Abstract
Electrolyzed reduced water, which is capable of scavenging reactive oxygen species, is attracting recent attention because it has shown improved efficacy against several types of diseases including diabetes mellitus. Alloxan produces reactive oxygen species and causes type 1 diabetes mellitus in experimental animals by irreversible oxidative damage to insulin-producing β-cells. Here, we showed that electrolyzed reduced water prevented alloxan-induced DNA fragmentation and the production of cells in sub-G1 phase in HIT-T15 pancreatic β-cells. Blood glucose levels in alloxan-induced type 1 diabetes model mice were also significantly suppressed by feeding the mice with electrolyzed reduced water. These results suggest that electrolyzed reduced water can prevent apoptosis of pancreatic β-cells and the development of symptoms in type 1 diabetes model mice by alleviating the alloxan-derived generation of reactive oxygen species.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-010-9317-6) contains supplementary material, which is available to authorized users.
Keywords: Electrolyzed reduced water, Alloxan, Type 1 diabetes mellitus, Reactive oxygen species, HIT-T15 cells
Introduction
Diabetes mellitus (DM) is classified into two major types, type 1 (T1DM) and type 2 (T2DM) (Kuzuya et al. 2002). DM is a metabolic disease characterized by chronic hyperglycemia, and is recognized as one of the major health problems in today’s society. Thirty thousand people are reported to be diagnosed with T1DM every year and several million people are affected worldwide (Bresson and von Herrath 2007). Apoptosis is believed to cause pancreatic β-cell loss in T1DM, while apoptosis or necrosis is implicated in T2DM (Cnop et al. 2005). In the case of T1DM, β-cell loss by apoptosis causes insulin deficiency leading to hyperglycemia, which often results in life threatening complications (Cnop et al. 2005). It has been reported that, under hyperglycemic conditions, oxidative stress by free radicals is markedly increased in tissues (Valko et al. 2007). In particular, pancreatic β-cells are highly sensitive to reactive oxygen species (ROS) attack because of their low expression of antioxidant enzyme genes which results in low levels of catalase, glutathione peroxidase and superoxide dismutase (SOD) activities (Lenzen et al. 1996; Sigfrid et al. 2004). In addition, several studies have suggested that ROS plays an important role in contributing to β-cell dysfunction and disease progression through direct free radical-mediated oxidative damage to bio-molecules including DNA, which leads to apoptosis, and causes various forms of tissue damage (Takasu et al. 1991; Kaneto et al. 1996; Pennathur and Heinecke 2007). These results indicate that ROS are the major triggering molecules to induce apoptosis in pancreatic β-cells. Therefore, the control of ROS levels could be the first step in preventing or reversing DM. In support of this idea, many reports suggest that ROS toxicity may be circumvented by overexpression of mitochondrial catalase and/or SOD in insulin-producing cells (Lortz and Tiedge 2003; Gurgul et al. 2004; Lortz et al. 2005).
Currently, the standard therapeutic approach for T1MD utilizes medications such as insulin as well as oral hypoglycemic agents like sulfonylureas, biguanides, thiazolidinediones (rosiglitazone, pioglitazone) and/or newly developed drugs (Nathan 2007). However, some of these drugs are often accompanied by side effects. For example, rosiglitazone increases the risk of myocardial infarction (Nissen and Wolski 2007) and decreases bone mineral density (Yaturu et al. 2007). Therefore, efforts have been made to discover effective and safer agents, and various agents have been found to be effective in laboratory animal trials. So far only three agents have shown safety and efficacy in phase II/III clinical trials in humans (Bresson and Von Herrath 2007). Thus, a continuous effort is still necessary to find drugs and/or reagents which fulfill both efficacy and safety requirements (Bresson and Von Herrath 2007). To meet this demand, a number of reports have been published regarding agents like Ganoderma lucidum polysaccharides (Zhang et al. 2003) and grape seeds proanthocyanidins (El-Alfy et al. 2005) with a range of efficacies against T1DM.
In recent years electrolyzed reduced water (ERW) has attracted increasing attention because it has been shown to possess antioxidative effects by functioning as a free-radical scavenger. ERW scanvenged ROS in vitro and protected DNA from oxidative damage (Shirahata et al. 1997), stimulating glucose uptake by muscle cells and adipocytes (Oda et al. 1999). It was demonstrated that ERW scavenged intracellular ROS, inhibited the decrease of pancreatic β-cell viability and enhanced glucose-stimulated insulin secretion in pancreatic β-cells damaged by alloxan (ALX) (Li et al. 2002). In addition, it was reported that ERW prepared from tap water could elevate blood insulin level as well as other indexes in genetically diabetic db/db mice, a model of human T2DM (Kim and Kim 2006). Thus ERW as a new ROS scavenger may be expected to show therapeutic efficacy against diabetes mellitus in general. However, the same authors have reported that ERW failed to elevate blood insulin levels in STZ-induced diabetic mice, an animal model of T1MD (Kim and Kim 2006), which raises the possibility that the anti-T1DM effect of ERW in vivo is different from that in vitro (Li et al. 2002). Also, the anti-apoptotic effect of ERW on β-cell death in vitro and in a T1DM animal model induced by ALX has not been investigated in detail.
Therefore, the aim of the present study was to evaluate the anti-T1DM effects of ERW on ALX-induced apoptotic β-cell (HIT-T15) death and ALX-induced diabetic male ICR (CD 1-strain) mice.
Materials and methods
Reagents
Roswell Park Memorial Institute (RPMI) 1640 medium was purchased from Nissui Pharmaceutical Co. (Tokyo, Japan). Propidium iodide (PI) and alloxan were purchased from SIGMA Chemical Co. (St. Louis, MO). DNase-free RNase, 4-[2-hydroxyethyl]-1-piperazineethane-sulfonic acid (HEPES), fetal bovine serum (FBS), bovine serum albumin (BSA), penicillin, streptomycin, and all other chemicals were obtained from Wako Pure Chemical Industries (Osaka, Japan).
Preparation of reduced water and medium
ERW was prepared by electrolysis of ultra pure water (UPW) (MilliQ, Millipore) containing 2 mM NaOH at 100 V for 60 min using a batch type electrolyzing device equipped with platinum-coated titanium electrodes (Type TI-200, Nihon Trim Co., Osaka, Japan). Further detail of the device is given elsewhere (Ye et al. 2008). Sample waters were stored in closed glass bottles at 4 °C and neutralized with 12 mM HEPES buffer (pH 7.4) when medium was prepared. The ERW prepared with this device has a high pH (11.47 ± 0.15), high dissolved hydrogen (DH) (0.93 ± 0.04 ppm), low redox potential (ORP) (−730.00 ± 91.65 mV) and low dissolved oxygen (DO) (6.15 ± 0.08 ppm) compared with UPW containing 2 mM NaOH (pH, 11.08 ± 0.11; DH, 0.00 ppm; ORP, 66.00 ± 5.7 mV; DO, 8.35 ± 0.52 ppm). The values were shown as Mean ± S.E.M. In order to investigate the effects of ERW on ALX-induced apoptosis in HIT-T15 cells, medium was prepared using ERW in place of UPW containing 2 mM NaOH (UPW(NaOH)). The pH, DH, ORP and DO of UPW(NaOH)- and ERW-based media were as follows: UPW(NaOH)-based medium (pH, 7.34 ± 0.02; DH 0.00 ppm; ORP, 53.0 ± 2.8 mV; DO, 8.59 ± 0.56 ppm) and ERW-based medium (pH, 7.61 ± 0.01; DH, 0.43 ± 0.1 ppm; ORP, −77.0 ± 10.5 inV; DO, 6.96 ± 0.63 ppm). DH was determined using a DH meter (type DHS-011) from ABLE Co. Ltd (Tokyo). ORP and DO were measured using a ORP meter (type HM-14P) and a DO meter (Type DO-14P) from Toa Electronics Ltd. (Tokyo). pH was measured using a pH meter (Beckman, Type pHI32). All measurements were performed at room temperature. The data on quality and component analysis of water samples are shown in a Supplementary Table (See Springer’s web site).
