Abstract
Mice with global deletion of one BDNF allele, or with forebrain-restricted deletion of both alleles show elevated aggression, but this phenotype is accompanied by other behavioral changes, including increases in anxiety and deficits in cognition. Here, we performed behavioral characterization of conditional BDNF knockout mice generated using a Cre recombinase driver line, KA1-Cre, which expresses Cre in few areas of brain: highly at hippocampal area CA3, moderately in dentate gyrus, cerebellum and facial nerve nucleus. The mutant animals exhibited elevated conspecific aggression and social dominance, but did not show changes in anxiety-like behaviors assessed using the elevated plus maze and open field test. There were no changes in depression like behaviors tested in the forced swim test, but small increase in immobility in the tail suspension test. In cognitive tasks, mutants showed normal social recognition and normal spatial and fear memory, but exhibited a deficit in object recognition. Thus, this knockout can serve as a robust model of BDNF-dependent aggression and object recognition deficiency.
Keywords: aggression, BDNF, animal model, knockout, Cre-recombinase
Introduction
BDNF is involved in several neuronal functions, including neuronal maturation, axonal and dendritic branching, and regeneration (Abidin et al., 2008, Binder & Scharfman, 2004, Yamada et al., 2002). This neurotrophin also affects synaptic transmission and plasticity (Jin et al., 2003, Poo, 2001, Tanaka et al., 2008) and has been implicated in modulation of learning (Bekinschtein et al., 2008, Gorski et al., 2003, Linnarsson et al., 1997) as well as anxiety- (Li et al., 2008, Rios et al., 2001) and depression-like behaviors (Duman & Monteggia, 2006, Shirayama et al., 2002). The effects of BDNF depend on the brain regions having altered secretion. For example, BDNF reduces depression-like behaviors when infused in the hippocampal dentate gyrus (Shirayama et al., 2002), but increases depression-like behavior (Eisch et al., 2003) and social aversion (Berton et al., 2006) when expressed in the nucleus accumbens. Thus, genetic manipulations of BDNF expression generally result in complex behavioral changes comprising several phenotypes.
Here, we made an attempt to dissociate BDNF-dependent phenotypes by generating mice with a more restricted BDNF knockout, which involves hippocampal area CA3, one of the few brain regions with high expression of BDNF. The BDNF knockout mice were generated using a kainate receptor promoter driven line of cre (KA1-Cre) for the first time. This line exhibits high levels of Cre-expression in the hippocampal area CA3, moderate expression in the dentate gyrus and facial nuclei, and low expression in the anterodorsal thalamus, cerebellar granule cell layers, and vestibular nuclei (Nakashiba et al., 2008). Surprisingly, despite significant loss of hippocampal BDNF, mutant mice did not display major deficits in several hippocampus-dependent tasks, except the object recognition task, but exhibited high levels of aggression and thus may serve as a robust animal model of BDNF-dependent aggressive behaviors and object recognition deficit.
Materials and methods
Generation of BDNF knockout mice
Mice with floxed BDNF gene were made from 129S6 embryonic stem cells (Zakharenko et al., 2003) and backcrossed to C57BL/6J background animals a minimum of 6 generations. The transgenic BAC KA1-driver line, which has intact endogenous KA-1 receptor alleles, was made from C57BL/6J embryonic stem cells (Nakazawa et al., 2002) and was maintained on C57BL/6 background. The two lines were crossed to obtain heterozygous BDNF floxed Cre-positive males (BDNF f+, Cre), which were then crossed to heterozygous BDNF floxed females (BDNF f+). The resulting homozygous BDNF floxed cre-positive (BDNF ff, Cre) males and homozygous BDNF floxed cre-negative (BDNF ff) females were crossed to obtain BDNF ff, Cre animals further referred to as KO, and BDNF ff animals referred to as WT. The presence of Cre and floxed BDNF alleles was determined as previously described (Zakharenko et al., 2003). Only male KO and WT mice were tested in experiments.
Biochemistry
In situ hybridization with BDNF antisense oligo-DNA probe was performed as previously described (Zakharenko et al., 2003). In situ images on X-ray films were quantified using ImageJ software as follows. Mean optical density of pixels in areas CA1, CA3, dentate gyrus and perirhinal cortex was determined. Following background subtraction, all values were normalized to the values for perirhinal cortex from the same section yielding arbitrary units of BDNF mRNA expression.
BDNF ELISA was done using BDNF EmaxR ImmunoAssay System (Promega, Madison, WI) following the manufacturer’s protocol. After mice were decapitated by cervical dislocation, hippocampi and frontal cortices were rapidly dissected and homogenized in 300 µl of extraction P-buffer (0.1 M sodium phosphate, pH 7.0, 500 mM NaCl, 0.2 % Triton X-100, 2 mM EDTA, 200 µM PMSF, 10 µM leupeptin, 0.3 µM aprotinin) by passing the tissue through a 27 G syringe needle 10 times, and separating the soluble fraction by 10 min centrifugation at 14000 g at 4 °C. The extracts from individual mice (60 µg of total protein per well) were tested in duplicates.
Free testosterone measurements in sera from the trunk blood were performed using Free Testosterone RIA kit (Beckman Coulter, Brea, CA) following manufacturer instructions.
Behavioral procedures
All mice were housed in a normal 12:12 h dark-light cycle with feed and water provided ad libitum. All experiments were approved by the National Institute of Mental Health Animal Care and Use Committee and NIH standards of housing and animal welfare were followed. Tests were performed on WT and KO male littermates at 2–4 months of age; each animal cohort was used only in one test. Ovariectomized wild type 129S6 (Taconic) females at >6 weeks of age were used as stimulus animals in the social recognition test. The experimenter was blind to the genotypes.
Intruder test
For the resident-intruder test (Winslow & Miczek, 1983), resident animals were housed in cages divided by a partition perforated with 1 mm holes, one animal per partition, beginning from p25. Such housing resulted in more stable levels of aggression when compared to single housing in a cage without a partition, possibly due to “the instigation effect” (De Almeida et al., 2005) of limited olfactory and auditory interaction between animals. Naïve resident animals were confronted with an intruder for 10 min in the home cage. The intruders were group-housed 8 week old C57BL/6J males and were always smaller than residents. Latency to the first aggressive bout and the numbers of aggressive bouts were quantified as described (Ogawa et al., 1999). An aggressive bout was defined as an attack that included either biting or wrestling. Offensive episodes separated by less than two seconds were considered as parts of the same aggressive bout. If no attack occurred during the 10 min period, the latency was recorded as 600 seconds.
