Abstract
The molecular mechanisms regulating vascular barrier integrity remain incompletely elucidated. We have previously reported an association between the GTPase R-Ras and repeat 3 of Filamin A (FLNa). Loss of FLNa has been linked to increased vascular permeability. We sought to determine whether FLNa’s association with R-Ras affects endothelial barrier function. We report that in endothelial cells endogenous R-Ras interacts with endogenous FLNa as determined by co-immunoprecipitations and pulldowns with the FLNa-GST fusion protein repeats 1–10. Deletion of FLNa repeat 3 (FLNa 3) abrogated this interaction. In these cells FLNa and R-Ras co-localize at the plasma membrane. Knockdown of R-Ras and/or FLNa by siRNA promotes vascular permeability, as determined by TransEndothelial Electrical Resistance and FITC-dextran transwell assays. Re-expression of FLNa restored endothelial barrier function in cells lacking FLNa whereas re-expression of FLNaΔ3 did not. Immunostaining for VE-Cadherin in cells with knocked down R-Ras and FLNa demonstrated a disorganization of VE-Cadherin at adherens junctions. Loss of R-Ras and FLNa or blocking R-Ras function via GGTI-2133, a selective R-Ras inhibitor, induced vascular permeability and increased phosphorylation of VE-Cadherin (Y731) and Src (Y416). Expression of dominant negative R-Ras promoted vascular permeability that was blocked by the Src inhibitor PP2. These findings demonstrate that maintaining endothelial barrier function is dependent upon active R-Ras and association between R-Ras and FLNa and that loss of this interaction promotes VE-Cadherin phosphorylation and changes in downstream effectors that lead to endothelial leakiness.
Keywords: R-Ras, Filamin A, vascular permeability, VE-Cadherin, TEER, endothelial barrier function
Introduction
The vascular endothelium acts as a restrictive barrier that regulates many biological processes such as protein and fluid transport and inflammation. Disruption of this barrier leads to vascular leakiness and results in inflammatory disease with enhanced severity in sepsis, cancer, diabetes and atherosclerosis (Gavard, 2009). However, the cellular mechanism(s) that regulate endothelial barrier function are not yet completely known and few specific therapies are currently available to counteract vascular leakage (Dejana et al., 2009; Groeneveld, 2002; van Nieuw Amerongen and van Hinsbergh, 2007).
Studies with vascular permeability inducing factors such as TNF-α have identified several signaling pathways that contribute to endothelial barrier dysfunction. These include VE-Cadherin phosphorylation on Tyr 731 (Potter et al., 2005), Ca2+-dependent activation of myosin light chain kinase (Petrache et al., 2001; Tinsley et al., 2008) and the RhoA/Rho kinase signaling pathway (Wojciak-Stothard and Ridley, 2002). Activation of the Rho-like small GTPase RhoA increases actomyosin contractility to induce the breakdown of intercellular junctions and promote permeability (Essler et al., 1998). The Rho-like GTPases Rac1 and Cdc42 abrogate the effects of RhoA and enhance barrier function (Andor et al., 2001; Petrache et al., 2003; Wojciak-Stothard et al., 2001; Wojciak-Stothard and Ridley, 2002). However, the role of Rac is complex and there are reports detailing both increases and decreases in Rac activity being associated with altered barrier function (Popoff and Geny, 2009). Recently, KRIT1 has been identified as a regulator of barrier integrity by blocking both RhoA activity and its downstream effector ROCK (Stockton et al., 2010). In addition, the small GTPase H-Ras contributes to endothelial barrier dysfunction by increasing vascular permeability through the activation of the PI3 Kinase-γ pathway (Serban et al., 2008).
Distinct from H-Ras, R-Ras induces cells that grow in suspension to become adherent (Zhang et al., 1996) by enhancing both the affinity and avidity of integrins. R-Ras differs from the other members of the Ras family in that it contains a proline-rich SH3 domain binding site (Wang et al., 2000) and a 26-amino acid extension at its N-terminus (Holly et al., 2005). While the contribution of R-Ras to endothelial barrier function has not been determined, R-Ras plays a central role in the vasculature. In vivo, R-Ras expression is restricted to smooth muscle cells and to endothelial cells (Komatsu and Ruoslahti, 2005). R-Ras enhances inflammatory responses to atherosclerotic lesions via a c-AMP/R-Ras/PI3K signaling pathway that leads to increased deposition of fibronectin on the apical surface of human arterial endothelial cells (Cole et al., 2003). In R-Ras null mice, arterial injury results in increased neo-intimal thickening, suggesting that R-Ras has an effect on how vessels respond to injury; a central role in regulating endothelial cell functions (Komatsu and Ruoslahti, 2005).
Filamin A (FLNa), a member of the non-muscle actin binding protein family, acts as a molecular scaffold protein that regulates signaling events involved in cell shape and cell motility by binding to β integrin tails, adaptor proteins and second messengers (Stossel and Hartwig, 2003). FLNa binds more than 30 proteins, however, the physiological processes relating to these interactions remain elusive. FLNa null mice are embryonic lethal with severe cardiac defects, aberrant vascular patterning, and vascular deficits such as dilated vasculature, hemorrhage, and edema. Loss of FLNa enhances LPS-induced endothelial permeability in lung microvascular cells (Bogatcheva et al., 2009).
We previously used yeast two-hybrid analysis to identify R-Ras interacting partners and reported that R-Ras binds to repeat 3 of FLNa (Gawecka et al, 2010). The prominent role of both R-Ras and FLNa in the vasculature in vivo suggested to us that these proteins may play a role in the regulation of barrier function. We show here that the mechanism whereby barrier integrity is achieved is, in part, dependent upon the interaction between R-Ras and FLNa and that loss of this interaction promotes vascular permeability by increasing phosphorylation of VE-Cadherin at Y731 and Src at Y416.
Materials and Methods
Cell Culture
Human Coronary Artery Endothelial Cells (HCAECs) were grown in supplemented EGM-2MV media (Lonza, Basel, Switzerland) at 37°C, in 5% CO2.
Collection of Cell Lysates
HCAECs were washed twice in cold PBS prior to lysis in 50–100 μl of M2 buffer. Lysates were sonicated, and subjected to centrifugation at 16,000 rpm to pellet cellular debris. The resulting supernatant was used for subsequent Western blotting analysis.
Permeability-Inducing Factor induced inflammation
HCAECs were subjected to inflammation via the addition of the permeability-inducing factor, TNFα, to a final concentration of 10 ng/ml (in supplemented EGM-2) for 4 hrs prior to obtaining cell lysates for Western blotting or FITC-dextran or ECIS analysis (Chen et al., 2008).
Immunoprecipitation
Immunoprecipitation assays were done using 500 μg of whole-celllysate as previously described (Schiestl and Gietz, 1989). Briefly, HCAECs were lysed in lysis buffer containing 50 mM Tris-HCL, 50 mM NaCl, 0.5% Triton X-100, 10% glycerol, 0.1% BSA, and protease inhibitors (0.1 unit/ml aprotinin, 10 μg leupeptin and 0.5 mM phenyl-methylsulfonyl fluoride). Lysates were centrifuged at 16,000 rpm for 10 min. Antibodies at a concentration of 2–4 μg were added to lysates containing an equal amount of protein and incubated overnight at 4°C. To precipitate the antibody-antigen complex, Protein A/G PLUS agarose beads (Santa Cruz) were added to lysates and incubated for 4 h at 4°C. Samples treated with IgG beads in the absence of antibody were used as a negative control. The immunoprecipitates were pelleted by centrifugation and washed twice with PBS (Cukier et al., 2007; Vaidyanathan et al., 2007). Beads were boiled in sample buffer and separated on SDS-PAGE gels. After electrophoresis, proteins were transferred to a nitrocellulose membrane and subjected to immunoblotting as described below.
Glutathione S-transferase (GST) protein construction and FLNa fragment pulldown assay
Human FLNa fragments were a gift from Dr. Jonathan Lee and fragments were purified as previously described (University of Ottawa;(Cukier et al., 2007). Briefly, the FLNa fragments were cloned into the pGEX-4T1 vector (Pharmacia). The FLNa fragments are FLNa 1–10, 110kDa, repeats 1–10; FLNa 11–16, 60 kDa, repeats 11–16, and FLNa 17–23 &D, 80 kDa, repeats 17–23 & dimerization domain. GST-FLNa fusion proteins were expressed in Escherichia coli BL-21 cells (Invitrogen) according to standard protocols, and affinity purified by glutathione-sepharose-4B beads (Amersham Biosciences). All constructs were sequenced before use. For pull down assays, FLNa fragment protein expression was confirmed via a 12% polyacrylamide gel and coomassie staining prior to initiation of the pull down assay. HCAEC lysate was incubated with the bound GST-FLNa fragment fusion proteins or with the GST vector and then run on a 12% SDS-PAGE gel, and immunoblotted for R-Ras.
