting greater GVL activity, without apparent increase in GVHD or NRM compared to MRD. Further studies are warranted. If verified, these findings could alter current clinical practice regarding selection of donors for RIC transplantation.
Acknowledgments
We would like of acknowledge Kimberly Phillips and all the data specialists and clinical research coordinators for their tireless work in maintaining the DFCI BMT clinical data repository, without which this project would not have been possible.
This study was supported, in part, by the Ted and Eileen Pasquarello Research Fund, NIH grants CA142106, PO1HL070149-02, and the Jock and Bunny Adams Education and Research Fund.
Footnotes
Data in this manuscript were presented, in part, at the Annual Meeting of the European Group for Blood and Marrow Transplant, Vienna 2010.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Slavin S, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91(3):756–763. [PubMed] [Google Scholar]
- 2.Giralt S, et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood. 1997;89:4531–4536. [PubMed] [Google Scholar]
- 3.McSweeney PA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97(11):3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 4.Marks DI, et al. Allogeneic bone marrow transplantation for chronic myeloid leukemia using sibling and volunteer unrelated donors. A comparison of complications in the first 2 years. Annals of Internal Medicine. 1993;119:207–214. doi: 10.7326/0003-4819-119-3-199308010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Beatty PG, et al. Marrow transplantation form unrelated donors for treatment of hematologic malignancies: Effect of mismatching for one HLA locus. Blood. 1993;81:249–253. [PubMed] [Google Scholar]
- 6.Ringden O, et al. The graft-versus-leukemia effect using matched unrelated donors is not superior to HLA-identical siblings for hematopoietic stem cell transplantation. Blood. 2009;113(13):3110–3118. doi: 10.1182/blood-2008-07-163212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eapen M, et al. Comparable long-term survival after unrelated and HLA-matched sibling donor hematopoietic stem cell transplantations for acute leukemia in children younger than 18 months. J Clin Oncol. 2006;24(1):145–151. doi: 10.1200/JCO.2005.02.4612. [DOI] [PubMed] [Google Scholar]
- 8.Weisdorf DJ, et al. Sibling versus unrelated donor allogeneic hematopoietic cell transplantation for chronic myelogenous leukemia: refined HLA matching reveals more graft-versus-host disease but not less relapse. Biol Blood Marrow Transplant. 2009;15(11):1475–1478. doi: 10.1016/j.bbmt.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horan J, et al. Reducing the Risk for Transplant Related Mortality After Allogeneic Hematopoietic Cell Transplantation: How Much Progress Has been Made? Blood. 2009;114(22):269–270. Asbstract 649. [Google Scholar]
- 10.Petersdorf EW, et al. Optimizing outcome after unrelated marrow transplantation by comprehensive matching of HLA class I and II alleles in the donor and recipient. Blood. 1998;92(10):3515–3520. [PubMed] [Google Scholar]
- 11.Ottinger HD, et al. Hematopoietic stem cell transplantation: contrasting the outcome of transplantations from HLA-identical siblings, partially HLA-mismatched related donors, and HLA-matched unrelated donors. Blood. 2003;102(3):1131–1137. doi: 10.1182/blood-2002-09-2866. [DOI] [PubMed] [Google Scholar]
- 12.Schetelig J, et al. Matched unrelated or matched sibling donors result in comparable survival after allogeneic stem-cell transplantation in elderly patients with acute myeloid leukemia: a report from the cooperative German Transplant Study Group. J Clin Oncol. 2008;26(32):5183–5191. doi: 10.1200/JCO.2007.15.5184. [DOI] [PubMed] [Google Scholar]
- 13.Walter RB, et al. Comparison of matched unrelated and matched related donor myeloablative hematopoietic cell transplantation for adults with acute myeloid leukemia in first remission. Leukemia. 2010 doi: 10.1038/leu.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yakoub-Agha I, et al. Allogeneic marrow stem-cell transplantation from human leukocyte antigen-identical siblings versus human leukocyte antigen-allelic-matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol. 2006;24(36):5695–5702. doi: 10.1200/JCO.2006.08.0952. [DOI] [PubMed] [Google Scholar]
- 15.Hegenbart U, et al. Treatment for acute myelogenous leukemia by low-dose, total-body, irradiation-based conditioning and hematopoietic cell transplantation from related and unrelated donors. J Clin Oncol. 2006;24(3):444–453. doi: 10.1200/JCO.2005.03.1765. [DOI] [PubMed] [Google Scholar]
- 16.Mielcarek M, et al. Comparable outcomes after nonmyeloablative hematopoietic cell transplantation with unrelated and related donors. Biol Blood Marrow Transplant. 2007;13(12):1499–1507. doi: 10.1016/j.bbmt.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gyurkocza B, et al. Nonmyeloablative Allogeneic Hematopoietic Cell Transplantation in Patients With Acute Myeloid Leukemia. J Clin Oncol. doi: 10.1200/JCO.2009.27.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorror ML, et al. Hematopoietic cell transplantation after nonmyeloablative conditioning for advanced chronic lymphocytic leukemia. J Clin Oncol. 2005;23(16):3819–3829. doi: 10.1200/JCO.2005.04.569. [DOI] [PubMed] [Google Scholar]
- 19.Maris MB, et al. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood. 2004;104(12):3535–3542. doi: 10.1182/blood-2004-06-2275. [DOI] [PubMed] [Google Scholar]
- 20.Alyea EP, et al. Comparative outcome of nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation for patients older than 50 years of age. Blood. 2005;105(4):1810–1814. doi: 10.1182/blood-2004-05-1947. [DOI] [PubMed] [Google Scholar]
- 21.Ho VT, et al. Comparison of Tacrolimus and Sirolimus (Tac/Sir) versus Tacrolimus, Sirolimus, and mini-methotrexate (Tac/Sir/MTX) as acute graft-versus-host disease prophylaxis after reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2009;15(7):844–850. doi: 10.1016/j.bbmt.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Przepiorka D, et al. Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1994;15(6):825–828. [PubMed] [Google Scholar]
- 23.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 24.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 25.Ruggeri L, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94(1):333–339. [PubMed] [Google Scholar]
- 26.Armand P, et al. Improved survival in lymphoma patients receiving sirolimus for graft-versus-host disease prophylaxis after allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning. J Clin Oncol. 2008;26(35):5767–5774. doi: 10.1200/JCO.2008.17.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cutler C, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109(7):3108–3114. doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richa E, et al. Older age but not donor health impairs allogeneic granulocyte colony-stimulating factor (G-CSF) peripheral blood stem cell mobilization. Biol Blood Marrow Transplant. 2009;15(11):1394–1399. doi: 10.1016/j.bbmt.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Baron F, et al. Unrelated donor status and high donor age independently affect immunologic recovery after nonmyeloablative conditioning. Biol Blood Marrow Transplant. 2006;12(11):1176–1187. doi: 10.1016/j.bbmt.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Kollman C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98(7):2043–2051. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- 31.Carreras E, et al. Donor age and degree of HLA matching have a major impact on the outcome of unrelated donor haematopoietic cell transplantation for chronic myeloid leukaemia. Bone Marrow Transplant. 2006;37(1):33–40. doi: 10.1038/sj.bmt.1705195. [DOI] [PubMed] [Google Scholar]