Abstract
Objective
To develop a standard operating procedure (SOP) for collection, transport, storage of human endometrial tissue and blood samples, subject and specimen annotation, and establishing sample priorities.
Design
The SOP synthesizes sound scientific procedures, the literature on ischemia research, sample collection and gene expression profiling, good laboratory practices, and the authors’ experience of workflow and sample quality.
Setting
The NIH University of California San Francisco Human Endometrial Tissue and DNA Bank.
Patients
Women undergoing endometrial biopsy or hysterectomy for non-malignant indications.
Intervention
Collecting, processing, storing, distributing endometrial tissue and blood samples under approved institutional review board (IRB) protocols and written informed consent from participating subjects.
Main outcome measure
SOP.
Results
The SOP addresses rigorous and consistent subject annotation, specimen processing and characterization, strict regulatory compliance, and a reference for researchers to track collection and storage times that may influence their research.
Conclusion
The comprehensive and systematic approach to the procurement of human blood and endometrial tissue in this SOP ensures the high quality, reliability, and scientific usefulness of biospecimens made available to investigators by the NIH University of California San Francisco Human Endometrial Tissue and DNA Bank. The detail and perspective in this SOP also provides a blueprint for implementation of similar collection programs at other institutions.
Keywords: endometrium, biobanking, standard operating procedure
In recent years volumes of discovery-driven scientific information and advances in bioinformatic technologies, together with expanding regulatory requirements have transformed the conduct of clinical and translational research, including diagnostic development. State of the art infrastructure has become essential, including standard operating procedures for collection, processing and storage of subject samples, rigorous biobanking protocols, encrypted electronic databases, extensive subject/sample annotation, and electronic tracking systems for specimen characteristics, storage, derivatization, and utilization (1, 2). Indeed, the National Cancer Institute (NCI) Best Practices for BioSpecimen Resources (2) comments that consistent and accurate annotation, processing and tracking of biospecimens are “crucial to the overall usefulness of the biospecimen resource as a tool for scientific research”.
The human endometrium, a steroid-hormone dependent tissue that undergoes cyclic proliferation, differentiation, and regeneration (3), is the subject of intense basic, clinical and translational research, focusing on normal/abnormal responses to steroid hormones, reproductive disorders, cancer (4), stem/progenitor cells (5), and development of diagnostics on endometrial tissue or peripheral blood. The success of investigations using endometrial tissue and blood samples critically depend on specimen quality, accurate determination of hormonal state of the subject at sampling, and standardized methods and quality indicators for processing, handling, storing, and tracking specimens. Herein, we describe a standard operating procedure (SOP) for collecting, receiving, processing, storing, handling, and distributing samples obtained under approved institutional review board (IRB) protocols and written informed consent from participating subjects. These procedures were established through the NIH University of California San Francisco Human Endometrial Tissue and DNA Bank - a national resource founded in 2000 serving as a repository for human endometrial tissue, DNA, and blood specimens for NIH investigators and collaborators. The tissues obtained, as described herein, have provided data to the NIH Endometrium Database Resource (http://endometrium.bcm.tmc.edu/edr/ome.seam), an evolving bioinformatics resource on genes associated with the uterus.
The full text SOP is available online (link). Core aspects addressed in this SOP include: 1) compliance with federal/state/institutional, safety, and human subjects regulations; 2) complete and accurate sample and subject annotation; 3) consistent and documented biospecimen processing; and 4) proper specimen storage, identity, and tracking. These aspects are integrated in the SOP and highlighted below.
Definition of purpose, scope, assigned responsibilities, references, definitions, materials, reagents, and forms
Sections 1 and 2 state purpose and scope, and section 3 defines responsibilities of the collection operator, the laboratory staff, and the project/program manager/director. Sections 4 and 5 contain references, definitions and abbreviations relevant to endometrial tissue and peripheral blood specimen collection, handling, processing, storage, and distribution. Section 6 includes a comprehensive list of materials, reagents and paperwork, complete and detailed formulation and procedural notes for reagent preparation, material/reagent vendors, and description of required materials and paperwork. These sections ensure proper and full implementation of the SOP.
Regulatory compliance and safety
In section 7, sample collection approvals address regulatory compliance with institutional biological safety guidelines and federally mandated internal IRB research protocols involving human subjects, including requirement for authorization from the institutional Biological Safety Committee, ensuring safeguard of personal and environmental safety in collection of human blood and tissues, and approval from the internal IRB, the UCSF Committee on Human Research, such that all activities under this SOP fully comply with applicable ethical standards, as well as patient safety and privacy protection. Also described are the procedural steps in obtaining informed consent from participating subjects, in compliance with local institutional, state, and federal regulations.