Cell culture
The hamster pancreatic β-cell line, HIT-T15, was purchased from Dainippon Pharmaceutical Co. (Tokyo, Japan). The cells were maintained and cultured on Falcon dishes with 10% FBS/RPMI 1640 medium supplemented with 2 mM L-glutamine, 100 IU penicillin-G, 100 μg/mL streptomycin and 12 mM HEPES buffer (pH 7.4) at 37 °C in a humidified atmosphere of 5% CO2. For the sub-G1 phase assay and TUNEL assay, the cells were sub-cultured in 6-well plates at a density of 2 × 105 cells/well. When the cell density reached 80% confluence, they were used for the various studies. The culture media were replaced every 48 h throughout the cell growth period.
In situ labeling of 3′-hydroxyl ends on DNA assay (TUNEL assay)
Apoptotic cells were determined using an ApoAlert DNA Fragmentation assay kit (Clontech Laboratories. Inc., Mountain View, CA) based on the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end-labeling (TUNEL) method. Briefly, the cells were harvested by gentle scraping, counted, washed in PBS, resuspended in fresh, prechilled 1% formaldehyde on ice and incubated for 20 min. The cells were collected by centrifugation and washed once with PBS and then fixed with 70% ethanol at −20 °C overnight. Following centrifugation, the cell samples were washed with PBS and treated with 0.2% Triton X-100 in PBS for 5 min at room temperature. The cells were washed again with PBS after centrifugation and resuspended in the TdT reaction buffer and incubated at 37 °C for 1 h, and then 20 mM EDTA was added to terminate the reaction. After washing with 0.1% Triton X-100/BSA/PBS solution, the cells were resuspended in 500 μL PBS containing 0.5 μg/mL of PI and 0.5 mg/mL of DNase-free RNase A, and incubated at room temperature for 20 min prior to measurement. Cellular fluorescence was measured using the flow cytometry.
Sub-G1 phase assay
In the present study, cell cycle analysis was carried out according to the methods of Nicoletti et al. (1991), which utilize PI staining and flow cytometry. Briefly, HIT-T15 cells were pre-incubated with medium containing various waters for 24 h, then the pre-incubation medium was replaced by Hank’s balanced salt solution (HBSS) (137 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 2 mM CaCl2) containing 1 g/L BSA, 12 mM HEPES (pH 7.4) and ERW followed by 30 min incubation. After the addition of 1 mM ALX, the cells were further incubated for 4 h. The cells were collected by trypsinization and centrifugation at 200 × g for 5 min. Then, the cell pellets were gently resuspended in 0.5 mL phosphate buffered saline (PBS) followed by overnight fixation in 2 mL of 70% ethanol at −20 °C. Fixed cells were centrifuged at 200 × g for 5 min to remove cell debris. The cell pellets were resuspended in 100 μL phosphate-citrate buffer (0.2 M Na2HPO4, 0.1 M citric acid), incubated for 20 min and then centrifuged at 200 × g for 5 min. The cell pellets were incubated in 1 mL PI/RNase A/PBS staining solution (10 μg/mL PI, 10 μg/mL RNase A) at 25 °C for 20 min in the dark. Stained cells were analyzed by a flow cytometer (EPICS XL System II-JK. Beckman Coulter, USA). An excitation source of 488 nm was used. Fluorescence emission was collected through a 610 nm band pass filter for PI. PI fluorescence data were collected on a linear scale. Ten thousand cells were evaluated for each sample. The percentage of apoptotic cells was determined using the Cell-Quest software.
Animals
Healthy, male ICR (CD 1-strain) mice were obtained from Charles River Japan Inc. (Tokyo, Japan). Animals were approximately 5 weeks of age, weighing about 30 g at receipt. Following a 7-day acclimation period, 36 mice were randomly assigned into three groups of 12 mice to ensure homogeneity of body weights across the groups. Mice were housed in a room maintained at an average daily temperature of 22–25 °C, an average daily relative humidity of 40–60% and on a 12 h light–dark cycle (light phase from 7:00 a.m. to 7:00 p.m.). Mice were fed balanced mice feed (CRF-2) obtained from Charles River Japan Inc. The feed and water were consumed ad libitum during the acclimation and study periods. Prior to each study, the mice were fasted for 15–18 h.
Animal treatments
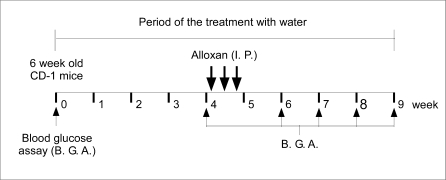
To study the effects of ERW on normal mice, all mice were fed daily with UPW (two groups) or freshly prepared ERW (one group) for 4 weeks. To induce diabetes, the group of ERW fed mice was fasted for 18 h, and ALX was administered by three consecutive intraperitoneal injections at intervals of 48 h at a dose of 100 mg/kg body weight (total dose of 300 mg/kg weight) (Fig. 2). Prior to injection, ALX was dissolved in citrate buffer (0.05 M, pH 4.0), because ALX is very unstable at neutral pH. One of the two UPW fed control groups was injected with the citrate buffer only, and the other control group was injected with ALX as described above. ERW and UPW was given as the source of drinking water and changed daily until the end of the study. All mice were observed for behavioral changes like polydipsia, polyphagia and polyuria. During the experimental periods, body weights were measured once weekly at a designated time and water and food consumption were measured thrice weekly. Daily water and food consumption were calculated for each mouse by combining the total weight of the water and food consumed every 2 day period. Details of the experimental protocol are given in Fig. 2.
Fig. 2.
Schematic representation of the in vivo study protocol. Six-week-old CD-1 mice were treated with UPW or ERW for up to 9 weeks. Blood samples were taken at the indicated time points and used for the blood glucose assay (B.G.A. indicated by upward arrow, ↑). Alloxan (ALX) was administered intraperitoneally (I.P.), as indicated by a downward arrow at the indicated time points (↓). Further detail is given in the Materials and methods
Ethical committee approval
This experiment was carried out following the guidelines for Animal Experiments in the Faculty of Agriculture and in the Graduate School of Kyushu University and the Law (No.105) and Notification (No.6) of the Government.
