Abstract
Incompatible blood group antigens are highly immunogenic and can cause graft rejections. Focusing on distinct carbohydrate- and protein-based membrane structures, defined by blood group antigens, we investigated human bone marrow-derived mesenchymal stem cells (MSCs) cultured in human serum. The presence of H (CD173), ABO, RhD, RhCE, RhAG, Kell, urea transporter type B (SLC14A1, previously known as JK), and Duffy antigen receptor of chemokines (DARC) was evaluated at the levels of genome, transcriptome and antigen. Fucosyltransferase-1 (FUT1), RHCE, KEL, SLC14A1 (JK) and DARC mRNA were transcribed in MSCs. FUT1 mRNA transcription was lost during differentiation. The mRNA transcription of SLC14A1 (JK) decreased during chondrogenic differentiation, while that of DARC increased during adipogenic differentiation. All MSCs synthesized SLC14A1 (JK) but no DARC protein. However, none of the protein antigens tested occurred on the surface, indicating a lack of associated protein function in the membrane. As A and B antigens are neither expressed nor adsorbed, concerns of ABO compatibility with human serum supplements during culture are alleviated. The H antigen expression by GD2dim+ MSCs identified two distinct MSC subpopulations and enabled their isolation. We hypothesize that GD2dim+H+ MSCs retain a better “stemness”. Because immunogenic blood group antigens are lacking, they cannot affect MSC engraftment in vivo, which is promising for clinical applications.
Keywords: stem cell transplantation, mesenchymal cells, blood groups, H antigen, CD173
Introduction
The bone marrow (BM) harbours a variety of adult stem cells, among which mesenchymal stem cells (MSCs), also called mesenchymal stromal cells, constitute less than 0.01% of all BM-derived mononuclear cells (Phinney & Prockop, 2007). Clinically useful for immunomodulation, multipotent differentiation and paracrine effector secretion (Dominici et al., 2001; Herzog et al., 2003; Satija et al., 2009; Schaefer et al., 2009; Siegel et al., 2009), MSCs are utilized in immunomodulatory therapies (Le Blanc et al., 2004; Uccelli et al., 2006; Ball et al., 2007; Le Blanc et al., 2008) and regenerative medicine (Horwitz et al., 1999; Horwitz et al., 2001). Characterization of MSC subpopulations and their involvement in graft compatibility and immunogenicity may be instrumental in better defining their therapeutic potential in clinical trials.
Several distinct subpopulations sharing various degrees of MSC-like properties are collectively labelled MSCs. Molecules encoding surface structures like CD271, CD49a, W7C5, W8B2, C15, CDCP1, CD340, CD349 and SSEA1/4 may be used to define such subpopulations, while the neural ganglioside GD2, expressed exclusively on MSCs in the BM, is particularly promising as a unifying marker (Quirici et al., 2002; Giesert et al., 2003; Buhring et al., 2004; Martinez et al., 2007; Phinney & Prockop, 2007).
Membrane structures of blood group antigens, like the Duffy (FY) antigen receptor of chemokines (DARC) (Hadley & Peiper, 1997; Swardson-Olver et al., 2002), the ammonia transporter RhAG (Marini et al., 2000) or the urea transporter type B (SLC14A1, previously termed JK) (Shayakul & Hediger, 2004; Wester et al., 2008), and the RhBG and RhCG glycoproteins (Zidi-Yahiaoui et al., 2005; Han et al., 2006), function as receptors or transporters. Investigating these structures may allow classification of MSCs by morphological or genetic criteria, possibly implying membrane functionality. Because alloantibodies play a pivotal role in organ-allograft rejection (Colvin & Smith, 2005; Terasaki & Cai, 2008; Tobian et al., 2008), immunization induced by MSC antigens may result in the alteration or even rejection of a cellular graft is of particular concern for promising MSC applications in regenerative medicine without immunosuppressive therapy.
Focusing on carbohydrate- and protein-based membrane structures that are defined as blood group antigens, we investigated the characteristics of undifferentiated and differentiated MSCs derived from BM and cultured in medium free of animal serum.
Materials and Methods
Isolation and differentiation of human MSCs
BM was obtained under sterile conditions from patients without metabolic or neoplastic diseases during orthopaedic operations. All patients gave written informed consent. The study was approved by the institutional review board (IRB) of the University Hospital Tübingen (IRB no. 95/2005V).
Detailed isolation and characterization protocols and further Materials and Methods specifications are available online (see Supplementary Material online) (Pittenger et al., 1999; Colter et al., 2001; Flegel et al., 2002; Ji et al., 2004; Kern et al., 2006; Muruganandan et al., 2009).
Characterization of MSCs by surface antigens
(i) CD antigens other than blood group antigens. MSCs were stained using monoclonal antibodies specific for 30 distinct MSC surface antigens and controls. (ii) Blood group antigens. MSCs were analysed using antibodies to H; A and B; D, C, E, c and e; K and k; Jka and Jkb; and Fya and Fyb. We evaluated patients who were known to be positive or negative for these antigens. (iii) Immunocytochemistry. To explore the expression of the H and GD2 antigens, the MSCs were cultivated and stained in chamber slides using anti-H and anti-GD2 antibodies. Fluorescence labelling was performed with Alexa 594-labelled (anti-H) and Alexa 488-labelled (anti-GD2) antibodies. Nuclei were stained with 4′,6-Diamidino-2-phenylindole (DAPI).
