Abstract
Cigarette smoking is common among patients in cocaine and opioid dependence treatment, and may influence treatment outcome. We addressed this issue in a secondary analysis of data from an outpatient clinical trial of buprenorphine treatment for concurrent cocaine and opioid dependence (13 weeks, N = 200). The association between cigarette smoking (lifetime cigarette smoking status, number of cigarettes smoked per day prior to study entry) and short-term treatment outcome (% of urine samples positive for cocaine or opioids, treatment retention) was evaluated with analysis of covariance, bivariate correlations, and multivariate linear regression. Nicotine-dependent smokers (66% of participants) had a significantly higher percentage of cocaine-positive urine samples than non-smokers (12% of participants) (76% vs. 62%), but did not differ in percentage of opioid-positive urine samples or treatment retention. Number of cigarettes smoked per day at baseline was positively associated with percentage of cocaine-positive urine samples, even after controlling for baseline sociodemographic and drug use characteristics, but was not significantly associated with percentage of opioid-positive urine samples or treatment retention. These results suggest that cigarette smoking is associated with poorer short-term outcome of outpatient treatment for cocaine dependence, but perhaps not of concurrent opioid dependence, and support the importance of offering smoking cessation treatment to cocaine-dependent patients.
Keywords: cigarettes, nicotine, cocaine, opioids, opiates, treatment outcome
1. Introduction
Cigarette smoking is substantially more prevalent among illicit drug users than in the general population (Budney et al., 1993; Richter et al., 2002), with current smoking rates as high as 92% reported for opioid-dependent patients (Clemmey et al., 1997) and 81% for cocaine-dependent patients (Patkar et al., 2002). Cigarette smoking typically precedes use of other substances (Chen and Kandel, 1995; Gorelick et al., 1997), supporting a conceptualization of cigarettes as a “gateway drug” (Lindsay and Rainey, 1997; Weinberger and Sofuoglu, 2009).
Cigarette smoking may also influence the outcome of drug abuse treatment, based on several published association studies. A study of 749 HMO patients (58.9% with alcohol dependence, 8.3% with cocaine dependence, 1.1% with heroin dependence) in substance abuse treatment reported less alcohol and illicit drug abstinence among smokers than among nonsmokers (Kohn et al., 2003), even at 5-year follow-up (Satre et al., 2007). A study of 105 cocaine-dependent outpatients found nicotine dependence (high scores on the Fagerstrom Test for Nicotine Dependence (FTND)) positively associated with cocaine use during treatment (cocaine-positive urine samples) in the subsample that was negative for cocaine use at baseline, but not among the full sample (Patkar et al., 2003). However, a smaller (N = 63) study of cocaine-dependent outpatients found a similar degree of cocaine abstinence during treatment in both smokers and non-smokers (Roll et al., 1996). Similar results have been observed in small studies of methadone-maintained outpatients participating in smoking cessation treatment, i.e., a significant positive association between cigarette smoking and heroin and cocaine use during treatment (Frosch et al., 2000; Frosch et al., 2002; Shoptaw et al., 2002). One small (N = 17) study found this association only for cocaine use, but not for opioid use (Shoptaw et al., 1996).
Animal and human laboratory studies suggest possible mechanisms for this association between cigarette smoking and poor drug abuse treatment outcome. Nicotine enhances cocaine self-administration in rats (Bechtholt and Mark, 2002; Horger et al., 1992) and rhesus monkeys (Freeman and Woolverton, 2009) and conditioned place preference for cocaine in mice, (Zachariou et al., 2001), while inactivation of the nicotinic receptor reduces cocaine self-administration (Hansen and Mark, 2007; Levin et al., 2000) and conditioned place preference for cocaine (Zachariou et al., 2001). Inactivation of the nicotine receptor in mice reduces the dopamine-releasing effect of cocaine (Zachariou et al., 2001), suggesting that the behavioral interaction of nicotine and cocaine is mediated through dopamine effects. In people with concurrent nicotine and cocaine dependence, nicotine can enhance cue-induced cocaine craving (Reid et al., 1998), while administration of a nicotinic receptor antagonist can reduce cue-induced cocaine craving (Reid et al., 1999). A study in dual heroin- and cocaine-dependent patients in methadone maintenance found a significant positive relationship between cigarette smoking and cocaine use, i.e., cigarette smoking was significantly lower during periods of cocaine abstinence compared to periods of cocaine use (Epstein et al., 2009). That study obtained frequent, contemporaneous reports from participants using the technique of ecological momentary assessment (EMA). The availability of cigarettes makes methadone seem more rewarding to patients (e.g., they will work harder for it and self-administer more) (Spiga et al., 2005; Spiga et al., 1998). In addition, heavy smokers require larger doses of methadone per day than chippers and non-smokers (Frosch et al., 2000).
The present study examined the association of cigarette smoking with short-term treatment response (retention, objective measures of cocaine and opioid use) in a large (N = 200) outpatient drug abuse treatment sample. It was predicted that greater cigarette smoking at baseline would be associated with poorer illicit substance abuse treatment outcomes.