Biospecimen handling
Sections 8–10 provide protocols for collection of blood and tissue specimens, with details of specimen handling, including sampling and processing, labeling, and required annotation of sample and subject information. Strict specimen segregation throughout processing and separate sets of sterile instruments are required, thus preventing potential acquisition of environmental contaminants or sample cross-contamination. Optimization of tissue collection and processing workflow ensures the quality of tissue preservation for molecular applications, and living cells and preserved intact tissue. The latter two samples are of particular importance for studies involving tissue localization, and functional/phenotype characterization using living cells. For molecular applications, particular attention is paid to the time between the excision of the tissue and its preservation (by snap freezing or submersion in RNAlater®), or warm ischemia time. Tissue ischemia time is critical in biospecimen processing for gene expression analysis, essential for reliable data in screening for molecular targets/diagnostic patterns, and important for data consistency in multicenter programs (6). The effects of tissue ischemia on gene expression microarray analysis have been documented for a variety of human tissues (6–12), and there is broad consensus on the importance of standardizing tissue procurement procedures and minimizing processing time (13). Our approach to tissue procurement is consistent with current consensus in the field, and under the current SOP, endometrial tissue samples allocated for RNA isolation are processed in the operating room and preserved typically within 10 minutes after excision. Validation of the procedure involved pre-chip and post-chip hybridization quality control (QC) measurements (14) on RNA isolated from 96 endometrial tissue specimens. Agilent Bioanalyzer RNA integrity number (RIN) data showed 92% of high-quality RNA samples (RIN scores >7) (Figure 1) suitable for microarray analysis (15) and substantially higher than reported for biobanking of human pancreatic cancer tissue (42%) (16). Microarray data from 85 samples were subjected to standard QC measures of individual and group array quality examining distribution of perfect-match (PM) and background signal intensity, and intensities of probes targeting spiked-in transcripts and control genes. All arrays showed comparable shape and range of PM signal intensity distributions, and good separation between PM signal and background and between positive and negative control mRNA probes. Means of across-array comparison including percent present probes, average background signal, MAS5 scaling factor, and 3′/5′ probe ratios for GAPDH and ACTIN showed few outliers in the percent of probes present and/or 3′/5′ ratios of GAPDH and ACTIN probes, none of which were severe outliers for PM signal intensity or within-group correlation. All arrays had comparable average background and scaling factors, and none of the arrays needed to be excluded for technical reasons. Thus, the high RNA and array quality obtained with the SOP procurement protocol ensures the reliability and scientific usefulness of the data derived thereof.
Figure 1. RNA integrity number (RIN) scores for endometrial tissue isolates.
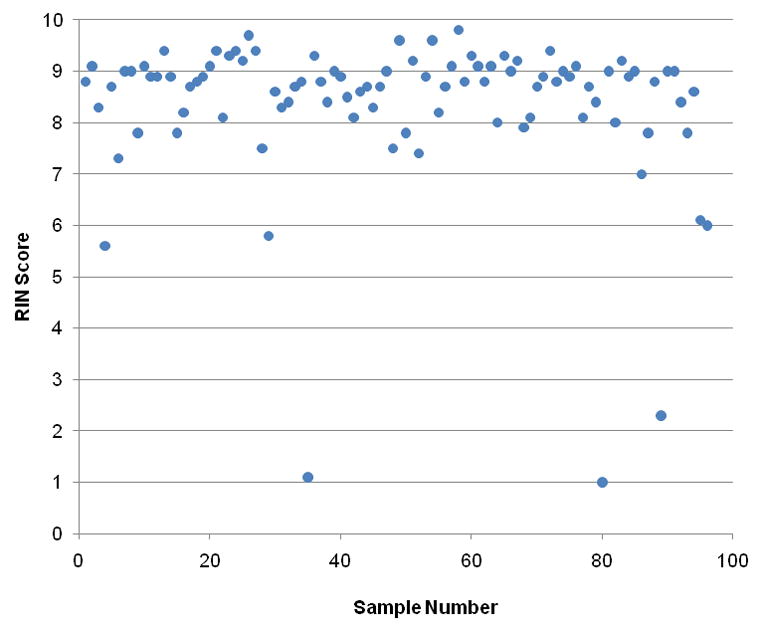
Pre-chip quality control data for total RNA samples isolated from 96 individual endometrial tissue specimens from the NIH UCSF Human Endometrial Tissue and DNA Bank. Values represent RNA integrity number (RIN) scores determined using an Agilent 2100 Bioanalyzer.