Western blotting for blood group proteins
The protein expression of SLC14A1 (JK) and DARC by undifferentiated MSCs and adipogenic, osteogenic, and chondrogenic differentiated MSCs was analysed by Western blot.
Molecular biology for blood group genes
(i) Blood group genotyping. Commercial blood group genotyping kits for ABO, CDE, Kell, Jk and Fy were used (Inno-Train Diagnostik, Kronberg, Germany). MSCs, peripheral blood mononuclear cells (PBMNCs), and KG-1a (myeloblast, chronic myeloid leukaemia) and SK-MEL-28 (melanoma) cell lines were genotyped for blood groups. Patient’s genotypes corresponded to their serological blood groups (data not shown). (ii) Qualitative reverse transcription polymerase chain reaction (RT-PCR). We determined the transcription of blood group genes by testing for the mRNA in MSCs using RT-PCR. (iii) Quantitative RT-PCR (qRT-PCR). To quantify the influence of differentiation on the blood group expression at the transcriptome level, the mRNA expression of SLC14A1 (JK) and DARC genes was evaluated by qRT-PCR in adipogenic, osteogenic and chondrogenic differentiated as well as in undifferentiated MSCs. The expression of peroxisome proliferator-activated receptor-γ (PPARG) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was determined as controls.
Immunohaematology
(i) Red blood cell (RBC) blood group phenotyping of the MSC donors. RBC samples of the MSC donors were typed for antigens of the blood group systems ABO (A and B), H (H antigen), Rhesus (D, C, E, c, e), Kell (K and k), Kidd (Jka and Jkb), and Duffy (Fya and Fyb) by standard serological methods. (ii) Adsorption of H, A or B antigens to MSCs. After 4 passages of cultivation in AB serum or O serum the MSCs from an O donor were analysed by flow cytometry using antibodies to the H, A and B antigens.
Statistics
We used the two-tailed Mann-Whitney U test and considered p < 0.05 statistically significant.
Results
MSCs were isolated from whole BM by a density gradient technique (Colter et al., 2001) and characterized functionally by in vitro differentiation assays for their potential to differentiate into the adipogenic, osteogenic or chondrogenic mesenchymal lineage (Fig. S1) (Pittenger et al., 1999). We confirmed the known surface antigen pattern by flow cytometry of undifferentiated MSCs. They were positive for CD10, CD29, CD44, CD59, CD71, CD73, CD90, CD105, CD106, CD130, CD140a, CD140b, CD146, CD166, CD 271, GD2, W8B2 and HLA class I; and negative for negative for CD14, CD15, CD31, CD34, CD43, CD45, CD56, CD93, CD117, CD133, CD243 and HLA class II (Fig. S2).
Transcription of blood group mRNA in MSCs
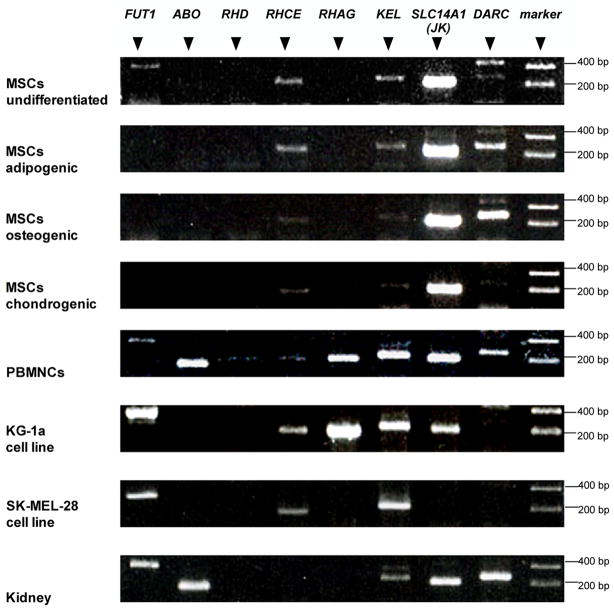
MSCs expressed FUT1, RHCE, KEL, SLC14A1 (JK) and DARC mRNA. No mRNA for ABO, RHD and RHAG was found (Fig. 1). The native, undifferentiated MSCs expressed FUT1 mRNA, whereas under adipogenic, osteogenic and chondrogenic differentiation no expression of FUT1 mRNA was detected.
Figure 1.
Blood group mRNA expression by undifferentiated MSCs and three differentiated human MSC lineages at Passages 1 and 2. RNA from peripheral blood mononuclear cells (PBMNCs), two human cell lines and kidney are tested as controls. Representative results are shown. Comparable results were obtained with 5 donors for the MSCs panels; 2 experiments for PBMNCs and the 2 cell lines; and more than 10 experiments with kidney RNA.
