Abstract
Transglutaminase (TGase) is a family of enzymes that catalyzes cross-linking reaction between glutamine- and lysine residue of substrate proteins in several mammalian biological events. Substrate proteins for TGase and their physiological relevance have been still in research, continuously expanding. In this study, we have established a novel screening system that enables identification of cDNA sequence encoding favorable primary structure as a substrate for tissue-type transglutaminase (TGase 2), a multifunctional and ubiquitously expressing isozyme. By the screening, we identified several T7 phage clones that displayed substrate peptides for TGase 2 as a translated product from human brain cDNA library. Among the selected clones, the C-terminal region of IKAP, IkappaB kinase complex associated protein, appeared as a highly reactive substrate sequence for TGase 2. This system will open possibility of rapid identification of substrate sequences for transglutaminases at a genetic level.
Keywords: Transglutaminase, cDNA library, T7 phage, IKAP, Calcium
Introduction
Transglutaminase (TGase: EC 2.3.2.13) is a family of enzymes that catalyze post-translational modification by cross-linking proteins between glutamine- and lysine residue. In the enzymatic reaction, glutamine residue is also modified by attachment of primary amine (Griffin et al. 2002; Lorand and Graham 2003). In the initiation of this catalytic reaction, formation of the intermediate between the enzyme and the limited glutamine residues is rate-limiting. Thus, the primary sequence and/or secondary structure around the reactive glutamine residue in substrates govern their reactivity.
TGase family consists of eight members, each of which shows unique tissue distribution, and plays a role in several biological events, such as apoptosis, extracellular matrix formation, blood coagulation and skin formation (Fésüs and Piacentini 2002; Ichinose 2001; Eckert et al. 2005; Hitomi 2005). To know physiological roles of TGases in these processes, investigation of substrates is essential research. So far, several approaches have revealed substrates for TGases by proteomics analyses of cross-linked/polymerized products, affinity purification using labeled primary amines, and screening from peptide display (Orru et al. 2003; Lee et al. 1992; Ikura et al. 1998; Ichikawa et al. 2008; Sugimura et al. 2006; Keresztessy et al. 2006). Based on the results of the studies described above, database for substrates and their sequences are available on the web site and summarized as several publications (Csosz et al. 2009; Esposito and Caputo 2005; Facchiano and Facchiano 2009). Although much information has been accumulated, researches to identify novel substrates are still ongoing.
To develop a more efficient system to search substrates for TGase, we attempted to establish a screening procedure using cDNA expression library. In recent years, T7 phage cDNA library, which displays translated products fused with their capsid protein, has been established and used for several studies such as identification of interacting proteins (Morohashi et al. 2005). We took advantage of the peptides displayed on T7 phage as a possible reactive substrate that incorporates labeled primary amine.
In this study, using human brain cDNA library, we identified several cDNAs of which translated products appeared favorable substrate for TGase 2 by established screening procedure. Among clones, translated products of partial cDNA encoding IKAP, IκB kinase complex associated protein (Cohen et al. 1998), demonstrated high reactivity as a substrate of TGase. Our examination shows successful screening that will generally enable rapid identification of substrates for TGase.
Materials and methods
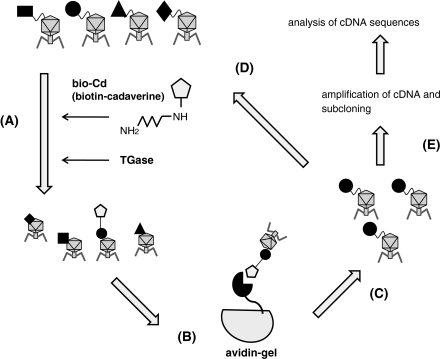
Screening of TGase substrate peptides from T7 phage-displayed cDNA library (Fig. 1)
Fig. 1.