Determination of Blood Glucose Level and Insulin
For the blood glucose assay (B.G.A in Fig. 2), blood samples from the control and experimental mice were collected at weeks 0, 4, 6, 7, 8 and 9 by puncturing a tail vein of the mice fasted for 15 h. The blood glucose concentration was determined using a glucose auto-analyzer (Bayer Corporation, Beaver Falls, PA). Mice with basal glucose levels under the fasting condition ranging between 150 and 350 mg/dL were considered mild ALX-induced diabetic mice, and higher than 350 mg/dL were considered as severe ALX-induced diabetic mice24). For determination of serum insulin levels, blood samples were obtained at the 9th week before sacrifice by orbital sinus puncture using capillary glass tubes. Sera were separated by centrifugation at 805 × g for 15 min at 4 °C. Serum insulin was estimated using an ELISA method insulin assay kit (Morinaga Seikagaku Corporation, Yokohama, Japan) following the manufacturer’s instructions.
Statistical analysis
Tukey-Kremen method was used except for the analysis on body weight to determine the levels of significance between the means of the control and the test values. Statistical analysis on body weight was performed using LSD method. Results are expressed as the mean ± standard error of the mean (S.E.M.) of independent experiments with one tail. A value of p ≤ 0.05 was considered as statistically significant.
Results
Effects of ERW on ALX-induced apoptosis of HIT-T15 cells
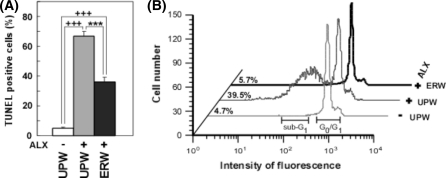
Previously, we reported that ERW scavenged the ALX-induced intracellular ROS in HIT-T15 cells and suppressed the death of HIT-T15 cells induced by ALX (Li et al. 2002). To examine the effects of ERW against ALX-induced apoptosis of HIT-T15 cells, the TUNEL (TdT) assay was performed. Because UPW (NaOH) solution and ERW had no buffering action, they were easily neutralized by 12 mM HEPES in media. The percentage of TUNEL positive HIT-T15 cells cultured in UPW based medium was 5.16 ± 0.44% (Fig. 1a, UPW(−)ALX), while the use of UPW based medium containing ALX increased the TUNEL positive cells to 66.93 ± 2.94% (Fig. 1a, cf. UPW(+)ALX vs. UPW (−)ALX, +++p < 0.001). However, TUNEL positive cells were reduced to 36.23 ± 3.19% when HIT-T15 cells were pretreated with ERW (Fig. 1a, cf. ERW(+)ALX vs. UPW(+)ALX, ***p < 0.001). Although ERW reduced the percentage of TUNEL positive cells, levels are still higher compared with that obtained from treatment with UPW (Fig. 1a, cf. UPW(−)ALX vs. ERW(+)ALX, +++p < 0.001) suggesting that most of the cells were irreversibly damaged by ALX.
Fig. 1.
Anti-apoptotic effects of ERW on alloxan (ALX)-treated HIT-T15 cells assessed by TUNEL assay (a), and sub-G1 analysis (b). (a) Anti-apoptotic effects of ERW on ALX-treated HIT-Ti 5 cells. HIT-T15 cells were treated with 1 mM ALX for 4 h after cultivation in ultrapure water (UPW) containing 2 mM NaOH-based medium or ERW-based medium for 24 h. The pHs of UPW containing 2 mM NaOH and ERW were neutralized by 12 mM HEPES in media. Apoptotic cells were then detected by the TUNEL procedure and analyzed by flow cytometry. UPW(−) means UPW containing 2 mM NaOH-based medium without ALX treatment; UPW(+) means ultrapure water containing 2 mM NaOH with 1 mM ALX treatment; ERW(+) means ERW-based medium with ALX treatment. Values are means ± S.E.M. from three independent experiments. Plus symbols represent significant differences observed between UPW(−) and UPW(+); p < 0.001. Asterisks represent significant differences observed between UPW(+) and ERW(+); *** p < 0.001. (b) Effects of ERW on the cell population in the sub-G1 phase. Cell cycle analysis was carried out using PI staining and flow cytometry, after HIT-T15 cells were treated in the same conditions as in Fig. 1a. Sub-diploidy apoptotic cells (sub-G1) are expressed as a percentage on the left side of each profile. The graph shown is a typical result of three independent experiments. Sample designations are the same as in Fig. 1a
To further confirm the effects of ERW, apoptotic cells resulting from ALX treatment for 4 h were detected quantitatively by measuring the PI-stained DNA content using flow cytometry (Fig. 1b). The distribution of the cell cycle phases in HIT-T15 cells cultured in the medium containing ERW or UPW(NaOH) was examined after exposure to ALX. The population of cells in the sub-G1 phase increased as high as 39.5% after exposure to ALX for 4 h (Fig. 1b, UPW(+)ALX), while that of the control cells treated with UPW(NaOH) alone was 4.7% (Fig. 1b, UPW(−)ALX). However, the population of cells in the sub-G1 phase in HIT-T15 cells pretreated with ERW-based medium, prior to ALX treatment, was decreased to 5.7% (Fig. 1b, ERW(+)ALX). Thus sub-G1 analysis further demonstrated that ERW prevented ALX-induced apoptosis in HIT-T15 cells. No significant difference was observed between UPW-based medium and UPW(NaOH)-based medium on their effects against the ALX-induced apoptosis of HIT-T15 cells (data not shown). Results of two separate experiments demonstrated consistently that ERW could prevent ALX induced apoptotic cell death.
Effect of ERW on ALX-induced type 1 diabetic mice
The results of the in vitro studies with HIT-T15 cells demonstrated the anti-apoptotic effects of ERW, suggesting the possibility that the ERW could exert a similar effect in vivo. To examine this possibility, ERW was applied to experimental mice following the study protocol shown in Fig. 2. All the animals received ALX (control) developed diabetes (100%) as early 2 weeks after final ALX treatment (i.e., 7th week from day 0, as shown in Table 1) and 25% died within 4 weeks (i.e., 9th week from day 0, as shown in Table 1) after ALX treatment, indicating that the study protocol is effective in inducing diabetes. On the other hand, the incidence of diabetes development for mice treated with ERW was reduced to 58.3% (Table 1). Notably, five mice fed with ERW survived with normoglycemic levels of less than 150 mg/dL. Therefore, ERW may partly protect animals from developing ALX-induced T1DM.
Table 1.