Quantitative SLC14A1 (JK) and DARC mRNA expression in MSCs
Because the largest mRNA amounts were detected for SLC14A1 (JK) and DARC (Fig. 1), we quantified these mRNAs by qRT-PCR. SLC14A1 (JK) mRNA was reduced in chondrogenic differentiated MSCs relative to undifferentiated MSCs (Table 1). Increased DARC mRNA was detected in adipogenic differentiated MSCs; some osteogenic differentiated MSCs had also hugely increased DARC mRNA, although there was much variation among the donors. For comparison, PPARG mRNA was increased, particularly in adipogenic differentiated MSCs. The overall SLC14A1 (JK) mRNA amounts exceeded that of PPARG mRNA, while DARC mRNA amounts were low.
Table 1.
Quantitative expression of SLC14A1 (JK), DARC and PPARG mRNA
| relative mRNA (mean ± SD)* |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MSCs | SLC14A1 (JK) (n =7†) | p | DARC (n = 6) | p | PPARG (n = 7) | p | ||||
| 1.0± 0.9 | 0.0 | 1.0 ± 0.9 | 0.002 | 1.0± 0.9 | 0.004 | |||||
| 01 | ||||||||||
| undifferentiated | ||||||||||
| 0.4± 0.2 | 5.6± 3.3 | 5.7± 4.8 | ||||||||
| adipogenic | ||||||||||
| 0.3± 0.4 | 123.9± 216.8 | 2.2± 1.9 | 0.018 | |||||||
| 0.0 | ||||||||||
| osteogenic | 38 | |||||||||
| 0.1± 0.1 | 0.3± 0.6 | 1.2± 1.9 | ||||||||
| chondrogenic | ||||||||||
The mRNA expression was normalized to a mean = 1.0 for undifferentiated MSCs in each of the 3 genes. mRNA in undifferentiated MSCs relative to GAPDH f expression was 1.4 × 10−2 ± 1.3 × 10−2 for SLC14A1 (JK), 4.8 × 10−6 ± 4.5 × 10−6 for DARC and 3.6 × 10−3 ± 3.4 × 10−3 for PPARG.
The two-tailed Mann-Whitney U test was used and p < 0.05 considered statistically significant.
SLC14A1 (JK) mRNA in osteogenic differentiation was n = 6
Intracellular SLC14A1 (JK) and DARC proteins in MSCs
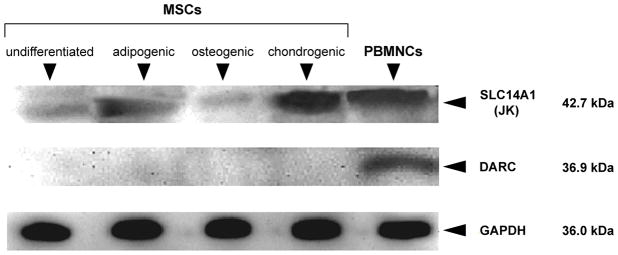
Following the detection of small quantities of SLC14A1 (JK) and DARC mRNA by qRT-PCR, we explored the presence of these proteins by Western blot. Some SLC14A1 (JK) protein was found in all MSC populations, while no DARC protein was detectable (Fig. 2).
Figure 2.
Expression of SLC14A1 (JK) and DARC protein in MSCs. Western blots for SLC14A1 (JK) and DARC proteins are depicted along with controls for the housekeeping protein GADPH. Representative results are shown. Comparable results were obtained in experiments with MSCs from 3 different donors.
Blood group antigens on the MSC surface
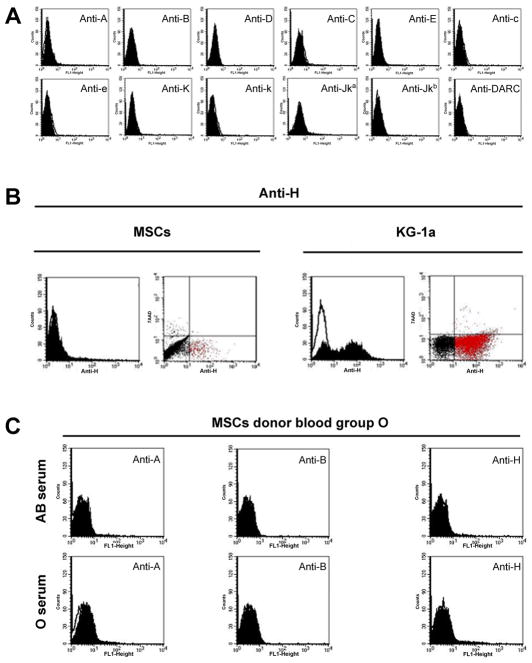
We examined the expression of blood group antigens during several passages. The antigens A, B, D, C, E, c, e, K, k, Jka, and Jkb, as well as the DARC protein could not be detected by flow cytometry (Fig. 3A), an established sensitive technique for RBCs (Flegel et al., 2002).