Procedure for screening of cDNAs that encode reactive substrate sequences for TGase using T7 phage-displayed library. (A) The enzymatic reaction of T7 phage particle with biotinylated cadaverine (bio-Cd) in the presence of guinea pig liver TGase. (B) Affinity purification of phage clones that catalytically incorporated bio-Cd using mono-avidin gel chromatography. (C) Amplification of phage particle in bacteria from the eluted fraction of affinity chromatography. (D) Subsequent reaction of concentrated phage particle. (E) Amplification of cDNAs by PCR, subcloning, and sequences analysis
The T7 phage solution that displays human brain cDNA library (T7 select 10-3b) was obtained from Novagen (Novagen-Merck, Germany). Phage solution of approximately 2–5 × 1010 pfu was incubated with 5 mM biotinylated cadaverine (bio-Cd) in the presence of 2 ng/μL of guinea pig liver TGase 2 (Sigma-Aldrich, MO, USA) in a buffer containing 10 mM Tris–HCl (pH 8.0), 150 mM NaCl, 5 mM CaCl2, and 1 mM DTT for 15 min at 37 °C. After termination of the enzymatic reaction by adding EDTA, the phage particle was precipitated with PEG/NaCl in the co-presence of salmon sperm DNA. The recovered phages were subjected to affinity purification using mono-avidin gel (SoftLink™ Soft Release Avidin Resin; Promega Corp., WI, USA), in which the phage particles incorporating bio-Cd were specifically captured and then eluted by the addition of excess amounts of biotin. The eluted phages were used for infection to pre-cultured BLT5403 and further cultured to amplify the phage particle. The amplified phages were harvested, precipitated by PEG/NaCl, and then subjected again to the enzymatic reaction in the presence of bio-Cd. From the next reaction cycle, the incubation time for the cross-linking reaction was gradually shortened (2nd, 10 min; 3rd–5th, 1 min). Panning of the bio-Cd conjugating phage particle was carried out by the same procedure in each round. In each step, DNA containing cDNAs encoding displayed protein was amplified by PCR using total phage DNA used as a template, whereas both the initial solutions for T7 phage cDNA library and the homemade peptide T7 phage library were used as negative control. The amplified DNA at final step (5th) was excised and cloned into the pCR-Blunt II TOPO vector (Zero-blunt Topo cloning kit, Invitrogen, CA, USA). Sequences for cDNAs were analyzed by a standard method.
Assessment of reactivities of peptides encoding cDNAs selected by screening
The obtained cDNA sequence in each clone was excised and inserted into pET24HisGST(QN) expression vector, which enables to attach each cDNA-derived protein at the C-terminus. In the expressed protein, hexahistidine was attached to the N-terminus to GST (glutathione-S-transferase), in which all glutamine residues were changed to asparagine prior to construction of the vector (Sugimura et al. 2006). Bacterial strain BL21(DE3)LysS was transformed with the expression vectors and then used for inducible expression by IPTG according to the established procedure. GST(QN) fusion-protein with the proteins encoding each cDNA, all of which was successfully expressed in a soluble form, was affinity-purified by TALON Metal Affinity resin (BD Bioscience, CA, USA) using the manufacture’s method.
Production of recombinant human IKAP in baculovirus-infected Sf9 cells
Partial cDNA encoding human IKAP (IKBKAP) was purchased from Openbiosystems (AL, USA). In order to obtain complete open reading frame for IKAP, the residual region was amplified from human pancreatic cDNA (TOYOBO, Japan) by PCR. Then, after construction of the full-length cDNA for IKAP, DNA sequence encoding hexahistidine-tag (6xHis) was attached at the 5′-terminus of the cDNA. The DNA fragment encoding 6xHis-IKAP was inserted into the baculovirus pFastBac™ 1 expression vector (Invitrogen) to express the recombinant protein in insect cells. Recombinant virus which enables the expression of the gene was produced and infected by manufacture’s method. Insect cells, Sf9, were infected in large scale culture and the expressed protein was purified using metal-ion affinity chromatography. In the case of the C-terminus truncated form of IKAP (1–1,280), the deleted gene was amplified by PCR, and then inserted into the expression vector.