2. Methods
2.1 Participants
Participants were research volunteers from an outpatient clinical trial conducted prior to 2005 for the treatment of concurrent opioid and cocaine dependence, conducted in the outpatient research clinic of the National Institute on Drug Abuse Intramural Research Program. The trial was a 13-week, randomized double-blind investigation of buprenorphine for participants with concurrent cocaine and opioid dependence (N = 200) (Montoya et al., 2004). Participants were eligible for the trial if they were 21–50 years old and met DSM-III-R criteria for current cocaine dependence and opioid dependence. Participants were excluded if they (1) fulfilled criteria for other current substance dependence besides caffeine or nicotine (approximately 62% reported current use of other drugs); (2) displayed current severe psychiatric symptoms, based on a clinical interview by a master’s-level drug abuse counselor, or a general distress score higher than 70 on the Symptom Checklist 90-R (Derogatis and Cleary, 1977); (3) demonstrated a current need for psychiatric treatment, as judged by the study psychiatrist; (4) had an active medical disorder or current need for medical treatment; (5) were currently nursing, pregnant, or of child-bearing potential and not using a medically accepted method of birth control; or (6) were unable to read or speak English. The study was approved by the NIDA Institutional Review Board. Written informed consent was obtained from all participants.
2.2 Procedures
All participants received a thorough medical and psychological evaluation at baseline, including the Diagnostic Interview Schedule (DIS) (Robins et al., 1982) to obtain information relating to psychiatric diagnoses and cigarette smoking behavior. Participants were randomized into 4 medication groups: 2 mg, 8 mg, or 16 mg daily or 16 mg every other day (Montoya et al., 2004).. In addition to study medication, all participants received weekly individual drug abuse counseling based on interpersonal psychotherapy. The two highest buprenorphine doses significantly reduced both cocaine and opioid use (Montoya et al., 2004).
Participants attended the outpatient clinic three times each week to provide urine samples under direct staff observation. Urine samples were tested for morphine and the cocaine metabolite benzoylecgonine by a screening immunoassay. The primary treatment outcome variables were proportion of cocaine-positive and opioid-positive urine samples. Urine drug test data were not available for 8 participants who did not attend their first clinic appointment. These data were treated as missing and not analyzed. Thirteen subjects dropped out before achieving their target buprenorphine dose on day 5; their urine data are included in the analyses. Ninety subjects completed the entire 10-week trial. There were no significant medication group differences in drop-out rate (Montoya et al., 2004). A secondary outcome variable was length of stay (retention) in treatment. This variable has been correlated with positive treatment outcome amongst cocaine-dependent outpatients in previous studies (e.g., Siqueland et al., 2002). Participants who consented to treatment but never appeared for the first visit were assigned a length of stay of 0.
2.3 Statistical analysis
For purposes of statistical analysis, cigarette smoking by participants at study entry was classified quantitatively by both number of cigarettes smoked per day and number of DSM-III-R nicotine dependence criteria satisfied. In addition, participants were placed into three mutually exclusive groups based on lifetime smoking status retrospectively reported at baseline: 1) non-smokers (NS; never smoked at least one cigarette a day for at least one month), 2) non-dependent smokers (NDS; qualified as a smoker, but did not meet DSM-III-R criteria for nicotine dependence), or 3) dependent smokers (DS; met DSM-III-R criteria for nicotine dependence).
Univariate comparisons of baseline characteristics for the three smoking status groups were conducted by one-way analyses of variance (ANOVA) for continuous variables and chi-square tests for categorical variables. Least Significant Difference (LSD) post-hoc tests were used to compare groups when ANOVA indicated significant differences (p < .05) overall. Variables showing a “trend” level of significance (p < .1) were used as covariates in analyses of covariance (ANCOVA) evaluating the association of cigarette smoking status on treatment outcomes. Associations between relevant baseline characteristics (cigarettes smoked per day, number of nicotine dependence criteria met, days of cocaine use in the 30 days prior to screening, days of opioid use in the 30 days prior to screening) and treatment outcome variables were initially evaluated using bivariate Pearson correlation coefficients. To control for potentially confounding variables, further evaluation attempted to predict cocaine and opioid treatment outcome by using multivariate linear regression models that included all demographic and baseline drug use characteristics except number of nicotine dependence criteria met. This variable was not included due to its high correlation with number of cigarettes smoked per day (r = 0.62, p < .05), thus raising multicollinearity issues if both variables were used in the same regression model (Morton, 1977).
All statistical analyses used SPSS v. 17.0 (SPSS Inc., Chicago, IL) with a two-tailed significance level set at p < .05, except where otherwise noted.
3. Results
3.1 Sociodemographic characteristics
Participant sociodemographic characteristics had few significant associations with lifetime smoking status (Table 1). African-Americans were more likely than others to be non-dependent smokers (26% vs. 10%) and less likely to be dependent smokers (62% vs. 78%). There was a trend towards less baseline days of cocaine use in the non-dependent smoker group (p < .07 for both post-hoc group comparisons). Therefore, race and baseline days of cocaine use were used as covariates when analyzing treatment outcome data.
Table 1.