Figure 3.
Flow cytometry analyses of blood group antigen expression on MSCs. Expression of all 12 blood group antigens tested was lacking from the surface of MSCs (panel A). Representative results are shown for undifferentiated MSCs. Comparable results were obtained with 3 donors for up to 6 passages each (P1 to P6). H antigen expression is detected in a small subpopulation of MSCs (panel B, red dots in the lower right quadrant of the flow cytometry dot plot), which is much more prominent in KG-1a cells tested for comparison. A representative result from more than 10 experiments with undifferentiated MSCs from different donors is shown. MSCs from a blood group O donor were cultivated in AB serum for 4 passages and tested for absorption of A, B and H substances (panel C); MSCs with O serum are shown as a control. Comparable results were obtained with MSCs from a blood group A donor. All histogram plots depict the specific antibody profile (solid) and the isotype control staining (line).
Because FUT1 mRNA, encoding the fucosyltransferase enzyme that synthesizes the H antigen, was found in native MSCs (Fig. 1), we explored H antigen expression by flow cytometry. Whereas the histogram plots were not indicative, the flow cytometric dot plots revealed a small subpopulation of native MSCs that was positive for the H antigen (anti-CD173; Fig. 3B, red dots in the lower right quadrant). The myeloblast KG-1a cells showed a similar wide variability in H antigen expression, although the fraction of strongly H positive cells was much larger than in MSCs. Thus, the flowcytometric dot plots showed a cell population stained by anti-H representing a distinct subpopulation of viable H antigen positive MSCs and KG-1a cells.
MSCs might adsorb A and B antigens to their cell surfaces from the standard culture media containing human serum with soluble A and B substances, as with AB serum. However, A and B antigens were not detected on MSCs after cultivation in AB serum after 4 passages, indicating no adsorption of the respective blood group substances to the surface of the MSCs (Fig. 3C).
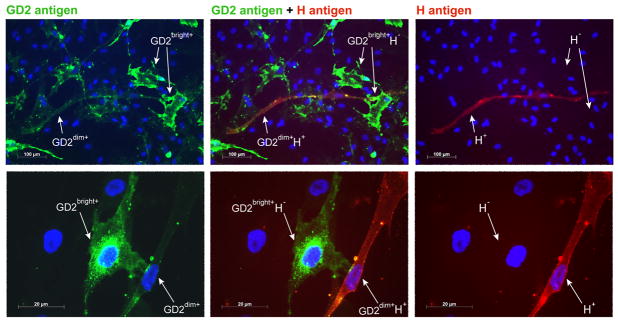
Antigen H is expressed on the surface of GD2dim+ MSCs
Immunocytochemical staining of undifferentiated MSCs identified MSC subpopulations differing by GD2 expression, which were dubbed GD2bright+ and GD2dim+ (Fig. 4). To corroborate our results with flow cytometry (Fig. 3B), we detected some MSCs that expressed the H antigen (CD173). Double staining of GD2 antigen by green fluorescence and the H antigen by red fluorescence revealed that the GD2bright+ cells expressed no H antigen, although a GD2dim+ H+ MSC subpopulation was identified.
Figure 4.
Identification of MSC subpopulations by GD2 and H antigen expression. Two immunocytochemistry slides from different donors (upper and lower panels) are stained for GD2 (green fluorescence) and H antigens (red fluorescence). The fluorescence for GD2 (left panels); GD2 and H antigens (middle panels); and H (right panels) are shown. Nuclei are stained blue (DAPI). Comparable results were obtained with MSCs from 2 additional donors.
Phenotyping of RBCs by flow cytometry
As a control for antigen detection by flow cytometry, blood group antigens on RBCs were confirmed using standard serological techniques (Fig. S3 and S4).
Discussion
The application of BM-derived MSCs, utilized with immunomodulating therapies and for repair of the musculoskeletal system, may provide a means for avoiding the use of immunosuppressive drugs that is often necessary with other cellular therapies. However, allogeneic MSCs can induce alloantibody production, as shown in an animal model (Beggs et al., 2006). Blood group antigens are potent activators of the host immune system and can lead to antibody-mediated destruction of not only RBCs, but also nucleated cells and organs (Colvin & Smith, 2005).
We investigated the expression of clinically important blood group antigens by MSCs at the levels of transcription, intracellular proteins and presence of antigens on the surface (Table 2). MSCs were isolated and cultivated using pooled allogeneic human serum, employed in several studies evaluating the clinical utility of MSCs (Bieback et al., 2009; Turnovcova et al., 2009; Bieback et al., 2010). Under Good Manufacturing Practice conditions, the ex vivo cultivation and expansion of MSCs should be free of animal serum, avoiding the immunization to fetal calf serum (FCS) known to occur in patients receiving MSCs cultured in FCS-containing media (Sundin et al., 2007).
Table 2.
