Abstract
Glycoprotein 130 (Gp130) cytokines are involved in the regulation of numerous biological processes, including hematopoiesis, immune response, inflammation, cardiovascular action, and neuronal survival. These cytokines share gp130 as a common signal transducer in their receptor complex and typically activate signal transducer and activator of transcription (STAT) 3. Studies have shown that several gp130 cytokines have differential effects on both adipogenesis and insulin-stimulated glucose uptake. Yet, the complex interactions of these cytokines in adipose tissue have not been studied. Gp130 cytokines are differentially regulated in multiple tissues due to the presence of additional receptor components that are required for signaling, including the leukemia inhibitory factor receptor (LIFR). Previous studies from our laboratory highlighted the ability of specific gp130 cytokines to crosstalk in adipocytes that correlated with LIFR degradation. Crosstalk is defined as the ability of one cytokine to modulate the signaling of another cytokine. Our novel studies reveal that white adipose tissue is highly responsive to gp130 cytokines, and we provide the first evidence that these cytokines can exert inhibitory crosstalk in adipose tissue in vivo. Moreover, several gp130 cytokines that use the LIFR, including cardiotrophin-1 (CT-1), LIF, and human oncostatin M (hOSM), can alter the subsequent signaling of other family members in adipocytes both in vitro and in vivo. Our data also show that murine OSM and neuropoietin do not crosstalk in the same manner as other gp130 cytokines, which likely results from their inability to activate the LIFR. Overall, we have observed distinctive patterns of crosstalk signaling by gp130 cytokines in adipocytes in vitro and in vivo and demonstrate the crosstalk is not dependent on new protein synthesis or extracellular-signal-regulated kinase activation.
INTRODUCTION
The Interleukin (IL)-6 family of cytokines is a group of functionally and structurally related proteins that consists of IL-6, IL-11, IL-27, leukemia inhibitory factor (LIF), oncostatin M (OSM), ciliary neurotrophic factor (CNTF), cardiotrophin-1 (CT-1), novel neurotrophin-1/B cell stimulating factor-3 or cardiotrophin-like cytokine, and neuropoietin (NP) (reviewed in ref. 1). The IL-6 cytokine family regulates a variety of complex cellular processes, such as hematopoiesis, immune response, inflammation, differentiation, mammalian reproduction, cardiovascular action, and neuronal survival (reviewed in ref. 2). Signal transduction involves activation of the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway, typically activating STAT3 and, to a lesser extent, STAT1 (reviewed in ref. 2). In addition, glycoprotein 130 (gp130) cytokines can also activate other signaling pathways, including the mitogen-activated protein kinase (MAPK) cascade, PI3K (phosphatidylinositol-3 kinase), mTOR (mammalian target of rapamycin/p70 S6 kinase), and AMPK (5′ adenosine monophosphate-activated protein kinase) (reviewed in ref. 3).
Because all members of this family use gp130 as a common signal transducer within their receptor complex for signaling, the IL-6 family is commonly referred to as the gp130 cytokines (reviewed in ref. 4). The shared usage of signal transducers in the receptor complexes likely accounts for the functional redundancy of gp130 cytokines in mediating biological effects. In addition, the ubiquitous expression of gp130 facilitates the pleiotropic nature of these cytokines. The differences in their activity can be partially attributed to the use of additional receptor components that are used by these cytokines. IL-6 and IL-11 first bind to the IL-6 receptor-α (5) and IL-11 receptor-α (6), respectively, and then the complex associates with a gp130 homodimer complex for signaling. IL-27 engages a gp130/WSX-1 (a subunit of the IL-27 receptor) heterodimeric receptor complex (7). LIF and CT-1 require the gp130/LIF receptor (LIFR) complex to mediate signal transduction (8,9). CT-1 has also been reported to recruit an α-receptor (10), which to date has not been characterized. OSM can alternatively use a gp130/OSM receptor (OSMR) complex (11), and has also been reported to use the LIFR (12). Both CNTF and cardiotrophin-like cytokine use a signaling complex, including CNTFRα, gp130, and LIFR for signal transduction (13,14). NP was also reported to use this tripartite receptor complex (15); however, recent studies from our laboratory demonstrate that NP-induced STAT3 activation in adipocytes is not mediated by LIFR signaling (16).
In recent years, gp130 cytokines have been implicated as potential therapeutic targets in obesity (reviewed in ref. 3). Although adipocytes are responsive to gp130 cytokines (17-22), the effects of these cytokines on adipose tissue function is largely unknown. Interestingly, some gp130 cytokines, like OSM and NP, can inhibit adipogenesis (22-24), while others, such as LIF (25), CT-1 (21), and CNTF (20), do not attenuate adipocyte differentiation. In addition, CT-1 and NP have been reported to inhibit insulin-stimulated glucose uptake (21,22), whereas LIF (25) and CNTF (20) do not impair insulin action in adipocytes. Notably, both CT-1 and LIF act through a signaling complex of LIFR/gp130, yet these cytokines exert different effects on insulin action. Hence, the mechanisms and signaling components involved in gp130 cytokine action are not fully understood.
Previous studies from our lab show that gp130 cytokines have the unique ability to crosstalk with other members of the gp130 family to attenuate one another’s signaling (26). Our findings in cultured adipocytes indicate that this crosstalk is correlated with the degradation of the LIFR via a lysosome-mediated pathway (26). To summarize, these studies demonstrated that LIF, CT-1, and human OSM had profound effects on LIFR degradation and supported the hypothesis that crosstalk among certain gp130 cytokines could be attributed to decreased expression of the LIFR. To further understand the signaling of this cytokine family, we have continued to investigate the crosstalk of gp130 cytokines in adipocytes in vitro and in vivo. Our novel data demonstrate that white adipose tissue (epididymal and retroperitoneal) is highly responsive to several gp130 cytokines. We also provide the first evidence of gp130 cytokine inhibitory crosstalk in adipose tissue in vivo. Moreover, NP and murine OSM (mOSM) do not exert the same inhibitory crosstalk that we have observed for CT-1 and LIF. Neither NP nor mOSM was capable of attenuating the signaling of other gp130 cytokines. Recent findings from our laboratory demonstrated that NP does not induce LIFR activation (16). Our current studies indicate that the unique crosstalk abilities of mOSM and NP can be attributed to their inability to activate the LIFR. Collectively, our data show that gp130 cytokines exert differential crosstalk signaling capabilities in both cultured adipocytes in vitro and adipose tissue in vivo, and the inhibitory crosstalk is not dependent on new protein synthesis or the activation of MAPK.