Immunocytochemical Analysis
Permeability inducing factor, TNF-α (inflamed) and quiescent non-treated (non-inflamed) HCAECs were plated on fibronectin coated coverslips (10 μg/ml) and allowed to adhere overnight at 4°C. Cells were subsequently fixed in 4% paraformaldehyde for 15 min, washed 1X in PBS, permeablized (.002% Triton-X, 5 min at 37° C), and blocked (PBS + 3% BSA for 30 min) prior to incubation in rabbit anti R-Ras Ab (1:100, Santa Cruz Biotechnology) for 1 hr at 37° C. After 3 PBS washes, cells were incubated with mouse anti-FLNa Ab (1:100, 1 hr at 37° C; Cell Signaling Technology, Danvers, MA). Cells were then washed 3X in PBS prior to incubation in Alexa Fluor anti-rabbit 564 (red) secondary antibody (1:500, 1 hr at 37° C; Molecular Probes) and 3X more before incubation in Alexa Fluor anti-mouse 488 (green) secondary antibody (1:500, 1 hr at 37° C; Molecular Probes). After 3 final washes in PBS, cells were mounted with fluoromount onto glass slides and subsequently imaged using a Zeiss Axiovert 200m fluorescent microscope (Matter et al., 1998). VE-Cadherin and Zo-1 staining was performed as described above using an anti-VE-Cad antibody or an anti-Zo-1 (Santa Cruz Biotechnology and Invitrogen respectively). Detection of F-actin was performed via Alexa fluor Phalloidin 488 (Invitrogen) staining as per manufactures instructions. Briefly, fixed and blocked cells were incubated for 20 min in 165 nM Alexa fluor Phalloidin 488 at room temperature, then washed 3 × in PBS prior to mounting and imaging as described above.
siRNA Knockdown of R-Ras and FLNa
HCEAC cells were transfected via the Amaxa nucleoporator with 10 nm of Smartpool (consisting of a mix of four separate siRNAs for each protein; Dharmacon, Lafayette CO) FLNa, R-Ras, or both siRNAs using the HCAEC Nucleofection kit (Lonza, Gaithersburg, MD). Cells transfected with scrambled siRNA were used as controls. Cells were then lysed (60 hr later) and immunoblotted for R-Ras and FLNa (see Figure 3A) or replated in Electric Cell Impedance Sensing (ECIS) chamber glass slides or transwell inserts and allowed to grow to confluence as described below. For barrier integrity rescue experiments endogenous FLNa was knocked down using 10 nM of FLNa siRNA and cells with knocked down FLNa were transfected with 3 μg of either full-length wild-type FLNa or FLNa that lacked repeat 3 (FLNaΔ3; Gawecka et al., 2010).
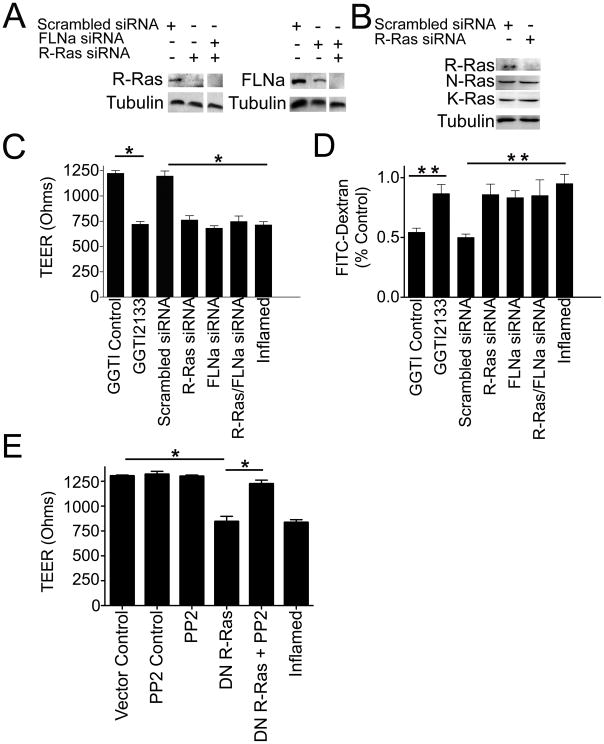
Figure 3. Loss of R-Ras/FLNa protein expression promotes vascular permeability.
A) Western blot demonstrating the knockdown of R-Ras, FLNa or both proteins after siRNA treatment. HCAECs were transfected with either R-Ras or FLNa siRNA individually or R-Ras/FLNa combined siRNAs (Dharmacon Smartpool that consists of 4 separate siRNAs for each protein). Results are representative of at least 3 independent experiments. B) Western blot demonstrating that knockdown of R-Ras siRNA does not alter K-Ras or M-Ras expression levels. HCAECS were transfected with either R-Ras siRNA or scrambled siRNA and immunoblotted for R-Ras, K-Ras or M-Ras using mouse monoclonal antibodies to R-, K- or M-Ras, respectively. C) Loss of R-Ras/FLNa promotes vascular permeability. Transendothelial resistance (TEER) measurements on HCAEC monolayers with a scrambled siRNA or siRNA against R-Ras, FLNa, R-Ras/FLNa or treated with the R-Ras GGTI-2133 inhibitor, the GGTI DMSO-carrier control or a permeability-inducing factor (inflamed). TEER levels (y axis) are the mean electrical resistance in ohms±SEM corrected for the resistance of a naked transwell. *P<0.0001 by two-way ANOVA, Bonferroni post-test (n=5,5,5,5,5,5). D) Assessment of vascular permeability of identically treated samples as above were determined by the FITC-dextran (40,000 MW) assay which measures the amount of FITC-dextran that passes through the cell monolayer from the upper to the lower chamber (%control). Vascular permeability induced by treatment with either the R-Ras specific inhibitor GGTI-2133, the GGTI DMSO-carrier control or a permeability-inducing factor (TNFα; inflamed) was also assessed. **P<0.001 relative to control. Each bar represents the mean ±SEM of 3 replicates (N=15). E) Dominant negative R-Ras promotes vascular permeability and the Src inhibitor PP2 suppresses it. Transendothelial resistance (TEER) measurements on HCAEC monolayers transfected with a pcDNA3 vector control or dominant negative R-Ras (DN R-Ras; T43N), or treated with the Src inhibitor PP2, the PP2 DMSO-carrier control or a permeability-inducing factor (TNFα; inflamed). *P<0.0001 by two-way ANOVA, Bonferroni post-test (n=5,5,5,5,5).
Immunoblotting
To determine the effects of loss of R-RAS and/or FLNa on downstream effectors, HCEAC cells were transfected with 10 nM of Smartpool (consisting of a mix of four siRNAs for each protein; Dharmacon, Lafayette CO) FLNa, R-Ras, or both siRNAs using the HCAEC Nucleofection kit (Lonza, Gaithersburg, MD). Cells transfected with scrambled siRNA were used as controls. Cells were lysed 60 hr later and immunoblotted for R-Ras, FLNa, VE-Cadherin, phospho VE-Cadherin (Y731, Y658, Y685), Src, phospho-Src (Y416), CSK, SH2P2, and ICAM. Tubulin was used as a loading control. The immunobloting protocol has been described in detail elsewhere (Vaidyanathan et al., 2007). Briefly, cells were lysed with lysis buffer containing 50 mM Tris-HCl, 3 mM EDTA, 0.5% Triton X-100, pH 7.0, 0.5 mM dithiothreitol, and protease inhibitors (0.1 units/ml aprotinin, 10 mg/ml leupeptin, and 0.5 mM phenylmethylsulfonyl fluoride). Samples of cell lysates were subjected to BCA analysis (Pierce, Rockford IL) to determine total protein concentration. Cell lysates were cleared by centrifugation at 16,000 rpm for 10 min at 4 °C. Equal protein concentrations of cell lysates were resolved on an 8% (probing for FLNa) or 12% (probing for R-Ras) SDS-polyacrylamide gel. After electrophoresis, proteins were transferred to a nitrocellulose membrane (Millipore Corp., Bedford, MA) and immunoblotted. Membranes were blocked for 1 hr with blocking buffer (3% BSA in TBS-T) and then incubated with an anti-FLNa antibody or anti-R-Ras, or anti-SHP2, anti-CSK, anti-ICAM, anti-VE-Cad, anti-K-Ras, or anti-N-Ras (all from Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or anti-phospho-Src (Y416; Cell Signaling), anti-c-Src (Cell Signaling), anti-VE-Cad (Y731; BioSource), or anti-tubulin (Biolegend) antibody at a 1:1000 dilution for 1 h. Membranes were washed and incubated with horseradish peroxidase-conjugated secondary antibodies (Bio-Rad, Hercules, CA) at 1:5000 (anti-rabbit) and 1:15,000 (anti-goat and anti mouse) dilution for 1 hr. Immunoblots were developed by ECL Plus (GE Health, United Kingdom).