Association of lifetime cigarette smoking status with baseline participant characteristics in 200 cocaine- and opioid-dependent outpatients
| NS | NDS | DS | F/χ2 | p | |
|---|---|---|---|---|---|
| n = 24 | n = 44 | n =132 | |||
| Age (M ± SD) | 32.2 ± 6.3 | 32.7 ± 6.4 | 34.2 ± 6.2 | 1.70 | 0.19 |
| Months Employed Past Year (M ± SD) | 4.2 ± 4.5 | 4.9 ± 4.4 | 4.2 ± 4.6 | 0.33 | 0.72 |
| Highest Grade Completed (M ± SD) | 12.6 ± 2.0 | 11.6 ± 1.7 | 11.6 ± 2.4 | 2.18 | 0.12 |
| Female [n (%)] | 7 (29) | 13 (30) | 47 (36) | 0.77 | 0.68 |
| Race [n (%)] | |||||
| African-American (128) | 16 (67) | 33 (83) | 79 (60) | 6.71 | 0.04 |
| Other (67) | 8 (33) | 7 (18) | 52 (40) | ||
| Marital Status [n (%)] | |||||
| Presently Married (24) | 2 (8) | 5 (11) | 20 (15) | 4.24 | 0.37 |
| Previously Married (44) | 3 (13) | 11 (25) | 36 (27) | ||
| Never Married (132) | 19 (79) | 28 (64) | 76 (58) | ||
| Days of cocaine use in Past 30 Days1 (M ± SD) | 24.0 ± 6.8 | 19.7 ± 9.0 | 22.5 ± 8.7 | 2.44 | 0.09 |
| Days of heroin use in Past 30 Days1 (M ± SD) | 29.2 ± 2.3 | 28.3 ± 3.8 | 28.4 ± 5.0 | 0.38 | 0.68 |
| Milligrams of Buprenorphine [n (%)] | 5.09 | 0.53 | |||
| 2 mg per day | 6 (25) | 14 (32) | 30 (23) | ||
| 8 mg per day | 5 (21) | 10 (23) | 33 (25) | ||
| 16 mg per day | 9 (38) | 12 (27) | 31 (24) | ||
| 16 mg every other day | 4 (17) | 8 (18) | 38 (29) | ||
prior to screening for study.
NS = Non-smokers, NDS = Non-dependent smokers, DS = Dependent Smokers (numbers do not always add up to column headings because of missing data)
3.2 Baseline substance use
As expected from the study eligibility criteria, participants were heavy users of cocaine and heroin in the month prior to study screening (Table 1). These characteristics were not significantly associated with number of cigarettes smoked per day at study entry (Table 2). Number of cigarettes smoked per day was highly correlated with number of nicotine dependence criteria met (Table 2).
Table 2.
Association of nicotine dependence and baseline substance use with short-term treatment outcome in 200 cocaine- and opioid-dependent outpatients.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1. % Opioid-positive urines | — | ||||||
| 2. % Cocaine-positive urines | .48** | — | |||||
| 3. % Treatment retention | −.33** | −.37** | — | ||||
| 4. Cigarettes smoked per day | −.02 | .19* | − .01 | — | |||
| 5. No. of nicotine dependence criteria met | −.00 | .16* | .05 | .64** | — | ||
| 6. Days of cocaine use in Past 30 Days1 | .05 | .15* | −.05 | .02 | .01 | — | |
| 7. Days of opioid use in Past 30 Days1 | −.04 | −.10 | .01 | −.04 | −.04 | .13 | — |
p < .05,
p < .01
prior to screening for study.
3.2 Lifetime smoking status and treatment outcome
Lifetime smoking status was not significantly associated with cocaine use during treatment, after adjusting for race and baseline days of cocaine use, F(1, 186) = 2.11, p = .124. However, non-smokers (NS) had a significantly lower proportion of cocaine-positive urine samples (61.2%, SE = 6.9) than dependent (DS) smokers (76.4%, SE = 3.0) (LSD p < .05), although they did not differ significantly from non-dependent smokers (NDS, Figure 1). The number of nicotine dependence criteria met was significantly positively correlated with proportion of cocaine-positive urine samples, r = .16, p < 0.05 (Table 2). Lifetime smoking status had no significant association with opioid use during treatment (Figure 1) nor with retention in treatment: NS length of stay = 50.8 (SE = 7.4) days, NDS = 52.0 (SE = 5.8) days, DS = 53.2 (SE = 3.2) days; F(1,194) = 0.06, p = .95.
Figure 1.
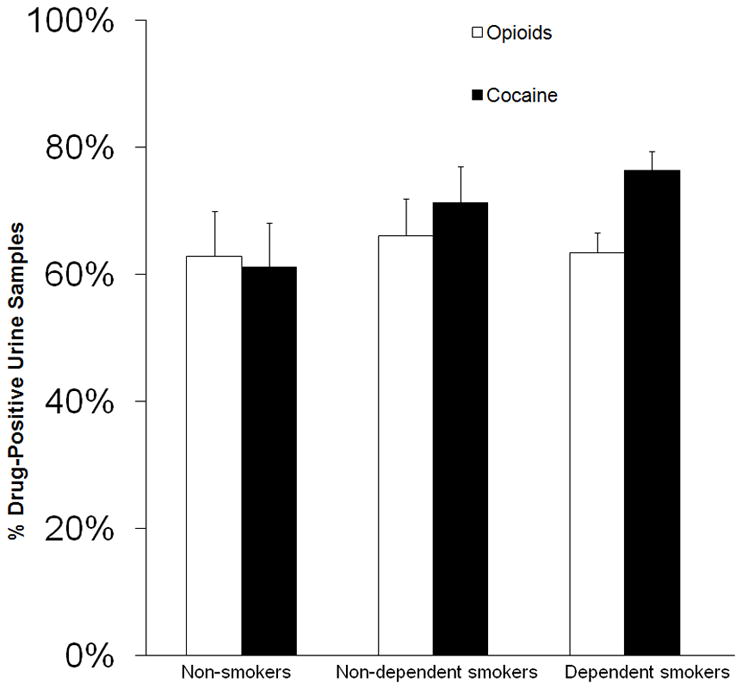
Association of cigarette smoking status with cocaine and opioid use during treatment. Mean (± SE) percentages of urine samples positive for cocaine or opioids during treatment as a function of cigarette smoking status. Urine samples were collected under direct staff observation three times each week over 13 weeks and tested for morphine and the cocaine metabolite benzoylecgonine by a screening immunoassay. Percentages adjusted for race and baseline cocaine use as covariates.