Summary of mRNA and protein expression by clinically important blood group genes in human bone marrow-derived mesenchymal stem cells (MSCs)
| Blood group system |
Parameter of MSCs |
||
|---|---|---|---|
| Gene | Major antigens | Transcriptome mRNA in cells | Proteome/ Antigen on cell surface |
| FUT1 | H | + | + |
| ABO | A and B | − | − |
| RHAG | RhAG | − | − |
| RHD | D | − | − |
| RHCE | C, E, c, and e | + | − |
| KEL | K and k | + | − |
| SLC14A1 (JK) | Jka and Jkb | + | − |
| DARC | Fya and Fyb | + | − |
Blood group status can be determined at the genomic level, a technology increasingly performed in the clinical setting using blood group genotyping platforms. Our serological and genotyping results were equivalent indicating blood group genotyping is reliable. Because most antigens are not expressed on MSCs, blood group genotyping is currently not needed to characterize undifferentiated BM-MSCs for clinical use. While we detected mRNA transcription of the FUT1, RHCE, KEL, SLC14A1 (JK) and DARC genes, no ABO, RHD or RHAG mRNA occurred in MSCs. mRNA transcription varied among subpopulations of MSCs that differentiated into adipogenic, osteogenic and chondrogenic lines.
Trypsin affects the detection of few clinically relevant blood group proteins. However, Rhesus, Kell, Kidd and Duffy, and the carbohydrate-based antigens are known to be resistant to trypsin treatment. Therefore, we used trypsin to detach the MSCs from the culture flasks in our study.
MSCs did not express ABO mRNA and consequently neither A nor B antigen was detectable on their surface. Both results indicate that ABO compatibility is not required for the human serum used in MSC culture. If supplies of AB serum become short with anticipated increases in clinical stem cell culture and its applications increase as anticipated, it can be replaced by serum of other blood groups. In addition, because the soluble A and B antigens occurring in AB serum were also not adsorbed onto MSCs, use of AB serum for culture is unlikely to interfere with the ABO compatibility of MSCs in vivo.
The key enzyme for synthesizing the H antigen is the β–D-galactoside 2-α-L-fucosyltransferase (FUT1), which is in turn a prerequisite for the generation of the A and B antigens. FUT1 and H antigen are expressed by KG-1a cells and immature CD34+ haematopoietic progenitor cells but absent in mature lymphocytes (Cao et al., 2001). We detected the H antigen on a GD2dim+ subpopulation of undifferentiated MSCs, whereas no FUT1 mRNA was found in differentiated MSCs. H is a strong sugar-based antigen and avid poly- and monoclonal antibodies are available, to facilitate the identification as well as the purification and clinical testing of the newly defined GD2dim+ H+ and GD2bright+ H- cells occurring among undifferentiated MSCs (Fig. 4). Based on this technical feature, the H antigen may become instrumental for the identification of immature MSCs, which may differ in “stemness” from the remaining MSCs in the BM. FUT1 is also involved in the biosynthesis of Globo H, a potential tumour-associated antigen in human breast cancer stem cells (Chang et al., 2008).
Among the three Rh-associated ammonia transporters, RhBG and RhCG are expressed on many nucleated cells, but not on RBCs (Handlogten et al., 2005). The third, RhAG, recently recognized as blood group system no. 30, is found on RBC and required for membrane integration of the two proper Rhesus proteins, RhD and RhCE (Huang, 1998; Mouro-Chanteloup et al., 2002; Daniels et al., 2009). Because RHAG is not transcribed in MSCs, the lack of the RhCE protein on the surface, despite some RHCE transcription, can be explained by the known post-translational dependence of RhCE on RhAG. Considering the erythroid-restricted expression of RhAG (Zidi-Yahiaoui et al., 2005), our data confirm the non-erythroid character of MSCs.
Prichett et al., (2000) detected the human urea transporter HUT11 (SLC14A1; JK), the protein carrying the Jk antigen, in "explant cultures of human bone" containing osteoblasts and an unknown fraction of MSCs. We confirmed a reduced SLC14A1 (JK) transcription in differentiating MSCs. However, there was no good correlation with PPARG transcription, which is a marker for adipogenic differentiation (Wu et al., 2010): SLC14A1 (JK) was least transcribed in chondrogenic MSCs, while PPARG transcription in chondrogenic MSCs equaled undifferentiated MSCs. In conclusion, PPARG and SLC14A1 (JK) seem to be regulated independently in MSCs. DARC transcription is increased under adipogenic and apparently osteogenic differentiation. It occurs however in such low copy numbers that, not surprisingly, no DARC protein was found on the MSC surface. The Kell blood group protein, a zinc endopeptidase, is expressed on erythroid and many non-erythroid cells (Russo et al., 2000). We found some KEL transcription in MSCs but no KEL protein on the MSC surfaces, which is consistent with the immature stem cell character of MSCs.