Incidence of diabetes in CD-1 mice treated with ERW and alloxan
| Weeks after ALX administration | 6th week | 7th week | 9th week | 9th week | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Blood sugar level (mg/dL) | 150–350 | >350 | Death | 150–350 | >350 | Death | 150–350 | >350 | Death | >150 |
| Control (n = 12) | 2 | 4 | 1 | 5 | 5 | 2 | 2 | 7 | 3 | 100% |
| ERW (n = 12) | 5 | 0 | 0 | 4 | 2 | 0 | 3 | 4 | 0 | 58.3% |
Male ICR(CD-1 strain) mice were administrated ultrapure water (control) and ERW. At the 4th week time point, ALX was injected intraperitoneally 3 times and number of mice exhibiting abnormal blood sugar levels and dead mice were determined. Mice with basal glucose levels under the fasting condition ranging between 150 and 350 mg/dL were considered mild ALX-induced diabetic mice, and higher than 350 mg/dL were as severe ALX-induced diabetic mice. At 9th week, the incidence rate of mice exhibiting blood sugar levels more than 150 mg/dL were also shown
Effects of ERW on blood glucose levels in ALX-induced type 1 diabetes model mice
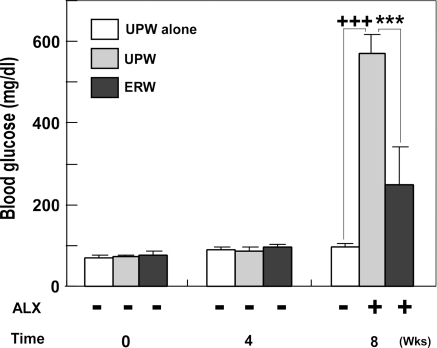
A useful marker to evaluate diabetic condition in mouse is blood glucose level. We first evaluated blood glucose levels, as described in the Materials and Methods section, after 0, 4 and 8 weeks with or without ALX administration. The normal mice fed with ERW for 4 weeks had slightly elevated blood glucose levels although they were statistically insignificant (Fig. 3, cf. 0 and 4th week). A similar trend was observed after 8 weeks of prolonged feeding with UPW (Fig. 3, UPW(−)ALX at 8th week) and this level of blood glucose (97.33 ± 9.63 mg/dL) was set as the basal level. Under the same conditions, administration of ALX elevated blood glucose levels to 569.33 ± 49.33 mg/dL (Fig. 3, UPW(+)ALX at 8th week, p < 0.001), substantiating that the study protocol is effective in inducing diabetes. However, when mice were fed with ERW prior to and after ALX administration, blood glucose levels were significantly lowered to 248.50 ± 92.10 mg/dL (Fig. 3, ERW(+)ALX at 8th week, *** p < 0.001). However, blood glucose levels of mice fed with UPW and ERW for 8th weeks with ALX were significantly higher than when compared with the basal level (97.33 ± 9.63 mg/dL) (+++p < 0.001 for UPW; p < 0.05 for ERW, respectively) (Fig. 3, 8th week). Together, the anti-hyperglycemic effects were concluded to be exerted by ERW. Although the reduction of blood glucose levels by ERW is effective, glucose levels are still considered to be mildly diabetic, suggesting that the anti-hyperglycemic effect of ERW is mild (Fig. 3, 8th week, cf. UPW(−)ALX vs. ERW+ALX, +p < 0.05).
Fig. 3.
The effects of ERW on blood glucose levels of normal and ALX-induced diabetic CD-1 mice. CD-1 mice were treated as described in the study protocol (Fig. 2). Blood glucose levels at the 0, 4 and 8th week time points are presented. Each value denotes the means ± S. E. M., calculated from the data of survived mice. Plus symbols represent significant differences observed between UPW-administrated mice without ALX treatment (UPW(−)ALX) and UPW-administrated mice with ALX treatment (UPW(+)ALX) (+++p < 0.001) or ERW-administrated mice with ALX treatment (ERW(+)ALX) (+p < 0.05). Asterisks represent significant differences observed between UPW(+)ALX and ERW(+)ALX (*** p < 0.001)
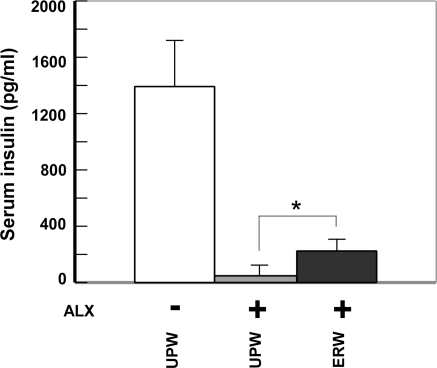
Effect of ERW on serum insulin levels in ALX-induced type 1 diabetes model mice
The serum insulin level is another important marker for monitoring pancreatic function, and could be used to evaluate the extent of tissue damage as well as physiological vitality. Again, using the mice treated as shown in the study protocol (Fig. 2), blood samples were collected via orbital sinus puncture before animals were sacrificed. Insulin levels in mice fed with UPW alone were 1,391.5 ± 325 pg/mL (Fig. 4, UPW(−)ALX). When the mice were fed with UPW and administered ALX, the serum insulin was drastically decreased to 47.5 ± 7.7 pg/mL (Fig. 4, cf. UPW(+)ALX vs. UPW(−)ALX). However, the levels of insulin in mice fed with ERW recovered to 222.5 ± 128.0 pg/mL (ERW(+)ALX, *** p < 0.001) in comparison with the suppressed level of insulin in ALX treated mice (UPW(+)ALX). Therefore, ERW was found to have preventive effects against ALX induced insulin reduction.
Fig. 4.
The effect of ERW on serum insulin levels of ALX-administered CD-1 mice. At the end of the study shown in Fig. 2, blood samples collected via orbital sinus puncture was centrifuged and sera were collected. The serum insulin concentration was determined using an insulin ELISA kit. Values are means ± S.E.M., calculated from the data of survived mice. Asterisks represent significant differences observed between UPW or ERW fed mice followed by ALX administration; *** p < 0.001. Sample designations are the same as in Fig. 3
Effects of ERW on body weight, and food and water consumption
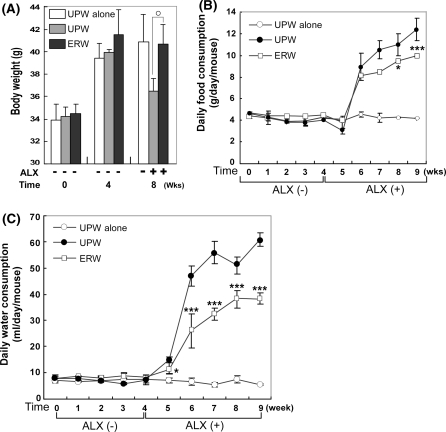
Body weights as well as food and water consumptions were monitored throughout the experimental period (Fig. 5). Body weights of mice fed with UPW and ERW for 4 weeks reached 39–42 g. The group of mice treated with UPW based medium alone reached 40.88 ± 2.46 g and maintained their body weight throughout the experimental period (Fig. 5a, 8th week, UPW(−)ALX). In ALX-induced diabetic mice, the group fed with ERW showed significantly restored body weight (Fig. 5a, 8th week, cf. ERW(+)ALX [40.67 ± 1.77 g] vs.UPW(+)ALX [36.45 ± 1.16 g], **p < 0.01). Food consumption of the three groups of mice fed with UPW (UPW(−)ALX (-○-) and UPW(+)ALX (-●-)) and ERW(+)ALX (-□-) was similar to each other up to the 5th week except that the UPW(+)ALX (-●-) group dropped slightly at the 5th week. The group fed with UPW without ALX-administration kept their food consumption fairly constant to the end of the experiment (Fig. 5b, -○-). However, food consumption of the mouse group fed with UPW was increased by approximately twofold within a week following ALX administration and gradually increased throughout the study period (Fig. 5b, -●-). Although, food consumption of the group fed with ERW was similarly increased within a week, as the UPW(+)ALX (-●-) group did, the amount of food consumed was suppressed compared with that of the UPW group (Fig. 5b, cf, -□- vs. -●-, *p < 0.05).