METHODS AND PROCEDURES
Materials
Dulbecco’s modified Eagle’s media was purchased from Sigma (St. Louis, MO). Bovine and fetal bovine sera were purchased from Hyclone (South Logan, Utah). Mouse recombinant NP, mouse recombinant CT-1, and mouse recombinant OSM were all purchased from R&D Systems (Minneapolis, MN). Recombinant human OSM was purchased from Invitrogen (Carlsbad, CA), and mouse recombinant LIF was purchased from BD Transduction (Franklin Lakes, NJ). Cycloheximide was purchased from Sigma. Both STAT3 and phospho-specific STAT3 (Y705) were monoclonal antibodies purchased from BD Transduction. Both the MAPK (extracellular-signal-regulated kinases (ERKs) 1 and 2) and LIFR polyclonal antibodies and the peroxisome proliferator–activated receptor-γ monoclonal antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The polyclonal anti-active MAPK (ERK1/2) antibody and U0126 inhibitor were purchased from Promega (Madison, WI). Nitrocellulose was purchased from Bio-Rad (Hercules, CA). The BCA kit and the enhanced chemiluminescence kit were purchased from Thermo Scientific (Waltham, MA). Horseradish peroxidase–conjugated secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
Cell culture
Murine 3T3-L1 preadipocytes were plated and grown to 2 days postconfluence in Dulbecco’s modified Eagle’s medium containing 10% bovine serum. Medium was changed every 48 h. Cells were induced to differentiate by changing the medium to Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, 0.5 mmol/l 3-isobutylmethylxanthine, 1 μmol/l dexamethasone, and 1.7 μmol/l insulin. After 48 h, this medium was replaced with Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, and cells were maintained in this medium until used for experimentation.
Preparation of whole cell extracts
Cell monolayers of 3T3-L1 adipocytes were harvested in a nondenaturing buffer containing 150 mmol/l NaCl, 10 mmol/l Tris pH 7.4, 1 mmol/l (ethylenebis(oxyethylenenitrilo)) tetraacetic acid, 1 mmol/l EDTA, 1% Triton-X 100, 0.5% Igepal CA-630, 1 μmol/l phenylmethylsulfonyl fluoride, 1 μmol/l pepstatin, 50 trypsin inhibitory milliunits of aprotinin, 10 μmol/l leupeptin, and 2 mmol/l sodium vanadate and frozen. Next, the samples were thawed and centrifuged at 14,000g at 4 °C for 10 min. Supernatants containing whole cell extracts were analyzed for protein content using a BCA kit according to the manufacturer’s instructions.
Rodent adipose tissue isolation
Six-week-old Sprague–Dawley rats were euthanized by cervical dislocation, and tissues were immediately removed and frozen in liquid nitrogen. Frozen tissues were homogenized in a buffer containing 150 mmol/l NaCl, 10 mmol/l Tris pH 7.4, 1 mmol/l EGTA, 1 mmol/l EDTA, 1% Triton-X 100, 0.5% Igepal CA-630, 1 μmol/l phenylmethylsulfonyl fluoride, 1 μmol/l pepstatin, 50 trypsin inhibitory milliunits of aprotinin, 10 μmol/l leupeptin, and 2 mmol/l sodium vanadate. Homogenates were centrifuged for 10 min at 6,500g to remove any debris and insoluble material and then analyzed for protein content. All animal studies were carried out with protocols that were reviewed and approved by institutional animal care and use committees.
Gel electrophoresis and western blot analysis
Proteins were separated in 7.5% polyacrylamide (acrylamide from National Diagnostics) gels containing sodium dodecyl sulfate (SDS) according to Laemmli (27) and transferred to nitrocellulose membrane in 25 mmol/l Tris, 192 mmol/l glycine, and 20% methanol. Following transfer, the membrane was blocked overnight in 4% milk at 4 °C. Results were visualized with horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence.
RESULTS
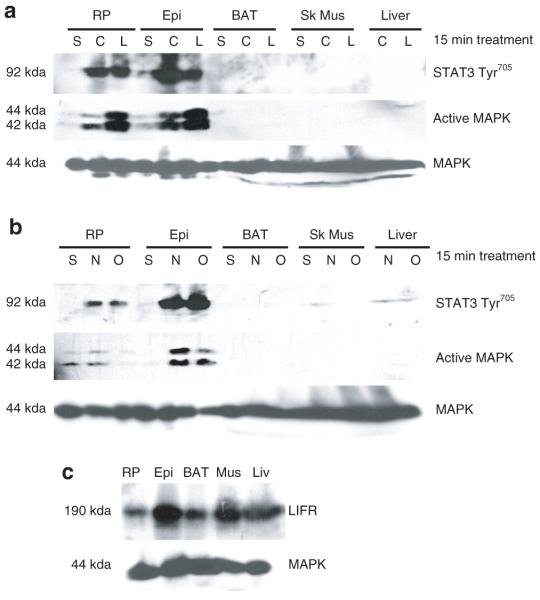
Although studies have shown that cultured adipocytes are responsive to gp130 cytokines (17-22,25), the responsiveness of these cytokines in vivo has not been investigated. Hence, we examined the ability of several gp130 cytokines to induce STAT3 and ERK phosphorylation in insulin sensitive tissues. Tissue samples were extracted from Sprague–Dawley rats 20 min after an intraperitoneal injection with either vehicle (saline), CT-1, LIF, NP, or OSM. As shown in Figure 1a,b, CT-1, LIF, NP, and OSM potently induce both STAT3 tyrosine phosphorylation and ERKs 1 and 2 activation in retroperitoneal and epididymal adipose tissue. We did not observe similar effects in brown adipose tissue, skeletal muscle, or liver. It should be noted that we used twice as much protein for our analysis of skeletal muscle and liver. These data indicate that gp130 signaling is robust in white adipose tissue as compared to other insulin sensitive tissues. MAPK protein levels are shown to demonstrate even protein loading. Since all of the g130 cytokines used in this experiment have been reported to act via LIFR signaling (reviewed in ref. 2), we examined LIFR expression in these tissues. As shown in Figure 1c, a substantial amount of the LIFR was present in each tissue. MAPK protein levels are shown to demonstrate even protein loading. Therefore, the varied responsiveness of the tissues to gp130 cytokines could not be explained by reduced LIFR expression.