Social dominance test
Social dominance relationships within pairs of animals in home cages were determined as described (Sa-Rocha et al., 2006). Mice were housed in pairs after weaning. On a testing day, mice were removed from their home cage, isolated in similar cages for 1 h and then returned to the home cage. Immediately after the return, animals were videotaped for 2 h and the duration of offensive (biting, wrestling, chasing and aggressive grooming), submissive (submissive posture and fleeing) and social interest behaviors (following and sniffing of partner) were scored offline for the first 10 min after the reunion. The latency to huddling was also recorded. A dominant-subordinate relationship was considered established if subordinate behaviors were predominantly expressed by one animal of a pair, while offensive behaviors were mainly expressed by the dominant animal. Based on these criteria, one out of 6 WT-WT pairs and 2 out of 13 WT-KO pairs did not establish dominant-subordinate relationships and were not included in the analysis. A single WT-KO pair did not huddle and was excluded from the huddling analysis.
Tests for depression-like behaviors
Forced swim test (Porsolt et al., 1977) was performed as described (Borsini et al., 1989, Dulawa et al., 2004). Mice were placed in plastic cylinders filled with water at 24 °C, and videotaped for 10 min. The test was repeated 24 h later. Immobility was scored offline using ForcedSwimScan software (Clever Sys., Inc, Reston, VA). The tail suspension test (Steru et al., 1985), was performed as described (Duman et al., 2007). Immobility time throughout a 6-min session was quantified using CleverSystem Tail Suspension module (Clever Sys., Inc, Reston, VA). Immobility was defined as lack of movement except respiration and whisker movement.
Anxiety tests
The elevated plus maze test (Handley & Mithani, 1984) was carried out as previously described (Ramboz et al., 1998). Naïve mice were placed in the center of the maze facing a closed arm and the following behaviors were recorded during a 5 min observation period: latency of the first entry to an open arm, number of entries into open and closed arms and time spent in the open arms. The open field test (Denenberg & Morton, 1962) was performed essentially as described (Dulawa et al., 2004) in 70 × 70 cm plastic boxes. Animal movement was tracked using Ethovision video tracking system (Noldus, Leesburg, VA) for 30 min. Center was defined as an area distant from each wall by more than 17.5 cm (one fourth of the field). Illumination was at 20 or 800 lux.
Cognitive tests
The Morris water task (Morris, 1984) was performed essentially as described (Zakharenko et al., 2003). Pool diameter was 1.4 m. Temperature was kept at 22 °C. During 12 consecutive days of testing, animals were trained four times per day with a visible platform from day 1 to day 2, and followed by hidden platform from day 3 to day 12. At day 12, the last trial was replaced with a probe trial (60 sec). The contextual fear conditioning test (Fanselow, 1980) was performed as described (Bourtchuladze et al., 1994). On day 1, animals were placed in the fear conditioning chamber (Med Associates Inc., St. Albans, VT), received a single 2 sec 0.7 mA foot shock at the time point of 150 sec, and removed from the chamber 30 sec later. On day 2, animals were tested for contextual freezing in the training context. The training and analysis was done by FreezeFrame software (ActiMetrics, Wilmette, IL). The social recognition test (Gheusi et al., 1994) was performed using ovariectomized females as stimulus mice (Ferguson et al., 2001, Ferguson et al., 2000). Male test mice were individually housed for 2 weeks. During a session, the subject was exposed in the home cage to an unfamiliar ‘initial’ female for 5 min. Thirty min later, mice were tested by introducing the 'testing' female, either the same familiar female or another unfamiliar female for 5 min. The procedure was repeated 3 days later, and the mice that were presented with familiar females during the first session were given unfamiliar females and vice versa, in such a way that the presentation order was balanced across sessions and genotypes. The duration of exploratory behaviors, which included nosing, sniffing, following and pursuit, was recorded; meanwhile, grooming, aggressive posturing, and sexual behaviors were excluded. For each animal we calculated a recognition index R = [1 − (ttest-F / tinitial-F) / (ttest-unF / tinitial-unF)] * 100, where tinitial-F and ttest-F are the exploration times of the initial/testing female when tested with a familiar animal, and tinitial-unF and ttest-unF are the times when tested with an unfamiliar animal. R equals to 100 if animal does not explore a familiar female, and R equals to 0 if exploration time of familiar and unfamiliar females does not differ when normalized to the exploration time of the respective ‘initial’ females. The object recognition test (Ennaceur & Delacour, 1988) was performed as described (Mansuy et al., 1998). Mice were single housed for 5 days. An opaque square box (36 × 36 cm) under red light was used. Two out of three objects (falcon tube, centrifuge bottle, and a beaker with a yellow cap) were chosen randomly and placed inside the box away from the corners. A mouse was tested in the box for 10 min or until the mouse jumped on top of an object, while time spent for investigating each object was counted. Thirty minutes after the first session, the same animal was tested again with one object replaced with the third one in a random fashion. The preference index (PI) was calculated as follows: time investigating a novel object was divided by total investigating time and multiplied by 200 (Mansuy et al., 1998).
Food consumption
Food consumption was determined by housing animals individually and weighing their food daily over 2 days.
Statistical methods
Statistical comparisons were performed using two-tail unpaired t-test (if not specified), one sample t-test (social / object recognition and Morris water maze), repeated measure ANOVA (Morris water maze) and the Mantel-Cox test (survival curve). The significance cut off criteria was p<0.05. Data represent means ± s.e.m.