3.3 Cigarette smoking at baseline and treatment outcome
Number of cigarettes smoked per day was significantly positively correlated with percentage of cocaine-positive urine samples (Table 2), even after controlling for baseline participant characteristics in the regression model (Table 3). Cocaine use during treatment had no significant association with any other baseline characteristic in the regression model, including with cocaine or heroin use in the 30 days prior to study screening (Table 3). Number of cigarettes smoked per day had no significant correlation with opioid use during treatment (Table 2), even after controlling for baseline participant characteristics in the regression model (Table 4). However, several other baseline characteristics (sex, marital status, education level) were significantly associated with opioid use (Table 4).
Table 3.
Participant characteristics as predictors of cocaine treatment outcome in 186 cocaine- and opioid-dependent outpatients.
| Predictor Variables | % of Cocaine-Positive Urine Samples |
||
|---|---|---|---|
| Standardized B | t | p | |
| Age | 0.02 | 0.23 | 0.74 |
| Female | 0.12 | 1.65 | 0.09 |
| African-American | 0.14 | 1.85 | 0.08 |
| Never Married | −0.01 | −0.15 | 0.92 |
| Highest Grade in School Completed | 0.13 | 1.72 | 0.08 |
| Number of Months Employed Past Year | −0.12 | −1.67 | 0.10 |
| # of Past 30 Days1 Used Cocaine | 0.13 | 1.75 | 0.09 |
| # of Past 30 Days1 Used Heroin | −0.11 | −1.52 | 0.12 |
| 8 Mg of Buprenorphine Per Day2 | −0.11 | −1.20 | 0.23 |
| 16 Mg of Buprenorphine Per Day2 | −.012 | −1.35 | 0.18 |
| 16 Mg of Buprenorphine Every Other Day2 | −0.01 | −0.16 | 0.87 |
| Number of Cigarettes Smoked Per Day | 0.22 | 2.84 | 0.005 |
Multivariate linear regression was used to evaluate the association between baseline characteristics and % of cocaine-positive urine samples during treatment
prior to screening.
Compared to 2 milligrams (mg) of buprenorphine per day
Table 4.
Participant characteristics as predictors of opioid treatment outcome in 186 cocaine- and opioid-dependent outpatients.
| Predictor Variables | % of Opioid-Positive Urine Samples |
||
|---|---|---|---|
| Standardized B | t | p | |
| Age | 0.00 | −0.03 | 0.98 |
| Female | 0.20 | −2.70 | 0.008 |
| African-American | 0.04 | −0.55 | 0.59 |
| Never Married | 0.16 | −1.97 | 0.05 |
| Highest Grade in School Completed | 0.16 | −2.07 | 0.04 |
| Number of Months Employed Past Year | −0.04 | −0.57 | 0.57 |
| # of Past 30 Days1 Used Cocaine | 0.06 | −0.81 | 0.42 |
| # of Past 30 Days1 Used Heroin | −0.04 | −0.61 | 0.54 |
| 8 Mg of Buprenorphine Per Day2 | −0.13 | −1.44 | 0.15 |
| 16 Mg of Buprenorphine Per Day2 | −0.14 | −1.54 | 0.12 |
| 16 Mg of Buprenorphine Every Other Day2 | −0.14 | −1.52 | 0.13 |
| Number of Cigarettes Smoked Per Day | 0.01 | −0.15 | 0.88 |
Multivariate linear regression was used to evaluate the association between baseline characteristics and % of opioid-positive urine samples during treatment
prior to screening.
Compared to 2 milligrams (mg) of buprenorphine per day
Number of cigarettes smoked per day was not significantly associated with treatment retention (Table 2). However, as expected from prior studies (Siqueland et al., 2002), treatment retention was negatively correlated with both cocaine use and opioid use during treatment (Table 2).
4. Discussion
This study found a significant association between cigarette smoking and short-term outcome of cocaine dependence treatment, with greater smoking involvement predicting poorer treatment outcome (Tables 1–2), even after controlling for potential confounding variables, such as pre-treatment drug use and baseline sociodemographic characteristics (Table 3). Such an association was not observed in two previous studies of cocaine-dependent outpatients (Patkar et al., 2003; Roll et al., 1996), possibly because smaller sample sizes (63 and 105, respectively) may have resulted in those studies being underpowered. Our observed association between cigarette smoking and cocaine use during treatment is consistent with prior studies showing nicotine enhancement of cocaine self-administration in rats (Bechtholt and Mark, 2002; Horger et al., 1992) and rhesus monkeys (Freeman and Woolverton, 2009), and of cue-induced cocaine craving in people with concurrent nicotine and cocaine dependence (Reid et al., 1998). It may also explain the significant association between cigarette abstinence and cocaine abstinence observed in a study using EMA (Epstein et al., 2009).
We did not observe a significant association between cigarette smoking and short-term outcome of opioid dependence treatment (in patients with concurrent cocaine dependence), despite prior human laboratory studies suggesting that cigarette smoking may make opioids (specifically, methadone) more rewarding (Spiga et al., 2005; Spiga et al., 1998). However, our findings are consistent with results of a prior study of seventeen methadone-maintained patients that found associations between reduced cigarette smoking and reduced cocaine use, but not with opioid use (Shoptaw et al., 1996). The EMA study also found a weaker short-term association of cigarette abstinence with opioid abstinence than with cocaine abstinence (Epstein et al., 2009). This pattern of findings suggests that cigarette smoking may have a stronger influence on cocaine use than on opioid use.
