Abstract
Fulvic acid (FA) is class of compounds of humic substances formed through the degradation of organic substances by chemical and biological processes. FA has been utilized in traditional Chinese medicine and possesses various pharmacological properties. Previously, we reported that FA extracted from solubilized excess sludge (SS-FA) had an inhibitory effect on β-hexosaminidase release in human leukemia basophilic (KU812) cells. In this study, we investigated the effects of SS-FA on the immediate-type allergic reaction and studied its possible mechanisms of action in KU812 cells following activation with phorbol myristate acetate (20 nmol L−1) plus calcium ionophore A23187 (1 μmol L−1) (PMACI). The inhibitory effect of SS-FA on degranulation in PMACI-stimulated KU812 cells was examined using histamine release assay. SS-FA significantly decreased the histamine release in KU812 cells at concentrations of 0.1–10.0 μg mL−1. To gain more information regarding the mechanism of the suppression of degranulation following SS-FA treatment, microarray was conducted to determine which genes were differentially expressed in response to SS-FA in PMACI-activated KU812 cells. From a total of 201 genes in the DNA chip, 28 genes were up-regulated and 173 genes were down-regulated in cells pretreated with SS-FA for 15 min and stimulated with PMACI. From the 71 genes that showed more than two fold change in expression, 16 genes were significantly down-regulated that were subjected to hierarchical clustering. SS-FA affected the expression of genes that were involved in the following pathways: signal transduction, cytokine–cytokine receptor interaction, immune response, cell adhesion molecules and IgE receptor β subunit response.
Keywords: Immediate-type allergy, Histamine release, Microarray analysis, Fulvic acid, Solubilized excess sludge
Introduction
Fulvic acid (FA) is a class of compounds of humic substances together with humic acid and humin, and is formed through the degradation of organic substances such as dead plants, microbes and animals by chemical and biological processes. Recently, the physiological action of FA on the human body has been studied. For example, the possible application of coal-derived FA as antimicrobials (van Rensburg et al. 2000), and the anti-inflammatory property of coal-derived FA have been reported (van Rensburg et al. 2001). In addition, the FA extracted from peat (Tachibana et al. 2004) had an antioxidative activity and the FA extracted from Canadian Sphagnum Peat (Yamada et al. 2007) had an inhibitory effect on chemical mediator release in rat basophilic leukemia cells (RBL-2H3).
Basophil activation has been implicated in allergic reaction and immune modulation. These events trigger the secretions of chemical mediators such as cytokines. Among the cytokines, IL4 and IL13 are the major cytokines for IgE production, facilitating Th2 differentiation and allergic responses (Gauchat et al. 1993). Basophils could rapidly secrete large amount of IL4 and IL13, and have been detected in organs affected by allergic reactions, such as bronchial and airway biopsies from asthma patients, nasal lavage fluids from allergic rhinitis patients, as well as skin biopsies from atopic and contact dermatitis. Human basophils, therefore, play a crucial role as a high priority source of IL4, IL13, and histamine that polarize the early type 2 immune response leading to allergy (Chen et al. 2009; Zhu et al. 1998; Yanagihara et al. 1997).
In our previous study, we extracted FA from solubilized excess sludge (SS-FA) and determined the structural properties of SS-FA using elemental analysis, fourier transform infrared spectroscopy and 1H-nuclear magnetic resonance spectroscopy (Motojima et al. 2009). Excess sludge is one of the components of sewage sludge, and Japan generates 1.7 million tons of excess sludge every year. Recently, there is a demand for the use of this excess sludge. Under the circumstances, we developed a pretreatment technique for the improvement of the extraction rate of FA using solubilization and carried out extraction following the standard method of the International Humic Substances Society. Moreover, to search for a utility for SS-FA, we examined its inhibitory effect on the chemical mediator release in rat basophilic leukemia (RBL-2H3) cells (Motojima et al. 2009). Our experiment showed that SS-FA had β-hexosaminidase release inhibitory effect on the antigen–antibody binding stage and the antigen-receptor binding stage in RBL-2H3 cells.
In the present study, in order to gain more information regarding the mechanism of the suppression of the degranulation following SS-FA treatment, we studied the effect of SS-FA on the immediate-type allergic reaction in human leukemia basophilic (KU812) cells. The inhibitory effect of SS-FA on the degranulation in PMA (20 nmol L−1) plus calcium ionophore A23187 (1 μmol L−1) (PMACI)-stimulated KU812 cells was examined using histamine release assay. The cytotoxicity of SS-FA at the concentration used for the histamine release assay was assessed by MTT assay. Microarray analysis is a technique that has been shown to be useful for the simultaneous profiling of global gene expression and uncovering new genes or new functions of known genes (Thornton et al. 2002). For these reasons, microarray assay was conducted to determine which genes were differentially expressed in response to SS-FA in PMACI-activated KU812 cells.
Materials and methods
Extraction and purification of SS-FA
Excess sludge was sampled from the return line of an aeration reactor at a municipal wastewater treatment plant in Japan. Extraction and isolation procedures were carried out following the protocol of the International Humic Substances Society. The procedure for the structural features analysis of SS-FA was described in the previous report (Motojima et al. 2009).