Figure 1.
The responsiveness to glycoprotein 130 (gp130) cytokines in insulin sensitive tissues in vivo. Six-week-old male Sprague–Dawley rats were given an intraperitoneal injection of (a) vehicle (saline) control, cardiotrophin-1 (0.1 μg/g animal), and leukemia inhibitory factor (LIF) (0.1 μg/g animal) or (b) vehicle (saline) control, neuropoietin (0.1 μg/g animal), and murine oncostatin M (mOSM) (0.1 μg/g animal). Fifteen min after the injection the rats were killed and the retroperitoneal (RP), epididymal (Epi), brown adipose tissue (BAT), skeletal muscle (Sk Mus), and liver extracts were immediately removed and frozen in liquid nitrogen. 220 μg of epididymal, retroperitoneal, and BAT extracts and 440 μg of Sk Mus and liver extracts were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose, and subjected to western blot analysis. This is a representative experiment independently performed three times. (c) Tissue extracts from these rats given only a vehicle (saline) injection were used to examine LIF receptor expression. 200 μg of each extract was separated by SDS-PAGE, transferred to nitrocellulose, and subjected to western blot analysis. This is a representative experiment independently performed two times. STAT, signal transducer and activator of transcription; MAPK, mitogen-activated protein kinase.
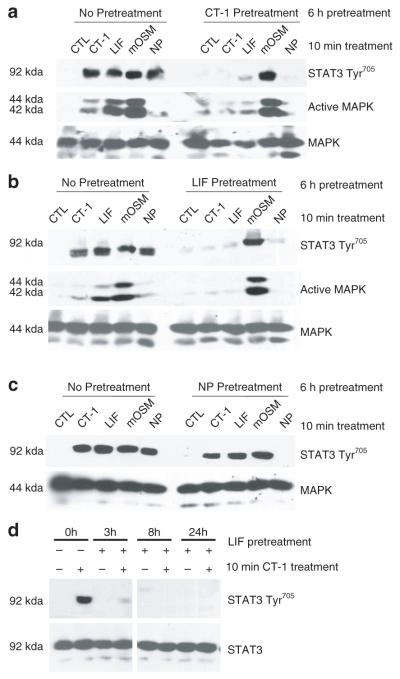
Previous studies from our laboratory demonstrated that LIF, CT-1 and OSM have the ability to crosstalk with one another in a manner that resulted in the attenuation of subsequent signaling (26). We have recently reported the most newly identified gp130 cytokine, NP, can activate STAT3 tyrosine phosphorylation in adipocytes both in vitro and in vivo (22). Hence, we examined the ability of NP to modulate the signaling of other gp130 cytokines. Of note, our previous crosstalk studies were performed using human oncostatin M (hOSM). Evidence shows that mOSM signals differently from hOSM in murine cells and uses the OSM receptor β (11) as opposed to LIFR, which is used by hOSM (16). In short, hOSM signals via the LIFR and not via OSM receptor β (11,16). To study the crosstalk capabilities of these cytokines, we examined the ability of LIF, CT-1, and NP pretreatments to affect the acute actions of LIF, CT-1, NP, and mOSM. In these experiments, fully differentiated 3T3-L1 adipocytes were pretreated with different gp130 cytokines for 6 h followed by an acute 10 min treatment with CT-1, LIF, mOSM, or NP. To ensure the efficacy of each cytokine, the activation of STAT3 Tyr705 was observed in the absence of any pretreatment. As shown in Figure 2a,b, a 6-h pretreatment with CT-1 or LIF completely blocked the STAT3 tyrosine phosphorylation induced by a 10-min treatment of CT-1, LIF, or NP, but did not attenuate mOSM signaling. The same pattern of inhibition was observed for MAPK (ERKs 1 and 2) activation. In contrast to CT-1 and LIF, the results in Figure 2c indicate that NP pretreatment did not crosstalk with or inhibit the action of any of the other gp130 cytokines. Taken together, these studies show that several gp130 cytokines have the ability to block NP signaling, but NP does not inhibit the signaling of any other gp130 family members. MAPK protein levels are shown to demonstrate even loading in each experiment. As shown in Figure 2d, we observed crosstalk at various periods of time. A 3, 8, or 24-h pretreatment with LIF resulted in an inhibition of subsequent CT-1 signaling, as shown by decreased STAT3 phosphorylation. We also obtained the same results at 4 h, 12 h, and 18 h (data not shown). These results indicate that gp130 cytokines exert inhibitory crosstalk over various pretreatment times. STAT3 protein levels are shown to demonstrate even loading.
Figure 2.
Crosstalk of glycoprotein 130 (gp130) cytokines in 3T3-L1 adipocytes. Fully differentiated 3T3-L1 adipocytes received no pretreatment (CTL, control) or were preincubated with 1 nmol/l cardiotrophin-1 (CT-1) (a), 1 nmol/l leukemia inhibitory factor (LIF) (b,d), or 1 nmol/l neuropoietin (NP) (c) for 3 h, 6 h, 8 h, or 24 h Next, the cells were treated for 10 min with 1 nmol/l CT-1, 1 nmol/l LIF, 1 nmol/l murine oncostatin M (mOSM), or 1 nmol/l NP. After the acute treatment, whole cell extracts were prepared and 150 μg of each extract was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose, and subjected to western blot analysis. The data in d is generated from the same exposure of the same blot. This is a representative experiment performed independently four times. MAPK, mitogen-activated protein kinase; STAT, signal transducer and activator of transcription.