In contrast to the significant association between cigarette smoking and cocaine use during treatment, we did not observe a significant association between cigarette smoking (either lifetime smoking status or number of cigarettes smoked per day prior to the study) and cocaine use prior to study entry (Table 2). This dissociation suggests that cigarette smoking may have a stronger influence on cocaine use when people are trying to cut down or stop such use (as during treatment) than at other times. We are not aware of any other data that bear on this possibility.
Both pharmacological and non-pharmacological mechanisms might explain the association between cigarette smoking and cocaine use. The reinforcing aspects of cocaine use are linked to direct activation of the mesolimbic dopamine system via blockade of the presynaptic dopamine transporter (Gorelick et al., 2004; Haile et al., 2009). Nicotine increases dopamine release in the mesolimbic system (Tanda and Di Chiara, 1998), thereby potentially augmenting the effect of cocaine (Zachariou et al., 2001). In contrast, opioids indirectly activate the mesolimbic dopamine system via activation of mu-opioid receptors (Gianoulakis, 2009). This difference in pharmacological mechanisms may account for the observed difference in associations between cigarette smoking and cocaine versus opioid use.
Psychological factors common to cigarette smoking and cocaine use might also be involved, such as depression (Dierker et al., 2002; Meyer et al., 1996), impulsivity (Moeller and Dougherty, 2002), lack of task persistence (Brandon et al., 2003), or positive expectancies for drug use (Brandon et al., 1999; Kirsch, 1999). However, it is not clear why any of these factors should differentially operate on cocaine use and not on opioid use. Clinically depressed participants were excluded from our study, so depression was unlikely to be a factor.
One possible differential factor between cocaine use and opioid use is stimulus generalization. A related possibility is that cigarette smoking serves as a conditioned cue for the availability of cocaine (Neugebauer et al., 2010), thus stimulating cocaine craving and seeking. Cigarette smoking and cocaine smoking both involve lighting a substance, hand-to-mouth action, and inhaling and exhaling (Sees and Clark, 1993). A majority of people using heroin do so by injection, while only a minority of cocaine users do so (Substance Abuse and Mental Health Services Administration, 2009). Smoking of cocaine is much more common in the US than smoking of heroin (Substance Abuse and Mental Health Services Administration, 2009), which was also the pattern in our study participants. This difference in drug route of administration would favor stimulus generalization from cigarette smoking promoting cocaine use more than heroin use. We are not aware of any human data on cigarette smoking serving as a conditioned cue for cocaine availability. A recent rat study found no evidence that subcutaneous nicotine served as a conditioned cue for methamphetamine self-administration (Neugebauer et al., 2010).
This study found a significant positive association between cocaine use prior to treatment entry and cocaine use during treatment, but no such association for opiate use (Table 2). This finding may be related to buprenorphine treatment reducing opiate use sufficiently to weaken the association.
This study has several limitations. External validity is limited because participants were concurrently dependent on both opioids and cocaine, so results may not generalize to people with only cocaine dependence, although co-use of opioids and cocaine is relatively common (Leri et al., 2003). All participants were taking buprenorphine, which causes a dose-dependent increase in cigarette smoking (Mello et al., 1985; Mutschler et al., 2002). It is possible that buprenorphine may have influenced the interaction between cigarette smoking and treatment outcome. Buprenorphine, at higher doses, reduced illicit opioid use and this reduction may have obscured any association between illicit opioid use during treatment and baseline cigarette use. However, because higher buprenorphine doses were also associated with better cocaine treatment outcome (Montoya et al., 2004), it is not clear how a buprenorphine influence could explain our observed finding of a negative association between higher levels of cigarette smoking and cocaine treatment outcome.
All data on cigarette smoking were based on participant self-report obtained at baseline. However, there were no adverse contingencies for reporting cigarette (or other drug) use at baseline, and self-reports of cigarette smoking are relatively accurate (Shiffman, 2009). Cigarette smoking was not measured during treatment, so it is possible that participants changed their smoking behavior during the course of the study. However, there is evidence that cigarette smoking patterns remain stable among outpatients in treatment for cocaine dependence (Patkar et al., 2006) and opioid dependence (Haas et al., 2008).
This study has several strengths. It is the largest study of which we are aware on the association of cigarette smoking and the outcome of cocaine dependence treatment. It assessed cigarette smoking in terms of both lifetime categorical (lifetime nicotine dependence) and quantitative (number of cigarettes smoked per day) measures, and treatment outcome by an objective measure of drug use (urine drug testing) and treatment retention.
Our findings, consistent with other evidence discussed above, suggest a substantial adverse association between cigarette smoking and cocaine dependence treatment outcome. This finding, coupled with the fact that cigarette smoking significantly damages the health of substance abusers (Hurt et al., 1996; Patkar et al., 2002), offers support for providing smoking cessation programs to cocaine-dependent patients. While this study did not measure cigarette smoking during treatment, it is likely that the cigarette smoking observed at baseline (along with its adverse association with short-term treatment outcome) continued during treatment (Patkar et al., 2006; Haas et al., 2008). Thus, enhancing smoking cessation is likely to improve the outcome of treatment for cocaine dependence by reducing this adverse association. Despite the concerns of some treatment providers that providing treatment for nicotine dependence would detract from treatment of the illegal drug of choice (Guydish et al., 2007; Weinberger et al., 2008), there is little evidence that smoking abstinence or smoking cessation attempts are associated with worse substance abuse treatment outcomes (Kalman, 1998; Reid et al., 2008). In fact, individuals who quit smoking during substance abuse treatment generally have better outcomes than those who do not (Friend and Pagano, 2005; Lemon et al., 2003; Shoptaw et al., 1996; Shoptaw et al., 2002; Sussman, 2002). Studies also suggest that patients in treatment for illicit drug use are interested in nicotine dependence treatment (Clemmey et al., 1997; McDonald et al., 2000).