Reagent, cell and cell culture
KU812 cells were purchased from the Riken Cell Bank, Japan and were maintained in RPMI1640 medium (Gibco BRL, Grand island, NY, USA) supplemented with 10% fetal bovine serum (FBS) (Hyclone Co. Japan) containing 50 U mL−1 of penicillin and 50 μg mL−1 of streptomycin, and were cultured at 37 °C in a humidified atmosphere with 5% CO2. RBL-2H3 cells were purchased from JCRB Bank, Japan, and were cultivated in Eagle’s Minimum Essential Medium with 10% FBS and 2 mmol L−1 of l-glutamine at 37 °C in 5% CO2 incubator. Calcium ionophore A23187 and phorbol 12-myristate 13-acetate (PMA) were obtained from Sigma, Japan (Tokyo) and dissolved in DMSO at a concentration of 10 mM. Dinitrophenylated bovine serum albumin (DNP-BSA) was obtained from Cosmo Biotechnology Co. (Japan), and anti-DNP-IgE was from Sigma (Japan).
Measurement of intracellular calcium [Ca2+]i level
[Ca2+]i measurement was performed using the method of Aase and Arna (1991) with some modifications. Cells (5 × 105 cells mL−1 in 100 μL of the medium) were preincubated at 37 °C for 24 h in 96-well plates in a medium containing 10% FBS and anti-DNP IgE (final concentration 0.3 μg mL−1). The cells were washed twice with 200 μl of PBS to eliminate the free IgE. The cells were then incubated with 100 μL per well of a loading buffer containing Fluo3-AM (Caladium Kit-Fluo3, Dojindo Co.) at 37 °C for 1 h in a 5% CO2 incubator. After washing the dye from the cell surface, cells were incubated with 60 μl per well of a recording medium (Caladium Kit-Fluo3, Dojindo Co.) containing the sample (0.1, 10.0 μg mL−1 SS-FA) for 15 min. The fluorescence intensity was determined 50 s, after adding 5 μL of the DNP-BSA antigen using a microplate reader at an excitation wavelength of 490 nm and emission wavelength of 530 nm.
Histamine release assay
KU812 cells at a density of 1 × 106 cells mL−1 were preincubated for 24 h in RPMI1640 medium supplemented with 10% FBS. The cells were treated with or without 25 μL per well of various concentrations of SS-FA (0.1–10 μg mL−1) for 15 min, and followed by stimulation with PMACI for 30 min at 37 °C. The cells were separated from the released histamine by centrifugation at 190×g for 3 min at 4 °C. The supernatant (200 μL) was transferred to a 96-well ELISA system. The fluorescence intensity was measured at 414 nm using a multidetection microplate reader (Power Scan HT. Dainippon Pharmaceutical Co.), and the histamine concentration determined by ELISA kit according to the manufactures’ instruction.
MTT assay
Cell proliferation was assessed by MTT assay which is a measure of the mitochondrial respiration of the cells (Mossam 1983). KU812 cells were seeded onto 96-well plates at 1 × 106 cells mL−1 in 80 μL of medium, respectively. After an overnight incubation, 10 μL of the SS-FA dissolved in the medium was added to obtain the final concentrations of 0.1, 1.0 and 10.0 μg mL−1. The cells were then incubated for 24 h before 10 μL of 5 mg mL−1 of MTT was added. After 24 h of incubation, 100 μL of 10% sodium dodecyl sulfate was added, followed by another 24 h of incubation to completely dissolve the formazan produced by the cells. The absorbance was then determined at 570 nm with a microplate reader. Blanks were prepared at the same time to correct for the absorbance caused by sample color and by the inherent ability of a sample to reduce MTT in the absence of cells. The optical density of the formazan produced by the untreated control cells represented 100% proliferation.
RNA extraction
KU812 cells at concentration of 1 × 106 cells mL−1 were treated with or without 0.1 μg mL−1 of SS-FA for 15 min and incubated at 37 °C. After exposure to PMACI for 30 min, total RNA was extracted from the cells using 1 mL of Isogen (Nippon Gene Co., Tokyo, Japan) following the manufactures instruction.
DNA microarray
Synthetic DNA oligonucleotide probes were installed as probes onto Genopal (Mitsubishi Rayon, Tokyo, Japan), which is composed of plastic hollow fibers. With this system oligonucleotide DNA probes are attached to a gel within the three-dimensional space of each hollow fiber (Hohjoh and Fukushima 2007).