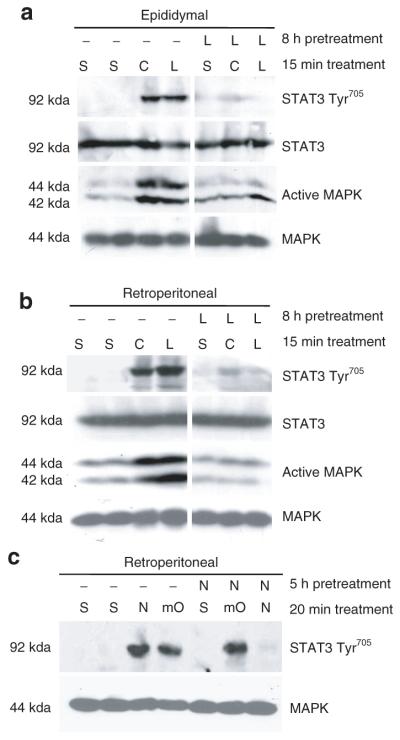
The results in Figure 2 and previous studies (26) demonstrate that the gp130 cytokines CT-1, LIF, and hOSM crosstalk to inhibit one another’s signaling. Our current studies indicate that NP and mOSM have distinct crosstalk capabilities. To further examine the distinct crosstalk abilities of mOSM and hOSM, we pretreated cells with LIF or CT-1 for 6 h, and then examined the induction of STAT3 tyrosine phosphorylation by hOSM and mOSM. As shown in Figure 3a, human, but not murine, OSM signaling was attenuated by CT-1 and LIF. Similar studies were performed to examine the crosstalk capabilities of OSM and NP. In these experiments, 3T3-L1 adipocytes were pretreated for 6 h with mOSM, hOSM or NP and then exposed to NP, mOSM, or hOSM for 10 min. The data in Figure 3b show that hOSM, but not mOSM, inhibited NP signaling, as evidenced by the decreased activation of STAT3 Tyr705 and MAPK. Conversely, NP did not attenuate either mOSM or hOSM. Yet a NP pretreatment was capable of inhibiting NP action. MAPK protein levels are shown to demonstrate even loading.To determine whether crosstalk of gp130 cytokines occurs in vivo, we injected Sprague–Dawley rats with vehicle (saline), CT-1, or LIF, with or without an 8-h preinjection of LIF. The animals were killed 15 min following the second injection. As shown in Figure 4a,b, both a CT-1 and a LIF injection induced the activation of STAT3 Tyr705 and MAPK in the epididymal and retroperitoneal adipose tissues. However, a preinjection with LIF inhibited the STAT3 tyrosine phosphorylation of both CT-1 and LIF in white adipose tissue. This is the first in vivo demonstration that LIF can inhibit the action of CT-1. The activation of ERKs 1 and 2 was also attenuated. STAT3 and MAPK protein levels are shown to demonstrate even loading.
Figure 3.
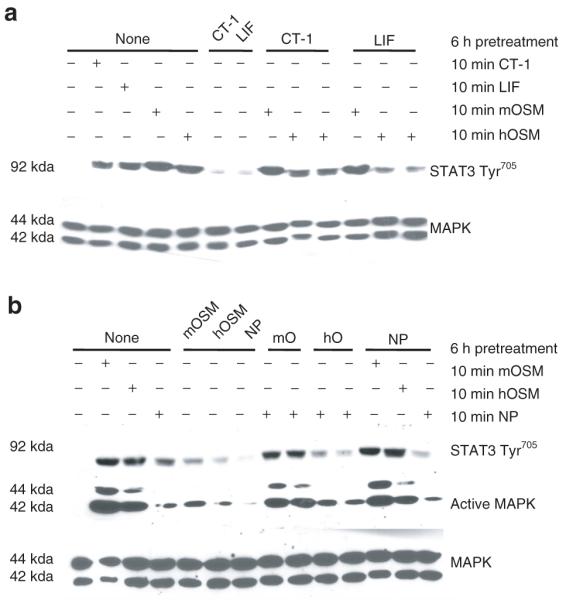
Neuropoietin (NP) and murine oncostatin M (mOSM) have distinct crosstalk abilities compared to other glycoprotein 130 cytokines in 3T3-L1 adipocytes. (a) Fully differentiated 3T3-L1 adipocytes were pretreated with 1 nmol/l cardiotrophin (CT) or 1 nmol/l leukemia inhibitory factor (LIF) for 6-h. Next, the cells were treated for 10 min with 1 nmol/l mOSM, or 1 nmol/l human oncostatin M (hOSM). (b) Fully differentiated 3T3-L1 adipocytes were pretreated with 1 nmol/l mOSM, 1 nmol/l hOSM, or 1 nmol/l NP for 6 h. Next, the cells were treated for 10 min with 1 nmol/l mOSM, 1 nmol/l hOSM, or 1 nmol/l NP. After the treatment, whole cell extracts were prepared, and 160 μg of each extract was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose, and subjected to western blot analysis. Each figure is a representative experiment performed independently three times. MAPK, mitogen-activated protein kinase; STAT, signal transducer and activator of transcription.
Figure 4.