The SOP workflow addresses parallel processing of tissue samples from individual specimens for three distinct applications (tissue sectioning/histology, RNA extraction, and cell isolation and culture), each having different handling requirements, and establishes an order of priority for processing samples for the different applications, in keeping with known requirements and recommendations (17), as follows: 1) RNA isolation (flash frozen, or immersed in RNAlater®); 2) histology/immunohistochemistry (fixed in 10% formalin and/or 4% paraformaldehyde); 3) frozen sectioning (OCT-embedded frozen ); 4) viable cell isolation (immersed in tissue transport medium). The order of priority reflects the critical importance of processing time in samples for RNA isolation compared to other applications. It is also important to ensure availability of adequate samples for histological evaluation of each endometrial specimen (18) since the endometrium undergoes major molecular and cellular cycle-dependent changes, and lack of reliable cycle phase determination for would compromise the accurate interpretation of molecular analyses.
Sample labeling processing and storage
Instructions in sections 8–11 for labeling, lab processing, and final storage of samples ensure consistent, accurate, and unambiguous identification of all specimens and their derivatives, enabling efficient sample tracking and inventory.
Concluding remaks
Since its inception, the NIH UCSF Human Endometrial Tissue and DNA Bank has accrued over 700 endometrial tissue and 150 blood samples, under approved IRB protocols, from women undergoing endometrial biopsy or benign gynecologic surgery, and is a national resource as a repository for human endometrial tissue, DNA, and blood specimens for NIH investigators and collaborators. The implementation of rigorous biobanking specimen protocols becomes essential to assure high quality specimens, sample consistency, accurate histologic characterization. Complete clinical annotation, and the development and implementation of this SOP for collecting, receiving, processing, storing, and handling samples are pivotal for high biospecimen quality essential for state of the art molecular applications. Functional genomics approaches applying microarray technologies to studying human endometrium (19) have led to discoveries of cycle phase-specific gene expression profiles (20–22) and previously unsuspected differences in gene expression in eutopic endometrium of women with endometriosis (23, 24). This underscores the importance of meticulous subject annotation, specimen procurement and phenotypic characterization for studies with human endometrium and exemplifies how control of biospecimen processing variables is essential to obtain reliable data in discovery of molecular targets and diagnostic molecular patterns. The current SOP addresses these critical issues - providing a synthesis of sound scientifically validated procedures, thorough documentation, and strict regulatory compliance relevant to the collection of endometrial samples in multiple clinical settings. The comprehensive and systematic approach to the procurement of human endometrial tissue and blood contained in this SOP, ensures the high degree of quality, reliability, and scientific usefulness of biospecimens procured and made available to investigators by the NIH UCSF Human Endometrial Tissue and DNA Bank. In addition, the detail and perspective in this SOP may provide a starting blueprint for implementing endometrial sample collection programs at other sites and institutions.
Supplementary Material
Acknowledgments
Supported by the NIH Eunice Kennedy Shriver National Institute of Child Health and Human Development through cooperative agreement U54HD055764-04 (LCG) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blow N. Biobanking. Nature Methods. 2009;6:173. [Google Scholar]
- 2.NCI Best Practices for Biospecimen Resources. www.nci.nih.gov.