Fig. 5.
The effects of ERW on body weight (a), food (b) and water (c) consumptions of ALX-induced type 1 diabetes model mice. (a) Body weight was measured at the time of experimental initiation (designated as 0 weeks), and the 4th and 8th week. Asterisks represent significant differences observed between UPW(−)ALX and ERW(+)ALX; ** p < 0.01. Values are means ± S.E.M., calculated from the data of survived mice. The time course of food (b) and water (c) consumptions in normal and ALX-administrated mice was measured at the indicated time points. In the case of food and water consumption measurements, three mice were placed in a cage and one group is composed of three cages, and food and water were measured as a total weight per cage. Thus the values are expressed as means ± S.E.M. (n = 3). * p < 0.05 and *** p < 0.001, compared with UPW(+)ALX. Sample designations are the same as in Fig. 3
Trends in daily water consumption in all groups were quite similar to those of food consumption without a decline in consumption at the end of the 5th week (Fig. 5c, -●-). It should be noted that water consumption of the group fed with UPW after ALX-administration was the highest which reflects the severity of hyperglycemic condition (Fig. 5c, -●-). In comparison to UPW fed mouse group, the ERW fed mouse group consumed less water and suggests that ERW exerts an anti-hyperglycemic effect (Fig. 5c, cf, -□- vs. -●-, *p < 0.05 and ***p < 0.001).
Discussion
Previously, Li et al. reported that ERW protects alloxan (ALX)-induced HIT-T15 cell death by scavenging intracellular ROS, and suggested that the effect of ERW could be applicable to treatment of in vivo T1DM (Li et al. 2002). The present studies further strengthen the results of Li et al. and provide evidence that ERW protects against apoptotic cell death induced by ALX. Furthermore, the in vivo studies have demonstrated a protective effect of ERW against ALX-induced T1DM development. Diabetes mellitus (T1DM, T2DM) is one of the most important health problems worldwide, showing high indices of prevalence and mortality (Kuzuya et al. 2002; Bresson and Von Herrath 2007). T1DM development is mainly due to immune-mediated destruction of β-cells through contact with activated macrophages and T-cells, and/or exposure to cytokines, nitric oxide and ROS. These primary factors are thought to be associated with environmental agents, such as viruses and/or various pathological conditions and/or chemotoxins which modulate the incidence of T1DM (Toniolo et al. 1980). Generation of ROS in response to such factors leads to apoptotic cell death (Valko et al. 2007). When the level of ROS overwhelms the capacity of intracellular defense systems, redox homeostasis will be hampered and builds up oxidative stress in the cell. In turn, such oxidative stress, contributes to β-cell dysfunction and death resulting in the development of diabetes mellitus (Eizirik and Darville 2001; Curtin et al. 2002). Taken together, the data indicate that apoptosis is the main mode of pancreatic β-cell destruction (Cnop et al. 2005; Kay et al. 2000; Jörns et al. 2005; Sakurai et al. 2001). It is known that DNA fragmentation is preceded by single-strand nicking in apoptosis. The TUNEL assay allows for the detection of the generated single strand breaks by labeling the 3′-hydroxyl ends of nicked DNA. The TUNEL positive cells enter either to the DNA repair pathway for survival or to apoptosis (Bernstein et al. 2002). Results of the TUNEL assay showed that ERW reduced TUNEL positive cells, indicating reduced single-strand nicking events. Sub-G1 analysis is also a way to detect genuine apoptotic cells and the results showed positive effects of ERW in reducing the sub-G1 population, i.e., apoptotic cells. The results from these independent assays strongly suggest that ERW has preventive effects against HIT-T15 cell apoptosis caused by ALX.
In order to find ways to prevent and/or treat DM, in vitro and in vivo DM models induced by ALX have been widely used. ALX is used as a diabetogenic compound because it is known to damage pancreatic β-cells specifically by ROS generation (Winterbourn and Munday 1989; Elsner et al. 2002, 2006). Although the half-life of ALX is ca. 1.5 min. at neutral pH and 37 °C in physiological solutions (Zhang et al. 1995), it is reported to generate both extra- and intra-cellular ROS (free radicals), which are responsible for its toxic effect. ALX is extracellularly reduced to dialuric acid through interaction with any available cysteine, GSH or sulfhydryl group at the extracellular plasma membrane sites, followed by the auto-oxidation of dialuric acid back to ALX resulting in redox-cycling. This is accompanied by superoxide anion radicals (O2·) and hydrogen peroxide (H2O2), of which the latter can diffuse into the intracellular compartment (Winterbourn and Munday 1989; Lenzen and Munday 1991; Zhang et al. 1995; Szkudelski 2001). Other studies have suggested that the basis for the toxicity of ALX to pancreatic β-cells is that it is taken up into the cells via the Glut 2 glucose transporter, where it is reduced to dialuric acid by intracellular cysteine and/or GSH (Elsner et al. 2002, 2006). Dialuric acid is then readily auto-oxidized back to ALX establishing a redox-cycle reaction for the generation of superoxide radicals and H2O2 (Lenzen and Munday; Schulte im Walde et al. 2002; Washburn and Wells 1997). Furthermore, recent studies have shown that enhanced ALX toxicity depends on the released iron from ferritin mediated by ascorbate (Sakurai et al. 2006), and the involvement of oxygen on the redox cycling between ALX and dialuric acid (Brömme et al. 2005). These experimental data indicate that various effectors are related to apoptotic β-cell death as a primary cause of T1DM. Thus the extent to which β-cell destruction is sufficient to elaborate the diabetic condition is an important criterion. In this respect, Jörns et al. reported that normoglycemia turns to hyperglycemia when β-cell losses exceed 60–70% in T1DM model rats (Jörns et al. 2005). Similar results have been reported by others suggesting that β-cell loss occurs gradually over years (Cnop et al. 2005; Klöppel and Clemens 1997). Therefore, these observations suggest the possibility that the onset of T1DM could be prevented and/or delayed by using agents that scavenge ROS. The present in vitro and in vivo studies showed that the mice fed with ERW throughout the experimental period had a reduced incidence of diabetes, which was accompanied by improved diabetic indexes. Therefore, our results together with the previous report by Li et al. suggest that β-cell loss occurring gradually over several years could be prevented or retarded by taking ERW for a long period of time (Li et al. 2002).