Leukemia inhibitory factor (LIF) treatment in vivo blocks subsequent cardiotrophin-1 (CT-1) and LIF signaling, but neuropoietin (NP) does not inhibit murine oncostatin M (mOSM) in rodent fat pads. Six week old male Sprague–Dawley rats were given an intraperitoneal injection of vehicle (saline) control, CT-1 (0.1 μg/g animal), or LIF (0.1 μg/g animal), following at 8-h preinjection of saline (S) or LIF. Fifteen min after the second injection the rats were killed and both (a) retroperitoneal and (b) epididymal fat pads were immediately removed and frozen in liquid nitrogen. 220 μg of retroperitoneal and 350 μg of epididymal tissue extract were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose, and subjected to western blot analysis. The data in each figure is generated from the same exposure of the same blot. Each figure is a representative experiment independently performed three times. (c) Six week old male Sprague–Dawley rats were given an intraperitoneal injection of vehicle (saline) control, NP (0.1 μg/g animal), or mOSM (.1 μg/g animal), with or without a 5-h preinjection of NP. Twenty min after the second injection the rats were killed and the retroperitoneal fat pad was immediately removed and frozen in liquid nitrogen. 150 μg of retroperitoneal tissue extract was separated by SDS-PAGE, transferred to nitrocellulose, and subjected to western blot analysis. This is a representative experiment independently performed three times. MAPK, mitogen-activated protein kinase; STAT, signal transducer and activator of transcription.
In addition to observing the interaction of CT-1 and LIF signaling in vivo, we examined the crosstalk signaling of NP and mOSM in adipose tissue. Although we have shown that NP can induce STAT3 tyrosine phosphorylation in fat tissue in vivo (22), the interaction of NP with other gp130 cytokines has not been previously observed in vitro or in vivo. In this study, rats were injected with vehicle (saline), NP, or mOSM, with or without a 5-h preinjection of NP. The animals were killed 20 min following the second injection. As shown in Figure 4c, both a NP and a mOSM injection induced the activation of STAT3 Tyr705 in retroperitoneal adipose tissue. Our data indicate that a NP pretreatment did not inhibit the mOSM induction of STAT3 tyrosine phosphorylation. Yet, the NP-induced STAT3 Tyr705 was abolished. These findings correlate with our in vitro data (Figure 2c). Similar observations were shown in epididymal fat pads (data not shown). MAPK protein levels are shown to demonstrate even loading.
The mechanisms underlying the crosstalk of gp130 cytokines have not been elucidated. Hence, we examined the ability of ERK phosphorylation and new protein synthesis to contribute to the observed interactions among gp130 cytokines. Numerous studies have shown that gp130 cytokines induce the expression of suppressors of cytokine signaling (SOCS) proteins (reviewed in ref. 2), and we have observed the ability of these cytokines to induce SOCS-3 mRNA in cultured fat cells (21-22,25). SOCS are known to negatively regulate cytokine signal transduction, and their induction is dependent on new protein synthesis. To determine whether the inhibitory crosstalk exerted by gp130 cytokines could be a result of newly synthesized proteins, fully differentiated 3T3-L1 adipocytes were pretreated with CT-1 or LIF in the presence or absence of cycloheximide, an inhibitor of protein synthesis. Next, the cells were acutely (10 min) exposed to CT-1. Adipocytes with no pretreatment were observed to show the efficacy of CT-1 signaling. As indicated in Figure 5, the same pattern of crosstalk was observed in the presence or absence of cycloheximide. Both CT-1 and LIF blocked the signaling of an acute CT-1 treatment, as evidenced by a substantial decrease in STAT3 Tyr705 and MAPK activation. Both STAT3 and MAPK protein levels are shown to demonstrate even loading. The expression of peroxisome proliferator–activated receptor-γ, a labile transcription factor, is included to show efficacy of the cycloheximide.
Figure 5.
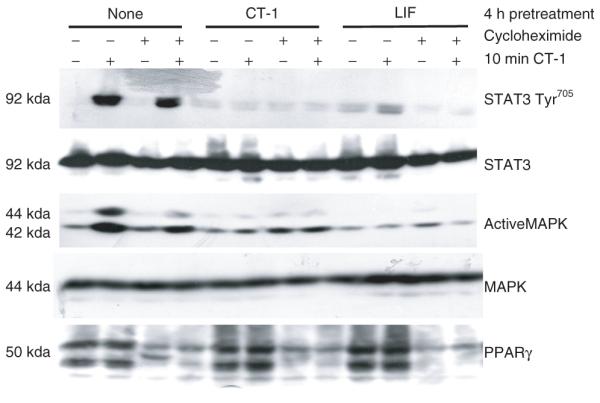
Crosstalk of glycoprotein 130 cytokines is not dependent on new protein synthesis. Fully differentiated 3T3-L1 adipocytes were pretreated with 1 nmol/l cardiotrophin-1 (CT-1) or 1nmol/l leukemia inhibitory factor (LIF) for 4-h in the presence or absence of a 30 min 5 μmol/l cycloheximide preincubation. The cells were then treated (+) for 10 min with 1 nmol/l CT-1. After the treatment, whole cell extracts were prepared and 150 μg of each extract was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose, and subjected to western blot analysis. This is a representative experiment performed independently four times. MAPK, mitogen-activated protein kinase, PPAR, peroxisome proliferator-activated receptor.
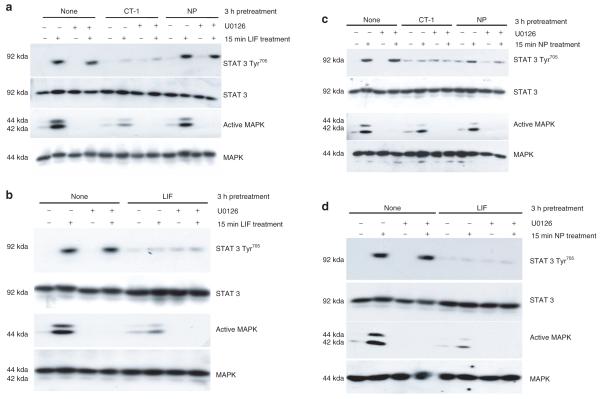
In addition to the JAK/STAT pathway, gp130 cytokines can also activate the MAPK cascade (ERKs 1 and 2) (reviewed in ref. 2). To assess whether the crosstalk of gp130 family members is dependent on the activation of ERKs 1 and 2, fully differentiated 3T3-L1 adipocytes were pretreated with CT-1, NP, or LIF in the presence or absence of U0126, a selective ERK 1 and 2 inhibitor. Next, cells were exposed to an acute treatment with LIF or NP. The efficacy of both LIF and NP signaling was observed in adipocytes with no pretreatment. As shown in Figure 6a,b, the ability of CT-1 and LIF to inhibit LIF signaling is independent of the presence of active ERKs 1 and 2. Also, as shown above, NP was incapable of inhibiting LIF action. An analysis of NP signaling in Figure 6c,d confirms that NP can be inhibited by CT-1 and LIF, and this crosstalk is unaffected by the ability to activate ERK. In each experiment, STAT3 and MAPK protein levels are shown to demonstrate even loading. The efficacy of the U0126 inhibitor was validated by the inhibition in ERK phosphorylation.