First, RNA was amplified using the MessageAmpII biotin-enhanced amplification kit (Applied Biosystems Japan, Tokyo, Japan), according to the manufacturer’s instructions, and column purified. Biotinylated aRNA (5 μg) was fragmented using fragmentation reagents (Applied Biosystems Japan) and then incubated at 94 °C for 7.5 min. The fragmentation reaction was terminated by the addition of stop solution. Hybridization was carried out with a DNA microarray (Genopal, Mitsubishi Rayon) in 180 μL of hybridization buffer (0.12 mol L−1 Tris–HCl/0.12 mol L−1 NaCl, 0.05% Tween-20) and 5 μg of fragmented biotinylated aRNA at 65 °C overnight. After hybridization, the DNA microarray was washed twice in wash solution A (0.12 mol L−1 Tris–HCl/0.12 mol L−1 NaCl, 0.05% Tween-20) followed by washing in wash solution B (0.12 mol L−1 Tris–HCl/0.12 mol L−1 NaCl). The DNA microarray was then labeled with streptavidin-Cy5 (GE Healthcare Bio-Science KK, Tokyo, Japan). The fluorescent labeled-DNA microarray was washed for 5 min four times in wash solution B at room temperature. Hybridization signal acquisition was performed using a DNA microarray reader adopting multibeam excitation technology (Yokogawa Electric Co., Tokyo, Japan) (Isozaki et al. 2006). The DNA microarrays were scanned at multiple exposure times ranging from 0.1 to 40 s and the intensity values with the best exposure condition for each spot were selected.
Statistical analysis
Statistical significance (p < 0.05) was evaluated by one-way ANOVA, and if significant, group means were compared using Bonferroni’s post hoc. Homogeneous subsets of significance were determined by Duncan’s post hoc test.
Results and discussion
Effect of SS-FA on histamine release in PMACI-stimulated KU812 cells
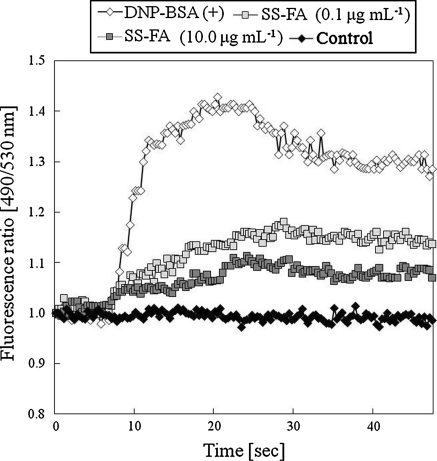
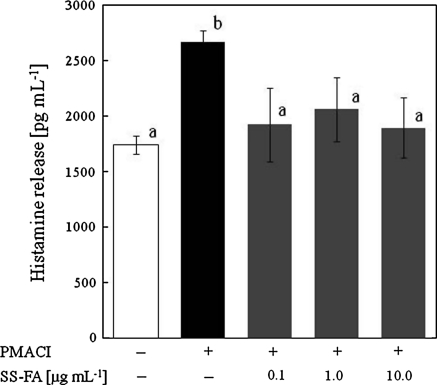
In immediate-type allergy, exposure to allergens causes the blood cells to produce a large amount of antibodies IgE. When IgE strongly binds to an IgE-specific (Fc) receptor on the surface of mast cells or basophiles, the receptors are crosslinked to transform the cell surfaces, various enzymes are activated and the cytosolic calcium level increase. The cells release ß-hexosaminidase, histamine, cytokines and other chemical mediators from their granules causing allergy. It is well recognized that these cells release histamine in response to an elevation of the concentration of cytosolic free Ca2+ resulting from PMACI-mediated stimulus. The PMA is the most commonly used phorbol ester; it binds and activates protein kinase C. A23187 is a calcimycin that greatly increases the ability of Ca2+ to cross biological membranes. Before the histamine release inhibition assay, we measured the [Ca2+]i mobilization by fluorometric analysis to examine the suppression of [Ca2+]i mobilization by SS-FA, and found that SS-FA suppressed [Ca2+]i elevation in the IgE-sensitized RBL-2H3 cells (Fig. 1). This result suggests that SS-FA suppressed various intracellular signals, which are associated with the elevation of [Ca2+]i. Therefore, the inhibitory effect of SS-FA on PMACI-induced histamine release was confirmed (Fig. 2). The SS-FA at different concentrations significantly inhibited the PMACI-induced histamine release. In our previous study, we showed that SS-FA had an anti-oxidative activity as determined by the DPPH radical scavenging assay. Suzuki et al. (2005) reported that radical scavenging activity correlates with inhibitory activities on histamine release and on the DPPH radical scavenging activity. In the current study, the radical scavenging activity of SS-FA probably affected the decrease of histamine release in KU812 cells. However, SS-FA was not cytotoxic in the tested concentrations during MTT assay and the inhibitory effect of SS-FA was not dose-dependent. FA consists of a mixture of closely related complex of aromatic polymer composites (Stevenson 1985). Macromolecular conformation of FA varies according to factors such as the concentration, composition, and pH of the solution (Yamada et al. 1987). These observations suggest that several stimulation mechanisms may be involved simultaneously in the cell in response to SS-FA.
Fig. 1.
Effect of fulvic acid extracted from solubilized excess sludge (SS-FA) on the intracellular [Ca2+]i levels with DNP-BSA activation in RBL-2H3 cells. The intracellular Ca2+ mobilization was measured using Calcium Kit-Fluo 3TM (Dojindo Laboratories, Kumamoto, Japan). IgE-sensitized RBL-2H3 cells (5.0 × 105 cells mL−1) were incubated with 100 μL of loading buffer including Fluo-3AM (Calcium Kit-Fluo 3TM) for 1 h. The treated cells were incubated in 60 μL of the recording medium and 5 μL of SS-FA for 15 min. Changes in the intracellular Ca2+ concentration induced by DNP-BSA were measured using a fluorometric imaging plate reader. Three addition trials show similar results
Fig. 2.