Results
Generation of mice lacking BDNF in restricted areas of brain using Cre driver line KA1-Cre
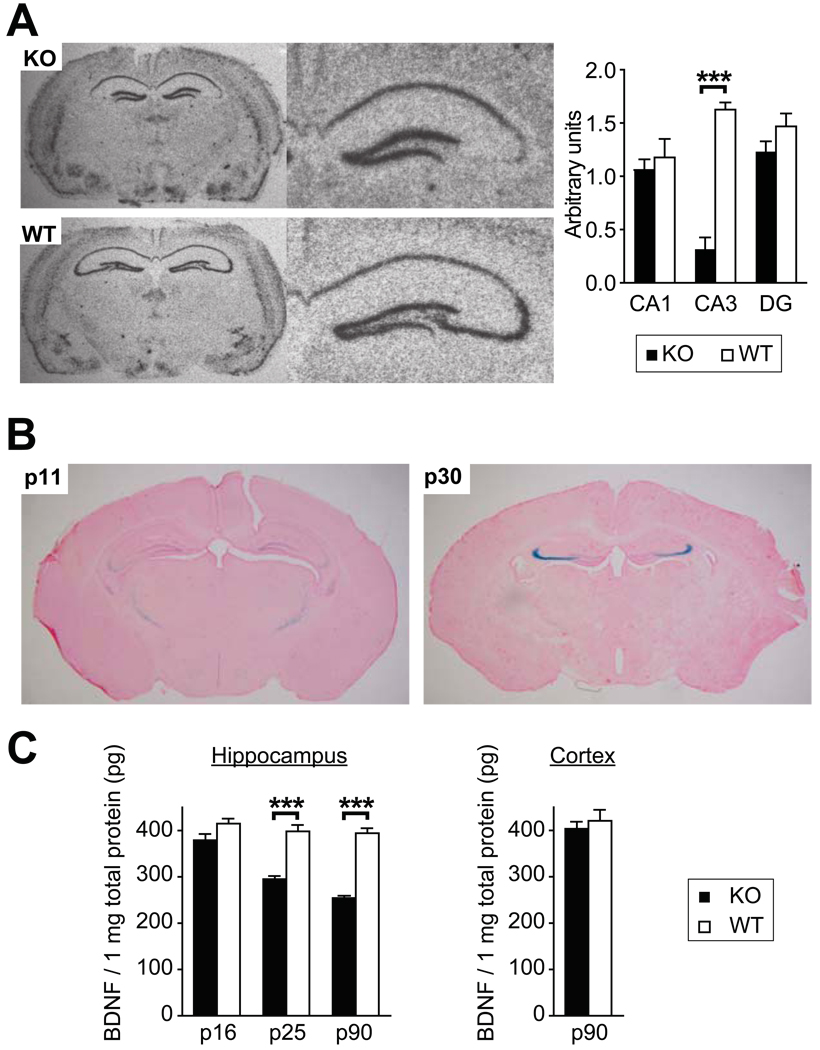
Mice carrying two floxed BDNF alleles were crossed with a Cre driver line KA1-Cre (Nakazawa et al., 2002), which has the most prominent Cre expression in the hippocampal area CA3, moderate expression in the dentate gyrus, cerebellum and facial nuclei and diffuse expression across the brain areas and peripheral tissues [see the online database (Gene_Expression_Database_(Gxd)) for comprehensive characterization of Cre activity in this line.] By postnatal day 60 (p60), expression of BDNF mRNA decreased in CA3 pyramidal layer (t9 = 10.55, p < 0.001), but did not change significantly in CA1 and dentate gyrus (Fig. 1A). There were no visible changes outside the hippocampus. To determine the onset period of Cre-recombinase activity, the KA1-Cre line was crossed with Rosa26 lacZ reporter mice (Soriano, 1999). As reported (Nakazawa et al., 2002), recombinase activity was detected in CA3 pyramidal layer at p30, but not at p11 (Fig. 1B). Accordingly, the amount of BDNF protein measured by ELISA in extracts from whole hippocampi remained similar between wild type (WT) and KO mice at p16, but became significantly lower in KO mice at p25 (t6 = 6.7, p < 0.001) and at p90 (t18 = 11.9, p < 0.001). However, the levels of BDNF protein in the cortex did not differ between genotypes even at p90 (Fig. 1C). Two and 3 month old KO mice were not different from WT littermates in body weight and food consumption per day (data not shown).
Figure 1.
KA1-CRE Cre-line deletes BDNF gene in the hippocampal area CA3. (A) Left: Loss of hippocampal CA3 BDNF mRNA transcripts as determined by in situ hybridization using brain slices from p60 KO (upper) and WT (lower) mice. Right: Quantification of in situ from 6 WT and 5 KO brains in areas CA1, CA3 and dentate gyrus (DG). (B) Activity of Cre-recombinase detected by Rosa-LacZ reporter in p11 (left) and p30 (right) mice. (C) Amounts of BDNF protein measured by ELISA in the total hippocampal extracts from p16, p25 and p90 animals (left) and in the cortical extracts from p90 animals (right). ***p < 0.001.
Elevated aggression in BDNF KO mice
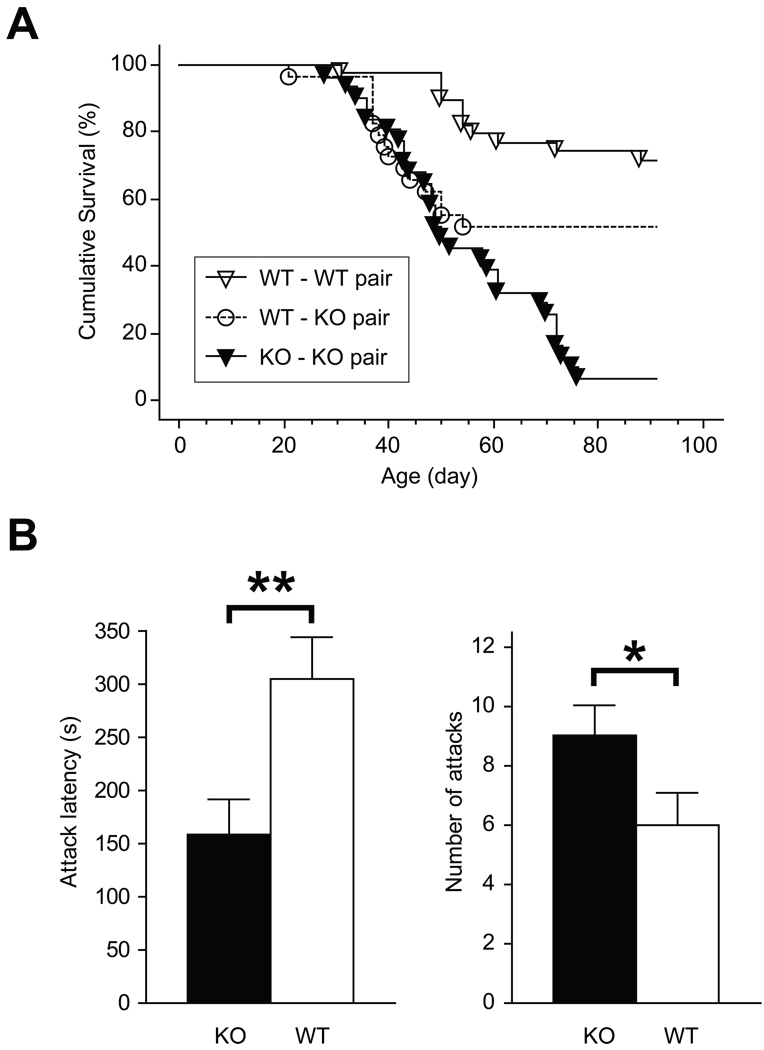
Male mice housed with their KO littermates manifested a high occurrence of bite injuries. No such injuries were observed in group-housed mice with female KO littermates. We tracked the appearance of injuries in littermates housed in pairs of three genotype combinations: WT-WT (39 pairs), WT-KO (30 pairs) and KO-KO (31 pairs). Animals were monitored daily until p91 and separated immediately following the appearance of bite wounds. By p50, 52 % of KO-KO, 48 % of KO-WT and 10 % of WT-WT pairs were engaged in injurious fights; by p91, the number of fighting pairs reached 94 % in KO-KO, 52% in KO-WT and 28% in WT-WT combinations. The aggressors in KO-WT pairs were always KO mice. Cumulative survival (the fraction of pairs without fighting injuries) in WT-WT pairs exceeded the cumulative survival in WT-KO and KO-KO pairs (WT-WT versus WT-KO: DF=1, χ2 = 4.7, p = 0.029; WT-WT versus KO-KO: DF=1, χ2 = 36.3, p < 0.001, the Mantel-Cox test), and the cumulative survival in WT-KO pairs exceeded that in KO-KO pairs (DF=1, χ2 = 7.9, p = 0.005) (Fig. 2A). In KO-KO pairs, fighting injuries were always observed only on one animal and attacks were initiated by only one animal too, which suggests that a weaker mouse in a pair acquired a submissive status and did not return the attacks.
