Abstract
Tea polyphenols, e.g., (-)-epigallocatechin-3-O-(3-O-methyl gallate (EGCG3”Me), (-)-epigallocatechin-3-O-gallate (EGCG), (-)-epigallocatechin (EGC), (-)-epicatechin-3-O-gallate (ECG), and (-)-epicatechin (EC), are believed to be responsible for the beneficial effects of tea. ‘Benifuuki’, a tea (Camellia sinensis L.) cultivar grown in Japan, is rich in the anti-allergic molecule epigallocatechin-3-O-(3-O-methyl) gallate (EGCG3”Me). Pulverized Benifuuki green tea powder (BGP) is more widely distributed than leaf tea in Japan. Japanese people mix their pulverized tea with water directly, whereas it is common to drink leaf tea after extraction. However, few studies of the effects of BGP particle size on polyphenol bioavailability have been performed. This study was conducted to investigate the absorption of catechins in rats after the intragastric administration of Benifuuki green tea. Therefore, we assessed the plasma concentrations of catechins following the ingestion of BGP with different mean particle sizes (2.86, 18.6, and 76.1 μm) or Benifuuki green tea infusion (BGI) as a control in rats. The bioavailabilities of EGCG3”Me, EGCG, ECG, EGC, and EC were analyzed after the oral administration of a single dose of Benifuuki green tea (125 mg/rat) to rats. The plasma concentrations of tea catechins were determined by HPLC analysis combined with of electrochemical detection (ECD) using a coulometric array. The AUC (area under the drug concentration versus time curve; min μg/mL) of ester-type catechins (EGCG3”Me, EGCG, and ECG) for the BGP 2.86 μm were significantly higher than those in the infusion and 18.6 and 76.1 μm BGP groups, but the AUC of free-type catechins (EGC and EC) showed no differences between these groups. Regarding the peak plasma level of EGCG3”Me adjusted for intake, BGP 2.86 μm and BGI showed higher values than the BGP 18.6 and 76.1 μm groups, and the peak plasma levels of the other catechins displayed the same tendency. The present study demonstrates that the bioavailability of ester-type catechins (EGCG and ECG) can be improved by reducing the particle size of green tea, but the plasma level of EGCG3”Me in the BGI group was similar to that in the BGP 2.86 μm group. This result suggests that drinking Benifuuki green tea with a particle size of around 2 μm would deliver the anti-allergic EGCG3”Me and the anti-oxidant EGCG efficiently.
Keywords: O-methylated EGCG, Absorption, ‘Benifuuki’ green tea powder particle size, Anti-allergic effect
Introduction
Tea (Camellia sinensis L.) is consumed all over the world, particularly in Japan and China, where it has been used for medicinal purposes for tens of centuries. It has been reported that tea has various bioregulatory activities, such as anti-carcinogenetic (Kuroda and Hara 1999; Lin et al. 1999; Suganuma et al. 1999; Cao and Cao 1999; Ahmad et al. 2000; Lambert and Yang 2003), anti-metastatic (Isemura et al. 1993; Sazuka et al. 1995; Sazuka et al. 1997; Maeda-Yamamoto et al. 1999; Maeda-Yamamoto et al. 2003), anti-oxidative (Okuda et al. 1983; Bors and Saran 1987; Kimura et al. 2002; Hashimoto et al. 2003), anti-hypertensive (Yokozawa et al. 1994), anti-hypercholesterolemic (Murase et al. 2002; Chisaka et al. 1988; Matsumoto et al. 1998), anti-dental caries (Hattori et al. 1990; Sakanaka et al. 1992), anti-bacterial (Fukai et al. 1991), and intestinal flora amelioration activity (Okubo et al. 1992). Catechins, a group of polyphenolic compounds, have been shown to be largely responsible for these activities. Epigallocatechin-3-O-gallate (EGCG) is the major catechin component of green tea and may be its major active constituent.
Allergies are defined as diseases that provoke excessive immune activity (Kinet 1999; Kawakami and Galli 2002), and in Japan, allergy morbidity is estimated to be about 30%. Many Japanese people have misgivings about the use of anti-allergic medicine because of the side effects and expense so there is a demand for physiologically-functional foods for allergy prevention to be developed. Catechins have been demonstrated to have anti-allergic effects (Matsuo et al. 1997; Yamashita et al. 2000). We have previously demonstrated that O-methylated EGCG (epigallocatechin-3-O-(3-O-methyl) gallate (EGCG3”Me), epigallocatechin-3-O-(4-O-methyl) gallate (EGCG4”Me) (Sano et al. 1999; Suzuki et al. 2000; Fujimura et al. 2002; Maeda-Yamamoto et al. 2004; Fujimura et al. 2007), and strictinin (Tachibana et al. 2001) have anti-allergic actions and that the Japanese tea cultivar Benifuuki is rich in EGCG3”Me, which is not present in black tea (Maeda-Yamamoto et al. 1998; Maeda-Yamamoto et al. 2001). The oral administration of these methylated catechins significantly and dose-dependently (5–50 mg/kg) inhibited type I allergic (anaphylactic) reactions in mice sensitized with ovalbumin and Freund’s incomplete adjuvant. These catechins also strongly inhibited mast cell activation by preventing the tyrosine phosphorylation (Lyn, Syk, and Btk) of cellular proteins, histamine/leukotriene release, and interleukin-2 secretion after FcεRI cross-linking (Maeda-Yamamoto et al. 2004).