The present studies also revealed that the serum insulin levels were maintained at significantly higher levels when mice were treated with ERW for 8 weeks (Fig. 4). Our interpretation of the higher insulin levels is that β-cell loss by apoptosis is lowered by scavenging ALX-induced ROS, consequently retarding DNA fragmentation and, thus delaying β-cell loss. Sakurai et al. also reported that vitamin E and butylated hydroxyanisol significantly prevented the inhibition of glucose-stimulated insulin release by treatment with 0.3 mM ALX (Sakurai et al. 2001). Other agents, including Ganoderma lucidum polysaccharides isolated from the fruiting body of a medicinal mushroom (Zhang et al. 2003) and red grape seeds proanthocyanidins (El-Alfy et al. 2005) are also reported to function as free radical scavengers in ALX-induced diabetic animals. Recently, Kim and Kim (2006) reported that ERW derived from tap water failed to affect blood insulin levels in streptozotocin (STZ)-induced diabetic mice. They speculated that ERW increases insulin sensitivity rather than increasing insulin release. Several possibilities available to explain the different observations obtained between our study and theirs. The β-cell responsiveness to ALX and STZ are likely different or may be due to different action mechanisms, although ROS is the common source of cytotoxicity (Szkudelski 2001). An in vitro study showed that a main target molecule for STZ is GLUT 2 protein, while that for ALX is GLUT 2 mRNA and GK mRNA (Gai et al. 2004). ALX causes the production of hydrogen peroxide via superoxide, while STZ is a nitric oxide (NO) donor that eventually causes DNA damage and also induces alkylation of DNA via the nitrosourea moiety of STZ (Szkudelski 2001).
Significant suppression of serum insulin levels were observed in ALX-induced diabetic mice compared with that of control mice. This ALX effect thus lead to elevated blood glucose levels which in turn, caused body weight loss due to low nutrient absorption even though food and water consumption were significantly increased to compensate poor nutrient utilization. On the other hand, the body weights of ERW fed mice were similar to control mice even though food and water consumptions were significantly reduced compared with control mice. This result could be explained by the possibility that ERW increased the levels of serum insulin which accelerated blood glucose uptake into tissues thus resulting in the requirement of less food and water. In agreement with our results, similar trends were reported in STZ-induced diabetic mice (Kim and Kim 2006). Diabetes is related to aging. We demonstrated that ERW reduced ROS level of C. elegans and elongated the lifespan of Caenorhabditis elegans (Yan et al. 2010).
Kim and Kim (2006) used ERW prepared from tap water to demonstrate the anti-diabetes effect of ERW, however, tap water contains dozen of components that make detection of active substance(s) awkward. Our studied ERW is composed of limited composition (UPW, hydrogen, oxygen, NaOH and possible containants from NaOH reagent and batch type electrolysis device). Despite this, our ERW exhibited significant anti-diabetes effect. This is very helpful for detecting active substance(s) of ERW. We show the result of water examination and analysis of components of NaOH solution, ERW and electrolyzed acidic water (EAW) in the Supplementary Tables (see Springer Web site). We show the data of EAW because there is a possibility that EAW produced nearby anode passes through separating semi-permeable membrane to ERW. As the result, we could not detect any organic components at each measurement range shown in supplementary Table 1. Compared to control NaOH solution which adjusted to the same pH as ERW, ERW exhibited changes within twofold in the concentration of almost all components (exception was noted as *). Among these components, only Pt and Pt components were ca. 20-fold increased to the concentration of 2.47 μg/L. The increase of Pt concentration is speculated to be due to elusion from Pt-coated titanium electrodes. Because Pt is coated on titanium electrode by electronic plating, electrolysis is supposed to elute a small amount of Pt into solution. We confirmed that stronger electrolysis produces larger amount of Pt in ERW. The eluted Pt is assumed to form Pt nanoparticles. From these results, the candidate active substance(s) may be dissolved hydrogen and Pt. We proposed an active hydrogen metal nanoparticle reduced water hypothesis that electrolysis of water produces metal nanopartilces from metal ions which directly scavenges ROS and also activate hydrogen molecule to active hydrogen (hydrogen atom) to scavenge ROS in ERW (Shirahata et al. 1997; Ye et al. 2008; Shirahata 2002, 2004). We demonstrated that Pt nanoparticles exhibited a good catalysis activity to scavenge O2·−, H2O2, and ·OH and suppressed oxygen-stress damages in cultured cells (Hamasaki et al. 2008). Pt nanoparticles can extend the lifespan of nematode by scavenging ROS (Kajita et al. 2007; Kim et al. 2008). Moreover, Pt nanoparticles are good catalysts to activate hydrogen molecule to atomic hydrogen, a potent reductant (Watzky and Finke 1997). During electrolysis of water, abundant hydrogen atoms are produced on the surface of Pt electrodes by the reduction of hydrogen ions by electron, then hydrogen atoms reduce metal ions in ERW possibly to produce metal nanoparticles (Aiken and Finke 1999). Thus there is a possibility that ERW derived from tap watcr contains more metal nanoparticles other than Pt nanoparticles. Recently, hydrogen molecules have been reported to prevent various oxidative stress-related diseases (Ohsawa et al. 2006; Fukuda et al. 2007; Hayashida et al. 2007), suggesting that hydrogen molecules may participate in the anti-diabetes effect of ERW. Taken altogether, at least one or all of hydrogen molecules, metal nanoparticles like platinum nanoparticles and atomic hydrogen may be responsible for the anti-diabetes effect of ERW.
The present study suggests that ERW can scavenge ROS and enhance anti-oxidant status in cells and animals, which then prevents ALX-induced apoptotic 13-cell death and the development of diabetes in experimental animals. Such anti-oxidative water like ERW is beneficial for the treatment of diabetes mellitus, because water has no energy and drinking a large quantity of water is possible.
In conclusion, ERW can prevent ALX-induced apoptosis in vitro and the effect is applicable to suppress T1DM in experimental animals by feeding for prolonged periods of time. The protective action of ERW may be related to their ability to prevent and/or quench the free radicals generated in the cells and tissues.
Supplementary materials available
The result of quality and component analysis of NaOH solution, ERW and electrolyzed acidic water (EAW) are available in a supplementary Table. See Springer Web site.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank Ms. Yuki Higuchi for excellent technical assistance.