Figure 2.
BDNF knockout exhibit elevated aggression. (A) Cumulative survival curves representing the percentage of littermate pairs (WT-WT, WT-KO, and KO-KO) without injuries from home cage fighting. (B) Attack latency (left) and number of attacks (right) of WT and KO mice tested in the resident-intruder paradigm. *p < 0.05, **p < 0.01.
To assess territorial aggression, we tested 34 KO and 34 WT mice, which were singly housed and naïve, in the resident-intruder paradigm. The KO animals were more aggressive than their wild type counterparts; KO mice showed a shorter latency to the first attack (t66 = 2.85, p = 0.006) and demonstrated more attacks during the 10 min observation time (t66 = 2.42, p = 0.018) (Fig. 2B).
Given that KA1-Cre mice exhibit Cre activity in the testis (Gene_Expression_Database_(Gxd)), where BDNF is highly expressed (Pruunsild et al., 2007), and that changes in testosterone levels may increase aggression (Siegel, 2005), we measured free testosterone in sera and found no differences between WT and KO mice (data not shown).
BDNF KO mice are dominant in the home cage even before the onset of aggression
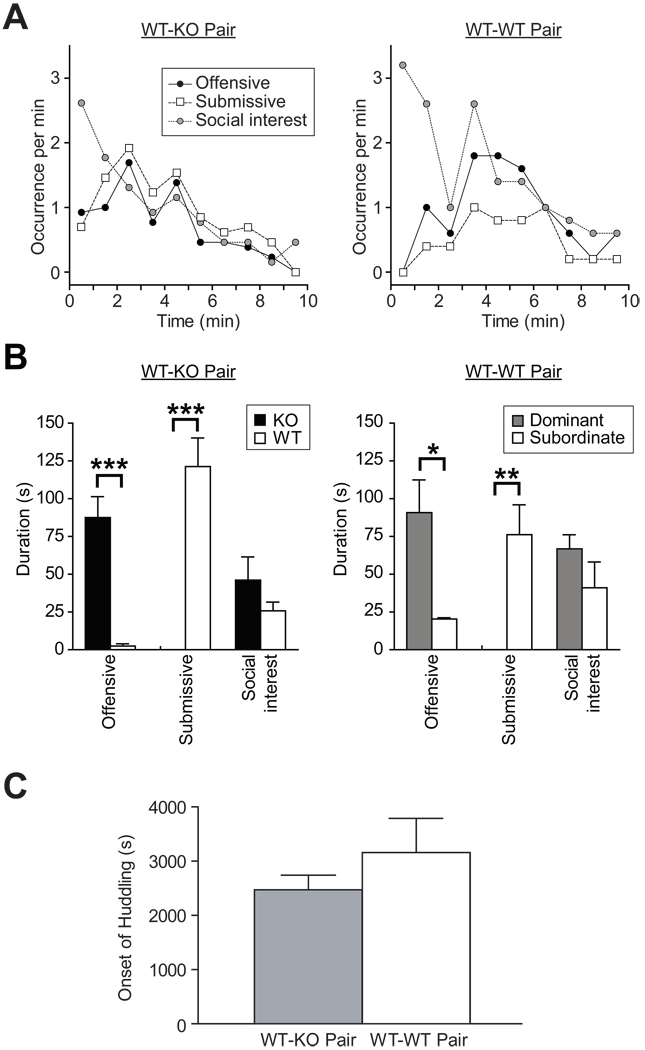
Next, we examined the social status, either dominant or subordinate, of mice housed in WT-KO pairs before the onset of injurious fights by observing brief confrontations between animals during reunion after temporary separation (1 hr) (Sa-Rocha et al., 2006). Upon reunion, mice were immediately engaged in behaviors that we classified as social interest, offensive, or submissive (see each definition in Materials and Methods) (Cairns & Nakelski, 1971, Crawley et al., 1975). Typically, social interest behaviors prevailed in both animals during the first 2 minutes. Offensive behaviors followed and were expressed initially by both mice as well; however, expression of submissive behaviors by one of the paired animal led to a cessation of aggression (Fig. 3A left). After about 10 minutes of these robust interactions, mice reduced their offensive/submissive and exploratory activities, began allo- or self-grooming, and huddled together (Movie S1). This typical sequence of behaviors allowed distinction between dominant and subordinate animals, of which subordinates showed little or no offensive behaviors and submitted to their opponents (Sa-Rocha et al., 2006).
Figure 3.
Increased dominance in BDNF knockout mice (A) Patterns of behavior induced by brief separation and reunion in WT-KO (left) and WT-WT (right) pairs: occurrence (per minute) of offensive (filled circles), submissive (open squares), and social interest (grey circles) behaviors is shown. (B) Durations of offensive, submissive, and social interest behaviors in WT-KO (left) and WT-WT (right) pairs. (C) Latency to huddling did not differ between WT-WT (open bar) and WT-KO (grey bar) pairs. *p < 0.05, **p < 0.01, ***p < 0.001.
Eleven out of 13 WT-KO pairs exhibited obvious dominant-subordinate relationships; the duration of offensive behaviors was higher in KO mice (t20 = −6.1, p < 0.001) and the expression of submissive behaviors was restricted to WT animals (t20 = 6.4, p < 0.001). The duration of social interest behaviors did not differ significantly between genotypes (Fig. 3B left).