Figure 6.
Crosstalk of glycoprotein 130 cytokines is not dependent on the activation of MAPK. Fully differentiated 3T3-L1 adipocytes were pretreated with 1 nmol/l cardiotrophin-1 (CT-1), 1 nmol/l leukemia inhibitory factor (LIF), or 1 nmol/l NP for 3-h in the presence or absence of a 30 min 5 μmol/l U0126 preincubation. The cells were then treated (+) for 10 min with (a,b) 1 nmol/l LIF or (c,d) 1 nmol/l neuropoietin (NP). After the treatment, whole cell extracts were prepared, and 150 μg of each extract was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose, and subjected to western blot analysis. This is a representative experiment performed independently three times. MAPK, mitogen-activated protein kinase; STAT, signal transducer and activator of transcription.
DISCUSSION
Although an understanding of the production, secretion, function, and mechanisms underlying the actions of gp130 cytokines have not been fully elucidated, it is known that several of these family members are measurable in circulation in humans (28-34). In addition, all gp130 cytokines activate the JAK/STAT pathway, and many also activate the MAPK (ERK) pathway. Previous studies from our laboratory highlighted the crosstalk of several gp130 cytokines in cultured adipocytes, which correlated with their ability to induce LIFR degradation. In these follow-up studies, we further explored the crosstalk of gp130 cytokines both in vitro and in vivo. Our novel observations demonstrate that white adipose tissue is highly sensitive to LIF, CT-1, NP, and OSM in a manner not shared by other insulin responsive tissues. In addition, we provide the first demonstration of gp130 cytokine inhibitory crosstalk in vivo. Overall, our results indicate that three gp130 family members—CT-1, LIF, and hOSM—exert inhibitory crosstalk both in vitro and in vivo. Moreover, this crosstalk is independent of the activation of ERKs 1 and 2 or new protein synthesis. Our findings also show that NP and mOSM lack the ability to crosstalk with other gp130 cytokines that are known LIFR activators.
A common feature of many gp130 cytokines is their shared use of not only gp130, but also LIFR proteins as components of their receptor complex. Our data support the idea that the crosstalk signaling may be due to differential usage of receptor components. Studies have shown that treatment with gp130 cytokines, including CT-1, LIF, and hOSM, results in LIFR internalization and degradation (26,35,36). Moreover, certain gp130 family members that use the LIFR crosstalk with one another in an antagonistic fashion, and this correlates with LIFR phosphorylation and degradation. Hence, this loss of LIFR from the plasma membrane appears to account for the inhibition of subsequent signaling by gp130 cytokines following an initial treatment.
Although NP was originally characterized as a cytokine that required the LIFR for signal transduction (15), our recent studies indicate that NP does not induce the phosphorylation or degradation of the LIFR in the same manner as other gp130 cytokines in adipocytes (16). Hence, these observations suggest that NP does not crosstalk and inhibit the signaling of other gp130 cytokines because it does not activate the LIFR. Because NP propagates its signal through the JAK/STAT and MAPK pathways in a manner similar to other gp130 cytokines, our data suggest that the lack of signal inhibition may reside at the level of the receptor. We postulate that NP uses a unique signal transducer in its receptor complex, in addition to gp130. Although we do not know the identity of this NP receptor(s), our data clearly indicate that the LIFR is not involved (16). Conversely, the unique crosstalk capabilities of mOSM can be readily hypothesized. Because this cytokine activates the OSM receptor β, it does not attenuate the signaling of gp130 cytokines that use the LIFR. This data is supported by studies of hOSM, which activates the murine LIFR (16) and can crosstalk with CT-1 and LIF (26). Our studies with mOSM also support the notion that NP likely interacts with a novel receptor that associates with gp130. A confounding observation is that several gp130 cytokines can block the signaling of NP (Figures 2 and 3b). These data indicate that LIFR activating cytokines can inhibit the action of NP, a gp130 cytokine that does not use the LIFR. In summary, our studies show that LIFR activating cytokines (LIF, CT-1, hOSM) are capable of inhibitory crosstalk with one another. Notably, these LIFR activating cytokines can also inhibit NP, whose signaling is independent of LIFR phosphorylation. NP pretreatment can attenuate NP signaling, but does not inhibit the action of other LIFR or OSM receptor β using cytokines.
Although the mechanisms underlying the crosstalk of gp130 cytokines are poorly understood, our studies indicate that two important factors, new protein synthesis and ERKs 1 and 2 activation, do not contribute to crosstalk among these cytokines. It is well-established that the expression of SOCS family members is highly induced by many different cytokines and these proteins are known to interfere with subsequent signaling via a classic negative feedback inhibition loop (reviewed in ref. 37). Many gp130 cytokines induce SOCS-3 expression, and LIF, CT-1, NP, and OSM induce SOCS-3 mRNA in adipocytes (21,22,25,38). The role of SOCS proteins, if any, in the crosstalk of gp130 cytokines is unknown. Nonetheless, our current studies clearly demonstrate that gp130 inhibitory crosstalk is not dependent on newly synthesized proteins (Figure 5) and suggests that SOCS proteins may not be involved. The activation of MAPK (ERKs 1 and 2) by gp130 cytokines can play important roles in cellular actions. However, our data show that the inhibitory crosstalk among several gp130 cytokines is not dependent on ERKs 1 and 2 activation (Figure 6).