Future research should evaluate smoking cessation interventions in relation to treatment outcomes for specific drugs of abuse. In particular, it would be useful to determine if concurrent treatment for nicotine dependence improves cocaine dependence treatment outcome, independent of opioid treatment outcome.
Acknowledgments
Role of Funding Source: This research was supported by the Intramural Research Program, National Institutes of Health, National Institute on Drug Abuse. The NIH played no role in the design or conduct of the study, data analysis, drafting of the manuscript, or the decision to submit for publication.
We thank Dr. David Epstein for help with statistical analysis.
Footnotes
Contributors: Author Gorelick originated the study, authors Montoya, Preston, and Gorelick wrote the clinical protocols which generated the study data, authors Harrell and Juliano performed the literature search, and author Harrell performed the statistical analysis and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of interest: The authors have no conflicting interests to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bechtholt AJ, Mark GP. Enhancement of cocaine-seeking behavior by repeated nicotine exposure in rats. Psychopharmacology. 2002;162:178–185. doi: 10.1007/s00213-002-1079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH, Herzog TA, Juliano LM, Irvin JE, Lazev AB, Simmons VN. Pretreatment task persistence predicts smoking cessation outcome. J Abnorm Psychol. 2003;112:448–456. doi: 10.1037/0021-843x.112.3.448. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Juliano LM, Copeland AL. How expectancies shape experience. Washington, DC, US: Am Psychol Assoc; 1999. Expectancies for tobacco smoking; pp. 263–299. [Google Scholar]
- Budney AJ, Higgins ST, Hughes JR, Bickel WK. Nicotine and caffeine use in cocaine-dependent individuals. J Subst Abuse. 1993;5:117–130. doi: 10.1016/0899-3289(93)90056-h. [DOI] [PubMed] [Google Scholar]
- Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. Am J Public Health. 1995;85:41–47. doi: 10.2105/ajph.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmey P, Brooner R, Chutuape MA, Kidorf M, Stitzer M. Smoking habits and attitudes in a methadone maintenance treatment population. Drug Alcohol Depend. 1997;44:123–132. doi: 10.1016/s0376-8716(96)01331-2. [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Cleary PA. Factorial invariance across gender for the primary symptom dimensions of the SCL-90. Br J Soc Clin Psychol. 1977;16:347–356. doi: 10.1111/j.2044-8260.1977.tb00241.x. [DOI] [PubMed] [Google Scholar]
- Dierker LC, Avenevoli S, Stolar M, Merikangas KR. Smoking and depression: an examination of mechanisms of comorbidity. Am J Psychiatry. 2002;159:947–953. doi: 10.1176/appi.ajp.159.6.947. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Marrone GF, Heishman SJ, Schmittner J, Preston KL. Tobacco, cocaine, and heroin: Craving and use during daily life. Addict Behav. 2010;35:318–324. doi: 10.1016/j.addbeh.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman KB, Woolverton WL. Self-administration of cocaine and nicotine mixtures by rhesus monkeys. Psychopharmacology (Berl) 2009;207:99–106. doi: 10.1007/s00213-009-1637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend KB, Pagano ME. Changes in cigarette consumption and drinking outcomes: findings from Project MATCH. J Subst Abuse Treat. 2005;29:221–229. doi: 10.1016/j.jsat.2005.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosch DL, Shoptaw S, Nahom D, Jarvik ME. Associations between tobacco smoking and illicit drug use among methadone-maintained opiate-dependent individuals. Exp Clin Psychopharmacol. 2000;8:97–103. doi: 10.1037//1064-1297.8.1.97. [DOI] [PubMed] [Google Scholar]
- Frosch DL, Stein JA, Shoptaw S. Using latent-variable models to analyze smoking cessation clinical trial data: an example among the methadone maintained. Exp Clin Psychopharmacol. 2002;10:258–267. doi: 10.1037//1064-1297.10.3.258. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem. 2009;9:999–1015. doi: 10.2174/156802609789630956. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Gardner EL, Xi ZX. Agents in development for the management of cocaine abuse. Drugs. 2004;64:1547–1573. doi: 10.2165/00003495-200464140-00004. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Simmons MS, Carriero N, Tashkin DP. Characteristics of smoked drug use among cocaine smokers. Am J Addict. 1997;6:237–245. [PubMed] [Google Scholar]
- Guydish J, Passalacqua E, Tajima B, Manser ST. Staff smoking and other barriers to nicotine dependence intervention in addiction treatment settings: A review. J Psycho Drugs. 2007;39:423–433. doi: 10.1080/02791072.2007.10399881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas AL, Sorensen JL, Hall SM, Lin C, Delucchi K, Sporer K, Chen T. Cigarette smoking in opioid-using patients presenting for hospital-based medical services. Am J Addict. 2008;17:65–69. doi: 10.1080/10550490701756112. [DOI] [PubMed] [Google Scholar]