To determine whether WT animals were able to become dominant we examined 6 WT-WT pairs, which showed similar behavioral repertoire and sequence with the WT-KO pairs (Fig. 3A right). Five of them exhibited clear dominant-subordinate relationships, in which offensive behaviors were expressed more by dominant mice (t8 = 3.2, p = 0.012), whereas submissive behaviors were found only in subordinate mice (t8 = 3.9, p = 0.005) (Fig. 3B right). The duration of social interest behaviors did not differ between dominant and subordinate WT mice (t8 = 1.3, p = 0.22). In addition, the latency to huddling did not differ between WT-WT (n = 5) and WT-KO (n = 10) pairs (Fig. 3C). Altogether, these observations indicate that KO mice dominate over WT animals even before the onset of injurious fighting, despite the capability of WT animals to become dominant over another mouse.
Depression- and anxiety-like behaviors in BDNF KO mice
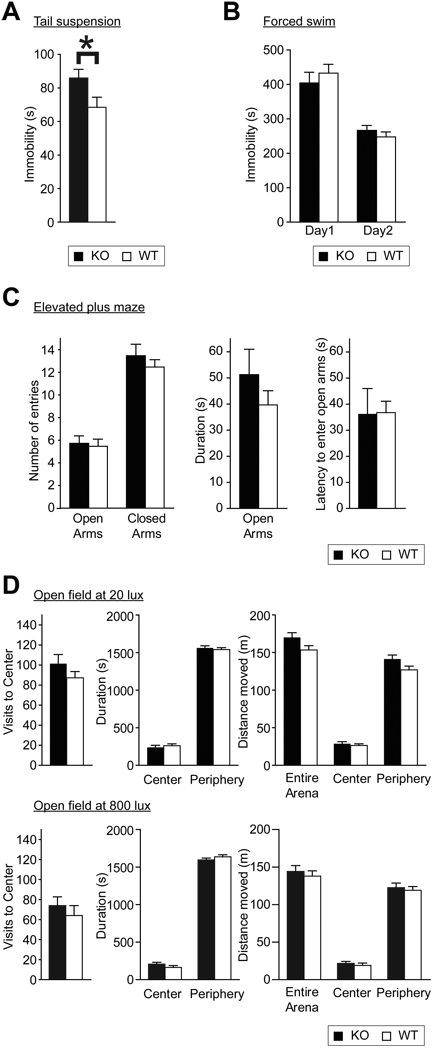
Depression-like behaviors were measured by the tail suspension and forced swim tests. In the tail suspension test, performed on a large group of animals (28 WT and 29 KO mice), KO mice showed 25 % higher immobility (t55 = 2.2, p=0.03); however, there was no difference between genotypes in the forced swim test (Fig. 4A, B). Anxiety-like behaviors were evaluated using the elevated plus maze and open field tests. WT and KO mice did not differ in the latency to enter open arms of the maze, number of visits to open arms, and the time spent in open arms (Fig. 4 C). The open field test was performed under 20 and 800 lux illumination. Under both levels of illumination, there was no difference between genotypes in the number of visits to center, in the distance traveled in center, in the time spent in center, and in total distance travelled during the first 10 min (data not shown) and during total 30 min of the test (Fig. 4 D), which indicated that anxiety-like behaviors and general locomotion were not altered by the mutation.
Figure 4.
Analysis of depression and anxiety-like behaviors in BDNF KO mice (A–B) Duration of immobility in KO and WT mice in tail-suspension test (A) and two consecutive days of forced swim test (B). (C) Numbers of entries into open and closed arms (left), time spent in open arms (middle) and latency to enter open arms (right) of KO and WT mice in elevated plus maze. (D) Number of visits into the center of the arena (left), time spent in the center and periphery (middle), and distances traveled in the center, periphery, and entire arena (right) of KO and WT mice in 30 min open field test under 20 (upper), or 800 (lower) lux illumination.
Mild cognitive deficit in BDNF KO mice
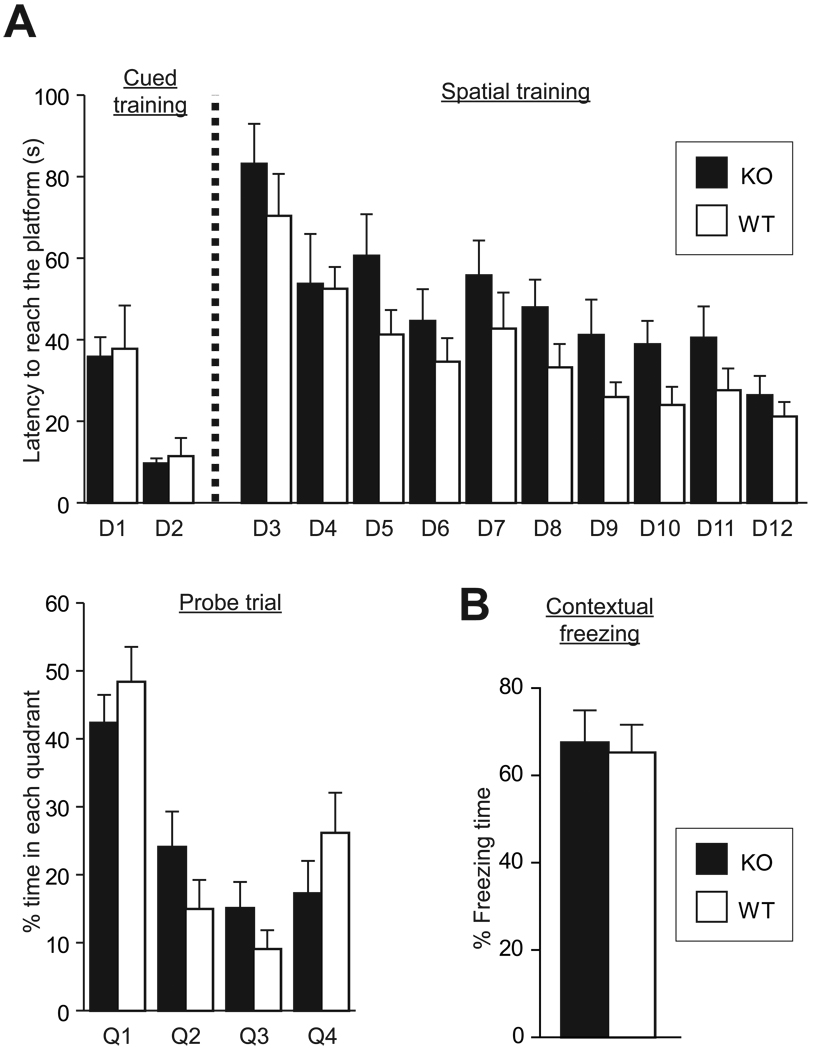
Next, we performed several cognitive tasks which depend on hippocampus. In the Morris water maze task, WT and KO mice showed similar robust learning during both cued and spatial training (repeated measure ANOVA, cued training, WT: n = 9, F(1,8) = 16.7, p = 0.004, KO: n = 8, F(1,7) = 25.0, p = 0.002; spatial training, WT: F(9, 8) = 6.9, p < 0.001 KO: F(9,7) = 4.1, p < 0.001), and ANOVA analysis did not detect a significant genotype*latency interaction). During probe trial, both genotypes spent more time in the target quadrant (one sample t-test, null hypothesis: % time in target quadrant = 25, WT: t8 = 4.52, p = 0.002, KO: p = 0.004), but did not differ from each other (Fig. 5A).