Dominant negative R-Ras expression and Src inhibition via PP2 inhibitor
HCAECs were transfected via the Amaxa nucleoporater with dominant negative R-Ras (T43N; DN R-Ras; 3μg) or pcDNA3 using the HCAEC Nucleofection kit (Lonza, Gaithersburg, MD) and then plated onto ECIS slides. After 47 hr, cells were treated for 1hr with either the Src inhibitor PP2 (100 nM) or carrier-control as previously described (Seye et al., 2004). Cells were then subjected to TEER analysis described below.
Selective Inhibitor of R-Ras: Geranylgeranyltransferase I (GGTI-2133)
HCAECs were plated on tissue culture coated plates, ECIS chamber slides, or transwell inserts and allowed to reach confluency prior to being treated for 8 hr with 38 nM GGTI-2133 (IC50 = 38 nM, Sigma), a cell-permeable non-thiol peptidomimetic that acts as a potent and selective inhibitor of geranylgeranyltransferase I (GGTase I) that inhibits R-Ras but not H-Ras (Sun et al., 2003). Control samples were treated with equal volumes of DMSO, the GGTI carrier (Kusama et al., 2006).
Electric Cell Impedance Sensing (ECIS): TransEndothelial Electrical Resistance (TEER) Assay
HCAECs were transfected with siRNA or the scrambled control as described above and plated on 8W10E+ ECIS chamber glass slides (Applied BioPhysics, Troy NY). Cells were plated and measured for TEER as previously described (Clark et al., 2007). Briefly, cells were plated at a concentration of 75,000 cells/well. They were allowed to grow to confluence for 54 hr prior to TNFα (or GGTI control) treatment and an additional 4 hr after that prior to ECIS analysis using ECIS ™ Model 1600R (Applied BioPhysics, Troy NY). Cells treated with R-Ras inhibitor GGTI-2133 were allowed to grow for 52 hr prior to blocking R-Ras function for 8 hr (as described above). TEER was assessed at the 30 min time point. Data from triplicate samples were pooled and averaged. Standard error and statistical analysis was determined via graphpad analysis using one-way ANOVA.
FITC-Dextran Permeability Assay
HCAECs were transfected with siRNAs as described above and plated into 24-well transwell inserts (Corning Lab Sciences Lowell, MA) as previously described (Schulz et al., 2008) and allowed to grow for 54 hr prior to treatment with permeability inducing factor TNFα (or DMSO control) treatment, and 50 hr prior to GGTI-2133 treatment or GGTI control treatment and an additional 4 or 8 hr after that respectively. To measure vascular permeability, all media was removed from the inner and outer chambers of each well and 200 μl of EGM-2 MV (+/− TNFα) was added to the outside chamber of each well. FITC-Dextran (MW 40,000) was added to EGM-2 MV media (1:25), and 100 μl of the FITC-Dextran EGM-2 MV media (+/− TNFα) was added to the inside chamber of each well. An aliquot (10 μl) of the media from the outside chamber was diluted 1:25 in 1X PBS and read via fluorometer (Wallac Victor3 1420 multilabel counter, Perkin Elmer). All values are depicted as percentage of control samples (10 μl of FITC-Dextran media diluted 1:2 in EGM-2 MV, diluted 1:25 in PBS). Standard error and statistical analysis was determined via graphpad analysis using one-way ANOVA.
Results
Endogenous R-Ras and FLNa Associate in Human Coronary Arterial Endothelial Cells
We previously identified FLNa as an R-Ras interacting protein via yeast two-hybrid screening using R-Ras as bait (Gawecka et al., 2010). In vivo, R-Ras is primarily expressed in endothelial cells and plays a role in regulating arterial endothelial cell function (Komatsu and Ruoslahti, 2005). FLNa null mice are embryonic lethal with vascular deficits and FLNa also is important for endothelial cell function (Zhou et al., 2007). Therefore, we examined whether R-Ras and FLNa were expressed in Human Coronary Arterial Endothelial Cells (HCAECs). Indeed, R-Ras and FLNa are expressed in HCAECs (Fig. 1A) as determined by Western blot analysis. Induction of vascular permeability did not alter expression levels of either protein (Fig. 1A).
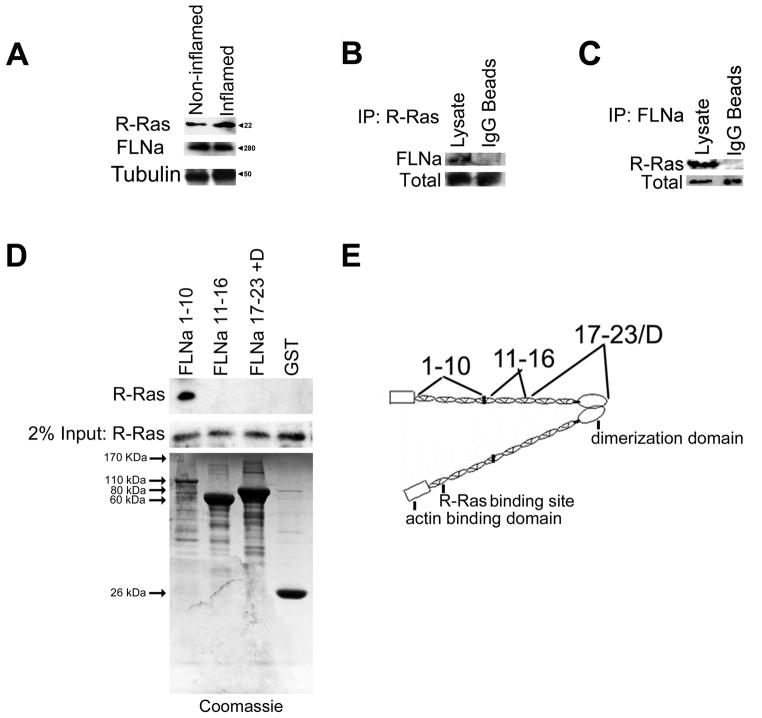
Figure 1.
Endogenous R-Ras and FLNa associate in arterial endothelial cells. A) Western blot of endogenous R-Ras/FLNa expression in quiescent endothelial cells (non-inflamed) and in cells treated with a permeability-inducing factor (TNFα; inflamed). B) R-Ras and FLNa interact in a complex in HCAECs. R-Ras and FLNa co-Immunoprecipitate in HCAECs. Cells were lysed, immunoprecipitated (IP) with anti-FLNa conjugated IgG beads or IgG beads alone and immunoprecipitates were analyzed by immunoblotting (IB) with an anti-R-Ras antibody. Input of R-Ras levels is shown (Bottom). Results are representative of 3 independent experiments. C) HCAECs were lysed, immunoprecipitated (IP) with anti-R-Ras conjugated IgG beads or IgG beads alone and immunoprecipitates were analyzed by immunoblotting (IB) with an anti-FLNa antibody. Input of FLNa levels is shown (Bottom). Results are representative of 3 independent experiments. D) R-Ras binds FLNa. GST fusion proteins derived from indicated FLNa fragments were incubated with HCAEC total lysates. Bound R-Ras was detected by Western blotting for R-Ras. Results are representative of 3 independent experiments. Coomassie stained gel containing purified FLNa fragments is shown to confirm FLNa fragment protein expression. E) Schematic of FLNa domains. The R-Ras binding site, the actin-binding domain and the dimerization domain at the C-terminus have been previously established (Gawecka et al., 2010; Gorlin et al., 1990). FLNa fragments 1–10, 11–16 17–23 & dimerization domain are shown on the top of the schematic.
Because both proteins are endogenously expressed in HCAECs, we next examined whether R-Ras and FLNa interact with one another in these cells. Using immunoprecipitations (IPs), we found that endogenous FLNa was detected in R-Ras IPs (Fig. 1B) and endogenous R-Ras was detected in FLNa IPs (Fig. 1C). We next used GST-FLNa fusion proteins that correspond to 3 different regions of FLNa to pulldown R-Ras from HCAECs. R-Ras bound to the fusion protein corresponding to repeats 1–10, whereas it did not bind to the other regions tested (Fig 1D). The region of interaction that we previously identified in the yeast two-hybrid screen is within repeat 3, which lies within the FLNa 1–10 fusion protein (Fig. 1E). Together these data support the hypothesis that R-Ras is in a complex with FLNa that requires filamin repeat 3. Moreover, these findings confirm that endogenous R-Ras and endogenous FLNa associate in arterial endothelial cells.