Previously, we reported on the bioavailability of EGCG3”Me and EGCG after the oral administration of Benifuuki green tea infusion in humans (Maeda-Yamamoto et al. 2007). Pulverized Benifuuki green tea powder (BGP) is more widely-distributed than leaf tea in Japan. Japanese people mix pulverized tea with water directly, whereas it is common to drink leaf tea after extraction. However, few studies of the effects of BGP particle size on catechin bioavailability have been performed. In this paper, we examine the blood levels of EGCG3”Me and other catechins after the administration of pulverized green tea (cv. Benifuuki) powders with different particle sizes or infused tea to rats.
Materials and methods
Chemicals
β-d-glucuronidase (G-7896, EC 3.2.1.31, from Escherichia coli with 9 × 106 U/g solids) and sulfatase (S-9754, EC 3.1.6.1, from abalone entrails with 0.2 × 105 U/g solids) were purchased from Sigma–Aldrich chemical (St. Louis, MO). All other reagents were of the highest grade commercially available.
Green tea sample
The green tea (cv. Benifuuki) was manufactured at the National Institute of Vegetable and Tea Science (NIVTS), Shizuoka, Japan, and was pulverized to fine powders with different mean particle sizes 2.86 μm (BGP 2.86 μm), 18.6 μm (BGP 18.6 μm), and 76.1 μm (BGP 76.1 μm) by Nisshin Engineering Limited (Tokyo, Japan). Mean green tea particle size was measured by the laser diffraction scattering method (Mastersizer 2000, Malvern Instruments Ltd., Worcestershire, UK).
To make the green tea infusion (BGI), 1.25 g of Benifuuki green tea were extracted for 10 min in 20 mL of boiled distilled water. After centrifugation, the supernatant was used as the infusion. The catechin (Fig. 1) contents of the infusion were measured by high performance liquid chromatography (HPLC).
Fig. 1.
Chemical structures of catechins in Benifuuki green tea
Rats
All animal care and experimental protocols were approved by NIVTS’s Animal Care and Use Committee. Female Sprague–Dawley rats (8 weaks; 168–189 g) were purchased from Charles River Laboratories (Yokohama, Japan). The rats were initially fed a nutritionally complete purified maintenance diet (CRF-1, Oriental Yeast, Tokyo, Japan) for 2 days prior to the study and were maintained in air-conditioned quarters with a room temperature of 22 ± 2 °C, a relative humidity of 50 ± 10%, and an alternating 12 h light: dark cycle. The rats were allowed ad libitum access to their diet and water. The rats were deprived of food for 12 h prior to the experiment.
Treatment of rats and sample collection
BGI (2 mL) and BGP (125 mg of powder was dispersed in 2 mL of distilled water just before use) were orally administrated to the rats (5 per group) by intragastric gavage. Following gavage, 500 μL of blood were collected at 1, 3, 6, 11, and 24 h from the tail vein. Blood samples were placed in Li-heparin tubes to prevent clotting and then centrifuged (5,000 rpm, 10 min, 4 °C). Following centrifugation, 100 μL of plasma were collected and stabilized by the addition of 25 μL of 1% aqueous ascorbic acid solution (W/V). The samples were then stored at −80 °C prior to analysis.
Analysis of catechins
The catechin contents of the BGP and BGI were analyzed as previously reported (Maeda-Yamamoto et al. 2005). In brief, the plasma levels of total (sulfatase/glucuronidase-treated) catechins were analyzed as previously reported (Chen et al. 1997). One hundred microliters of plasma samples were thawed at room temperature and 50 μL of 0.4 M sodium phosphate buffer (pH 7.4) were added. The sample was mixed with 10 μL of β-glucuronidase (250 units) and 10 μL of sulfatase (10 units) and then incubated at 37 °C for 45 min. Two hundred and 50 μL of dichloromethane were added to the reaction mixture and then vortexed. After centrifugation at 15,000 g for 10 min, the supernatant (aqueous phase) was extracted with fivefold ethyl acetate twice. The combined ethyl acetate solutions were evaporated to dryness in a vacuum centrifuge concentrator. The residues were redissolved in 100 μL of a NaH2PO4 buffer (pH 2.5) supplemented with 0.1 mM EDTA-acetonitrile (87:13) solution. The resultant solution was centrifuged, and a 20 μL sample was injected into the HPLC. The plasma level of free (uncongugated) EGCG3”Me was analyzed as previously described without enzyme treatment, because methylated EGCG was one of metabolites. The overall recoveries during the sample preparation procedure of EGCG, EGC, ECG, and EC were 84 ± 6, 80 ± 8, 90 ± 7, and 82 ± 4%, respectively (Lee et al. 2000).