Figure 5.
Spatial and contextual fear memory are normal in BDNF KO mice. (A) Escape latency during cued and spatial training in the Morris water maze test (upper) and % time in four maze quadrants during probe trial on day 12 (lower). Q1 is the target quadrant. (B) % freezing time during testing phases of contextual fear conditioning.
During contextual fear conditioning, either genotype did not show significant freezing in the training context before the shock (data not shown). 24 h later in testing, both genotypes showed similar freezing to the training context (Fig. 5B).
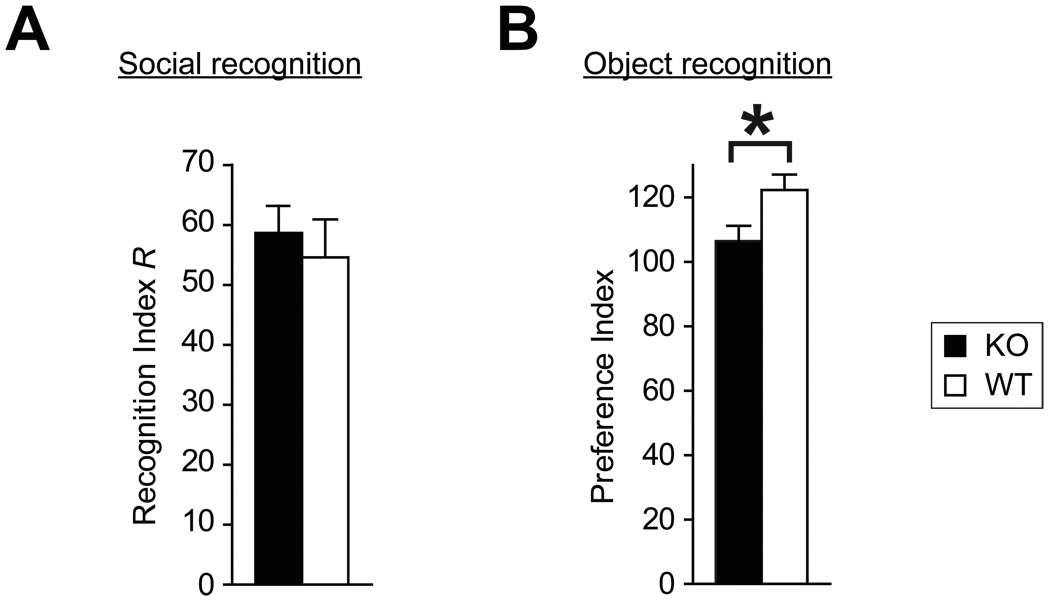
The social recognition test compares the duration of social investigation of a familiar versus unfamiliar ovariectomized female (Ferguson et al., 2001, Ferguson et al., 2000). Mice of both genotypes reduced exploration time when presented with a familiar female (one sample t-test, null hypothesis: recognition index R = 0, WT: t11 = 8.59, p < 0.001, KO: t11 = 13.04, p < 0.001), and R did not differ between genotypes, indicating that social recognition was not altered by the mutation (Fig. 6A).
Figure 6.
Normal social recognition but impaired object recognition in BDNF KO mice. (A) Recognition index R for both genotypes in social recognition test. (B) Preference index for both genotypes in object recognition test. *p < 0.05.
In the object recognition test, the initial exploration time of novel objects was 27 ± 3 seconds in WT and 30 ± 3 seconds in KO mice, which did not differ significantly. When presented with one new and one familiar objects, WT mice showed a significant preference to a new object, while KO mice did not (one sample t-test, null hypothesis: preference index = 100, WT: t13 = 4.67, p < 0.001, KO: t12 = 1.36, p = 0.19), and the preference index was higher in WT (t25 = 2.36, p = 0.027) (Fig. 6B).
Discussion
The present study characterizes a line of region-restricted BDNF knockout mice with a Cre driver line KA1-Cre, that exhibit no major behavioral changes except elevated aggression, a mild deficit in object recognition and higher immobility in the tail suspension, but not in the forced swim test for depression-like behaviors.
As BDNF acts in different brain areas, manipulation of BDNF expression in the brain has been found to alter several behaviors. Heterozygous BDNF knockout mice showed increased aggression (Lyons et al., 1999), but no changes in depression or anxiety-like behaviors (MacQueen et al., 2001). Forebrain-restricted knockout mice with EMX-Cre line showed similar phenotypes: increased aggression, no changes in depression or anxiety-like behaviors, and learning deficits (Gorski et al., 2003). Another forebrain-restricted BDNF knockout mouse with CaMKII-promoter driven Cre line exhibited hyperactivity, aggression, and elevated anxiety-like behaviors (Rios et al., 2001). In those mutants, it is difficult to dissociate aggression from other phenotypes, because of limited spatial and temporal restriction of the mutation. Recently published animal models, in which BDNF expression is altered in a more restricted manner, allow more targeted analysis of BDNF functions. For example, animals that received local delivery of BDNF protein or Cre-virus for BDNF deletion, showed more limited behavioral changes. Knockdown of BDNF in the nucleus accumbens prevented development of social aversion (Berton et al., 2006). BDNF infusion into the dentate gyrus had an antidepressant effect (Shirayama et al., 2002), whereas BDNF knockout from dentate gyrus or CA1 by AAV-Cre attenuated antidepressant actions of desipramine and citalopram (Adachi et al., 2008). Likewise, using inducible knockout of BDNF revealed that early developmental BDNF deletion results in more pronounced cognitive deficits (Monteggia et al., 2004), and analysis of BDNF Val66Met mutants showed deficit in the extinction, but not in acquisition or retention of the aversive memories (Yu et al., 2009). Recent work by Sakata et al. (Sakata et al., 2008) showed that elimination of promoter IV-driven BDNF transcription increases depression-like behaviors, reduces locomotion, but does not cause anxiety-like phenotype, which is another example of dissociation between BDNF-dependent behaviors achieved by selective alteration of BDNF expression.