To our knowledge, no other laboratories have investigated the interactions of gp130 cytokines in vivo. Our data indicate that gp130 family members crosstalk in vivo in the same manner as in vitro. Hence, these effects are likely very important in white adipose tissue since it is highly responsive to gp130 cytokine exposure (Figure 1). LIF pretreatment inhibited both LIF and CT-1 subsequent treatments in vitro and in vivo (Figures 2 and 4). This is the first evidence to show that a gp130 cytokine can exert negative inhibitory crosstalk in vivo. CT-1 pretreatment inhibited the signaling of CT-1 and LIF in vitro and in vivo (data not shown). In addition, NP pretreatment had no effect on an acute treatment of mOSM in vitro or in vivo (Figures 2 and 4). The fact that gp130 cytokines not only activate signaling pathways in vivo, but also inhibit subsequent signaling of other gp130 family members confirms that a detailed analysis of crosstalk mechanisms is relevant for a more in-depth perspective of gp130 cytokine secretion and action. In addition, circulating levels of many gp130 cytokines have been examined in humans and shown to have divergent effects on metabolism. Recent studies suggest that CT-1 is produced from adipose tissue, and may be modulated in obesity (28,29). Numerous studies have also shown that IL-6 levels are greatly elevated in obese individuals and strongly correlated with increased BMI (32,39,40). Increased serum concentrations of other gp130 cytokines, such as LIF and OSM, are also associated with metabolic abnormalities (30,31). Therefore, it is necessary to understand the functions and interactions of these cytokines, as well as the inhibitory crosstalk that gp130 cytokines exert on their respective family members. Collectively, our data provide a basis for future analyses of cytokine interaction, and we speculate that these interactions may greatly affect the physiological action of these cytokines, which can modulate both adipocyte development and insulin action. Nonetheless, further studies are needed to examine how these processes could possibly contribute to or affect metabolic disease states that are characterized by the expression of multiple STAT3 activating cytokines, including those belonging to the gp130 family.
ACKNOWLEDGMENTS
This work was supported by grant R01 DK52968 from the NIH to J.M.S.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Fasnacht N, Müller W. Conditional gp130 deficient mouse mutants. Semin Cell Dev Biol. 2008;19:379–384. doi: 10.1016/j.semcdb.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Heinrich PC, Behrmann I, Haan S, et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Febbraio MA. gp130 receptor ligands as potential therapeutic targets for obesity. J Clin Invest. 2007;117:841–849. doi: 10.1172/JCI30453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 5.Taga T, Hibi M, Hirata Y, et al. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58:573–581. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- 6.Hilton DJ, Hilton AA, Raicevic A, et al. Cloning of a murine IL-11 receptor alpha-chain; requirement for gp130 for high affinity binding and signal transduction. EMBO J. 1994;13:4765–4775. doi: 10.1002/j.1460-2075.1994.tb06802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pflanz S, Hibbert L, Mattson J, et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 8.Gearing DP, Thut CJ, VandeBos T, et al. Leukemia inhibitory factor receptor is structurally related to the IL-6 signal transducer, gp130. EMBO J. 1991;10:2839–2848. doi: 10.1002/j.1460-2075.1991.tb07833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pennica D, Shaw KJ, Swanson TA, et al. Cardiotrophin-1. Biological activities and binding to the leukemia inhibitory factor receptor/gp130 signaling complex. J Biol Chem. 1995;270:10915–10922. doi: 10.1074/jbc.270.18.10915. [DOI] [PubMed] [Google Scholar]
- 10.Robledo O, Guillet C, Chevalier S, et al. Hepatocyte-derived cell lines express a functional receptor for cardiotrophin-1. Eur Cytokine Netw. 1997;8:245–252. [PubMed] [Google Scholar]
- 11.Ichihara M, Hara T, Kim H, Murate T, Miyajima A. Oncostatin M and leukemia inhibitory factor do not use the same functional receptor in mice. Blood. 1997;90:165–173. [PubMed] [Google Scholar]
- 12.Gearing DP, Comeau MR, Friend DJ, et al. The IL-6 signal transducer, gp130: an oncostatin M receptor and affinity converter for the LIF receptor. Science. 1992;255:1434–1437. doi: 10.1126/science.1542794. [DOI] [PubMed] [Google Scholar]
- 13.Davis S, Aldrich TH, Stahl N, et al. LIFR beta and gp130 as heterodimerizing signal transducers of the tripartite CNTF receptor. Science. 1993;260:1805–1808. doi: 10.1126/science.8390097. [DOI] [PubMed] [Google Scholar]
- 14.Elson GC, Lelièvre E, Guillet C, et al. CLF associates with CLC to form a functional heteromeric ligand for the CNTF receptor complex. Nat Neurosci. 2000;3:867–872. doi: 10.1038/78765. [DOI] [PubMed] [Google Scholar]
- 15.Derouet D, Rousseau F, Alfonsi F, et al. Neuropoietin, a new IL-6-related cytokine signaling through the ciliary neurotrophic factor receptor. Proc Natl Acad Sci USA. 2004;101:4827–4832. doi: 10.1073/pnas.0306178101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White UA, Stephens JM. Neuropoietin activates STAT3 independent of LIFR activation in adipocytes. Biochem Biophys Res Commun. 2010;395:48–50. doi: 10.1016/j.bbrc.2010.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balhoff JP, Stephens JM. Highly specific and quantitative activation of STATs in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 1998;247:894–900. doi: 10.1006/bbrc.1998.8890. [DOI] [PubMed] [Google Scholar]