After being filtrated through a membrane filter (DISMIC-13HP-PTFE, pore size 0.45 μm, ADVANTEC, Tokyo, Japan), 20 μL of the filtrate were injected using an autosampler (SIL-10Avp, Shimadzu, Kyoto, Japan) into the HPLC apparatus (Shimadzu class VP HPLC system). HPLC was performed with a Shimadzu LC-10A pump coupled with a Coulochem-III electrode array detector (ESA Inc., Bedford, MA, USA) using a reverse-phase Wakopak Navi C18-5 column (150 × 4.6 mm i.d., particle size; 5 μm, Wako Chemical, Tokyo, Japan) with a Wakopak Navi C18-5 column (10 × 4.6 mm i.d., particle size; 5 μm, Wako Chemical) as a guard column eluted with the eluent described below at a flow rate of 1 mL/min at 40 °C. HPLC analysis was performed using a linear gradient system with mobile phase A (H2O-acetonitrile-H3PO4, 400:10:1) and mobile phase B (methanol-mobile phase A, 1:2). Linear gradient elution was performed as follows: 100% mobile phase A for 2 min; 20% mobile phase A for 27 min; 20% mobile phase A for a further 10 min; and return to 100% mobile phase A for 7 min. The eluent was monitored using the Coulochem-III electrode array system with potential settings at −200 (E1) and 400 mV(E2). Quantification was carried out using the external standard method. Catechin quantification was performed after data acquisition using an LC workstation (Class VP system, Shimadzu, Kyoto, Japan).
Pharmacokinetic analysis
The AUC (area under the plasma concentration vs. time curve), Cmax (the maximum plasma concentration), and Tmax (time to reach the maximum plasma concentration) were determined using Microsoft Excel (Office 2007) and KaleidaGraph v.3.6 J (Synergy software, PA, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation (SD) of five rats.
Multigroup comparisons were conducted one-way analysis of variance (ANOVA) followed by the Tukey–Kramer multiple comparison test, assuming a significance level of 5 or 1% using Statcel software (ver. 2).
Results
Pharmacokinetics of plasma catechins after the intragastric administration of Benifuuki green tea
To study the bioavailability of EGCG3”Me and other catechins after the administration of green tea, BGP with different particle sizes or BGI (control) was given to rats i.g. After the Benifuuki green tea had been i.g. administered to the rats at 125 mg/body equivalency, blood samples were collected at different time points (1, 3, 6, 11, and 24 h). Table 1 shows a comparison of the intake, Tmax, Cmax, and AUC for plasma catechins after the intragastric administration of the Benifuuki green teas (3 powders with different particle sizes and an infusion).
Table 1.
Comparison of the Intake, Tmax, Cmax, and AUC of plasma catechins after the i.g. administration of green teas (cv. Benifuuki)
| BGP 2.86 μm | BGP 18.6 μm | BGP 76.1 μm | BGI | |
|---|---|---|---|---|
| EGCG3”Me | ||||
| Intake (mg/rat) | 1.88 ± 0.05a | 1.81 ± 0.04ab | 1.74 ± 0.02b | 0.84 ± 0.04c |
| Tmax (h) | 1.57 ± 0.58 | 1.17 ± 0.09 | 1.85 ± 0.95 | 1.47 ± 0.10 |
| Cmax (μg/L) | 224.8 ± 98.9a | 40.5 ± 47.3b | 64.8 ± 40.6b | 74.5 ± 24.3b |
| AUC (μg h/L) | 760.9 ± 273.4a | 229.9 ± 152.3b | 319.8 ± 123.7b | 288.2 ± 112.6b |
| EGCG | ||||
| Intake (mg/rat) | 7.71 ± 0.15a | 7.46 ± 0.06a | 7.41 ± 0.05a | 3.60 ± 0.17b |
| Tmax (h) | 1.74 ± 0.57 | 2.89 ± 1.89 | 1.85 ± 0.88 | 2.76 ± 0.75 |
| Cmax (μg/L) | 642.7 ± 263.6a | 148.3 ± 97.1b | 739.3 ± 275.2a | 130.9 ± 63.4b |
| AUC (μg h/L) | 2763.5 ± 1158.9a | 968.3 ± 169.6b | 1157.7 ± 417.7b | 1166.4 ± 312.8b |
| ECG | ||||
| Intake (mg/rat) | 1.95 ± 0.04a | 1.88 ± 0.02a | 1.87 ± 0.01a | 0.80 ± 0.04b |
| Tmax (h) | 1.42 ± 0.09 | 2.87 ± 2.04 | 1.18 ± 0.62 | 2.11 ± 1.08 |
| Cmax (μg/L) | 171.2 ± 82.6a | 44.2 ± 34.2b | 79.4 ± 61.6a | 58.0 ± 24.5b |
| AUC (μg h/L) | 660.8 ± 191.9a | 305.2 ± 151.2b | 315.4 ± 150.9b | 222.1 ± 87.2b |
| EGC | ||||