Evaluation of reactivities of recombinant IKAP proteins for TGase 2
The concentration of the recombinant proteins was determined by the Bradford method (Bio-Rad Laboratories Inc., CA, USA) or by quantification of the intensity for the Coomassie brilliant blue stained-bands in SDS–PAGE gel using imaging software (Multigauge software, Fujifilm, Japan). The reactivities of recombinant proteins were evaluated by the incorporation of monodansylcadaverine (Dansyl-Cd) (Sigma), a fluorescence-labeled pentylamine. Recombinant protein (200 ng/μL) and 0.5 mM Dansyl-Cd were incubated in TBS buffer containing 5 mM CaCl2 and 1 mM DTT in the presence of TGase 2 (2 ng/μl). The reaction mixture was incubated at 37 °C and then separated by 7.5% SDS–PAGE. A fluorograph of the gel was obtained by UV irradiation (254 nm) to visualize the amount of incorporated Dansyl-Cd. To quantify the results, the fluorescence intensity of each product was analyzed (Multigauge software, Fujifilm).
Results
Screening of T7 phage clones that display cDNAs encoding substrate proteins for TGase
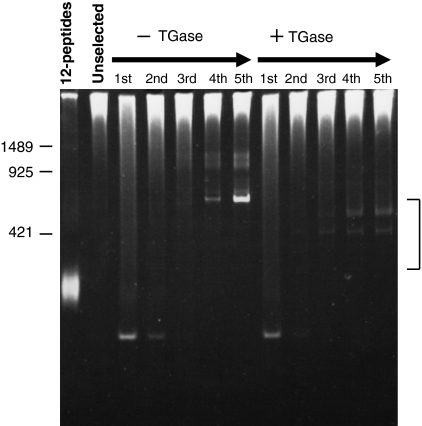
The procedure is shown in Fig. 1. In the presence of guinea pig liver TGase (TGase 2), T7 phage clones were incubated with biotin-labeled cadaverine (bio-Cd). Phage clones that were covalently bound to bio-Cd were selected by avidin affinity purification. The selected phages were amplified and then subjected to four additional cycles, as carried out in the screening using M13 phage-displayed random peptide library (Sugimura et al. 2006). During the procedure, in each cycle, DNA from the amplified phage clones was prepared and then used as a template to amplify their encoding cDNA containing region by PCR, which were then analyzed by polyacrylamide electrophoresis (Fig. 2). We paralleled the procedure in the absence of TGase and analyzed the amplified cDNAs from the phages. In the case of catalytic reaction (right), several faint bands were gradually increasing around the size of 300–500 bp. This is also in the case of absence of the enzyme, however, the sizes of the fragments were different. Next, we amplified the cDNAs form phage particles of the 5th cycle by PCR and the amplified fragments were cloned into pCR-Blunt II TOPO vector.
Fig. 2.
PCR analysis of cDNAs of which sequence was translated and displayed in identified phage particles. In the presence or absence of TGase, phage particles from the eluate (Fig. 1 step c) were harvested in each round. By PCR, DNA fragments containing selected cDNAs from the phage library were amplified using phage DNA as a template and subjected to 4.5% polyacrylamide gel electrophoresis. Each lane (1st–5th) shows the products in the panning cycle number that was used as template for PCR reaction. As negative controls, T7 phage DNA library encoding random 12-mer oligopeptide (12-peptide) or containing human brain cDNA that was used for this screening (Unselected) were put into parallel. The numbers at the left side of the gel corresponds base pairs. The bands in the indicated area were cloned and analyzed
By DNA sequencing analysis of these clones, three cDNAs were selected. As shown in Table 1, cDNAs were identified as highly homologous to partial regions of following corresponding proteins by translated blast search engine (NCBI): AAC64258.1 (IκB kinase complex associated protein; IKAP), AAH04231.2 (ring finger protein 157; RNF157), and EAW90874.1 (hCG2024782). These proteins have not been reported as prominent TGase substrates so far. In each sequence, several glutamine residues were observed (3–4 residues) in total 46–51 residues. IKAP, which was firstly reported to act as a scaffolding protein for the IκB complex, has been studied in neuronal defective diseases. In contrast, less information has been obtained for RNF and hCG2024782.