Allogeneic MSCs can be immunogenic and contribute to rejection of haematopoietic stem cells, inducing a memory T-cell response in the host, as shown in a nonmyeloablative murine transplantation setting (Nauta et al., 2006). Interferon-γ induced human leucocyte antigen (HLA) class II antigen expression may contribute to alloimmunization by MSCs (Chan et al., 2006), which may warrant further investigation. However, we could not detect such HLA class II antigen expression on undifferentiated MSCs during our culture with human serum. Among the clinically most relevant and potentially immunizing blood group proteins, only the SLC14A1 (JK) protein could be detected in the cytoplasm, but notably not on the surface of MSCs. Our data suggest that, even in the non-immunosuppressed graft recipient, a rejection or malfunction of the cellular graft due to an immunization against these blood group proteins and their antigens may not be expected. This lack of immunogenic blood group antigens on MSCs is a plus for promising clinical applications.
In further characterizing human MSCs, the data presented here suggests not only practical aspects of current GMP production, but also compatibility of these cells in the clinical setting of stem cell transplantation and regenerative medicine.
Supplementary Material
Acknowledgments
We gratefully acknowledge Martina Wölfle, née Maisel, Dept. Neurodegenerative Diseases, Hertie-Institute for Clinical Brain Research, University of Tübingen for the RHAG primer design; Rainer Kehlbach, Department of Diagnostic Radiology, University Hospital Tübingen for discussing flow cytometry data; Elizabeth J. Furlong, Dept. Transfusion Medicine, Clinical Center, NIH for excellent editing of the manuscript; and the medical technologists at IKET, University Hospital Tübingen for general technical support.
Footnotes
Conflict of interest disclosure: The authors declare no competing interests relevant to this article.
Statement of Disclaimer: The views expressed do not necessarily represent the view of the National Institutes of Health, the Department of Health and Human Services, or the U.S. Federal Government.
Authorship contribution: R.S. initiated the study and supervised the performance of the experiments; R.S., Ma.S. and W.A.F. provided the study concept and design; R.S., R.A.K., G.S. and Mi.S. collected samples; T.K. provided study material; C.G. designed primers; R.S., G.S., M.A.R., U.H.-K., M.A. and M.B. collected data; R.S., T.K., Mi.S. assembled data; L.D. performed statistical analyses; R.S., Ma.S., R.A.K., G.S., C.G. and W.A.F. interpreted data; R.S., H.N. and W.A.F. discussed data; C.G., L.D. and H.N. contributed to manuscript writing; R.S. wrote drafts and W.A.F. the final version of the manuscript.
References
- Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM, Locatelli F, Fibbe WE. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110:2764–2767. doi: 10.1182/blood-2007-04-087056. [DOI] [PubMed] [Google Scholar]
- Beggs KJ, Lyubimov A, Borneman JN, Bartholomew A, Moseley A, Dodds R, Archambault MP, Smith AK, McIntosh KR. Immunologic consequences of multiple, high-dose administration of allogeneic mesenchymal stem cells to baboons. Cell Transplant. 2006;15:711–721. doi: 10.3727/000000006783981503. [DOI] [PubMed] [Google Scholar]
- Bieback K, Hecker A, Kocaomer A, Lannert H, Schallmoser K, Strunk D, Kluter H. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells. 2009;27:2331–2341. doi: 10.1002/stem.139. [DOI] [PubMed] [Google Scholar]
- Bieback K, Ha VA, Hecker A, Grassl M, Kinzebach S, Solz H, Sticht C, Kluter H, Bugert P. Altered Gene Expression in Human Adipose Stem Cells Cultured with Fetal Bovine Serum Compared to Human Supplements. Tissue Eng Part A. 2010;16:3467–3484. doi: 10.1089/ten.TEA.2009.0727. [DOI] [PubMed] [Google Scholar]
- Buhring HJ, Kuci S, Conze T, Rathke G, Bartolovic K, Grunebach F, Scherl-Mostageer M, Brummendorf TH, Schweifer N, Lammers R. CDCP1 identifies a broad spectrum of normal and malignant stem/progenitor cell subsets of hematopoietic and nonhematopoietic origin. Stem Cells. 2004;22:334–343. doi: 10.1634/stemcells.22-3-334. [DOI] [PubMed] [Google Scholar]
- Cao Y, Merling A, Karsten U, Schwartz-Albiez R. The fucosylated histo-blood group antigens H type 2 (blood group O, CD173) and Lewis Y (CD174) are expressed on CD34+ hematopoietic progenitors but absent on mature lymphocytes. Glycobiology. 2001;11:677–683. doi: 10.1093/glycob/11.8.677. [DOI] [PubMed] [Google Scholar]