R-Ras and FLNa Co-Localize in Arterial Endothelial Cells
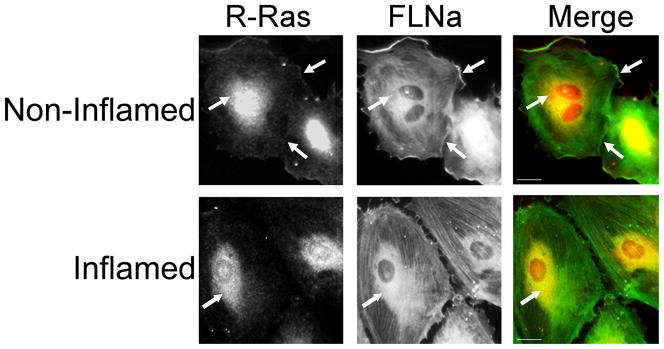
R-Ras associates with purified FLNa and they co-immunoprecipitate in endothelial cell lysates. To determine where this interaction occurs in cells, we examined their subcellular localization by immunofluorescence. We examined quiescent endothelial cells, and found that a portion of endogenous R-Ras and endogenous FLNa co-localized at the plasma membrane. In these cells R-Ras is also found in the perinuclear region, in agreement with previous reports that R-Ras is localized and active in endosomes (Takaya et al., 2007). Upon induction of permeability, endogenous R-Ras and FLNa are no longer localized at the plasma membrane (Fig. 2). These findings suggest that FLNA and R-Ras associate at the plasma membrane in quiescent arterial endothelial cells.
Figure 2. Endogenous R-Ras and FLNa co-localize in arterial endothelial cells.
In quiescent endothelial cells, R-Ras and FLNa partially co-localize as examined by immunofluorescence microscopy at 40X. Co-localization of R-Ras and FLNa is displayed in yellow in the merged image. Arrows indicate co-localization. HCAECs were plated on fibronectin coated glass slides. Quiescent cells (non-inflamed) were untreated whereas inflamed cells were treated with a permeability-inducing factor (TNFα). Upon inflammation R-Ras/FLNa co-localization at the plasma membrane is reduced. R-Ras was detected by immunostaining with an anti-R-Ras antibody followed by a Rhodamine conjugated secondary. FLNa was detected by immunostaining with an anti-FLNa antibody followed by a fluorescene 488 nm conjugated secondary. Data shown is representative of 3 independent experiments. Scale bar = 20 μm.
siRNA knockdown of R-Ras/FLNa causes barrier dysfunction
The observation that FLNa null mice have vascular deficits such as dilated vasculature, hemorrhage, and edema (Feng et al., 2006; Kyndt et al., 2007) suggested to us that FLNa is important in endothelial barrier function. In vivo, R-Ras also plays a role in arterial endothelial function and response to vascular injury (Komatsu and Ruoslahti, 2005). Therefore we investigated the role of the R-Ras/FLNa interaction in endothelial barrier function. To examine this, we knocked down R-Ras and FLNa either independently or simultaneously using siRNA (Fig. 3A). Knockdown of R-Ras did not affect expression of other Ras proteins (Fig. 3B). HCAEC monolayers with reduced expression of R-Ras and/or FLNa demonstrated a significant decrease in TransEndothelial Electrical Resistance (TEER) compared to a scrambled siRNA control (Fig. 3C), indicative of the increased permeability of the endothelial cell monolayer for water and ions (Kolosova et al., 2008). As expected induction of permeability in quiescent control endothelial cells by a permeability-inducing factor (TNFα) promoted a significant decrease in TEER (Fig. 3C).
We further examined whether inhibiting endogenous R-Ras function using the R-Ras inhibitor GGTI-2133 would impact barrier integrity. This inhibitor is a cell-permeable non-thiol peptidomimetic that acts as a potent and selective inhibitor of geranylgeranyltransferase I (GGTI) and acts on R-Ras but not H-Ras, which is farnesylated (Sun et al., 2003). Indeed, blocking R-Ras function with GGTI-2133 significantly decreased TEER and thus increased endothelial vascular permeability compared to the GGTI DMSO-carrier control (Fig. 3C). HCAECs also express the geranylgeranylated K-Ras and N-Ras. Therefore, the GGTI-2133 inhibitor may also act on K-Ras and N-Ras in these cells. However siRNA knock-down of R-Ras did not affect N- or K-Ras levels (Fig. 3B) while both R-Ras knock-down and GGTI-2133 treatment induced permeability to a similar extent. This suggests that the effect of GGTI-2133 on permeability is not due to effects on N- or K-Ras. Taken together, these findings indicate that loss of R-Ras/FLNa interaction promotes increased endothelial permeability.
In order to confirm these findings, we evaluated endothelial barrier function by measuring the macromolecular flux across endothelial cell monolayers grown on transwell membranes via the FITC-dextran transwell assay that measures endothelial permeability. Loss of R-Ras and FLNa significantly increased endothelial cell permeability compared to control cells (Fig. 3D). Treatment with the R-Ras inhibitor GGTI-2133 increased endothelial permeability whereas the GGTI DMSO carrier control did not (Fig. 3D). These results confirm that loss of R-Ras and FLNa interaction increases endothelial permeability.
R-Ras is a GTPase that binds to effector proteins when it is GTP loaded. We therefore sought to determine if GTP loading of R-Ras is required for its effects on vascular permeability. R-Ras T43N is unable to bind GTP and thus acts as a dominant negative when expressed in cells. We found that expression of this dominant negative R-Ras (T43N) in HCAEC monolayers induced vascular permeability compared to vector control as determined by TEER (Fig. 3E). Inhibiting Src function, by the Src inhibitor PP2, blocked dominant negative R-Ras-mediated vascular permeability and restored barrier function. These findings indicate that GTP loading of R-Ras is required for it to maintain endothelial barrier function and that loss of R-Ras function promotes vascular permeability through increased Src activity.
R-Ras association with FLNa is required for maintaining endothelial barrier function
We previously identified that R-Ras binds to FLNa repeat 3 (Gawecka et al., 2010). We therefore examined whether R-Ras binding to repeat 3 of FLNa influenced endothelial barrier integrity. We made an expression construct of FLNa in which repeat 3 was deleted (FLNaΔ3). We next determined whether FLNaΔ3 was the site of association between R-Ras and FLNa. Cells were transfected with His-tagged FLNaΔ3 or His-tagged wild type FLNa. R-Ras was immunoprecipitated from cell lysates and examined for co-precipitation of His by immunoblotting. His was detected in FLNa immunoprecipitations confirming that R-Ras and FLNa are in a complex in HCAECs (Fig 4A). Whereas R-Ras did not co-immunoprecipitate His tagged FLNa lacking repeat 3 (Fig 4A), confirming that this is the site of association.
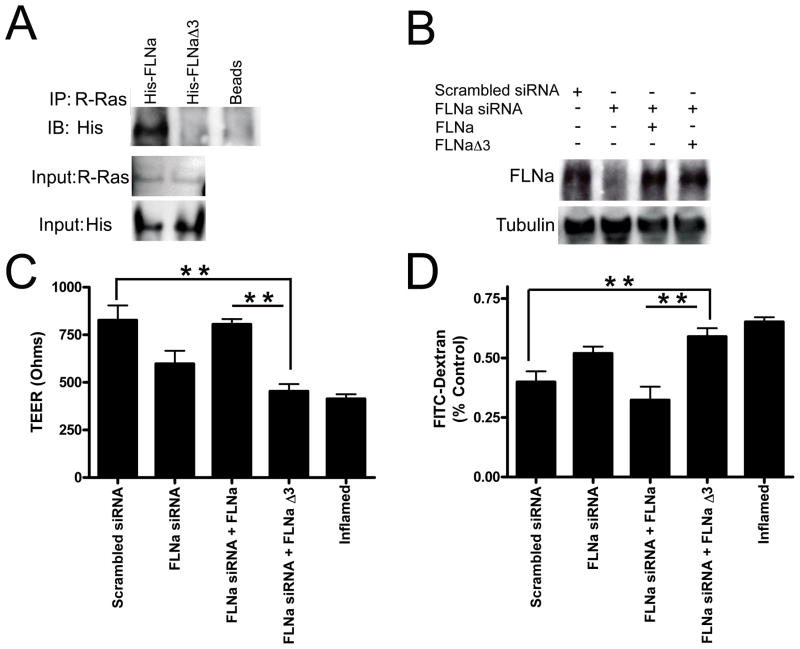
Figure 4. The association of R-Ras with FLNa maintains barrier function.
A) Deletion of FLNa repeat 3 abrogates the FLNa/R-Ras interaction. HCAECs were co-transfected with expression vectors for His-tagged wild type FLNa or His-tagged FLNaΔ3 (deletion of FLNa repeat 3). 48 hr post transfection cells were lysed, immunoprecipitated (IP) with anti-R-Ras conjugated IgG beads or IgG beads alone and immunoprecipitates were analyzed by immunoblotting with an anti-His antibody. Input of R-Ras and His levels are shown (Bottom). Results are representative of 3 independent experiments. B) Western blot demonstrating siRNA knockdown of FLNa and re-expression of either wild-type FLNa or FLNa lacking repeat 3 (FLNaΔ3). C) Transendothelial resistance (TEER) values from HCAEC monolayers that have reduced FLNa, re-expressed wild-type FLNa or FLNa lacking repeat 3 (FLNaΔ3). Quiescent endothelial cells expressing scrambled siRNA are used as a positive control for maintaining barrier function. Endothelial cells treated with a permeability-inducing factor (TNFα) are used as a negative control (Inflamed) for barrier function. *P<0.0001 by two-way ANOVA, Bonferroni post-test (n=5,5,5,5,5,5). D) Vascular permeability of identically treated samples as determined by the FITC-dextran (40,000 MW) assay. **P<0.001 relative to control. Each bar represents the mean ±SEM of 3 replicates (N=15).