- Haile CN, Kosten TR, Kosten TA. Pharmacogenetic treatments for drug addiction: cocaine, amphetamine and methamphetamine. Am J Drug Alcohol Abuse. 2009;35:161–177. doi: 10.1080/00952990902825447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen ST, Mark GP. The nicotinic acetylcholine receptor antagonist mecamylamine prevents escalation of cocaine self-administration in rats with extended daily access. Psychopharmacology (Berl) 2007;194:53–61. doi: 10.1007/s00213-007-0822-z. [DOI] [PubMed] [Google Scholar]
- Horger BA, Giles MK, Schenk S. Preexposure to amphetamine and nicotine predisposes rats to self-administer a low dose of cocaine. Psychopharmacology (Berl) 1992;107:271–276. doi: 10.1007/BF02245147. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, Melton LJ., 3rd Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA. 1996;275:1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- Kalman D. Smoking cessation treatment for substance misusers in early recovery: a review of the literature and recommendations for practice. Subst Use Misuse. 1998;33:2021–2047. doi: 10.3109/10826089809069815. [DOI] [PubMed] [Google Scholar]
- Kirsch I. How expectancies shape experience. Washington, DC, US: Am Psychol Assoc; 1999. [Google Scholar]
- Kohn CS, Tsoh JY, Weisner CM. Changes in smoking status among substance abusers: baseline characteristics and abstinence from alcohol and drugs at 12-month follow-up. Drug Alcohol Depend. 2003;69:61–71. doi: 10.1016/s0376-8716(02)00256-9. [DOI] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Lemon SC, Friedmann PD, Stein MD. The impact of smoking cessation on drug abuse treatment outcome. Addict Behav. 2003;28:1323–1331. doi: 10.1016/s0306-4603(02)00259-9. [DOI] [PubMed] [Google Scholar]
- Leri F, Bruneau J, Stewart J. Understanding polydrug use: review of heroin and cocaine co-use. Addiction. 2003;98:7–22. doi: 10.1046/j.1360-0443.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- Levin ED, Mead T, Rezvani AH, Rose JE, Gallivan C, Gross R. The nicotinic antagonist mecamylamine preferentially inhibits cocaine vs. food self-administration in rats. Physiol Behav. 2000;71:565–570. doi: 10.1016/s0031-9384(00)00382-6. [DOI] [PubMed] [Google Scholar]
- Lindsay GB, Rainey J. Psychosocial and pharmacologic explanations of nicotine’s “gateway drug” function. J Sch Health. 1997;67:123–126. doi: 10.1111/j.1746-1561.1997.tb03430.x. [DOI] [PubMed] [Google Scholar]
- McDonald CA, Roberts S, Descheemaeker N. Intentions to quit smoking in substance-abusing teens exposed to a tobacco program. J Subst Abuse Treat. 2000;18:291–308. doi: 10.1016/s0740-5472(99)00067-7. [DOI] [PubMed] [Google Scholar]
- Mello NK, Lukas SE, Mendelson JH. Buprenorphine effects on cigarette smoking. Psychopharmacology (Berl) 1985;86:417–425. doi: 10.1007/BF00427902. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Lin MM, Brown LS., Jr Nicotine dependence and depression among methadone maintenance patients. J Natl Med Assoc. 1996;88:800–804. [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM. Impulsivity and substance abuse: What is the connection? Addictive Disorders & Their Treatment. 2002;1:3–10. [Google Scholar]
- Montoya ID, Gorelick DA, Preston KL, Schroeder JR, Umbricht A, Cheskin LJ, Lange WR, Contoreggi C, Johnson RE, Fudala PJ. Randomized trial of buprenorphine for treatment of concurrent opiate and cocaine dependence. Clinical Pharmacology & Therapeutics. 2004;75:34–48. doi: 10.1016/j.clpt.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton TG. Factor analysis, multicollinearity, and regression appraisal models. Appraisal Journal. 1977;45:578. [Google Scholar]
- Mutschler NH, Stephen BJ, Teoh SK, Mendelson JH, Mello NK. An inpatient study of the effects of buprenorphine on cigarette smoking in men concurrently dependent on cocaine and opioids. Nicotine Tob Res. 2002;4:223–228. doi: 10.1080/14622200210124012. [DOI] [PubMed] [Google Scholar]
- Neugebauer NM, Harrod SB, Bardo MT. Nicotine elicits methamphetamine-seeking in rats previously administered nicotine. Drug Alcohol Depend. 2010;106:72–78. doi: 10.1016/j.drugalcdep.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patkar AA, Lundy A, Leone FT, Weinstein SP, Gottheil E, Steinberg M. Tobacco and alcohol use and medical symptoms among cocaine dependent patients. Subst Abus. 2002;23:105–114. doi: 10.1080/08897070209511480. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Mannelli P, Peindl K, Murray HW, Meier BR, Leone FT. Changes in tobacco smoking following treatment for cocaine dependence. Am J Drug Alcohol Abuse. 2006;32:135–148. doi: 10.1080/00952990500479209. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Vergare MJ, Thornton CC, Weinstein SP, Murray HW, Leone FT. Nicotine dependence and treatment outcome among African American cocaine-dependent patients. Nicotine Tob Res. 2003;5:411–418. doi: 10.1080/1462220031000094178. [DOI] [PubMed] [Google Scholar]