- Chan JL, Tang KC, Patel AP, Bonilla LM, Pierobon N, Ponzio NM, Rameshwar P. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-gamma. Blood. 2006;107:4817–4824. doi: 10.1182/blood-2006-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WW, Lee CH, Lee P, Lin J, Hsu CW, Hung JT, Lin JJ, Yu JC, Shao LE, Yu J, Wong CH, Yu AL. Expression of Globo H and SSEA3 in breast cancer stem cells and the involvement of fucosyl transferases 1 and 2 in Globo H synthesis. Proc Natl Acad Sci U S A. 2008;105:11667–11672. doi: 10.1073/pnas.0804979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci U S A. 2001;98:7841–7845. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin RB, Smith RN. Antibody-mediated organ-allograft rejection. Nat Rev Immunol. 2005;5:807–817. doi: 10.1038/nri1702. [DOI] [PubMed] [Google Scholar]
- Daniels G, Castilho L, Flegel WA, Fletcher A, Garratty G, Levene C, Lomas-Francis C, Moulds JM, Moulds JJ, Olsson ML, Overbeeke M, Poole J, Reid ME, Rouger P, van der Schoot E, Scott M, Sistonen P, Smart E, Storry JR, Tani Y, Yu LC, Wendel S, Westhoff C, Yahalom V, Zelinski T. International Society of Blood Transfusion Committee on terminology for red blood cell surface antigens: Macao report. Vox Sang. 2009;96:153–156. doi: 10.1111/j.1423-0410.2008.01133.x. [DOI] [PubMed] [Google Scholar]
- Dominici M, Hofmann TJ, Horwitz EM. Bone marrow mesenchymal cells: biological properties and clinical applications. J Biol Regul Homeost Agents. 2001;15:28–37. [PubMed] [Google Scholar]
- Flegel WA, Curin-Serbec V, Delamaire M, Donvito B, Ikeda H, Jorgensen J, Kumpel B, Le Pennec PY, Pisacka M, Tani Y, Uchikawa M, Wendel S, Wagner FF. Section 1B: Rh flow cytometry. Coordinator's report. Rhesus index and antigen density: an analysis of the reproducibility of flow cytometric determination. Transfus Clin Biol. 2002;9:33–42. doi: 10.1016/s1246-7820(01)00213-0. [DOI] [PubMed] [Google Scholar]
- Giesert C, Marxer A, Sutherland DR, Schuh AC, Kanz L, Buhring HJ. Antibody W7C5 defines a CD109 epitope expressed on CD34+ and CD34- hematopoietic and mesenchymal stem cell subsets. Ann N Y Acad Sci. 2003;996:227–230. doi: 10.1111/j.1749-6632.2003.tb03250.x. [DOI] [PubMed] [Google Scholar]
- Hadley TJ, Peiper SC. From malaria to chemokine receptor: the emerging physiologic role of the Duffy blood group antigen. Blood. 1997;89:3077–3091. [PubMed] [Google Scholar]
- Han KH, Croker BP, Clapp WL, Werner D, Sahni M, Kim J, Kim HY, Handlogten ME, Weiner ID. Expression of the ammonia transporter, rh C glycoprotein, in normal and neoplastic human kidney. J Am Soc Nephrol. 2006;17:2670–2679. doi: 10.1681/ASN.2006020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handlogten ME, Hong SP, Zhang L, Vander AW, Steinbaum ML, Campbell-Thompson M, Weiner ID. Expression of the ammonia transporter proteins Rh B glycoprotein and Rh C glycoprotein in the intestinal tract. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1036–1047. doi: 10.1152/ajpgi.00418.2004. [DOI] [PubMed] [Google Scholar]
- Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483–3493. doi: 10.1182/blood-2003-05-1664. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Gordon PL, Koo WW, Fitzpatrick LA, Neel MD, McCarville ME, Orchard PJ, Pyeritz RE, Brenner MK. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 2001;97:1227–1231. doi: 10.1182/blood.v97.5.1227. [DOI] [PubMed] [Google Scholar]
- Huang CH. The human Rh50 glycoprotein gene. Structural organization and associated splicing defect resulting in Rh(null) disease. J Biol Chem. 1998;273:2207–2213. doi: 10.1074/jbc.273.4.2207. [DOI] [PubMed] [Google Scholar]
- Ji JF, He BP, Dheen ST, Tay SS. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells. 2004;22:415–427. doi: 10.1634/stemcells.22-3-415. [DOI] [PubMed] [Google Scholar]
- Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringden O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- Marini AM, Matassi G, Raynal V, Andre B, Cartron JP, Cherif-Zahar B. The human Rhesus-associated RhAG protein and a kidney homologue promote ammonium transport in yeast. Nat Genet. 2000;26:341–344. doi: 10.1038/81656. [DOI] [PubMed] [Google Scholar]
- Martinez C, Hofmann TJ, Marino R, Dominici M, Horwitz EM. Human bone marrow mesenchymal stromal cells express the neural ganglioside GD2: a novel surface marker for the identification of MSCs. Blood. 2007;109:4245–4248. doi: 10.1182/blood-2006-08-039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouro-Chanteloup I, D'Ambrosio AM, Gane P, Le Van Kim C, Raynal V, Dhermy D, Cartron JP, Colin Y. Cell-surface expression of RhD blood group polypeptide is posttranscriptionally regulated by the RhAG glycoprotein. Blood. 2002;100:1038–1047. [PubMed] [Google Scholar]
- Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci. 2009;66:236–253. doi: 10.1007/s00018-008-8429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Prichett WP, Patton AJ, Field JA, Brun KA, Emery JG, Tan KB, Rieman DJ, McClung HA, Nadeau DP, Mooney JL, Suva LJ, Gowen M, Nuttall ME. Identification and cloning of a human urea transporter HUT11, which is downregulated during adipogenesis of explant cultures of human bone. J Cell Biochem. 2000;76:639–650. [PubMed] [Google Scholar]
- Quirici N, Soligo D, Bossolasco P, Servida F, Lumini C, Deliliers GL. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30:783–791. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- Russo D, Wu X, Redman CM, Lee S. Expression of Kell blood group protein in nonerythroid tissues. Blood. 2000;96:340–346. [PubMed] [Google Scholar]
- Satija NK, Singh VK, Verma YK, Gupta P, Sharma S, Afrin F, Sharma M, Sharma P, Tripathi RP, Gurudutta GU. Mesenchymal stem cell-based therapy: a new paradigm in regenerative medicine. J Cell Mol Med. 2009;13:4385–4402. doi: 10.1111/j.1582-4934.2009.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer R, Dominici M, Muller I, Horwitz E, Asahara T, Bulte JW, Bieback K, Le Blanc K, Buhring HJ, Capogrossi MC, Dazzi F, Gorodetsky R, Henschler R, Handgretinger R, Kajstura J, Kluger PJ, Lange C, Luettichau I, Mertsching H, Schrezenmeier H, Sievert KD, Strunk D, Verfaillie C, Northoff H. Basic research and clinical applications of non-hematopoietic stem cells, 4-5 April 2008, Tubingen, Germany. Cytotherapy. 2009;11:245–255. doi: 10.1080/14653240802582117. [DOI] [PubMed] [Google Scholar]
- Shayakul C, Hediger MA. The SLC14 gene family of urea transporters. Pflugers Arch. 2004;447:603–609. doi: 10.1007/s00424-003-1124-x. [DOI] [PubMed] [Google Scholar]
- Siegel G, Schafer R, Dazzi F. The immunosuppressive properties of mesenchymal stem cells. Transplantation. 2009;87:S45–49. doi: 10.1097/TP.0b013e3181a285b0. [DOI] [PubMed] [Google Scholar]
- Sundin M, Ringden O, Sundberg B, Nava S, Gotherstrom C, Le Blanc K. No alloantibodies against mesenchymal stromal cells, but presence of anti-fetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipients. Haematologica. 2007;92:1208–1215. doi: 10.3324/haematol.11446. [DOI] [PubMed] [Google Scholar]
- Swardson-Olver CJ, Dawson TC, Burnett RC, Peiper SC, Maeda N, Avery AC. Plasmodium yoelii uses the murine Duffy antigen receptor for chemokines as a receptor for normocyte invasion and an alternative receptor for reticulocyte invasion. Blood. 2002;99:2677–2684. doi: 10.1182/blood.v99.8.2677. [DOI] [PubMed] [Google Scholar]
- Terasaki PI, Cai J. Human leukocyte antigen antibodies and chronic rejection: from association to causation. Transplantation. 2008;86:377–383. doi: 10.1097/TP.0b013e31817c4cb8. [DOI] [PubMed] [Google Scholar]
- Tobian AA, Shirey RS, Montgomery RA, Ness PM, King KE. The critical role of plasmapheresis in ABO-incompatible renal transplantation. Transfusion. 2008;48:2453–2460. doi: 10.1111/j.1537-2995.2008.01857.x. [DOI] [PubMed] [Google Scholar]
- Turnovcova K, Ruzickova K, Vanecek V, Sykova E, Jendelova P. Properties and growth of human bone marrow mesenchymal stromal cells cultivated in different media. Cytotherapy. 2009;11:874–885. doi: 10.3109/14653240903188947. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Zappia E, Benvenuto F, Frassoni F, Mancardi G. Stem cells in inflammatory demyelinating disorders: a dual role for immunosuppression and neuroprotection. Expert Opin Biol Ther. 2006;6:17–22. doi: 10.1517/14712598.6.1.17. [DOI] [PubMed] [Google Scholar]
- Wester ES, Johnson ST, Copeland T, Malde R, Lee E, Storry JR, Olsson ML. Erythroid urea transporter deficiency due to novel JKnull alleles. Transfusion. 2008;48:365–372. doi: 10.1111/j.1537-2995.2007.01532.x. [DOI] [PubMed] [Google Scholar]
- Wu L, Cai X, Dong H, Jing W, Huang Y, Yang X, Wu Y, Lin Y. Serum regulates adipogenesis of mesenchymal stem cells via MEK/ERK-dependent PPARgamma expression and phosphorylation. J Cell Mol Med. 2010;14:922–932. doi: 10.1111/j.1582-4934.2009.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidi-Yahiaoui N, Mouro-Chanteloup I, D'Ambrosio AM, Lopez C, Gane P, Le van Kim C, Cartron JP, Colin Y, Ripoche P. Human Rhesus B and Rhesus C glycoproteins: properties of facilitated ammonium transport in recombinant kidney cells. Biochem J. 2005;391:33–40. doi: 10.1042/BJ20050657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.