We next assessed the effect of loss of R-Ras binding to FLNa on barrier function via TEER. HCAECs with knocked down FLNa levels were transfected with either wild type FLNa, or FLNaΔ3 (repeat 3 deleted; Fig. 4B). Cells expressing both R-Ras and wild type FLNa maintained barrier function approximately 2-fold more than in cells expressing R-Ras and FLNaΔ3 (Fig. 4C). Re-expression of FLNa restored endothelial barrier function in cells with knocked down FLNa whereas expression of FLNaΔ3 did not rescue barrier function in these cells (Fig. 4C).
In order to confirm these findings, we evaluated endothelial barrier function via a FITC-dextran transwell assay that measures endothelial permeability. Again, cells expressing both R-Ras and wild type FLNa maintained barrier function approximately 2-fold more than cells expressing R-Ras and FLNaΔ3 (Fig. 4D). Re-expression of FLNa rescued endothelial barrier function in cells with knocked down FLNa whereas expression of FLNaΔ3 in these cells did not restore barrier function (Fig. 4D). Taken together, these results suggest that the association of R-Ras with FLNa repeat 3 is required for maintaining endothelial barrier function.
siRNA Knockdown of R-Ras and FLNa promotes changes in the VE-Cadherin immunostaining pattern
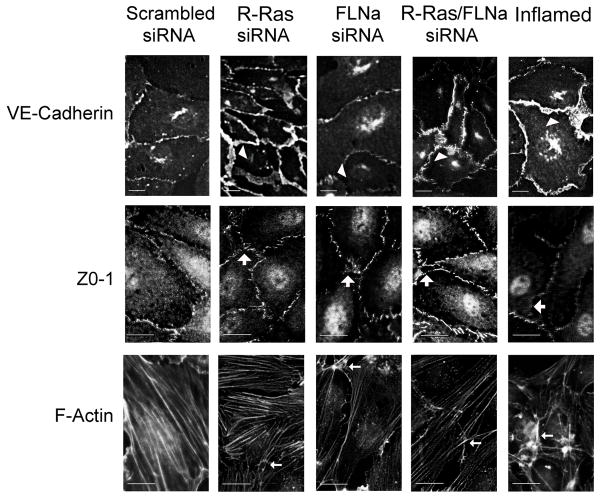
The staining pattern of VE-Cadherin at adherens junctions is altered upon induction of vascular permeability. Because we observed an increase in vascular permeability upon loss of R-Ras association with FLNa that was similar to permeability obtained by treating cells with a permeability-inducing factor, we next examined whether the staining patterns of VE-Cadherin might be affected by loss of the R-Ras/FLNa complex. In cells with reduced R-Ras and FLNa expression the VE-Cadherin immunostaining pattern demonstrated a zigzag appearance with enhanced thickness along the pericellular boundaries that is similar to cells treated with a permeability-inducing factor (Fig. 5). This is in contrast to scrambled control siRNA transfected cells. Immunostaining for F-actin in endothelial monolayers in which R-Ras and FLNa were knocked down also revealed that loss of R-Ras/FLNa induced moderate stress fiber formation (Fig. 5). These changes suggest that R-Ras and FLNa coordinately prevent vascular permeability by maintaining VE-cadherin function in adherens junctions.
Figure 5. R-Ras and FLNa loss alter cytoskeletal protein rearrangement in HCAECs.
Cells with knocked down R-Ras and/or FLNa were grown on fibronectin coated glass coverslips, fixed and stained with Alexa488 phalloidin, anti-VE-Cadherin or anti-ZO-1 antibodies. Arrowheads indicate the zigzag appearance due to the loss of R-Ras/FLNa or treatment of the cells with a permeability-inducing factor (TNFα; Inflamed). Large arrows point to the gaps formed between the cells as a result of loss of R-Ras/FLNa or inflamed control. Small arrows indicate moderate stress fiber formation upon loss of R-Ras/FLNa or inflamed control. Scale bar = 20 μm. Results are representative of 3 or more independent experiments.
To clarify the cytoskeletal events leading to HCAEC hyper-permeability, we next analyzed the distribution of the tight junction component Zo-1 in endothelial cells in which R-Ras and/or FLNa were knocked down by siRNA transfection. Loss of R-Ras and FLNa altered Zo-1 localization at cellular borders showing a disruption in Zo-1 continuity and induction of gap-like areas at the points of multiple cell contacts similar to cells treated with a permeability-inducing factor (Fig. 5). The scrambled siRNA control cells showed normal staining for Zo-1 as a strong cell border signal, which appeared as a continuous line of varied thickness. Loss of R-Ras/FLNa levels also caused moderate stress fiber formation compared to scrambled siRNA controls (Fig. 5).
siRNA Knockdown of R-Ras/FLNa Promotes Phosphorylation of VE-Cadherin at Y731
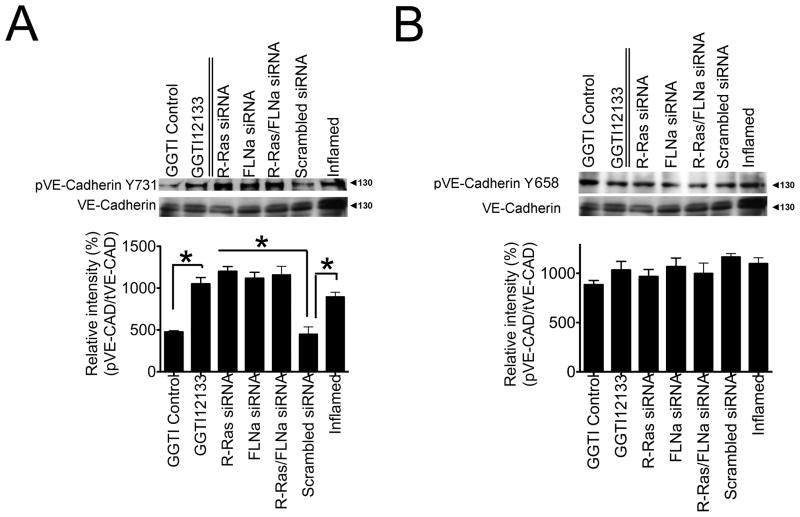
VE-Cadherin contains nine putative phospho-tyrosine sites, among which phosphorylation of either Y658, Y685, or Y731 results in increased endothelial cell permeability (Dejana et al., 2008). We examined whether loss of the R-Ras/FLNa complex induced VE-Cadherin phosphorylation at any of these sites. We found that phosphorylation at Y731 increased in endothelial cells in which R-Ras and/or FLNa had been knocked down as compared to cells receiving the scrambled siRNA control (Fig. 6A). The increased phosphorylation was similar to that in control cells treated with a permeability-inducing factor (TNFα). The increase in Y731 was also observed in cell monolayers treated with the R-Ras inhibitor GGTI-2133 but not in a DMSO-carrier treated control (Fig. 6A). Total VE-Cadherin expression was similar under all conditions (Fig. 6A). We did not observe any changes to Y658 (Fig. 6B) or Y685 (data not shown) under the conditions tested. These results suggest that loss of the R-Ras/FLNa complex induces phosphorylation of VE-Cadherin at Y731, contributing to endothelial cell permeability and endothelial barrier dysfunction.
Figure 6. Loss of the R-Ras/FLNa complex increases the phosphorylation of vascular permeability effector VE-Cadherin at Y731 but not at Y658.