Abbreviations
- ALX
Alloxan
- BSA
Bovine serum albumin
- EDTA
Ethylenediaminetetraacetic acid
- ERW
Electrolyzed reduced water
- FBS
Fetal bovine serum
- DM
Diabetes mellitus
- HBSS
Hank’s balanced salt solution
- HEPES
4-[2-hydroxyethyl]-1-piperazineethane-sulfonic acid
- PBS
Phosphate buffered saline
- PI
Propidium iodide
- ROS
Reactive oxygen species
- T1DM
Type 1 diabetes mellitus
- T2DM
Type 2 diabetes mellitus
- TdT
Terminal deoxynucleotidyl transferase
References
- Aiken JD, III, Finke RG. A review of modern transition-metal nanoclusters: their synthesis, characterization, and application in catalysis. J Mol Catal A Chem. 1999;145:1–44. [Google Scholar]
- Bernstein C, Bernstein H, Payne CM, Garewal H. DNA repair/pro-apoptotic dual-role proteins in five major DNA repair pathways: fail-safe protection against carcinogenesis. Mutat Res. 2002;511:145–178. doi: 10.1016/s1383-5742(02)00009-1. [DOI] [PubMed] [Google Scholar]
- Bresson D, Herrath M. Moving towards efficient therapies in type 1 diabetes: to combine or not to combine? Autoimmun Rev. 2007;6:315–322. doi: 10.1016/j.autrev.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brömme HJ, Weinandy R, Peschke E. Influence of oxygen concentration on redox cycling of alloxan and dialuric acid. Horm Metab Res. 2005;37:729–733. doi: 10.1055/s-2005-921093. [DOI] [PubMed] [Google Scholar]
- Cnop M, Welsh N, Jonas J-C, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic β-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54:97–107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- Curtin JF, Donovan M, Cotter TG. Regulation and measurement of oxidative stress in apoptosis. J Immunol Methods. 2002;265:49–72. doi: 10.1016/s0022-1759(02)00070-4. [DOI] [PubMed] [Google Scholar]
- Eizirik DL, Darville MI. β-cell apoptosis and defense mechanisms. Lessons from type 1 diabetes. Diabetes. 2001;50:S64–S69. doi: 10.2337/diabetes.50.2007.s64. [DOI] [PubMed] [Google Scholar]
- El-Alfy AT, Ahmed AAE, Fatani AJ. Protective effect of red grape seeds proanthocyanidins against induction of diabetes by alloxan in rats. Pharmacol Res. 2005;52:264–270. doi: 10.1016/j.phrs.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Elsner M, Tiedge M, Guldbakke B, Munday R, Lenzen S. Importance of the GLUT2 glucose transporter for pancreatic beta cell toxicity of alloxan. Diabetologia. 2002;45:1542–1549. doi: 10.1007/s00125-002-0955-x. [DOI] [PubMed] [Google Scholar]
- Elsner M, Gurgul-Convey E, Lenzen S. Relative importance of cellular uptake and reactive oxygen species for the toxicity of alloxan and dialuric acid to insulin-producing cells. Free Radic Biol Med. 2006;41:825–834. doi: 10.1016/j.freeradbiomed.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Asoh S, Ishikawa M, Yamamoto Y, Ohsawa I, Ohta S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem Biophys Res Commun. 2007;361:670–674. doi: 10.1016/j.bbrc.2007.07.088. [DOI] [PubMed] [Google Scholar]
- Gai W, Schott-Ohly P, Schulte im Walde S, Gleichmann H. Differential target molecules for toxicity induced by streptozotocin and alloxan in pancreatic islets of mice in vitro. Exp Clin Endocrinol Diabetes. 2004;112:29–37. doi: 10.1055/s-2004-815724. [DOI] [PubMed] [Google Scholar]
- Gurgul E, Lortz S, Tiedge M, Jörns A, Lenzen S. Mitochondrial catalase overexpression protects insulin-producing cells against toxicity of reactive oxygen species and proinflammatory cytokines. Diabetes. 2004;53:2271–2280. doi: 10.2337/diabetes.53.9.2271. [DOI] [PubMed] [Google Scholar]
- Hamasaki T, Kashiwagi T, Imada T, Nakamichi N, Aramaki S, Toh K, Morisawa S, Shimakoshi H, Hisaeda Y, Shirahata S. Kinetic analysis of superoxide anion radical-scavenging and hydroxyl radical-scavenging activities of platinum nanoparticles. Langmuir. 2008;24:7354–7364. doi: 10.1021/la704046f. [DOI] [PubMed] [Google Scholar]
- Hayashida K, Sano M, Ohsawa I, Shinmura K, Tamaki K, Kimura K, Endo J, Katayama T, Kawamura A, Kohsaka S, Makino S, Ohta S, Ogawa S, Fukuda K. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun. 2007;373:30–35. doi: 10.1016/j.bbrc.2008.05.165. [DOI] [PubMed] [Google Scholar]
- Jörns A, Günther A, Hedrich H-J, Wedekind D, Tiedge M, Lenzen S. Immune cell infiltration, cytokine expression, and β-cell apoptosis during the development of type 1 diabetes in the spontaneously diabetic LEW.1AR1/Ztm- iddm rat. Diabetes. 2005;54:2041–2052. doi: 10.2337/diabetes.54.7.2041. [DOI] [PubMed] [Google Scholar]
- Kajita M, Hikosaka K, Iitsuka M, Kanayama A, Toshima N, Miyamoto Y. Platinum nanoparticle is a useful scavenger of superoxide anion and hydrogen peroxide. Free Radical Res. 2007;41:615–626. doi: 10.1080/10715760601169679. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Fujii J, Myint T, Miyazawa N, Islam KN, Kawasaki Y, Suzuki K, Nakamura M, Tatsumi H, Yamasaki Y, Taniguchi N. Reducing sugars trigger oxidative modification and apoptosis in pancreatic β-cells by provoking oxidative stress through the glycation reaction. Biochem J. 1996;320:855–863. doi: 10.1042/bj3200855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay TW, Thomas HE, Harrison LC, Allison J. The beta cell in autoimmune diabetes: many mechanisms and pathways of loss. Trends Endocrinol Metab. 2000;11:11–15. doi: 10.1016/s1043-2760(99)00210-6. [DOI] [PubMed] [Google Scholar]
- Kim M-J, Kim HK. Anti-diabetic effects of electrolyzed reduced water in streptozotocin-induced and genetic diabetic mice. Life Sci. 2006;79:2288–2292. doi: 10.1016/j.lfs.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Kim J, Takahashi M, Shimizu T, Shirasawa T, Kajita M, Kanayama A, Miyamoto Y. Effects of a potent antioxidant, platinum nanoparticle, on the lifespan of Caenorhabditis elegans. Mech Ageing Dev. 2008;129:322–331. doi: 10.1016/j.mad.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Klöppel G, Clemens A. Insulin-dependent diabetes mellitus: islet changes in relation to etiology and pathogenesis. Endocr Pathol. 1997;8:273–282. doi: 10.1007/BF02739929. [DOI] [PubMed] [Google Scholar]
- Kuzuya T, Nakagawa S, Satoh J, Kanazawa Y, Iwamoto Y, Kobayashi M, Nanjo K, Sasaki A, Seino Y, Ito C, Shima K, Nonaka K, Kadowaki T. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res Clin Pract. 2002;55:65–85. doi: 10.1016/s0168-8227(01)00365-5. [DOI] [PubMed] [Google Scholar]
- Lenzen S, Munday R. Thiol-group reactivity, hydrophilicity and stability of alloxan, its reduction products and its n-methyl derivatives and a comparison with ninhydrin. Biochem Pharmacol. 1991;42:1385–1391. doi: 10.1016/0006-2952(91)90449-f. [DOI] [PubMed] [Google Scholar]
- Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20:463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- Li YP, Nishimura T, Teruya K, Maki T, Komatsu T, Hamasaki T, Kashiwagi T, Kabayama S, Shim SY, Katakura Y, Osada K, Kawahara T, Otsubo K, Morisawa S, Ishii Y, Gadek Z, Shirahata S. Protective mechanism of reduced water against alloxan-induced pancreatic β-cell damage: scavenging effect against reactive oxygen species. Cytotechnology. 2002;40:139–149. doi: 10.1023/A:1023936421448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lortz S, Tiedge M.Importance of mitochondrial superoxide dismutase expression in insulin-producing cells for the toxicity of reactive oxygen species and proinflammatory cytokines Free Radic Biol Med 200334683–688.12633745 [Google Scholar]
- Lortz S, Gurgul-Convey E, Lenzen S, Tiedge M. Importance of mitochondrial superoxide dismutase expression in insulin-producing cells for the toxicity of reactive oxygen species and proinflammatory cytokines. Diabetologia. 2005;48:1541–1548. doi: 10.1007/s00125-005-1822-3. [DOI] [PubMed] [Google Scholar]
- Nathan DM. Finding new treatments for diabetes—how many, how fast…how good? N Engl J Med. 2007;356:437–440. doi: 10.1056/NEJMp068294. [DOI] [PubMed] [Google Scholar]
- Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- Oda M, Kusumoto K, Teruya K, Hara T, Maki S, Kabayama S, Katakura Y, Otsubo K, Morisawa S, Hayashi H, Ishii Y, Shirahata S. Electrolyzed and natural reduced water exhibit insulin-like activity on glucose uptake into muscle cells and adipocytes. In: Bernard A, Griffiths B, Noe W, Wurm F, editors. Animal cell technology: products from cells, Cells as Products. The Netherlands: Kluwer Academic Publishers; 1999. pp. 425–427. [Google Scholar]
- Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nature Med. 2006;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- Pennathur S, Heinecke JW. Mechanisms for oxidative stress in diabetic cardiovascular disease. Antioxid Redox Signal. 2007;9:955–969. doi: 10.1089/ars.2007.1595. [DOI] [PubMed] [Google Scholar]
- Sakurai K, Katoh M, Someno K, Fujimoto Y. Apoptosis and mitochondrial damage in INS-1 cells treated with alloxan. Biol Pharm Bull. 2001;24:876–882. doi: 10.1248/bpb.24.876. [DOI] [PubMed] [Google Scholar]
- Sakurai K, Nabeyama A, Fujimoto Y. Ascorbate-mediated iron release from ferritin in the presence of alloxan. Biometals. 2006;19:323–333. doi: 10.1007/s10534-005-1300-x. [DOI] [PubMed] [Google Scholar]
- Schulte im Walde S, Dohle C, Schott-Ohly P, Gleichmann H. Molecular target structures in alloxan-induced diabetes in mice. Life Sci. 2002;71:1681–1694. doi: 10.1016/s0024-3205(02)01918-5. [DOI] [PubMed] [Google Scholar]
- Shirahata S. Reduced water for prevention of diseases. In: Shirahata S, Teruya K, Katakura Y, editors. Animal cell technology: basic & applied aspects. The Netherlands: Kluwer Academic Publishers; 2002. pp. 25–30. [Google Scholar]
- Shirahata S (2004) Reduced water. In: The characteristic and advanced technology of water—for agriculture, foods, and medicines (in Japanese). N.T.S., Tokyo, pp. 33–45
- Shirahata S, Kabayama S, Nakano M, Miura T, Kusumoto K, Gotoh M, Hayashi H, Otsubo K, Morisawa S, Katakura Y. Electrolyzed-reduced water scavenges active oxygen species and protects DNA from oxidative damage. Biochem Biophys Res Commun. 1997;234:269–274. doi: 10.1006/bbrc.1997.6622. [DOI] [PubMed] [Google Scholar]
- Sigfrid LA, Cunningham JM, Beeharry N, Borg LAH, Hernandez ALR, Carlsson C, Bone AJ, Green IC. Antioxidant enzyme activity and mRNA expression in the islets of Langerhans from the BB/S rat model of type 1 diabetes and an insulin-producing cell line. J Mol Med. 2004;82:325–335. doi: 10.1007/s00109-004-0533-4. [DOI] [PubMed] [Google Scholar]
- Szkudelski T. The mechanism of alloxan and streptozotocin action in β-cells of the rat pancreas. Physiol Res. 2001;50:536–546. [PubMed] [Google Scholar]
- Takasu N, Asawa T, Komiya I, Nagasawa Y, Yamada T. Alloxan-induced DNA strand breaks in pancreatic islets. J Biol Chem. 1991;266:2112–2114. [PubMed] [Google Scholar]
- Toniolo A, Onodera T, Yoon J-W, Notkins AL. Induction of diabetes by cumulative environmental insults from viruses and chemicals. Nature. 1980;288:383–385. doi: 10.1038/288383a0. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncola J, Cronin MTD, Mazura M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Washburn MP, Wells WW. Glutathione dependent reduction of alloxan to dialuric acid catalyzed by thioltransferase (glutaredoxin): a possible role for thioltransferase in alloxane toxicity. Free Radic Biol Med. 1997;23:563–570. doi: 10.1016/s0891-5849(97)00001-4. [DOI] [PubMed] [Google Scholar]
- Watzky MA, Finke RG. Transition metal nanocluster formation kinetic and mechanistic studies. A new mechanism when hydrogen is the reductant: slow, continuous nucleation and fast autocatalytic surface growth. J Am Chem Soc. 1997;119:10382–10400. [Google Scholar]
- Winterbourn CC, Munday R. Glutathione-mediated redox cycling of alloxan. Biochem Pharmacol. 1989;38:271–2771. doi: 10.1016/0006-2952(89)90037-3. [DOI] [PubMed] [Google Scholar]
- Yan H, Tian H, Kinjo T, Hamasaki T, Tomimatsu K, Nakamichi N, Teruya K, Kabayama S, Shirahata S (2010) Extension of the lifespan of Caenorhabditis elegans by the use of electrolyzed reduced water. Biosci Biotech Biochem 74:2011–2015 [DOI] [PubMed]
- Yaturu S, Bryant B, Jain SK. Thiazolidinedione treatment decreases bone mineral density in type 2 diabetic men. Diabetes Care. 2007;30:1574–1576. doi: 10.2337/dc06-2606. [DOI] [PubMed] [Google Scholar]
- Ye J, Li Y, Hamasaki T, Nakamichi N, Komatsu T, Kashiwagi T, Teruya K, Nishikawa R, Kawahara T, Osada K, Toh K, Abe M, Tian H, Kabayama S, Otsubo K, Morisawa S, Katakura Y, Shirahata S. Inhibitory effect of electrolyzed reduced water on tumor angiogenesis. Biol Pharm Bull. 2008;31:19–26. doi: 10.1248/bpb.31.19. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ollinger K, Brunk U. Insulinoma cells in culture show pronounced sensitivity to alloxan-induced oxidative stress. Diabetologia. 1995;38:635–641. doi: 10.1007/BF00401832. [DOI] [PubMed] [Google Scholar]
- Zhang H-N, Hea J-H, Yuanb L, Lin Z-B. In vitro and in vivo protective effect of Ganoderma lucidum polysaccharides on alloxan-induced pancreatic islets damage. Life Sci. 2003;73:2307–2319. doi: 10.1016/s0024-3205(03)00594-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.