| Intake (mg/rat) | 4.42 ± 0.09a | 4.30 ± 0.02a | 4.31 ± 0.04a | 3.75 ± 0.16b |
| Tmax (h) | 1.86 ± 0.61 | 1.55 ± 0.27 | 1.83 ± 0.93 | 1.47 ± 0.27 |
| Cmax (μg/L) | 903.1 ± 202.0 | 819.0 ± 251.0 | 757.6 ± 247.1 | 674.4 ± 213.1 |
| AUC (μg h/L) | 3577.0 ± 793.2 | 3447.5 ± 841.5 | 2887.0 ± 923.2 | 2463.2 ± 730.1 |
| EC | ||||
| Intake (mg/rat) | 1.38 ± 0.03a | 1.34 ± 0.02a | 1.33 ± 0.02a | 1.16 ± 0.05b |
| Tmax (h) | 1.83 ± 0.66 | 1.78 ± 0.75 | 1.83 ± 0.88 | 1.45 ± 0.28 |
| Cmax (μg/L) | 420.8 ± 148.2 | 571.2 ± 299.5 | 405.5 ± 168.3 | 479.9 ± 174.7 |
| AUC (μg h/L) | 1288.3 ± 315.7 | 1300.8 ± 462.3 | 1192.6 ± 839.9 | 1091.7 ± 443.0 |
All values represent the mean ± SD of 5 measurements except for the intake values (3 measurements)
EGCG3”Me, (-)-epigallocaetchin-3-O-(3-O-methyl) gallate; EGCG, (-)-epigallocatechin-3-O-gallate; ECG, (-)-epicatechin-3-O-gallate; EGC, (-)-epigallocatechin; EC, (-)-epicatechin. The catechin content was analyzed by HPLC with a binary gradient and ultraviolet visible detection (Intake) or a Coulochem-III electrode array system (Tmax, Cmax, and AUC). In each row, means with different superscript letters are significantly different, P < 0.05 (ANOVA and Tukey-Kramer’s method)
Lambert et al. (2003) demonstrated that in a comparison of the EGCG concentration of sulfatase/glucuronisdase-treated plasma (total EGCG) with that of free plasma (unconjugated EGCG), 50–90% of EGCG was present in the conjugated form in mouse plasma after oral administration of EGCG. Lee et al. (2002) indicated that after 1 h ingestion, 77% of the EGCG was present in the free (unconjugated) form whereas 31% of EGC and 21% of EC were in the free form. O-methylated EGCG was one of metabolite. So, EGCG, ECG, EGC and EC were shown as total (sulfatase/glucuronidase-treated) catechin.
The mean intake concentrations of ester-type catechins in the BGI group were about half of those in the other BGP groups, and the differences between BGI group and the other BGP groups were significant.
The intragastric administration of the green teas (BGP and BGI) did not result in significant changes in the Tmax of any catechin (1.17 ± 0.09–2.89 ± 1.89 h). In the BGP 2.86 μm group, the Cmax of ester-type catechins (free EGCG3”Me, total EGCG, and total ECG) were significantly higher than those of the other groups (BGP 2.86 μm > BGI > BGP 76.4 μm = BGP 18.6 μm). Furthermore, the AUC of ester-type catechins in the BGP 2.86 μm group were significantly higher than those of the other groups (approximately 2–3 fold higher).
Absorption of catechins after intragastric administration
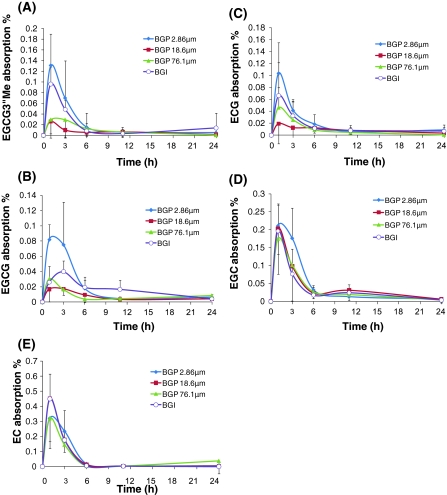
Figure 2 shows the mean absorption of EGCG3”Me (A), EGCG (B), ECG (C), EGC (D), and EC (E) concentration–time profiles after Benifuuki green tea administration. The peak absorption levels of EGC, EGCG, EC, EGCG3”Me, and ECG were 0.13 ± 0.06%, 0.08 ± 0.01%, 0.1 ± 0.05%, 0.21 ± 0.06%, and 0.32 ± 0.15%, respectively. The EGCG3”Me absorption rates of BGP 2.86, 18.6, 76.4 μm, and BGI were 0.52, 0.16, 0.23, and 0.42%, respectively. The EGCG absorption rates of BGP 2.86, 18.6, 76.4 μm, and BGI were 0.46, 0.16, 0.19, and 0.39%, respectively. The ECG absorption rates of BGP 2.86, 18.6, 76.4 μm, and BGI were 0.43, 0.20, 0.21, and 0.40%, respectively. The EGC absorption rates of BGP 2.86, 18.6, 76.4 μm, and BGI were 1.05, 0.96, 0.82, and 0.80%, respectively. The EC absorption rates of BGP 2.86, 18.6, 76.4 μm, and BGI were 1.21, 1.20, 1.12, and 1.17%, respectively. Similar to the AUC of the ester-type catechins, the absorptions of ester-type catechins were highest in the BGP 2.86 μm group. However, among the tested groups, the peak absorption level of EGCG3”Me was highest in the BGP 2.86 μm and BGI groups (BGP 2.86 μm ≥ BGI > BGP 76.4 μm = BGP 18.4 μm). The peak absorption levels of free-type catechins (EGC and EC) did not differ between the groups.