Western blot analyses of endothelial cells with siRNA knock down of R-Ras, FLNa or R-Ras/FLNa, scrambled siRNA control or cells treated with either the R-Ras inhibitor GGTI-2133, GGTI DMSO carrier control or a permeability-inducing factor (TNFα; Inflamed). Cell lysates (30 μg total protein/lane) were processed to detect A) phosphorylated VE-Cadherin (Y731) and total VE-Cadherin. B) phosphorylated VE-Cadherin (Y658) and total VE-Cadherin. Relative intensity of pVE-Cad (Y731)/tVE-Cad and pVE-Cad (Y658)/tVE-CAD were determined. *P=<0.05 (Student’s t test). Tubulin was used as a loading control. The values represent the average of three independent experiments.
siRNA Knockdown of R-Ras and FLNa Promotes Phosphorylation of c-Src
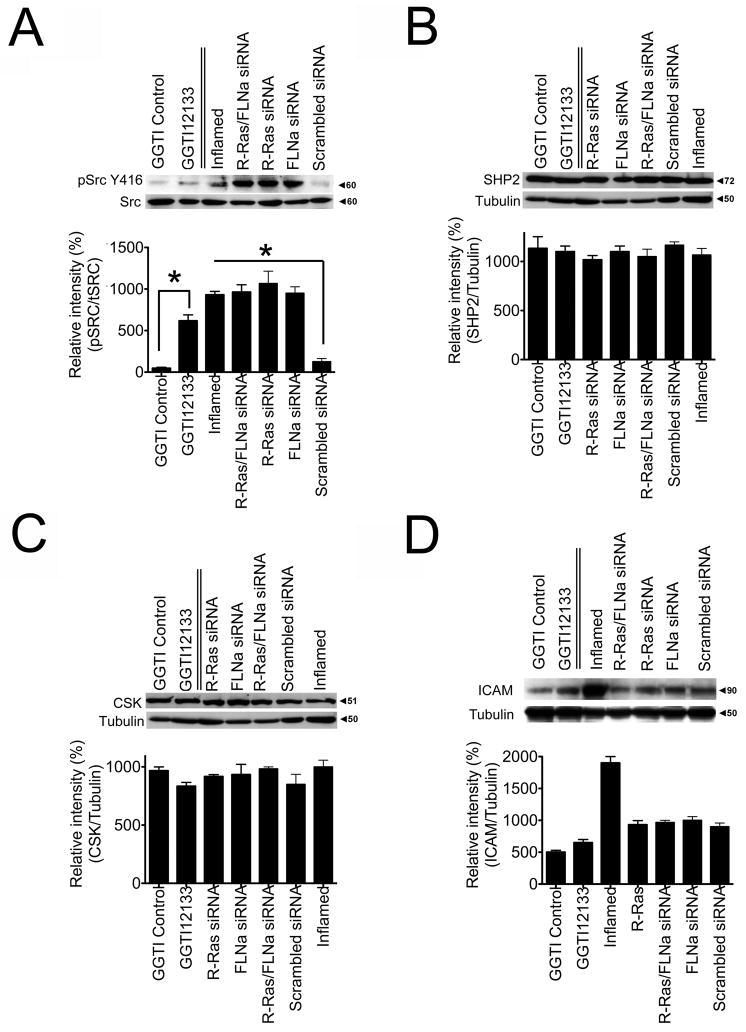
How tyrosine phosphorylation of VE-Cadherin promotes vascular leakage is not yet clear, however the non-receptor tyrosine kinases of the Src family are known to be involved. Src-deficient mice have decreased vascular permeability (Weis and Cheresh, 2005) and VE-Cadherin modulates c-Src signaling in response to permeability-inducing factors (Ha et al., 2008). Therefore, we examined if Src is involved in VE-Cadherin phosphorylation in cells lacking the R-Ras/FLNa complex. In HCAECs in which R-Ras and/or FLNa were knocked down phosphorylation of Src at Y416 was upregulated in a manner similar to control cells treated with a permeability-inducing factor (TNFα). HCAECs expressing the scrambled siRNA control maintained low levels of phospho-Src (Fig. 7A). Phospho-Src (Y416) levels were also increased by the R-Ras inhibitor GGTI-2133 but not by the vehicle DMSO (Fig. 7A). The levels of SH2-containing phospho-tyrosine phosphatase (SHP2), which promotes c-Src activation by controlling CSK access to Src kinases, and CSK (C terminal Src kinase) a negative regulator of Src, were not significantly altered (Fig. 7B,C). These findings demonstrate that upon loss of R-Ras and FLNa or treatment with the R-Ras inhibitor GTI-2133, phospho-Src levels are increased.
Figure 7. Loss of the R-Ras/FLNa complex increases the phosphorylation of vascular permeability effector Src (Y416).
Western blot analyses of endothelial cells with siRNA knock down of R-Ras, FLNa or R-Ras/FLNa, scrambled siRNA control or cells treated with either the R-Ras inhibitor GGTI-2133, GGTI DMSO carrier control or a permeability-inducing factor (TNFα; Inflamed). Cell lysates (30 μg total protein/lane) were processed to detect: A) phosphorylated Src (Y416) and total Src. B) SHP2 C) CSK or D) ICAM protein levels. Relative intensity of pSrc (Y416)/tSrc were determined. Relative intensity of SHP2-1/Tubulin, CSK/Tubulin, and ICAM/Tubulin were measured. *P=<0.05 (Student’s t test). Tubulin was used as a loading control. The values represent the average of three independent experiments.
Because increased vascular permeability is often associated with increased ICAM expression we examined whether ICAM levels were altered upon loss of the R-Ras/FLNa complex. ICAM expression did not change upon loss of R-Ras and/or FLNa (Fig. 7D). ICAM expression increased only upon treatment with a permeability-inducing factor (Fig. 7D), in agreement with published reports (Clark et al., 2007). Thus R-Ras and FLNa levels do not appear to alter ICAM expression.
Discussion
The major findings reported here are that endogenous R-Ras and FLNa associate in arterial endothelial cells and partially co-localize. We further show that the complex is functional in that quiescent arterial endothelial cells expressing endogenous R-Ras and FLNa demonstrate significant barrier function while deletion of R-Ras and/or FLNa abrogates barrier function. Moreover R-Ras binds FLNa repeat 3 and this region of FLNa is required for maintenance of barrier function. Finally, chemical inhibition of R-Ras and inhibition by expression of dominant negative R-Ras led to increased vascular permeability. These studies are the first to implicate R-Ras as a positive regulator of barrier integrity. These findings also indicate that FLNa modulates barrier function and provide a mechanistic insight into the function of the R-Ras/FLNa complex on the positive regulation of barrier integrity.
Many cardiovascular defects are associated with the loss of FLNa function in humans, including cardiac valvular anomalies, and vascular disorders (Zhou et al., 2007). FLNa null mice are embryonic lethal with vascular deficits such as dilated vasculature, hemorrhage, and edema (Zhou et al., 2007). Moreover, FLNa phosphorylation by CaM kinase II has been implicated in thrombin-induced cytoskeletal reorganization and endothelial barrier dysfunction (Borbiev et al., 2001). Recently, Bogatcheva and colleagues (2009) found that knockdown of FLNa in human lung microvascular endothelial cells exacerbated LPS-induced barrier dysfunction, suggesting a barrier protective role of FLNa. Our findings are in agreement with that data and suggest further that it is the R-Ras/FLNa interaction at FLNa repeat 3 that promotes a barrier protective role in human coronary arterial endothelial cells.
Our data indicate that maintaining endothelial barrier function may be, in part, dependent upon the direct interaction between R-Ras and FLNa. We find that at the plasma membrane approximately 30% of FLNa co-localized with R-Ras as determined by ImageJ analysis (Bolte and Cordelieres, 2006). While only a portion of total FLNa co-localized with total R-Ras this interaction occurs at the plasma membrane where it is most likely able to directly influence vascular permeability. Disrupting this interaction at the plasma membrane could therefore alter barrier function. Indeed, loss of this interaction (eg by deletion of FLNa repeat 3) promotes cytoskeletal-initiated vascular permeability through changes in VE-Cadherin phosphorylation and downstream signaling that mediate endothelial leakiness. Interestingly, we note that there is a cooperative knock-down of FLNa when both R-Ras and FLNa are targeted by siRNA compared to targeting FLNa alone. While we do not know why this occurs, it might partly be due to decreased stability of FLNa when one of its binding partners (R-Ras) is removed. This occurs with other proteins that function as members of closely associated non-covalently linked complexes such as integrins. For example, the integrin beta 1 subunit can affect other subunits by increasing subunit proteolysis or decreasing their mRNA stability (Gonzalez et al.; Retta et al., 2001). Thus, if there is no R-Ras present, some of the remaining FLNa in the double knock-down may be degraded. Nevertheless, we cannot exclude other possibilities.
R-Ras is a member of the Ras family and can antagonize H-Ras signaling to cell adhesion receptors (Kinbara et al., 2003). While H-Ras promotes vascular permeability by the activation of the PI3Kγ/δ pathway (Serban et al., 2008) our data indicate that R-Ras is involved in maintaining endothelial barrier integrity. Hence our data suggest at least another instance where these two Ras family members can have opposing effects on cell behavior. This novel role for R-Ras as a positive regulator of barrier function fits well with the in vivo data that R-Ras is restricted to the endothelium and regulates arterial endothelial cell function (Komatsu and Ruoslahti, 2005). Loss of R-Ras expression increased vascular permeability. This is corroborated by the fact that treatment of endothelial cells with an R-Ras inhibitor also increased permeability. Treatment with GGTI-2133, a selective inhibitor that acts on geranylgeranylated R-Ras but not the farnesylated H-Ras (Sun et al., 2003), induced vascular permeability and increased phospho-Src (Y416) levels. This inhibitor can also affect K-Ras and N-Ras, which are also geranylgeranylated (Caraglia et al., 2005) and are expressed in arterial endothelial cells. However, both the siRNA knock-down of R-Ras and the GGTI-2133 inhibitor induced vascular permeability to a similar extent and neither K-Ras nor N-Ras expression changed upon R-Ras knock-down. Therefore, it is most likely that only the inhibition of R-Ras geranylgeranylation is causing the effects on permeability. Moreover, expressing dominant negative R-Ras demonstrated increased vascular permeability similar to loss of R-Ras expression. Taken together, these findings suggest that blocking R-Ras function promotes barrier dysfunction.