Table 1.
Deduced primary sequences for the cDNAs that were screened from the T7 phage-displayed library
| Accession no. | Protein | Peptide sequences from identified cDNAs |
|---|---|---|
| AAC64258.1 | IkappaB kinase complex associated protein (IKBKAP) | NSATPVLGPNSTANSIMASYQQQTSVPVLDAELFIPPKINR RTQWKLSLLD |
| AAH04231.2 | Ring finger protein 157 (RNF 157) | SSKQKTSWAWWQAPVVPAIWEAEAGEWREPGRRSLQ |
| EAW90874.1 | hCG2024782 | SRPAWPTWQNPISTKNTKISRVCWQAPVIPATWEAEAG ESLKPRRQRLQ |
Characterization of the selected cDNAs as assessment of the reactivities of their encoding proteins
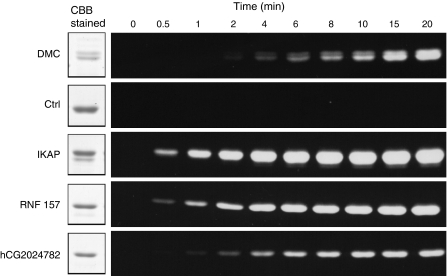
Next, we investigated whether the obtained primary sequences were indeed substrate sequences for TGase. In order to evaluate the reactivities, each cDNA was expressed as fusion proteins with GST, glutathione-S-transferase, in which all the glutamine residues were substituted to asparagine, designated as GST(QN). The cDNAs were inserted into the expression vector to enable expression at the C-terminus site of GST, which corresponds to the displayed position in the case of T7 phage capsid protein. Then, the fusion proteins, purified by taking advantage of the hexahistidine-tag at the N-terminus, were reacted with monodansyl-cadaverine (Dansyl-Cd), a fluorescent-labeled primary amine, in the presence of TGase. Dimethyl casein (DMC) and no glutamine containing-peptide GST(QN)-fusion protein were used as positive and negative controls, respectively. The time-dependent reaction products were analyzed by SDS–PAGE and UV illumination, and then aligned (Fig. 3). In all GST(QN)-fusion proteins, significant increase in the incorporation of Dansyl-Cd was observed, suggesting the expressed cDNA products revealed favored substrates for TGase 2. Among the cDNAs, IKAP showed the most prominent reactivity.
Fig. 3.
Evaluation of the reactivity of the GST(QN) fusion proteins with the protein that are encoded by the identified cDNAs. Each GST(QN)-fusion proteins containing the translation products of the identified cDNA were produced and subjected to the enzymatic reaction. The enzymatic reaction by guinea pig TGase 2 was carried out with 5 mM monodansylcadaverine (MDC) in an appropriate calcium-containing buffer. The reaction products were analyzed by 7.5% SDS–PAGE analysis in a time-dependent manner. Then, the gels were analysed under UV (254 nm). Dimethylcasein (DMC) and purified GST(QN) protein were used as a positive and negative control (Ctrl), respectively
Production of recombinant proteins and reactivities for the truncated and full-length forms of IKAP
As shown in the result of Fig. 3, all selected cDNAs revealed to encode a protein being a favored substrate for TGase. Since there is less information on physiological significance or tissue distribution as for RNF157 and hCG2024782, we did not further analyze the cDNAs for these proteins. Regarding IKAP, in contrast, several physiological significance and related diseases have been reported. Thus, in the further study, we focused on the analysis of the identified cDNA, which encodes the C-terminal region of IKAP (1,281–1,332).