Fig. 2.
Absorption of catechins versus time profiles after the oral administration of 4 types of Benifuuki green tea in rats i.g. BGP 2.86 μm: Benifuuki green tea powder with a mean particle size of 2.86 μm, BGP 18.6 μm: Benifuuki green tea powder 18.6 μm, BGP 76.1 μm: Benifuuki green tea powder 76.1 μm, BGI: Benifuuki green tea infusion. Absorption was calculated as follows; Absorption = (plasma concentration (μg/L)/1,000 * blood volume (rat weight(g) * 70/1,000))/(intake (mg) * 1,000) * 100). Each point represents the mean of five rats, and the cross-vertical bars represent the SD of the mean. All rats were given Benifuuki green tea within 1 min via gavage. a EGCG3”Me, b EGCG, c ECG, d EGC, e EC
Discussion
We studied the bioavailability of EGCG3”Me and other catechins after the i.g. administration of BGP with different particle sizes (2.86, 18.4, and 76.4 μm) or BGI (infusion, control) to rats. The mean intake concentrations of all catechins in the BGI group were about half of those of the other BGP groups, and the differences were significant. The reason was that the extraction rate of the BGI was lower than those of the BGP groups, despite the same green tea leaf quantity being administered in all groups. After the administration of BGP 2.86 μm, the Cmax of ester-type catechins were significantly higher than the values seen in the other groups, and the following order was observed: BGP 2.86 μm > BGI > BGP 76.4 μm = BGP 18.6 μm. The AUC of ester-type catechins in the BGP 2.86 μm groups were significantly higher than those in the other groups, and the differences were approximately 2–3 fold.
The plasma levels of ester-type catechins (EGCG3”Me, EGCG, ECG) were highest in the BGP 2.86 μm, but those of free-type catechins (EGC and EC) showed no difference among the 4 groups. The absorption of free-type catechins in rats was higher than that of ester-type catechins. In ester-type catechins, absorption was highest in the BGP 2.86 μm group, followed by the BGI, BGP 18.6 μm, and BGP 76.4 μm groups. This result suggested that a Benifuuki green tea powder particle size of around 2 μm would be good for efficiently delivering the anti-allergic EGCG3”Me or the anti-oxidant EGCG.
The mean particle size of Matcha green tea, which is commonly used, was reported to be approximately 20 μm (Haraguchi et al. 2003; Sawamura et al. 2010). However, in this study the ester-type catechin plasma level was higher in the BGP 2.86 μm group than in the infusion or larger particle size groups. The present study demonstrated that the bioavailability of the beneficial components of Benifuuki green tea, especially ester-type catechins, could be significantly improved by reducing the particle size (11 ⇒ 2.8 μm). Li et al. (2008) demonstrated that reducing the particle size (3.5 μm ⇒ 220 nm) produced significant increases (two-fold) in the antioxidant and antitumor activities of green tea particles in vitro. Deng et al. (2001) demonstrated that Realgar with a smaller particle size of 150 or 100 nm markedly reduced cell viability through apoptosis compared with that with a particle size of 200 or 500 nm. Suzuki et al. (2003) demonstrated that synthesized catechins were localized to restricted regions within the large central vacuoles (5–15 μm) or some small vacuoles (0.5–3 μm) in tea leaf mesophyll cells. It is conceivable that catechin form complexes with metal ions such as Ca(II) and Mg(II) and special proteins in central vacuole. Suzuki et al. (2003) supposed that synthesized catechins in ER or Golgi apparatus are packed in the formation of a small vacuole and small vacuoles fuse with each other and as a result, catechins are transported into the large central vacuole. However, localization of individual catechin was not fully elucidated. We supposed that ester type catechins were absorbed at 2.86 μm well by these catechins being located in small vacuole mainly.
On the other hand, Lambert et al. (2004, 2008) demonstrated that genistein (2008) from soybean and piperine (2004) from black pepper enhanced EGCG availability. This study revealed the modulation of EGCG bioavailability by a second dietary component and the plasma concentrations of free-type catechin, EGC, and EC, and there were no differences between any of the groups.
In our study, we found that the absorption of the beneficial components of Benifuuki green tea, especially the absorption of ester-type catechins, was significantly improved by reducing the particle size. It was previously shown that an extremely small size particle (e.g., nano-scale powder) showed nano-specific toxicological actions (O’brien and Cummins 2010), so it is important to clarify the optimal particle size and food components for modulating bioavailability in humans in future.
The present results regarding the pharmacokinetic properties of EGCG3”Me, EGCG, ECG, EGC, and EC provide a base for understanding the size effects of Benifuuki green tea powder in rats. To understand the health effects of this tea in humans, we are studying the pharmacokinetics of Benifuuki catechins in human volunteers.
Acknowledgments
This work ‘The Kakegawa Study’ was supported by Research and development projects for application in promoting new policy of agriculture forestry and fisheries, Ministry of Agriculture, Forestry and Fisheries, Japan. (Grant No. 21028).