In contrast to several mouse models with alterations in BDNF expression, our BDNF knockout had normal locomotion, several forms of memory, or anxiety-like behaviors, all of which are known to be modulated by BDNF. In the depression-like behaviors, our KO mice did not show changes in the forced swim test, but had 25 % higher immobility in the tail suspension test, which was also observed in mice lacking BDNF promoter IV (Sakata et al., 2008). Mice with the forebrain-restricted BDNF knockouts showed even greater immobility in the same test, but surprisingly low immobility in the forced swim test, possibly resulting from hyperactivity (Chan et al., 2006).
The elevated aggression in the mutant animals was evident from two observations: first, KO mice attacked and injured their home cage partners; second, singly housed KO mice exhibited shorter latency and more frequent attacks against an intruder. In addition, KO mice became dominant over WT home cage partners before the onset of the injurious attacks.
Certain memory deficits may lead to aggression. For example, loss of social memory, which requires hippocampus (Broadbent et al., 2004, Maaswinkel et al., 1996, Van Wimersma Greidanus & Maigret, 1996), can compromise the recognition between cage partners and cause confrontation. At the same time, loss of contextual memory, which also depends on hippocampus (Anagnostaras et al., 2001), may result in a perception of a familiar environment as more novel and thus hostile (Nelson & Trainor, 2007). However, it was surprising that KO mice, which lack BDNF gene in CA3 pyramidal cells, a major BDNF source in the hippocampus (Conner et al., 1997), did not show cognitive deficit when tested in social recognition, contextual fear conditioning, or spatial version of the Morris Water maze. Thus, the elevated aggression does not appear to be secondary to other deficits.
The only cognitive phenotype in KO mice was a deficit in object recognition, which is a hippocampus-dependent task (Mansuy et al., 1998, Myhrer, 1988a, Myhrer, 1988b). This finding, together with the observations of pattern completion deficiency in CA3-restricted NMDA receptor knockout mice (Nakazawa et al., 2002), suggests that interfering with the function of CA3 results in rather selective cognitive deficits.
It is intriguing that despite this deficit, KO mice displayed normal social recognition of ovariectomized females. Since social recognition in rodents utilizes olfaction as a key factor (Matochik, 1988) and involves distinct circuitries from object recognition (Caffe et al., 1987), this segregation might be due to differences of the modalities involved in each task and smaller contribution of the hippocampus in processing olfactory information.
Besides hippocampus, KA1-Cre line exhibits moderate Cre activity in the cerebellum and facial nerve nuclei (Nakashiba et al., 2008), which have not been implicated in aggression. Yet, our study does not exclude a possibility that Cre activity observed in the thalamus, brain stem or peripheral organs, even though diffuse and affecting small number of cells (Gene_Expression_Database_(Gxd)), may influence aggressive behaviors by deleting BDNF gene.
Several studies implicated hippocampus in aggression. In cats, electrical stimulation of the ventral hippocampus increases aggressive response to the electrical stimulation of hypothalamus (Siegel & Flynn, 1968). In mice, density of mossy fibers negatively correlates with aggression (Guillot et al., 1994). Moreover, hippocampus projects to the lateral septum, which suppress aggression, and to the medial hypothalamus, which enhances aggression (Siegel, 2005). Evidence of hippocampal involvement in aggression in rodents and cats has been recently complemented by a study in humans showing that hippocampal activity positively correlates with anger ruminations following verbal insults (Denson et al., 2009). Since deletion of BDNF gene in our KO mice is so prominent in the hippocampal CA3 pyramidal cells, the decrease in hippocampal BDNF could be one of the direct causes for the aggression. Finally, BDNF KO mice with relatively restricted behavioral phenotypes can be used as a tool to search for compounds with selective behavioral effects.
Supplementary Material
Movie S1: Representative sequence of behaviors in the social dominance test.
Acknowledgements
This research was supported by the NIMH Intramural Research Program. We thank Kazu Nakazawa and Susumu Tonegawa for KA1-Cre transgenic line and Daniel Sukato and Chao Yang for editorial work.
Footnotes
Supporting Information
Additional Supporting Information may be found online.
References
- Abidin I, Eysel UT, Lessmann V, Mittmann T. Impaired GABAergic inhibition in the visual cortex of brain-derived neurotrophic factor heterozygous knockout mice. J Physiol. 2008;586:1885–1901. doi: 10.1113/jphysiol.2007.148627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi M, Barrot M, Autry AE, Theobald D, Monteggia LM. Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol Psychiatry. 2008;63:642–649. doi: 10.1016/j.biopsych.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, Izquierdo I, Medina JH. BDNF is essential to promote persistence of long-term memory storage. Proc Natl Acad Sci U S A. 2008;105:2711–2716. doi: 10.1073/pnas.0711863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsini F, Lecci A, Sessarego A, Frassine R, Meli A. Discovery of antidepressant activity by forced swimming test may depend on pre-exposure of rats to a stressful situation. Psychopharmacology (Berl) 1989;97:183–188. doi: 10.1007/BF00442247. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffe AR, van Leeuwen FW, Luiten PG. Vasopressin cells in the medial amygdala of the rat project to the lateral septum and ventral hippocampus. J Comp Neurol. 1987;261:237–252. doi: 10.1002/cne.902610206. [DOI] [PubMed] [Google Scholar]
- Cairns RB, Nakelski JS. On fighting in mice: ontogenetic and experiential determinants. J Comp Physiol Psychol. 1971;74:354–364. doi: 10.1037/h0030584. [DOI] [PubMed] [Google Scholar]
- Chan JP, Unger TJ, Byrnes J, Rios M. Examination of behavioral deficits triggered by targeting Bdnf in fetal or postnatal brains of mice. Neuroscience. 2006;142:49–58. doi: 10.1016/j.neuroscience.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Schleidt WM, Contrera JF. Does social environment decrease propensity to fight in male mice? Behav Biol. 1975;15:73–83. doi: 10.1016/s0091-6773(75)92105-7. [DOI] [PubMed] [Google Scholar]
- De Almeida RM, Ferrari PF, Parmigiani S, Miczek KA. Escalated aggressive behavior: dopamine, serotonin and GABA. Eur J Pharmacol. 2005;526:51–64. doi: 10.1016/j.ejphar.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, Morton JR. Effects of environmental complexity and social groupings upon modification of emotional behavior. J Comp Physiol Psychol. 1962;55:242–246. doi: 10.1037/h0041116. [DOI] [PubMed] [Google Scholar]
- Denson TF, Pedersen WC, Ronquillo J, Nandy AS. The angry brain: neural correlates of anger, angry rumination, and aggressive personality. J Cogn Neurosci. 2009;21:734–744. doi: 10.1162/jocn.2009.21051. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry. 2007;61:661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Bolanos CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, Verhaagen J, Nestler EJ. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiatry. 2003;54:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Gene_Expression_Database_(GXD) Mouse Genome Informatics Web Site. [Retrieved on Sep. 2010];World Wide Web. (URL: http://www.informatics.jax.org/javawi2/servlet/WIFetch?page=alleleDetail&key=8631).