Then, we examined whether the identified region in IKAP act as favorable substrate efficiently even in the full-length form. Since IKAP is a large protein composed of 1,332 amino acid residues distributed in a variety of tissues in human, we attempted to express full-length recombinant protein in baculovirus-infected insect cell system. In addition to the full-length protein, the C-terminus truncated product (Δ1,281–1,332) was also prepared. Since both recombinant proteins were designed with an attached hexahistidine-tag, the successfully expressed proteins were purified using metal-ion affinity chromatography (data not shown).
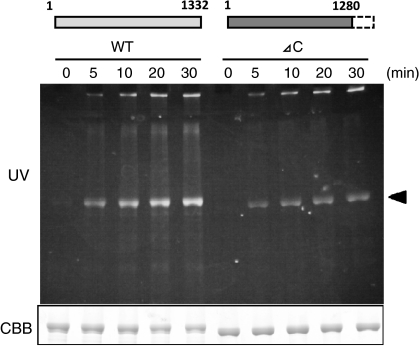
For evaluation, Dansyl-Cd was reacted with each purified recombinant protein for IKAP in the presence of guinea pig liver TGase (Fig. 4). The reaction products for wild type (full-length) IKAP, analyzed by SDS–PAGE and UV illumination, showed higher time-dependent incorporation of Dansyl-Cd than the case of C-terminus truncated form (Δ1,281–1,332). This result suggests that the peptide region isolated in this screening appeared to be the favourable substrate for the wild-type form of IKAP.
Fig. 4.
Reactivities of the recombinant proteins for wild-type and the C-terminus deleted IKAP for TGase. The recombinant proteins for wild type and truncated form with the identified deleted region were expressed by the baculovirus system, purified and then subjected to the enzymatic reaction in the presence of MDC and TGase in an appropriate buffer. The time-dependent reaction products were analyzed by 7.5% SDS–PAGE following UV illumination as described in the legend to Fig. 3. The protein band corresponding to IKAP is indicated by arrowhead
Discussion
TGases cross-link unique substrate proteins in several biological events, by catalyzing covalent binding between glutamine- and lysine residue. Upon these reactions, not all glutamine residues participate in the formation of enzyme-substrate intermediate, which is a limiting step for initiation of the reaction. Therefore, in order to clarify substrate specificity, the possible sequence around the reactive glutamine residues should be investigated. In recent years, we have established a screening system to identify the reactive substrate sequence around the glutamine residue using M13 phage-displayed random peptide library (Sugimura et al. 2006, 2008a, b; Hitomi et al. 2009). Regarding several isozymes including TGase 2, the preferred 12-mer amino acid sequences specific for each isozyme have been identified.
During a series of experiments using a phage-displayed system, we developed this approach to identify substrate peptide sequences from the cDNA library. In this case, rather than M13 phage, T7 phage library has been developed to express longer peptides or proteins on their surface. T7 phage particle, moreover, is extremely stable and replicates more rapidly than M13 phage after infection. As for commercially available T7 phage-display library, several kinds of expression systems and cDNA sources for library have been established. In this study, we used T7 select 10-3, which displayed peptide (or protein) fused with capsid protein because of appropriate length of encoding peptide (limited size in amino acid is 1,200 residues). As a cDNA source, human brain was targeted since several neuronal diseases are caused by aberrant TGase reactions, of which the mechanisms still remain unclear (Jeitner et al. 2009).
Screening system based on the enzymatic incorporation of biotin-labeled cadaverine into phages, which can be affinity-purified, revealed successful to identify favored substrate protein displayed on the phage. Instead of analysis of isolated phage DNA, selected cDNAs were amplified and cloned by taking advantage of PCR. This procedure appeared efficient because the amplified products gradually increased depending on the panning cycle (Fig. 2, right), suggesting cDNAs encoding favored substrate were specifically screened in the system.