Abbreviations
- EGCG
(-)-Epigallocatechin-3-O-gallate
- EGCG3”Me
(-)-Epigallocatechin-3-O-(3-O-methyl) gallate
- ECG
(-)-Epicatechin-3-O-gallate
- EGC
(-)-Epigallocatechin
- EC
(-)-Epicatechin
- BGI
Benifuuki green tea infusion
- BGP
Benifuuki green tea powder
- HPLC
High-performance liquid chromatography
- AUC
Area under the plasma concentration versus time curve
- Cmax
The maximum plasma concentration
- Tmax
Time to reach the maximum plasma concentration
- i.g.
Intragastric(ally)
References
- Ahmad N, Cheng P, Mukhtar H. Cell cycle dysregulation by green tea polyphenol epigallocatechin-3-gallate. Biochem Biophys Res Commun. 2000;275:328–334. doi: 10.1006/bbrc.2000.3297. [DOI] [PubMed] [Google Scholar]
- Bors W, Saran M. Radical scavenging by flavonoid antioxidants. Free Radic Res Commun. 1987;2:289–294. doi: 10.3109/10715768709065294. [DOI] [PubMed] [Google Scholar]
- Cao Y, Cao R. Angiogenesis inhibited by drinking tea. Nature. 1999;398:381–382. doi: 10.1038/18793. [DOI] [PubMed] [Google Scholar]
- Chen L, Lee MJ, Li H, Yang CS. Absorption, distribution, and elimination of tea polyphenols in rat. Drug metabol disposit. 1997;25:1045–1050. [PubMed] [Google Scholar]
- Chisaka T, Matsuda H, Kubomura Y, Mochizuki M, Yamamura J, Fujimura H. The effect of crude drugs on experimental hypercholesteremia: mode of action of (-)-epigallocatechin gallate in tea leaves. Chem Pharm Bull. 1988;36:227–233. doi: 10.1248/cpb.36.227. [DOI] [PubMed] [Google Scholar]
- Deng Y, XU H, Huang K, Yang X, Xie C, Wu J. Size effects of realgar particles on apotosis in a humna umbilical vein emdothelial cell line; ECV-304. Pharmacol Res. 2001;44:513–518. doi: 10.1006/phrs.2001.0885. [DOI] [PubMed] [Google Scholar]
- Fujimura Y, Tachibana H, Maeda-Yamamoto M, Miyase T, Sano M, Yamada K. Antiallergic tea catechin: (-)-epigallocatechin-3-o-(3-o-methyl)-gallate, suppresses fcepsilonri expression in human basophilic KU812 Cells. J Agric Food Chem. 2002;50:5729–5730. doi: 10.1021/jf025680z. [DOI] [PubMed] [Google Scholar]
- Fujimura Y, Umeda D, Yano S, Maeda-Yamamoto M, Yamada K, Tachibana H. The 67 kDa laminin receptor as a primary determinant of anti-allergic effects of o-methylated EGCG. Biochem Biophys Res Commun. 2007;364:79–85. doi: 10.1016/j.bbrc.2007.09.095. [DOI] [PubMed] [Google Scholar]
- Fukai K, Ishigami T, Hara Y. Antibacterial activity of tea polyphenols against phytopathogenic bacteria. Agric Biol Chem. 1991;55:1895–1897. [Google Scholar]
- Haraguchi Y, Imada Y, Sawamura S. Production of characterization of fine matcha for processed food. Nippon Shokuhin Kagaku Kougaku Kaishi. 2003;50:468–473. [Google Scholar]
- Hashimoto F, Ono M, Masuoka C, Ito Y, Sakata Y, Shimizu K, Nonaka G, Nishioka I, Nohara T. Evaluation of the anti-oxidative effect (in vitro) of tea polyphenols. Biosci Biotechnol Biochem. 2003;67:396–401. doi: 10.1271/bbb.67.396. [DOI] [PubMed] [Google Scholar]
- Hattori M, Kusumoto I, Namba T, Ishigami T, Hara Y. Effect of tea polyphenols on glucan synthesis by glucosyltransferase from Streptococcus mutans. Chem Pharm Bull (Tokyo) 1990;38:717–720. doi: 10.1248/cpb.38.717. [DOI] [PubMed] [Google Scholar]
- Isemura M, Suzuki Y, Satoh K, Narumi K, Motomiya M. Effects of catechins on the mouse lung carcinoma cell adhesion to the endothelial cells. Cell Biol Int. 1993;17:559–564. doi: 10.1006/cbir.1993.1099. [DOI] [PubMed] [Google Scholar]
- Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nat Rev Immunol. 2002;2:773–786. doi: 10.1038/nri914. [DOI] [PubMed] [Google Scholar]
- Kimura M, Umegaki K, Kasuya Y, Sugisawa A, Higuchi M. The relation between single/double or repeated tea catechin ingestions and plasma antioxidant activity in humans. Eur J Clin Nutr. 2002;56:1186–1193. doi: 10.1038/sj.ejcn.1601471. [DOI] [PubMed] [Google Scholar]