- Reid MS, Fallon B, Sonne S, Flammino F, Nunes EV, Jiang H, Kourniotis E, Lima J, Brady R, Burgess C, Arfken C, Pihlgren E, Giordano L, Starosta A, Robinson J, Rotrosen J. Smoking cessation treatment in community-based substance abuse rehabilitation programs. J Subst Abuse Treat. 2008;35:68–77. doi: 10.1016/j.jsat.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Berger SP. A nicotine antagonist, mecamylamine, reduces cue-induced cocaine craving in cocaine-dependent subjects. Neuropsychopharmacology. 1999;20:297–307. doi: 10.1016/S0893-133X(98)00076-1. [DOI] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Hall SM, Berger SP. An acute dose of nicotine enhances cue-induced cocaine craving. Drug Alcohol Depend. 1998;49:95–104. doi: 10.1016/s0376-8716(97)00144-0. [DOI] [PubMed] [Google Scholar]
- Richter KP, Ahluwalia HK, Mosier MC, Nazir N, Ahluwalia JS. A population-based study of cigarette smoking among illicit drug users in the United States. Addiction. 2002;97:861–869. doi: 10.1046/j.1360-0443.2002.00162.x. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Ratcliff KS, Seyfried W. Validity of the diagnostic interview schedule, version II: DSM-III diagnoses. Psychol Med. 1982;12:855–870. doi: 10.1017/s0033291700049151. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Budney AJ, Bickel WK, Badger GJ. A comparison of cocaine-dependent cigarette smokers and non-smokers on demographic, drug use and other characteristics. Drug Alcohol Depend. 1996;40:195–201. doi: 10.1016/0376-8716(96)01219-7. [DOI] [PubMed] [Google Scholar]
- Satre DD, Kohn CS, Weisner C. Cigarette smoking and long-term alcohol and drug treatment outcomes: a telephone follow-up at five years. Am J Addict. 2007;16:32–37. doi: 10.1080/10550490601077825. [DOI] [PubMed] [Google Scholar]
- Sees KL, Clark HW. When to begin smoking cessation in substance abusers. J Subst Abuse Treat. 1993;10:189–195. doi: 10.1016/0740-5472(93)90044-3. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. NSDUH Series H-36, HHS Publication No. SMA 09-4434. Office of Applied Studies; Rockville, MD: 2009. [accessed February 10, 2010]. Results from the 2008 National Survey on Drug Use and Health: National Findings. Available at http://www.oas.samhsa.gov/nsduh/2k8nsduh/2k8Results.pdf. [Google Scholar]
- Shiffman S. How many cigarettes did you smoke? Assessing cigarette consumption by global report, Time-Line Follow-Back, and ecological momentary assessment. Health Psychol. 2009;28:519–526. doi: 10.1037/a0015197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Jarvik ME, Ling W, Rawson RA. Contingency management for tobacco smoking in methadone-maintained opiate addicts. Addict Behav. 1996;21:409–412. doi: 10.1016/0306-4603(95)00066-6. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Rotheram-Fuller E, Yang X, Frosch D, Nahom D, Jarvik ME, Rawson RA, Ling W. Smoking cessation in methadone maintenance. Addiction. 2002;97(10):1317. doi: 10.1046/j.1360-0443.2002.00221.x. [DOI] [PubMed] [Google Scholar]
- Siqueland L, Crits-Christoph P, Gallop R, Barber JP, Griffin ML, Thase ME, Daley D, Frank A, Gastfriend DR, Blaine J, Connolly MB, Gladis M. Retention in psychosocial treatment of cocaine dependence: predictors and impact on outcome. Am J Addict. 2002;11:24–40. doi: 10.1080/10550490252801611. [DOI] [PubMed] [Google Scholar]
- Spiga R, Martinetti MP, Meisch RA, Cowan K, Hursh S. Methadone and nicotine self-administration in humans: a behavioral economic analysis. Psychopharmacology (Berl) 2005;178:223–231. doi: 10.1007/s00213-004-2020-6. [DOI] [PubMed] [Google Scholar]
- Spiga R, Schmitz J, Day J., 2nd Effects of nicotine on methadone self-administration in humans. Drug Alcohol Depend. 1998;50:157–165. doi: 10.1016/s0376-8716(98)00020-9. [DOI] [PubMed] [Google Scholar]
- Sussman S. Smoking cessation among persons in recovery. Subst Use Misuse. 2002;37:1275–1298. doi: 10.1081/ja-120004185. [DOI] [PubMed] [Google Scholar]
- Tanda G, Di Chiara G. A dopamine-mu1 opioid link in the rat ventral tegmentum shared by palatable food (Fonzies) and non-psychostimulant drugs of abuse. Eur J Neurosci. 1998;10:1179–1187. doi: 10.1046/j.1460-9568.1998.00135.x. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Reutenauer EL, Vessicchio JC, George TP. Survey of clinician attitudes toward smoking cessation for psychiatric and substance abusing clients. J Addict Dis. 2008;27:55–63. doi: 10.1300/J069v27n01_06. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Sofuoglu M. The impact of cigarette smoking on stimulant addiction. Am J Drug Alcohol Abuse. 2009;35:12–17. doi: 10.1080/00952990802326280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Domino EF. Tobacco/nicotine and endogenous brain opioids. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1131–1138. doi: 10.1016/j.pnpbp.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Caldarone BJ, Weathers-Lowin A, George TP, Elsworth JD, Roth RH, Changeux JP, Picciotto MR. Nicotine receptor inactivation decreases sensitivity to cocaine. Neuropsychopharmacology. 2001;24:576–589. doi: 10.1016/S0893-133X(00)00224-4. [DOI] [PubMed] [Google Scholar]