VE-Cadherin is an important determinant of barrier function within the vascular endothelium. It is linked through its cytoplasmic tail to the adherens junction proteins p120, β-catenin and plakoglobin. Phosphorylation of VE-Cadherin at Y731 results in a loss of β-catenin binding and increases endothelial permeability (Potter et al., 2005). This complex is also influenced by the actin cytoskeleton, however the molecular mechanism of this interaction is not yet defined (Dejana et al., 2008; Gavard, 2009). Our data point to a role for the R-Ras/FLNa complex in maintaining VE-Cadherin at the adherens junctions. We found that loss of FLNa/R-Ras induced a zipper like distortion of VE-Cadherin, increased phosphorylation of VE-Cadherin at Y731 but not at Y658 or Y685, and increased endothelial permeability similar to control cells that had been treated with a permeability-inducing factor (TNFα). This site can be phosphorylated by the tyrosine kinase Src. Here we found that phospho-Src levels also increased in cells with reduced R-Ras/FLNa expression to a similar level as cells treated with a permeability-inducing factor. Thus it may be Src that is responsible for the increase in VE-Cadherin phosphorylation that we see when R-Ras function is impaired.
While the mechanism of how tyrosine phosphorylation of VE-Cadherin promotes vascular leakage is not yet clear, the non-receptor tyrosine kinases of the Src family may play an active role. Src-deficient mice have decreased vascular permeability and VE-Cadherin tyrosine phosphorylation in response to permeability-inducing factors (Eliceiri et al., 2002; Ha et al., 2008). Our data indicate that phospho-Src (Y416) levels are upregulated in endothelial cells that have reduced R-Ras/FLNa expression. Furthermore, addition of a Src inhibitor blocked vascular permeability induced by dominant negative R-Ras expression suggesting that Src is downstream of R-Ras in this pathway. This is in agreement with reports that Src activation promotes cytoskleletal-induced endothelial cell barrier dysfunction (Mucha et al., 2003; Sallee et al., 2006). FLNa acts to anchor filamentous actin to plasma membrane proteins and thus may play a pivotal role in maintaining barrier function. The actin cross-linking function of FLNa is altered by CaM kinase II-dependent phosphorylation that promotes a decrease in FLNa-mediated actin filament cross-linking (Ohta and Hartwig, 1995). Loss of R-Ras or FLNa may therefore mimic dissolution of this complex in response to induction of vascular permeability.
Regulation of endothelial barrier function and vascular permeability is controlled by modulation of a number of GTPases. We now add R-Ras to the list. The association between R-Ras and FLNa may provide the link between endothelial barrier function, the cytoskeleton and signals initiated by permeability factors.
Acknowledgments
This work was supported by NIH/NCRR P20RR016453 (M.L.M.), The Geist Foundation 200842993 (M.L.M.) and GI2 RR0030161-21 (J.S.A.).
We thank Joe W. Ramos for critical review of the manuscript.
MLM designed the research; GSG and MG performed research; MLM GSG and JSA analyzed data. MLM and GSG wrote the paper.
Footnotes
Disclosures: NONE
Literature Cited
- Andor A, Trulzsch K, Essler M, Roggenkamp A, Wiedemann A, Heesemann J, Aepfelbacher M. YopE of Yersinia, a GAP for Rho GTPases, selectively modulates Rac-dependent actin structures in endothelial cells. Cell Microbiol. 2001;3(5):301–310. doi: 10.1046/j.1462-5822.2001.00114.x. [DOI] [PubMed] [Google Scholar]
- Bogatcheva NV, Zemskova MA, Kovalenkov Y, Poirier C, Verin AD. Molecular mechanisms mediating protective effect of cAMP on lipopolysaccharide (LPS)-induced human lung microvascular endothelial cells (HLMVEC) hyperpermeability. J Cell Physiol. 2009;221(3):750–759. doi: 10.1002/jcp.21913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224(Pt 3):213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- Borbiev T, Verin AD, Shi S, Liu F, Garcia JG. Regulation of endothelial cell barrier function by calcium/calmodulin-dependent protein kinase II. Am J Physiol Lung Cell Mol Physiol. 2001;280(5):L983–990. doi: 10.1152/ajplung.2001.280.5.L983. [DOI] [PubMed] [Google Scholar]
- Caraglia M, Budillon A, Tagliaferri P, Marra M, Abbruzzese A, Caponigro F. Isoprenylation of intracellular proteins as a new target for the therapy of human neoplasms: preclinical and clinical implications. Curr Drug Targets. 2005;6(3):301–323. doi: 10.2174/1389450053765833. [DOI] [PubMed] [Google Scholar]
- Chen C, Jamaluddin MS, Yan S, Sheikh-Hamad D, Yao Q. Human stanniocalcin-1 blocks TNF-alpha-induced monolayer permeability in human coronary artery endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28(5):906–912. doi: 10.1161/ATVBAHA.108.163667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PR, Manes TD, Pober JS, Kluger MS. Increased ICAM-1 expression causes endothelial cell leakiness, cytoskeletal reorganization and junctional alterations 2. JInvest Dermatol. 2007;127(4):762–774. doi: 10.1038/sj.jid.5700670. [DOI] [PubMed] [Google Scholar]
- Cole AL, Subbanagounder G, Mukhopadhyay S, Berliner JA, Vora DK. Oxidized phospholipid-induced endothelial cell/monocyte interaction is mediated by a cAMP-dependent R-Ras/PI3-kinase pathway 2. ArteriosclerThrombVascBiol. 2003;23(8):1384–1390. doi: 10.1161/01.ATV.0000081215.45714.71. [DOI] [PubMed] [Google Scholar]
- Cukier IH, Li Y, Lee JM. Cyclin B1/Cdk1 binds and phosphorylates Filamin A and regulates its ability to cross-link actin. FEBS Lett. 2007;581(8):1661–1672. doi: 10.1016/j.febslet.2007.03.041. [DOI] [PubMed] [Google Scholar]
- Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121(Pt 13):2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16(2):209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Eliceiri BP, Puente XS, Hood JD, Stupack DG, Schlaepfer DD, Huang XZ, Sheppard D, Cheresh DA. Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling 2. JCell Biol. 2002;157(1):149–160. doi: 10.1083/jcb.200109079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essler M, Amano M, Kruse HJ, Kaibuchi K, Weber PC, Aepfelbacher M. Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. J Biol Chem. 1998;273(34):21867–21874. doi: 10.1074/jbc.273.34.21867. [DOI] [PubMed] [Google Scholar]
- Feng Y, Chen MH, Moskowitz IP, Mendonza AM, Vidali L, Nakamura F, Kwiatkowski DJ, Walsh CA. Filamin A (FLNA) is required for cell-cell contact in vascular development and cardiac morphogenesis 2. Proc Natl Acad Sci USA. 2006;103(52):19836–19841. doi: 10.1073/pnas.0609628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavard J. Breaking the VE-cadherin bonds. FEBS Lett. 2009;583(1):1–6. doi: 10.1016/j.febslet.2008.11.032. [DOI] [PubMed] [Google Scholar]
- Gawecka JE, Griffiths GS, Ek-Rylander B, Ramos JW, Matter ML. R-Ras regulates migration through an interaction with filamin A in melanoma cells. PLoS One. 2010;5(6):e11269. doi: 10.1371/journal.pone.0011269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez AM, Bhattacharya R, deHart GW, Jones JC. Transdominant regulation of integrin function: mechanisms of crosstalk. Cell Signal. 22(4):578–583. doi: 10.1016/j.cellsig.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlin JB, Yamin R, Egan S, Stewart M, Stossel TP, Kwiatkowski DJ, Hartwig JH. Human endothelial actin-binding protein (ABP-280, nonmuscle filamin): a molecular leaf spring. J Cell Biol. 1990;111(3):1089–1105. doi: 10.1083/jcb.111.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneveld AB. Vascular pharmacology of acute lung injury and acute respiratory distress syndrome. Vascul Pharmacol. 2002;39(4–5):247–256. doi: 10.1016/s1537-1891(03)00013-2. [DOI] [PubMed] [Google Scholar]