- Kinet JP. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- Kuroda Y, Hara Y. Antimutagenic and anticarcinogenic activity of tea polyphenols. Mutat Res. 1999;436:69–97. doi: 10.1016/S1383-5742(98)00019-2. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Yang CS. Cancer chemopreventive activity and bioavailability of tea and tea polyphenols. Mutat Res. 2003;523:201–208. doi: 10.1016/s0027-5107(02)00336-6. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Lee AJ, Lu H, Meng X, Hong JJJ, Seril DN, Sturgill MG, Yang CS. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J Nutrition. 2003;133:4172–4177. doi: 10.1093/jn/133.12.4172. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Hong J, Kim DH, Misin VM, Yand CS. Piperine enhsances the bioavailabiliyu of the tea polyphenol (-)-epigallocatechin-3-gallate in mice. J Nutr. 2004;134:1948–1952. doi: 10.1093/jn/134.8.1948. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Kwon SJ, Ju J, Bose M, Lee MJ, Hong J, Hao X, Yang CS. Effect of genistein on the bioavailability and intestinal cancer chemoprevention activity of (-)-epigallocatechi-3-gallate. Carcinogenesis. 2008;29:2019–2024. doi: 10.1093/carcin/bgn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Prabhe S, Meng X, Li C, Yang CS. An improved method for the determination of green and black tea polyphenols in biomatrices by high-performance liquid chromatography with coulometric array detection. Anal Biochem. 2000;279:164–169. doi: 10.1006/abio.2000.4487. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, Lambert G, Mohr S, Yang CS. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: formation of different metabolites and variability. Cancer Epidemiol Biomarkers Prev. 2002;11:1025–1032. [PubMed] [Google Scholar]
- Li H, Li F, Yang F, Fang Y, Xin Z, Zhao L, Hu Q. Size effect of Se-enriched green tea particles on in vitro antioxidant and antitumor activities. J Agric Food Chem. 2008;56:4529–4533. doi: 10.1021/jf0731200. [DOI] [PubMed] [Google Scholar]
- Lin JK, Liang YC, Lin-Shiau SY. Cancer chemoprevention by tea polyphenols through mitotic signal transduction blockade. Biochem Pharmacol. 1999;58:911–915. doi: 10.1016/S0006-2952(99)00112-4. [DOI] [PubMed] [Google Scholar]
- Maeda-Yamamoto M, Kawahara H, Matsuda N, Nesumi K, Sano M, Tsuji K, Kawakami Y, Kawakami T. Effects of tea infusions of various varieties or different manufacturing types on inhibition of mouse mast cell activation. Biosci Biotechnol Biochem. 1998;62:2277–2279. doi: 10.1271/bbb.62.2277. [DOI] [PubMed] [Google Scholar]
- Maeda-Yamamoto M, Kawahara H, Tahara N, Tsuji K, Isemura M, Hara Y. Effects of tea polyphenols on the invasion and matrix metalloproteinases activities of human fibrosarcoma HT1080 cells. J Agric Food Chem. 1999;47:2350–2354. doi: 10.1021/jf9811525. [DOI] [PubMed] [Google Scholar]
- Maeda-Yamamoto M, Sano M, Matsuda N, Miyase T, Kawamoto K, Suzuki N, Yoshimura M, Tachibana H, Hakamata K. The change of epigallocatechin-3-o-(3-o-methyl) gallate contents in tea of different varieties, tea seasons of crop and processing method. J Jpn Food Sci Tech. 2001;48:64–68. [Google Scholar]
- Maeda-Yamamoto M, Suzuki N, Sawai Y, Miyase T, Sano M, Hashimoto-Ohta A, Isemura M. Association of suppression of ERK phosphorylation by EGCG with the reduction of matrix metalloproteinase activities in human fibrosarcoma HT1080 cells. J Agric Food Chem. 2003;51:1858–1863. doi: 10.1021/jf021039l. [DOI] [PubMed] [Google Scholar]
- Maeda-Yamamoto M, Inagaki N, Kitaura J, Chikumoto T, Kawahara H, Kawakami Y, Sano M, Miyase T, Tachibana H, Nagai H, Kawakami T. O-methylated catechins from tea leaves, inhibit multiple protein kinases in mast cells. J Immunology. 2004;172:4486–4492. doi: 10.4049/jimmunol.172.7.4486. [DOI] [PubMed] [Google Scholar]
- Maeda-Yamamoto M, Nagai H, Suzuki Y, Ema K, Mitsuda H. Changed in–methylated catechin and chemical component contents of ‘Benifuuki’ green tea (Camellia sinensis L.) beverage under various extraction conditions. Food Sci Technol Res. 2005;11:248–253. doi: 10.3136/fstr.11.248. [DOI] [Google Scholar]
- Maeda-Yamamoto M, Ema K, Shibuichi I. In vitro and in vivo anti-allergic effects of ‘benifuuki’ green tea containing o-methylated catechin and ginger extract enhancement. Cytotechnology. 2007;55:135–142. doi: 10.1007/s10616-007-9112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto N, Okushio K, Hara Y. Effect of black tea polyphenols on plasma lipids in cholesterol-fed rats. J Nutr Sci Vitaminol (Tokyo) 1998;44:337–342. doi: 10.3177/jnsv.44.337. [DOI] [PubMed] [Google Scholar]