Effect of SS-FA on the PMACI-induced histamine release from KU812 cells. KU812 cells (1 × 106 cells mL−1) were incubated in the presence of different concentration of SS-FA (0.1, 1.0 and 10.0 μg mL−1) for 15 min, before the cells were stimulated with PMA (20 nmol−1) and calcium ionophore A23187 (1 μmol−1) for 30 min. Histamine concentration was determined by an ELISA kit according to the manufacturer’s instruction. Statistically significant tests (p < 0.05) were determined by one-way analysis of variance followed by Duncan’s post hoc test. Means without a common letter within the same graph differ significantly. SS-FA: fulvic acid extracted from solubilized excess sludge. PMACI: phorbol 12-myristate 13-acetate plus calcium ionophore A23187
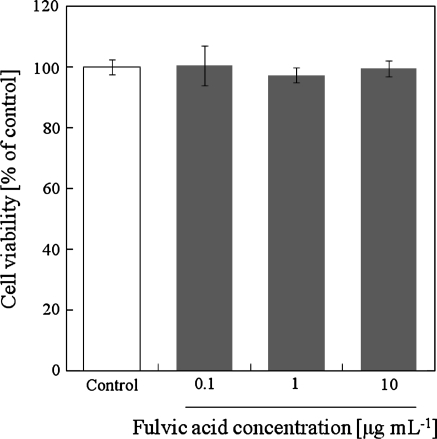
Cytotoxicity effect of SS-FA on KU812 cells
MTT assay was used to evaluate the effect of SS-FA on the proliferation of KU812 cells. KU812 cells were treated with SS-FA (0.1, 1.0 and 10.0 μg mL−1) for 24 h and incubated in 37 °C. This is the same concentration that was used for histamine release assay. As shown in Fig. 3, SS-FA at the different concentrations tested did not cause cytotoxicity.
Fig. 3.
Cytotoxic effect of fulvic acid extracted from solubilized excess sludge (SS-FA) on KU812 cells as determined by MTT assay. The percent cell viability was calculated relative to the untreated control. The cells (1 × 106 cells mL−1 in 100 μL) were treated with SS-FA at 37 °C for 24 h in 5% CO2. Results represent one trial (n = 4). Two additional trials show similar result. *Significantly different from the control (p < 0.05, Student’s t test)
Gene expression profile of SS-FA treated cells
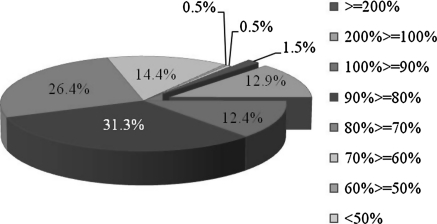
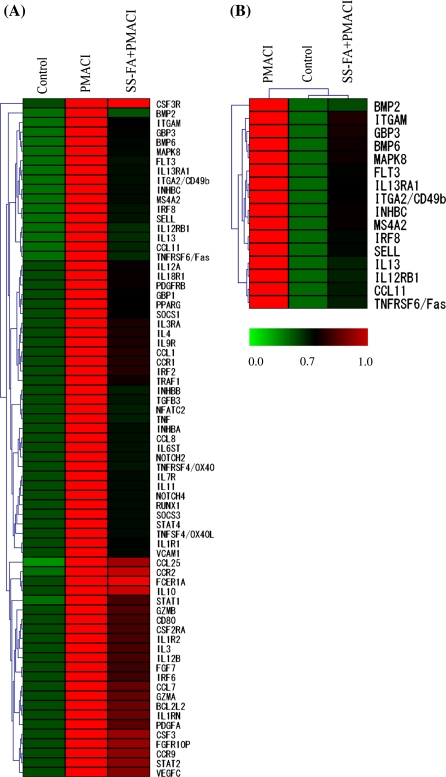
To elucidate the molecular mechanisms controlled by SS-FA, we determined the gene expression profile of SS-FA plus PMACI-treated cells compared to PMACI alone using DNA microarray analysis. From the 201 total genes in the DNA chip, 28 genes were up-regulated and 173 genes were down-regulated in cells that were pretreated with SS-FA for 15 min and treated with PMACI. The percentages of up- or down-regulated genes in cells by SS-FA plus PMACI are shown in Fig. 4. About 86% of the total genes were down-regulated by SS-FA treatment in PMACI-stimulated KU812 cells. After that, the 2-differentially expressed genes in cells treated with PMACI alone (compared to control) were selected. The analysis of the 71 genes with two-fold expression was done using the hierarchical clustering program of the TIGR’s MultiExperiment Viewer v4.0 software (Fig. 5A). The expression ratios of treated (PMACI or SS-FA plus PMACI) to untreated (control) are shown in the heat map using red and green color codes for up- and down-regulation, respectively. From the hierarchical clustering, 16 genes that were significantly down-regulated by SS-FA treatment were identified (Fig. 5B). Cluster analysis in microarray technology is to group genes or experiments into groups with similar profiles. The sample tree yielded two groups wherein SS-FA was grouped with the control (unstimulated) and the other group contained PMACl (stimulated) which may indicate that SS-FA has an inhibitory effect on immediate-type allergy. The 16 significantly down-regulated genes (BMP2, BMP6, CCL11, FLT3, GBP3, IL13, IL12RB1, L13RA1, INHBC, ITGA2/CD49b, ITGAM, IRF8, MAPK8, MS4A2, SELL, TNFRSF6/Fas) were mapped to their relevant pathways in Table 1 and the gene expression profile of cells treated with PMACI or SS-FA alone is also shown in the same table. KEGG pathways were used to identify the enriched function-related gene groups characterizing the different datasets generated (http://www.genome.jp/dbget-bin/get_linkdb?-t+2+path:hsa04060). SS-FA affected genes that are involved in the following: signal transduction, cytokine–cytokine receptor interaction, immune response, cell adhesion and IgE receptor β subunit.