The obtained cDNAs of favored substrates consist of three proteins: IKAP, RNG157, and hCG2024782. Each of them contained several glutamine residues showing high reactivities as GST-fusion proteins. Although the precise reactive glutamine residue(s) have not been identified, there are possible primary sequences: glutamine residue “island” (QQQ) or Q-x-P-ψ (x and ψ are any and hydrophobic amino acids, respectively) that was proposed as preferred consensus sequence for TGase 2 (Sollid 2002; Sugimura et al. 2006). Interestingly, the same sequence (WQAPV) is observed in RNG157 and hCG2024782, suggesting the possibility of a novel primary sequence as favored substrate. For further study of these obtained proteins, however, we selected neither RNG157 nor hCG2024782, since there is no molecular and biochemical information in publication with respect to these two proteins.
IKAP, encoded by the gene IKABKAP, is 150 kDa protein that does not belong to any known protein family. Originally this protein was described as a scaffold protein for the IκB kinase (IKK) complex. In recent reports, IKAP appeared to be involved in actin organization and embryonic development as an interacting molecule to filamin A and as a subunit of elongator, respectively (Johansen et al. 2008; Chen et al. 2009). Additionally, loss of function mutations in this gene cause familial dysautonomia (FD), with defective neuronal development (Slaugenhaupt et al. 2001; Anderson et al. 2001). In this mutation, the truncated form was expressed in which the C-terminal half region (572–1,332) was deleted by aberrant splicing due to a single point mutation. The precise mechanism by which the truncated form of IKAP causes symptoms in FD patients is still unknown. Our results demonstrated that a highly reactive sequence is located at the C-terminus in IKAP (Fig. 4), which is potent to cross-link to other proteins and/or to be modified by polyamination or conversion to glutamic acid. It would be interesting to know if such modification in IKAP might affect their stability or physiological functions, which could be relevant to FD.
In conclusion, we established a screening system to identify cDNA using phage-displayed library. Among the obtained clones, the C-terminus region of IKAP revealed favored substrates for TGase. Different from the other systems, inducible and even non-inducible substrate proteins can be accessed in screening of our procedure, independent to the protein expression level. However, improvements are still required because both the number and length of isolated cDNAs were less than expected. We suppose that the enzymatic reaction condition was favored only for highly reactive sequences resulting in a reduced number of clones. Longer substrate peptides on phage capsids might cross-link between phage particles, resulting in a reduced infection efficiency. Working on this area is in progress to improve the procedure.
Acknowledgments
We greatly appreciate Dr. Masatoshi Maki and Dr. Hideki Shibata in our laboratory for providing valuable suggestions. This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas (No. 20200072) from the Ministry of Education, Sports, Science and Technology (MEXT, Japan) (to K. H.) and also Grant-in-Aid for Young Scientists Research (No. 186701) from the Japan Society for the Promotion of Science (JSPS) (to Y. S.).