- Matsuo N, Yamada K, Shoji K, Mori M, Sugano M. Effect of tea polyphenols on histamine release from rat basophilic leukemia (RBL-2H3) cells; structure-inhibitory activity relationship. Allergy. 1997;55:58–64. doi: 10.1111/j.1398-9995.1997.tb02546.x. [DOI] [PubMed] [Google Scholar]
- Murase T, Nagasawa A, Suzuki J, Hase T, Tokimitsu I. Beneficial effects of tea catechins on diet-induced obesity: stimulation of lipid catabolism in the liver. Int J Obes Relat Metab Disord. 2002;26:1459–1464. doi: 10.1038/sj.ijo.0802141. [DOI] [PubMed] [Google Scholar]
- O’Brien N, Cummins E. Ranking initial environmental and human health risk resulting from environmentally relevant nanomaterials. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2010;45:992–1007. doi: 10.1080/10934521003772410. [DOI] [PubMed] [Google Scholar]
- Okubo T, Ishihara N, Okura A, Serit M, Kim M, Yamamoto T, Mitsuoka T. In vitro effects of tea polyphenols intake on human intestinal microflora and metabolism. Biosci Biotechnol Biochem. 1992;56:588–591. doi: 10.1271/bbb.56.588. [DOI] [PubMed] [Google Scholar]
- Okuda T, Kimura Y, Yoshida T, Hatano T, Okuda H, Arichi S. Studies on the activities of tannins and related compounds from medicinal plants and drugs I. Inhibitory effects on lipid peroxidation in mitochondria and microsomes of liver. Chem Pharm Bull. 1983;32:1625–1631. doi: 10.1248/cpb.31.1625. [DOI] [PubMed] [Google Scholar]
- Sakanaka S, Shiumua N, Masumi M, Kim M, Yamamoto T. Preventive effect of green tea polyphenols against dental caries in conventional rats. Biosci Biotechnol Biochem. 1992;56:592–594. doi: 10.1271/bbb.56.592. [DOI] [PubMed] [Google Scholar]
- Sano M, Suzuki M, Miyase T, Yoshino K, Maeda-Yamamoto M. Novel antiallergic catechin derivatives isolated from oolong tea. J Agric Food Chem. 1999;47:1906–1910. doi: 10.1021/jf981114l. [DOI] [PubMed] [Google Scholar]
- Sawamura S, Haraguchi Y, Ikeda H, sonoda J. Properties and shapes of Matcha with various milling method. Nippon Shokuhin Kagaku Kogoku Kaishi. 2010;57:304–309. doi: 10.3136/nskkk.57.304. [DOI] [Google Scholar]
- Sazuka M, Murakami S, Isemura M, Satoh K, Nukiwa T. Inhibitory effects of green tea infusion on in vitro invasion and in vivo metastasis of mouse lung carcinoma cells. Cancer Lett. 1995;98:27–31. [PubMed] [Google Scholar]
- Sazuka M, Imazawa H, Shoji Y, Mita T, Hara Y, Isemura M. Inhibition of zcollagenases from mouse lung carcinoma cells by green tea catechins and black tea theaflavins. Biosci Biotechnol Biochem. 1997;61:1504–1506. doi: 10.1271/bbb.61.1504. [DOI] [PubMed] [Google Scholar]
- Suganuma M, Okabe S, Sueoka N, Sueoka E, Matsuyama S, Imai K, Nakachi K, Fujiki H. Green tea and cancer chemoprevention. Mutat Res. 1999;428:339–344. doi: 10.1016/s1383-5742(99)00059-9. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Yoshino K, Maeda-Yamamoto M, Miyase T, Sano M. Inhibitory effects of tea catechins and o-methylated derivatives of (-)-epigallocatechin-3-o-gallate on mouse type-IV allergy. J Agric Food Chem. 2000;48:5649–5653. doi: 10.1021/jf000313d. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Yamazaki N, Sada Y, Oguni I, Moriyasu Y. Tissue distribution and intracellular localization of catechin in tea leaves. Biosci Biotechnol Biochem. 2003;67:2683–2868. doi: 10.1271/bbb.67.2683. [DOI] [PubMed] [Google Scholar]
- Tachibana H, Kubo T, Miyase T, Tanino S, Yoshimoto M, Sano M, Maeda-Yamamoto M, Yamada K. Identification of an Inhibitor for interleukin 4-induced e germline transcription and antigen-specific IgE production in vivo. Biochem Biophys Res Commun. 2001;280:53–60. doi: 10.1006/bbrc.2000.4069. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Suzuki Y, Matsui T, Yoshimura T, Yamaki M, Suzuki-Karasaki M, Hayakawa S, Shimizu K. Epigallocatechin gallate inhibits histamine release from rat basophilic leukemia (RBL-2H3) cells; role of tyrosine phophorylation pathway. Biochem Biophysic Res Commun. 2000;274:603–608. doi: 10.1006/bbrc.2000.3200. [DOI] [PubMed] [Google Scholar]
- Yokozawa T, Okura H, Sakanaka S, Ishigaki S, Kim M. Depressor effect of tannin in green tea on rats with renal hypertension. Biosci Biotechnol Biochem. 1994;58:855–858. doi: 10.1271/bbb.58.855. [DOI] [Google Scholar]