- Gheusi G, Blluthe RM, Goodall G, Dantzer R. Social and individual recognition in rodents: methodological aspects and neurobiological basis. Behavioral Processes. 1994;33:59–88. doi: 10.1016/0376-6357(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Gorski JA, Balogh SA, Wehner JM, Jones KR. Learning deficits in forebrain-restricted brain-derived neurotrophic factor mutant mice. Neuroscience. 2003;121:341–354. doi: 10.1016/s0306-4522(03)00426-3. [DOI] [PubMed] [Google Scholar]
- Guillot PV, Roubertoux PL, Crusio WE. Hippocampal mossy fiber distributions and intermale aggression in seven inbred mouse strains. Brain Res. 1994;660:167–169. doi: 10.1016/0006-8993(94)90852-4. [DOI] [PubMed] [Google Scholar]
- Handley SL, Mithani S. Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of 'fear'-motivated behaviour. Naunyn Schmiedebergs Arch Pharmacol. 1984;327:1–5. doi: 10.1007/BF00504983. [DOI] [PubMed] [Google Scholar]
- Jin X, Hu H, Mathers PH, Agmon A. Brain-derived neurotrophic factor mediates activity-dependent dendritic growth in nonpyramidal neocortical interneurons in developing organotypic cultures. J Neurosci. 2003;23:5662–5673. doi: 10.1523/JNEUROSCI.23-13-05662.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnarsson S, Bjorklund A, Ernfors P. Learning deficit in BDNF mutant mice. Eur J Neurosci. 1997;9:2581–2587. doi: 10.1111/j.1460-9568.1997.tb01687.x. [DOI] [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaswinkel H, Baars AM, Gispen WH, Spruijt BM. Roles of the basolateral amygdala and hippocampus in social recognition in rats. Physiol Behav. 1996;60:55–63. doi: 10.1016/0031-9384(95)02233-3. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Ramakrishnan K, Croll SD, Siuciak JA, Yu G, Young LT, Fahnestock M. Performance of heterozygous brain-derived neurotrophic factor knockout mice on behavioral analogues of anxiety, nociception, and depression. Behav Neurosci. 2001;115:1145–1153. doi: 10.1037//0735-7044.115.5.1145. [DOI] [PubMed] [Google Scholar]
- Mansuy IM, Mayford M, Jacob B, Kandel ER, Bach ME. Restricted and regulated overexpression reveals calcineurin as a key component in the transition from short-term to long-term memory. Cell. 1998;92:39–49. doi: 10.1016/s0092-8674(00)80897-1. [DOI] [PubMed] [Google Scholar]
- Matochik JA. Role of the main olfactory system in recognition between individual spiny mice. Physiol Behav. 1988;42:217–222. doi: 10.1016/0031-9384(88)90073-x. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, Meuth S, Nagy A, Greene RW, Nestler EJ. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci U S A. 2004;101:10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Myhrer T. Exploratory behavior and reaction to novelty in rats with hippocampal perforant path systems disrupted. Behav Neurosci. 1988a;102:356–362. doi: 10.1037//0735-7044.102.3.356. [DOI] [PubMed] [Google Scholar]
- Myhrer T. The role of medial and lateral hippocampal perforant path lesions and object distinctiveness in rats' reaction to novelty. Physiol Behav. 1988b;42:371–377. doi: 10.1016/0031-9384(88)90279-x. [DOI] [PubMed] [Google Scholar]
- Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science. 2008;319:1260–1264. doi: 10.1126/science.1151120. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Chester AE, Gustafsson JA, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor beta gene-deficient (betaERKO) male and female mice. Proc Natl Acad Sci U S A. 1999;96:12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Pruunsild P, Kazantseva A, Aid T, Palm K, Timmusk T. Dissecting the human BDNF locus: bidirectional transcription, complex splicing, and multiple promoters. Genomics. 2007;90:397–406. doi: 10.1016/j.ygeno.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci U S A. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, Lechan RM, Jaenisch R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- Sa-Rocha VM, Sa-Rocha LC, Palermo-Neto J. Variations in behavior, innate immunity and host resistance to B16F10 melanoma growth in mice that present social stable hierarchical ranks. Physiol Behav. 2006;88:108–115. doi: 10.1016/j.physbeh.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Sakata K, Jin L, Jha S. Lack of promoter IV-driven BDNF transcription results in depression-like behavior. Genes Brain Behav. 9:712–721. doi: 10.1111/j.1601-183X.2010.00605.x. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel A. The Neurobilogy of Aggression and Rage. Boca Raton: CRC Press; 2005. [Google Scholar]
- Siegel A, Flynn JP. Differential effects of electrical stimulation and lesions of the hippocampus and adjacent regions upon attack behavior in cats. Brain Res. 1968;7:252–267. doi: 10.1016/0006-8993(68)90102-9. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Horiike Y, Matsuzaki M, Miyazaki T, Ellis-Davies GC, Kasai H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008;319:1683–1687. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wimersma Greidanus TB, Maigret C. The role of limbic vasopressin and oxytocin in social recognition. Brain Res. 1996;713:153–159. doi: 10.1016/0006-8993(95)01505-1. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Miczek KA. Habituation of aggression in mice: pharmacological evidence of catecholaminergic and serotonergic mediation. Psychopharmacology (Berl) 1983;81:286–291. doi: 10.1007/BF00427564. [DOI] [PubMed] [Google Scholar]
- Yamada MK, Nakanishi K, Ohba S, Nakamura T, Ikegaya Y, Nishiyama N, Matsuki N. Brain-derived neurotrophic factor promotes the maturation of GABAergic mechanisms in cultured hippocampal neurons. J Neurosci. 2002;22:7580–7585. doi: 10.1523/JNEUROSCI.22-17-07580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Wang Y, Pattwell S, Jing D, Liu T, Zhang Y, Bath KG, Lee FS, Chen ZY. Variant BDNF Val66Met polymorphism affects extinction of conditioned aversive memory. J Neurosci. 2009;29:4056–4064. doi: 10.1523/JNEUROSCI.5539-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharenko SS, Patterson SL, Dragatsis I, Zeitlin SO, Siegelbaum SA, Kandel ER, Morozov A. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1–CA3 synapses. Neuron. 2003;39:975–990. doi: 10.1016/s0896-6273(03)00543-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1: Representative sequence of behaviors in the social dominance test.