Fig. 4.
Percentages of up- or down-regulated genes in cells after SS-FA plus PMACI treatment compared to PMACI alone. SS-FA: fulvic acid extracted from solubilized excess sludge. PMACI: phorbol 12-myristate 13-acetate plus calcium ionophore A23187
Fig. 5.
Hierarchical clustering of down-regulated genes in KU812 cells treated with SS-FA plus PMACI (control compared to PMACI). (A) Seventy-one differentially expressed genes (2.0-fold) analyzed using TIGR’s MultiExperiment Viewer v4.0 software. The clustering of 71 genes using an average linkage algorithm, underscores the overall suppression of the genes by SS-FA plus PMACI relative to PMACI. Distance metric is 1 correlation. Horizontal stripes represent genes while columns show experimental samples. Red denotes gene up-regulation while green denotes gene downregulation relative to the median. (B) Sixteen of significantly downregulated genes were identified from the hierarchical cluster
Table 1.
Down-regulated genes in KU812 cells that were treated with SS-FA plus PMACI
| Function | Symbol | Accession no | Gene name | Fold change | ||
|---|---|---|---|---|---|---|
| PMACI | SS-FA plus PMACI ((SS-FA plus PMACI)/PMACI)a | SS-FA | ||||
| Cytokine- Cytokine receptor interaction | IL13 | NM_002188 | Homo sapiens interleukin 13 | 2.83 | 1.67 (0.59) | 1.31 |
| IL12RB1 | NM_005535 | Homo sapiens interleukin 12 receptor, beta 1 | 2.51 | 1.49 (0.59) | 1.31 | |
| IL13RA1 | NM_001560 | Homo sapiens interleukin 13 receptor, alpha 1 | 2.62 | 1.77 (0.68) | 1.44 | |
| CCL11 | NM_002986 | Homo sapiens chemokine(C–C motif) ligand 11 | 2.30 | 1.41 (0.61) | 1.17 | |
| INHBC | NM_005538 | Homo sapiens inhibin, beta C | 2.42 | 1.67 (0.69) | 1.37 | |
| BMP2 | NM_001200 | Homo sapiens bone morphogenetic protein 2 | 2.35 | 1.12 (0.48) | 1.22 | |
| BMP6 | NM_001718 | Homo sapiens bone morphogenetic protein 6 | 2.33 | 1.63 (0.70) | 1.05 | |
| FLT3 | NM_004119 | Homo sapiens fms-related tyrosine kinase 3 | 2.24 | 1.50 (0.67) | 1.21 | |
| MAPK signaling | MAPK8 | NM_139049 | Homo sapiens mitogen-activated protein kinase 8 | 2.30 | 1.62 (0.70) | 1.37 |
| TNFRSF6/Fas | NM_000043 | Homo sapiens Fas (TNF receptor superfamily, member 6 | 2.30 | 1.37 (0.60) | 1.34 | |
| Immune response | IRF8 | NM_002163 | Homo sapiens interferon regulatory factor 3 | 2.26 | 1.48 (0.66) | 1.42 |
| GBP3 | NM_018284 | Homo sapiens guanylate binding protein 3 | 2.31 | 1.65 (0.71) | 1.22 | |
| Cell adhesion molecules | ITGA2/CD49b | NM_002203 | Homo sapiens integrin, alpha 2 | 2.25 | 1.53 (0.68) | 1.13 |
| ITGAM | NM_000632 | Homo sapiens integrin, alpha M | 2.40 | 1.73 (0.72) | 1.37 | |
| SELL | NM_000655 | Homo sapiens selectin L | 2.26 | 1.49 (0.66) | 1.34 | |
| IgE receptor β subunit | MS4A2 | NM_000139 | Homo sapiens membrane-spanning 4-domains, subfamily A, member 2 | 2.25 | 1.55 (0.69) | 1.2 |
aThe number shown in parentheses indicates inhibition ratio of SS-FA plus PMACI compared to PMACI stimulation
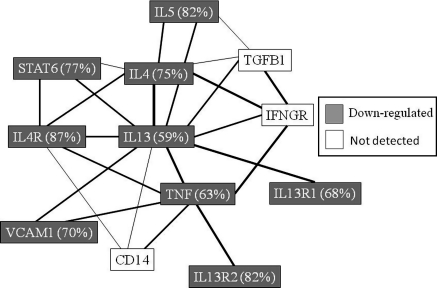
IL13 and IL4 participate in allergic inflammation, share a receptor subunit (IL4R) are structurally similar, and share a functional signaling receptor chain (de Vries 1998). IL13 and IL4 are required for optimal induction of IgE synthesis. IL13R is usually present at 200–3,000 sites per cell and binds IL13 with high affinity. Two different cDNAs encoding IL13-binding proteins have been cloned recently and designated IL13RA1 and IL13RA2 (Hilton et al. 1996; Aman et al. 1996). Our experiment showed that IL13 and IL13RA1 were up-regulated following PMACI stimulation. However, treatment with SS-FA suppressed their expression significantly and down-regulated IL4 by 75% (SS-FA plus PMACI/PMACI alone). The inhibitory effect of SS-FA on the expression of IL13-related genes in KU812 cells are shown in Fig. 6. Signal transducer and activator of transcription 6 (STAT6) is involved in the IL4 and IL13 signaling pathway (Lin et al. 1995). STAT6-deficient mice were reported to fail to develop IL4-mediated functions, including Th2 differentiation, the expression of cell surface markers, and Ig class switching to IgE (Shimoda et al. 1996). In addition to its ability to induce IgE synthesis, IL13 also contributes to allergic-inflammatory processes through its capacity to induce vascular cell adhesion molecule (VCAM)-1 expression on human umbilical vein endothelial cells (Bochner et al. 1995). The cytokine IL5 is a marker for the Th2 subset of effector T cells and is often expressed together with IL4 and IL13. As shown in Fig. 6, treatment with SS-FA suppressed these IL13-related genes in KU812 cells (IL4, IL4 receptor, IL13 receptor, IL5, STAT6, TNF, VCAM).