- 3.Hess A, Nayak N, Giudice LC. Oviduct and endometrium: cyclic changes in primate oviduct and endometrium. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 3. Academic Press; San Diego: 2005. pp. 337–381. [Google Scholar]
- 4.Aplin J, Fazleabas A, Glasser S, Giudice LC. The Endometrium: Molecular, Cellular & Clinical Perspectives. 2. Informa Healthcare; London: 2008. [Google Scholar]
- 5.Gargett CE. Uterine stem cells: what is the evidence? Hum Reprod Update. 2007;13:87–101. doi: 10.1093/humupd/dml045. [DOI] [PubMed] [Google Scholar]
- 6.Spruessel A, Steimann G, Jung M, Lee SA, Carr T, Fentz AK, et al. Tissue ischemia time affects gene and protein expression patterns within minutes following surgical tumor excision. Biotechniques. 2004;36:1030–7. doi: 10.2144/04366RR04. [DOI] [PubMed] [Google Scholar]
- 7.Dash A, Maine IP, Varambally S, Shen R, Chinnaiyan AM, Rubin MA. Changes in differential gene expression because of warm ischemia time of radical prostatectomy specimens. Am J Pathol. 2002;161:1743–8. doi: 10.1016/S0002-9440(10)64451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang J, Qi R, Quackenbush J, Dauway E, Lazaridis E, Yeatman T. Effects of ischemia on gene expression. J Surg Res. 2001;99:222–7. doi: 10.1006/jsre.2001.6195. [DOI] [PubMed] [Google Scholar]
- 9.Blackhall FH, Pintilie M, Wigle DA, Jurisica I, Liu N, Radulovich N, et al. Stability and heterogeneity of expression profiles in lung cancer specimens harvested following surgical resection. Neoplasia. 2004;6:761–7. doi: 10.1593/neo.04301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Cecco L, Musella V, Veneroni S, Cappelletti V, Bongarzone I, Callari M, et al. Impact of biospecimens handling on biomarker research in breast cancer. BMC Cancer. 2009 Nov 24;9:409. doi: 10.1186/1471-2407-9-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popova T, Mennerich D, Weith A, Quast K. Effect of RNA quality on transcript intensity levels in microarray analysis of human post-mortem brain tissues. BMC Genomics. 2008;9:91. doi: 10.1186/1471-2164-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong V, Wang DY, Warren K, Kulkarni S, Boerner S, Done SJ, et al. The effects of timing of fine needle aspiration biopsies on gene expression profiles in breast cancers. BMC Cancer. 2008;8:277. doi: 10.1186/1471-2407-8-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medeiros F, Rigl CT, Anderson GG, Becker SH, Halling KC. Tissue handling for genome-wide expression analysis: a review of the issues, evidence, and opportunities. Arch Pathol Lab Med. 2007;131(12):1805–16. doi: 10.5858/2007-131-1805-THFGEA. [DOI] [PubMed] [Google Scholar]
- 14.Jones L, Goldstein DR, Hughes G, Strand AD, Collin F, Dunnett SB, et al. Assessment of the relationship between pre-chip and post-chip quality measures for Affymetrix GeneChip expression data. BMC Bioinformatics. 2006;7:211. doi: 10.1186/1471-2105-7-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson KL, Pine PS, Rosenzweig BA, Turpaz Y, Retief J. Characterization of the effect of sample quality on high density oligonucleotide microarray data using progressively degraded rat liver RNA. BMC Biotechnol. 2007;7:57. doi: 10.1186/1472-6750-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudloff U, Bhanot U, Gerald W, Klimstra DS, Jarnagin WR, Brennan MF, et al. Biobanking of human pancreas cancer tissue: impact of ex-vivo procurement times on RNA quality. Ann Surg Oncol. 2010;17(8):2229–36. doi: 10.1245/s10434-010-0959-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handling, Transport, and Storage of Specimens for Molecular Methods, CLSI Approved Guideline (MM13-A), Aug 2007
- 18.Murray MJ, Meyer WR, Zaino RJ, Lessey BA, Novotny DB, Ireland K, et al. A critical analysis of the accuracy, reproducibility, and clinical utility of histologic endometrial dating in fertile women. Fertil Steril. 2004;81:1333. doi: 10.1016/j.fertnstert.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 19.Giudice LC. Application of functional genomics to primate endometrium: insights into biological processes. Reprod Biol Endocrinol. 2006;4 (Suppl 1):S4. doi: 10.1186/1477-7827-4-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147:1097–121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- 21.Ponnampalam AP, Weston GC, Trajstman AC, Susil B, Rogers PA. Molecular classification of human endometrial cycle stages by transcriptional profiling. Mol Hum Reprod. 2004;10:879–93. doi: 10.1093/molehr/gah121. [DOI] [PubMed] [Google Scholar]
- 22.Riesewijk A, Martín J, van Os R, Horcajadas JA, Polman J, Pellicer A, et al. Gene expression profiling of human endometrial receptivity on days LH+2 versus LH+7 by microarray technology. Mol Hum Reprod. 2003;9:253–64. doi: 10.1093/molehr/gag037. [DOI] [PubMed] [Google Scholar]
- 23.Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144:2870–81. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- 24.Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–26. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