- 18.Tenney R, Stansfield K, Pekala PH. Interleukin 11 signaling in 3T3-L1 adipocytes. J Cell Physiol. 2005;202:160–166. doi: 10.1002/jcp.20100. [DOI] [PubMed] [Google Scholar]
- 19.Stephens JM, Lumpkin SJ, Fishman JB. Activation of signal transducers and activators of transcription 1 and 3 by leukemia inhibitory factor, oncostatin-M, and interferon-gamma in adipocytes. J Biol Chem. 1998;273:31408–31416. doi: 10.1074/jbc.273.47.31408. [DOI] [PubMed] [Google Scholar]
- 20.Zvonic S, Cornelius P, Stewart WC, Mynatt RL, Stephens JM. The regulation and activation of ciliary neurotrophic factor signaling proteins in adipocytes. J Biol Chem. 2003;278:2228–2235. doi: 10.1074/jbc.M205871200. [DOI] [PubMed] [Google Scholar]
- 21.Zvonic S, Hogan JC, Arbour-Reily P, Mynatt RL, Stephens JM. Effects of cardiotrophin on adipocytes. J Biol Chem. 2004;279:47572–47579. doi: 10.1074/jbc.M403998200. [DOI] [PubMed] [Google Scholar]
- 22.White UA, Stewart WC, Mynatt RL, Stephens JM. Neuropoietin attenuates adipogenesis and induces insulin resistance in adipocytes. J Biol Chem. 2008;283:22505–22512. doi: 10.1074/jbc.M710462200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyaoka Y, Tanaka M, Naiki T, Miyajima A. Oncostatin M inhibits adipogenesis through the RAS/ERK and STAT5 signaling pathways. J Biol Chem. 2006;281:37913–37920. doi: 10.1074/jbc.M606089200. [DOI] [PubMed] [Google Scholar]
- 24.Song HY, Jeon ES, Kim JI, Jung JS, Kim JH. Oncostatin M promotes osteogenesis and suppresses adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells. J Cell Biochem. 2007;101:1238–1251. doi: 10.1002/jcb.21245. [DOI] [PubMed] [Google Scholar]
- 25.Hogan JC, Stephens JM. Effects of leukemia inhibitory factor on 3T3-L1 adipocytes. J Endocrinol. 2005;185:485–496. doi: 10.1677/joe.1.05980. [DOI] [PubMed] [Google Scholar]
- 26.Zvonic S, Baugh JE, Jr, Arbour-Reily P, Mynatt RL, Stephens JM. Crosstalk among gp130 cytokines in adipocytes. J Biol Chem. 2005;280:33856–33863. doi: 10.1074/jbc.M508020200. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Malavazos AE, Ermetici F, Morricone L, et al. Association of increased plasma cardiotrophin-1 with left ventricular mass indexes in normotensive morbid obesity. Hypertension. 2008;51:e8–9. doi: 10.1161/HYPERTENSIONAHA.107.105346. author reply e10. [DOI] [PubMed] [Google Scholar]
- 29.Natal C, Fortuño MA, Restituto P, et al. Cardiotrophin-1 is expressed in adipose tissue and upregulated in the metabolic syndrome. Am J Physiol Endocrinol Metab. 2008;294:E52–E60. doi: 10.1152/ajpendo.00506.2007. [DOI] [PubMed] [Google Scholar]
- 30.Hirota H, Izumi M, Hamaguchi T, et al. Circulating interleukin-6 family cytokines and their receptors in patients with congestive heart failure. Heart Vessels. 2004;19:237–241. doi: 10.1007/s00380-004-0770-z. [DOI] [PubMed] [Google Scholar]
- 31.Pradeep AR, S TM, Garima G, Raju A. Serum levels of oncostatin M (a gp 130 cytokine): an inflammatory biomarker in periodontal disease. Biomarkers. 2010;15:277–282. doi: 10.3109/13547500903573209. [DOI] [PubMed] [Google Scholar]
- 32.Hansen D, Dendale P, Beelen M, et al. Plasma adipokine and inflammatory marker concentrations are altered in obese, as opposed to non-obese, type 2 diabetes patients. Eur J Appl Physiol. 2010;109:397–404. doi: 10.1007/s00421-010-1362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heizmann O, Koeller M, Muhr G, Oertli D, Schinkel C. Th1- and Th2-type cytokines in plasma after major trauma. J Trauma. 2008;65:1374–1378. doi: 10.1097/TA.0b013e31818b257d. [DOI] [PubMed] [Google Scholar]
- 34.Miyagaki T, Sugaya M, Shibata S, et al. Serum interleukin-27 levels in patients with cutaneous T-cell lymphoma. Clin Exp Dermatol. 2010;35:e143–e144. doi: 10.1111/j.1365-2230.2009.03684.x. [DOI] [PubMed] [Google Scholar]
- 35.Blanchard F, Duplomb L, Wang Y, et al. Stimulation of leukemia inhibitory factor receptor degradation by extracellular signal-regulated kinase. J Biol Chem. 2000;275:28793–28801. doi: 10.1074/jbc.M003986200. [DOI] [PubMed] [Google Scholar]
- 36.Blanchard F, Wang Y, Kinzie E, et al. Oncostatin M regulates the synthesis and turnover of gp130, leukemia inhibitory factor receptor alpha, and oncostatin M receptor beta by distinct mechanisms. J Biol Chem. 2001;276:47038–47045. doi: 10.1074/jbc.M107971200. [DOI] [PubMed] [Google Scholar]
- 37.Croker BA, Kiu H, Nicholson SE. SOCS regulation of the JAK/STAT signalling pathway. Semin Cell Dev Biol. 2008;19:414–422. doi: 10.1016/j.semcdb.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stross C, Radtke S, Clahsen T, et al. Oncostatin M receptor-mediated signal transduction is negatively regulated by SOCS3 through a receptor tyrosine-independent mechanism. J Biol Chem. 2006;281:8458–8468. doi: 10.1074/jbc.M511212200. [DOI] [PubMed] [Google Scholar]
- 39.Gnacinska M, Malgorzewicz S, Guzek M, Lysiak-Szydlowska W, Sworczak K. Adipose tissue activity in relation to overweight or obesity. Endokrynol Pol. 2010;61:160–168. [PubMed] [Google Scholar]
- 40.Khaodhiar L, Ling PR, Blackburn GL, Bistrian BR. Serum levels of interleukin-6 and C-reactive protein correlate with body mass index across the broad range of obesity. JPEN J Parenter Enteral Nutr. 2004;28:410–415. doi: 10.1177/0148607104028006410. [DOI] [PubMed] [Google Scholar]