Fig. 6.
A scheme showing the inhibitory effect of fulvic acid (extracted from solubilized excess sludge or SS-FA) on the expression of IL13-related genes in KU812 cells. IL13 related genes and other genes that were differentially expressed by 1.5-fold were used in the analysis. The numbers shown in parentheses indicate the inhibition ratio of SS-FA plus PMACI compared to PMACI-stimulation only
Mitogen-activated protein kinase 8 (MAPK8) was up-regulated following PMACI stimulation but SS-FA treatment suppressed its expression. The encoded protein is a member of the serine/threonine protein kinase family. This kinase can activate both the MAP kinase and JNK kinase pathways. Moreover, MAP3K8 promotes the production of tumor necrosis factor (TNF)-α. Rasheed et al. (2009) reported that activation of MAPKs was intimately associated with the expression of pro-inflammatory cytokines, reduction of pro-inflammatory cytokines expression, and inhibited PMACI-induced phosphorylation of JNKp54/p46- and ERKp44/p42 in KU812 cell. In our experiment, pro-inflammatory cytokines TNF (63% SS-FA plus PMACI/PMACI alone) and the cytokine receptors such as tumor necrosis factor receptor superfamily, member 6 (TNFRSF6) (FAS) might be down-regulated for suppression of MAPK8 gene expression following SS-FA treatment.
The IgE-receptor, a tetramer composed of an alpha, beta and 2 disulfide-linked gamma chains, is found on the surface of mast cells and basophils. MS4A2 gene encodes the beta subunit of the high affinity IgE receptor which is a member of the membrane-spanning 4A gene family. In this study, MS4A2 expression was highly increased by PMACI treatment, but decreased by SS-FA treatment. Moreover, the expression of the gene for Fc fragment of IgE, high affinity I, receptor for gamma polypeptide (FCER1G) which was increased two-fold by PMACI-treatment for 30 min when compared to the control, was down-regulated by SS-FA treatment by 84%. In our previous study, SS-FA showed an inhibitory effect on β-hexosaminidase release at the antibody-receptor binding stage (Motojima et al. 2009). SS-FA affected not only the binding of IgE and FcεRI receptor but also the expression of the IgE-receptor.
Interferon regulatory factor (IRF) and integrin alpha M (ITGAM) are systemic lupus erythematosus (SLE) candidate genes (Hom et al. 2008; Isaac et al. 2009). SLE is a potentially deadly systemic illness, and is sometimes considered a model for systemic humoral autoimmune diseases. Autoantibodies play an important role in the pathogenesis of SLE, and the diverse clinical manifestations of the disease are caused by the deposition of antibody-containing immune complexes in blood vessels, leading to inflammation in the kidney, brain and skin. In this study, IRF and ITGAM gene expression were increased two fold by PMACI-treatment but decreased by SS-FA treatment.
Bone morphogenetic proteins (BMPs) are closely correlated with tumor differentiation and skeletal metastasis. It has been reported that BMP-2 and -6 gene expression can activate breast cancer and prostate cancer (Zhang et al. 2007; Yamamoto et al. 2002; Alarmo et al. 2008). Generally, FA and humic acid has been known as material that has anti-oxidative and anti-cancer effect (Tachibana et al. 2004; Wang et al. 1996; Yang et al. 2004). In our previous study, SS-FA had anti-oxidative activity as determined by the results of the DPPH radical scavenging assay. These results may indicate that SS-FA has an anti-cancer effect.