- Ha CH, Bennett AM, Jin ZG. A novel role of vascular endothelial cadherin in modulating c-Src activation and downstream signaling of vascular endothelial growth factor. J Biol Chem. 2008;283(11):7261–7270. doi: 10.1074/jbc.M702881200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly SP, Larson MK, Parise LV. The unique N-terminus of R-ras is required for Rac activation and precise regulation of cell migration 1. Mol Biol Cell. 2005;16(5):2458–2469. doi: 10.1091/mbc.E03-12-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinbara K, Goldfinger LE, Hansen M, Chou FL, Ginsberg MH. Ras GTPases: integrins’ friends or foes? Nat Rev Mol Cell Biol. 2003;4(10):767–776. doi: 10.1038/nrm1229. [DOI] [PubMed] [Google Scholar]
- Kolosova IA, Mirzapoiazova T, Moreno-Vinasco L, Sammani S, Garcia JG, Verin AD. Protective effect of purinergic agonist ATPgammaS against acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;294(2):L319–324. doi: 10.1152/ajplung.00283.2007. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Ruoslahti E. R-Ras is a global regulator of vascular regeneration that suppresses intimal hyperplasia and tumor angiogenesis 2. Nat Med. 2005;11(12):1346–1350. doi: 10.1038/nm1324. [DOI] [PubMed] [Google Scholar]
- Kusama T, Mukai M, Tatsuta M, Nakamura H, Inoue M. Inhibition of transendothelial migration and invasion of human breast cancer cells by preventing geranylgeranylation of Rho. Int J Oncol. 2006;29(1):217–223. [PubMed] [Google Scholar]
- Kyndt F, Gueffet JP, Probst V, Jaafar P, Legendre A, Le BF, Toquet C, Roy E, McGregor L, Lynch SA, Newbury-Ecob R, Tran V, Young I, Trochu JN, Le MH, Schott JJ. Mutations in the gene encoding filamin A as a cause for familial cardiac valvular dystrophy 2. Circulation. 2007;115(1):40–49. doi: 10.1161/CIRCULATIONAHA.106.622621. [DOI] [PubMed] [Google Scholar]
- Matter ML, Zhang Z, Nordstedt C, Ruoslahti E. The alpha5beta1 integrin mediates elimination of amyloid-beta peptide and protects against apoptosis. J Cell Biol. 1998;141(4):1019–1030. doi: 10.1083/jcb.141.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha DR, Myers CL, Schaeffer RC., Jr Endothelial contraction and monolayer hyperpermeability are regulated by Src kinase. Am J Physiol Heart Circ Physiol. 2003;284(3):H994–H1002. doi: 10.1152/ajpheart.00862.2002. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Hartwig JH. Actin filament cross-linking by chicken gizzard filamin is regulated by phosphorylation in vitro. Biochemistry. 1995;34(20):6745–6754. doi: 10.1021/bi00020a020. [DOI] [PubMed] [Google Scholar]
- Petrache I, Crow MT, Neuss M, Garcia JG. Central involvement of Rho family GTPases in TNF-alpha-mediated bovine pulmonary endothelial cell apoptosis. Biochem Biophys Res Commun. 2003;306(1):244–249. doi: 10.1016/s0006-291x(03)00945-8. [DOI] [PubMed] [Google Scholar]
- Petrache I, Verin AD, Crow MT, Birukova A, Liu F, Garcia JG. Differential effect of MLC kinase in TNF-alpha-induced endothelial cell apoptosis and barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1168–1178. doi: 10.1152/ajplung.2001.280.6.L1168. [DOI] [PubMed] [Google Scholar]
- Popoff MR, Geny B. Multifaceted role of Rho, Rac, Cdc42 and Ras in intercellular junctions, lessons from toxins. Biochim Biophys Acta. 2009;1788(4):797–812. doi: 10.1016/j.bbamem.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Potter MD, Barbero S, Cheresh DA. Tyrosine phosphorylation of VE-cadherin prevents binding ofp120-and beta-catenin and maintains the cellular mesenchymal state. J Biol Chem. 2005;280(36):31906–31912. doi: 10.1074/jbc.M505568200. [DOI] [PubMed] [Google Scholar]
- Retta SF, Cassara G, D’Amato M, Alessandro R, Pellegrino M, Degani S, De Leo G, Silengo L, Tarone G. Cross talk between beta(1) and alpha(V) integrins: beta(1) affects beta(3) mRNA stability. Mol Biol Cell. 2001;12(10):3126–3138. doi: 10.1091/mbc.12.10.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallee JL, Wittchen ES, Burridge K. Regulation of cell adhesion by protein-tyrosine phosphatases: II. Cell-cell adhesion. J Biol Chem. 2006;281(24):16189–16192. doi: 10.1074/jbc.R600003200. [DOI] [PubMed] [Google Scholar]
- Schiestl RH, Gietz RD. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989;16(5–6):339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- Schulz B, Pruessmeyer J, Maretzky T, Ludwig A, Blobel CP, Saftig P, Reiss K. ADAM10 regulates endothelial permeability and T-Cell transmigration by proteolysis of vascular endothelial cadherin. Circ Res. 2008;102(10):1192–1201. doi: 10.1161/CIRCRESAHA.107.169805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serban D, Leng J, Cheresh D. H-ras regulates angiogenesis and vascular permeability by activation of distinct downstream effectors. Circ Res. 2008;102(11):1350–1358. doi: 10.1161/CIRCRESAHA.107.169664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seye CI, Yu N, Gonzalez FA, Erb L, Weisman GA. The P2Y2 nucleotide receptor mediates vascular cell adhesion molecule-1 expression through interaction with VEGF receptor-2 (KDR/Flk-1) J Biol Chem. 2004;279(34):35679–35686. doi: 10.1074/jbc.M401799200. [DOI] [PubMed] [Google Scholar]
- Stockton RA, Shenkar R, Awad IA, Ginsberg MH. Cerebral cavernous malformations proteins inhibit Rho kinase to stabilize vascular integrity. J Exp Med. 2010;207(4):881–896. doi: 10.1084/jem.20091258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel TP, Hartwig JH. Filling gaps in signaling to actin cytoskeletal remodeling. Dev Cell. 2003;4(4):444–445. doi: 10.1016/s1534-5807(03)00098-4. [DOI] [PubMed] [Google Scholar]
- Sun J, Ohkanda J, Coppola D, Yin H, Kothare M, Busciglio B, Hamilton AD, Sebti SM. Geranylgeranyltransferase I inhibitor GGTI-2154 induces breast carcinoma apoptosis and tumor regression in H-Ras transgenic mice. Cancer Res. 2003;63(24):8922–8929. [PubMed] [Google Scholar]
- Takaya A, Kamio T, Masuda M, Mochizuki N, Sawa H, Sato M, Nagashima K, Mizutani A, Matsuno A, Kiyokawa E, Matsuda M. R-Ras regulates exocytosis by Rgl2/Rlf-mediated activation of RalA on endosomes. Mol Biol Cell. 2007;18(5):1850–1860. doi: 10.1091/mbc.E06-08-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley JH, Hunter FA, Childs EW. PKC and MLCK-Dependent, Cytokine-Induced Rat Coronary Endothelial Dysfunction. J Surg Res. 2008 doi: 10.1016/j.jss.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan H, Opoku-Ansah J, Pastorino S, Renganathan H, Matter ML, Ramos JW. ERK MAP kinase is targeted to RSK2 by the phosphoprotein PEA-15 1. ProcNatlAcadSciUSA. 2007;104(50):19837–19842. doi: 10.1073/pnas.0704514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nieuw Amerongen GP, van Hinsbergh VW. Endogenous RhoA inhibitor protects endothelial barrier. Circ Res. 2007;101(1):7–9. doi: 10.1161/CIRCRESAHA.107.156513. [DOI] [PubMed] [Google Scholar]
- Wang B, Zou JX, Ek-Rylander B, Ruoslahti E. R-Ras contains a proline-rich site that binds to SH3 domains and is required for integrin activation by R-Ras. J Biol Chem. 2000;275(7):5222–5227. doi: 10.1074/jbc.275.7.5222. [DOI] [PubMed] [Google Scholar]
- Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437(7058):497–504. doi: 10.1038/nature03987. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci. 2001;114(Pt 7):1343–1355. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vascul Pharmacol. 2002;39(4–5):187–199. doi: 10.1016/s1537-1891(03)00008-9. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Vuori K, Wang H, Reed JC, Ruoslahti E. Integrin activation by R-ras 2. Cell. 1996;85(1):61–69. doi: 10.1016/s0092-8674(00)81082-x. [DOI] [PubMed] [Google Scholar]
- Zhou X, Boren J, Akyurek LM. Filamins in cardiovascular development 1. Trends Cardiovasc Med. 2007;17(7):222–229. doi: 10.1016/j.tcm.2007.08.001. [DOI] [PubMed] [Google Scholar]