In conclusion, SS-FA decreased the histamine release and the expression of various genes related to the allergy responses which occur in PMACI-stimulated KU812 cells. Our study suggests that FA obtained from excess sludge may be useful as a new resource of FA production. SS-FA may be of therapeutic use as a treatment for immediate-type allergy by suppressing basophils activation, and this effect may occur as a result of the decreased expressions of the following genes-BMP2, BMP6, CCL11, FLT3, GBP3, IL13, IL12RB1, L13RA1, INHBC, ITGA2/CD49b, ITGAM, IRF8, MAPK8, MS4A2, SELL and TNFRSF6/Fas. However, the precise mechanisms by which the anti-inflammatory effects occur, as well as the major bioactive component remains to be elucidated.
Acknowledgments
This research was partially supported by the Mitsui & Co., Ltd. Environment Fund.
Abbreviations
- DNP-BSA
Dinitrophenylated bovine serum albumin
- FA
Fulvic acid
- FBS
Fetal bovine serum
- KU812
Human leukemia basophilic
- PMA
Phorbol 12-myristate 13-acetate
- PMACI
PMA plus calcium ionophore A23187
- RBL-2H3
Rat basophilic leukemia
- SLE
Systemic lupus erythematosus
- SS-FA
Fulvic acid extracted from solubilized sludge
References
- Aase F, Arna S. Dantrolene prevents glutamate cytotoxicity and Ca+2 releases from intracellular stores in cultured cerebral cornical neurons. J Neurochem. 1991;56:1075–1078. doi: 10.1111/j.1471-4159.1991.tb02031.x. [DOI] [PubMed] [Google Scholar]
- Alarmo EL, Korhonen T, Kuukasja T, Huhtala H, Holli K, Kallioniemi A. Bone morphogenetic protein 7 expression associates with bone metastasis in breast carcinomas. Ann Oncol. 2008;19:308–314. doi: 10.1093/annonc/mdm453. [DOI] [PubMed] [Google Scholar]
- Aman MJ, Tayebi N, Obiri NI, Puri RK, Modi WS, Leonard WJ. cDNA cloning and characterization of the human interleukin 13 receptor a chain. J Biol Chem. 1996;271:29265–29270. doi: 10.1074/jbc.271.46.29265. [DOI] [PubMed] [Google Scholar]
- Bochner BS, Klunk DA, Sterbinsky SA, Coffman RL, Schleimer RP. IL-13 selectively induces vascular cell adhesion molecule-1 expression in human endothelial cells. J Immunol. 1995;154:799–803. [PubMed] [Google Scholar]
- Chen TY, Lu YH, Li JY, Chrn CJ, Hsu WH, Lin HY, HWANG DF, Chen ST. Comparative proteomics on resting and activated human basophilic leukemic KU812 upon stimulation with PMA plus A23187. J Food Drug Anal. 2009;17:363–375. [Google Scholar]
- Vries JE. The role of IL-13 and its receptor in allergy and inflammatory responses. J Allergy Clin Immunol. 1998;102:165–169. doi: 10.1016/S0091-6749(98)70080-6. [DOI] [PubMed] [Google Scholar]
- Gauchat JF, Henchoz S, Mazzei G, Aubry JP, Brunner T, Blasey H, Life P, Talabot D, Flores RL, Thomopson J, Kishi K, Butterfield J, Dahinden C, Bonnefoy JY. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature. 1993;365:340–343. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]
- Harley ITW, Kaufman KM, Langefeld CD, Harley JB, Kelly JA. Genetic susceptibility to SLE: new insights from fine mapping and genome-wide association studies. Nat Rev Genet. 2009;10:285–290. doi: 10.1038/nrg2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton DJ, Zhang JG, Metcalf D, Alexander WS, Nicola NA, Willson TA. Cloning and characterization of a binding subunit of the interleukin 13 receptor that is also a component of interleukin 4 receptor. Proc Natl Acad Sci USA. 1996;93:497–501. doi: 10.1073/pnas.93.1.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohjoh H, Fukushima T. Expression profile analysis of microRNA (miRNA) in mouse central nervous system using a new miRNA detection system that examines hybridization signals at every step of washing. Gene. 2007;391:39–44. doi: 10.1016/j.gene.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Hom G, Graham R, Modrek B, Taylor K, Word O, Garnier S, Lee AT, Chung SA, Ferreira RC, et al. Association of systemic lupus erythematosus with C8orf13–BLK and ITGAM–ITGAX. N Engl J Med. 2008;358:900–909. doi: 10.1056/NEJMoa0707865. [DOI] [PubMed] [Google Scholar]
- Isozaki K, Imamura M, Fukushima K, Tanaami T, Kawamur S, Negishi H, Otsuki S, Tomosada N. Micro-measurement and manipulation technologies. Yokogawa Tech Rep Engl Ed. 2006;41:7–18. [Google Scholar]
- Lin JX, Migone TS, Tsang M, Friedmann M, Weatherbee JA, Zhou L, Yamauch A, Bloom ET, Mietz J, John S. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- Mossam T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assay. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Motojima H, Yamada P, Han JK, Ozaki M, Shigemori H, Isoda H. Properties of fulvic acid extracted from excess sludge and its β-hexosaminidase release inhibition effect. Biosci Biotechnol Biochem. 2009;73:2210–2216. doi: 10.1271/bbb.90316. [DOI] [PubMed] [Google Scholar]
- Rasheed Z, Akhtar N, Anbazhagan AN, Ramamurthy S, Shukla M, Haqqi TM (2009) Polyphenol-rich pomegranate fruit extract (POMx) suppresses PMACI-induced expression of pro-inflammatory cytokines by inhibiting the activation of MAP Kinases and NF-κB in human KU812 cells. J Inflamm 6:1. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2625340/pdf/1476-9255-6-1.pdf [DOI] [PMC free article] [PubMed]
- Shimoda K, Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DAA, Doherty PC, Grosveld G, Paul WE, Ihle JN. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- Stevenson FJ. Geochemistry of soil humic substances. In: Aiken GR, McKnight DM, Wershaw RL, Maccarthy P, editors. Humic substances in soil, sediment, water. New York: Wiley; 1985. pp. 13–52. [Google Scholar]
- Suzuki M, Nakamura T, Iyoki S, Fujiwara A, Watanabe Y, Mohri K, Isobe K, Ono K, Yano S. Elucidation of anti-allergic activities of crucumin-release compounds with a special reference to their anti-oxidative activities. Biol Pharm Bull. 2005;28:1433–1438. doi: 10.1248/bpb.28.1438. [DOI] [PubMed] [Google Scholar]
- Tachibana Y, Hiribe S, Tawa R. Studies of antioxidative activity of humic substances in peat (1) Trace Nutrients Res. 2004;23:104–108. [Google Scholar]
- Thornton S, Sowders D, Aronow B, Witte DP, Brunner HI, Giannini EH, Hirsch R. DNA microarray analysis reveals novel gene expression profiles in collagen-induced arthritis. Clin Immunol. 2002;105:155–168. doi: 10.1006/clim.2002.5227. [DOI] [PubMed] [Google Scholar]
- Rensburg CEJ, Straten A, Dekker J. An in vitro investigation of the antimicrobial activity of oxifulvic acid. J Antimicrob Chemother. 2000;46:853–854. doi: 10.1093/jac/46.5.853. [DOI] [PubMed] [Google Scholar]
- Rensburg CEJ, Malfeld SCK, Dekker J. Topical application of oxifulvic acid suppresses the cutaneous immune response in mice. Drug Develop Res. 2001;53:29–32. doi: 10.1002/ddr.1166. [DOI] [Google Scholar]
- Wang C, Wang Z, Peng A, Hou J, Xin W. Interaction between fulvic acids of different origins and active oxygen radicals. Sci China C Life Sci. 1996;39:267–275. [PubMed] [Google Scholar]
- Yamada H, Miyata Y, Hattori T. Studies on the complexing capacity of soil humic substances. II. pH dependence of the metal complexing capacity of soil humic substance. Dojouhiryougakkaishi (Japanese) 1987;58:205–208. [Google Scholar]
- Yamada P, Isoda H, Han JK, Talorete TPN, Yamaguchi T, Abe Y. Inhibitory effect of fulvic acid extracted from Canadian sphagnum peat on chemical mediator release by RBL-2H3 and KU812 cells. Biosci Biotechnol Biochem. 2007;71:1294–1305. doi: 10.1271/bbb.60702. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Saatcioglu F, Matsuda T. Cross-talk between bone morphogenic proteins and estrogen receptor signaling. Endocrinology. 2002;143–7:2635–2642. doi: 10.1210/en.143.7.2635. [DOI] [PubMed] [Google Scholar]
- Yanagihara Y, Kajiwara K, Basaki Y, Ikizawa K, Akiyama K, Saito H. Induction of human IgE synthesis in B cells by a basophilic cell line, KU812. Clin Exp Immunol. 1997;108:295–301. doi: 10.1046/j.1365-2249.1997.d01-1001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HL, Hseu YC, Hseu YT, Lu FJ, Lin E, Lai JS. Humic acid induces apoptosis in human premyelocytic leukemia HL-60 cells. Life Sci. 2004;75:1817–1831. doi: 10.1016/j.lfs.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Zhang M, Wang Q, Yuan W, Yang S, Wang X, Yan JD, Du J, Yin J, Gao SY, Sun BC, Zhu TH. Epigenetic regulation of bone morphogenetic protein-6 gene expression in breast cancer cells. J Steroid Biochem Mol Biol. 2007;105:91–97. doi: 10.1016/j.jsbmb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Zhu FG, Gomi K, Marshall JS. Short-term and long-term cytokine release by mouse bone marrow mast cells and the differentiated KU-812 cell line are inhibited by Brefeldin A1. J Immunol. 1998;161:2541–2551. [PubMed] [Google Scholar]