References
- Anderson SL, Coli R, Daly IW, Kichula EA, Rork MJ, Volpi SA, Ekstein J, Rubin BY. Familial dysautonomia is caused by mutation of the IKAP gene. Am J Hum Genet. 2001;68:753–758. doi: 10.1086/318808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-T, Hims MM, Shetty RS, Mull J, Liu L, Leyne M, Slaugenhaupt SA. Loss of mouse Ikbkap, a subunit of elongator, leads to transcriptional deficits and embryonic lethality that can be rescued by human IKAKAP. Mol Cell Biol. 2009;29:736–744. doi: 10.1128/MCB.01313-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Henzel WJ, Baeuerie PA. IKAP is a scaffold protein of the IkB kinase complex. Nature. 1998;395:292–296. doi: 10.1038/26254. [DOI] [PubMed] [Google Scholar]
- Csosz E, Mesko B, Fésüs L. Transdab wiki: the interactive transglutaminase substrate database on web 2.0 surface. Amino Acids. 2009;36:615–617. doi: 10.1007/s00726-008-0121-y. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Sturniolo MT, Broome AM, Ruse M, Rorke EA. Transglutaminase function in epidermis. J Invest Dermatol. 2005;124:481–492. doi: 10.1111/j.0022-202X.2005.23627.x. [DOI] [PubMed] [Google Scholar]
- Esposito C, Caputo I. Mammalian transglutaminases: identification of substrates as a key to physiological function and physiological relevance. FEBS J. 2005;272:615–631. doi: 10.1111/j.1742-4658.2004.04476.x. [DOI] [PubMed] [Google Scholar]
- Facchiano A, Facchiano F. Transglutaminases and their substrates in biology and human diseases: 50 years of growing. Amino Acids. 2009;36:599–614. doi: 10.1007/s00726-008-0124-8. [DOI] [PubMed] [Google Scholar]
- Fésüs L, Piacentini M. Transglutaminase 2: an enigmatic enzyme with diverstic functions. Trends Biochem Sci. 2002;27:534–539. doi: 10.1016/S0968-0004(02)02182-5. [DOI] [PubMed] [Google Scholar]
- Griffin M, Casadio R, Bergamini CM. Transglutaminases: nature’s biological glues. Biochem J. 2002;368:377–396. doi: 10.1042/BJ20021234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitomi K. Transglutaminase in skin epidermis. Eur J Dermatol. 2005;15:313–319. [PubMed] [Google Scholar]
- Hitomi K, Kitamura M, Sugimura Y. Preferred substrate sequences for transglutaminase 2: screening using a phage-displayed peptide library. Amino Acids. 2009;36:619–624. doi: 10.1007/s00726-008-0126-6. [DOI] [PubMed] [Google Scholar]
- Ichikawa A, Ishizaki J, Morita M, Tanaka K, Ikura K. Identification of new amine acceptor protein substrate candidate of transglutaminase in rat liver extract: use of 5-(biotinamido)pentylamine as a probe. Biosci Biotechnol Biochem. 2008;72:1056–1062. doi: 10.1271/bbb.70796. [DOI] [PubMed] [Google Scholar]
- Ichinose A. Physiopathology and regulation of factor XIII. Thromb Haemost. 2001;86:57–65. [PubMed] [Google Scholar]
- Ikura K, Kita K, Fujita I, Hashimoto H, Kawabata N. Idetification of amine acceptor protein substrates of transglutaminase in liver extracts: use of 5-(biotinamido)pentylamine as a probe. Arch Biochem Biophys. 1998;356:280–286. doi: 10.1006/abbi.1998.0775. [DOI] [PubMed] [Google Scholar]
- Jeitner TM, Pinto JT, Krasnikov BF, Horswill M, Cooper AJL. Transglutaminase and neurogeneration. J Neurochem. 2009;109(1):160–166. doi: 10.1111/j.1471-4159.2009.05843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen LD, Naumanen T, Knudsen A, Westerlund N, Gromova I, Junttilla M, Nielsen C, Bottzauw T, Tolkovsky A, Wastermarck J, Coffey ET, Jaattela M, Kallunki T. IKAP localizes to membrane ruffles with filamin A and regulates actin cytoskeleton organization and cell migration. J Cell Sci. 2008;121:854–864. doi: 10.1242/jcs.013722. [DOI] [PubMed] [Google Scholar]
- Keresztessy Z, Csosz É, Hársfalvi J, Csomós K, Gray J, Lightowlers RN, Lakey JH, Balajthy Z, Fésüs L. Phage display selection of efficient glutamine-donor substrate peptides for transglutaminase 2. Protein Sci. 2006;15:2466–2480. doi: 10.1110/ps.051818406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KN, Maxwell MD, Patterson MK, Birckbichler PJ, Conway E. Identification of transglutaminase substrates in HT29 colon cancer cells: use of 5-(biotinamido)pentylamine as a transglutaminase-specific probe. Biochim Biophys Acta. 1992;1136:12–16. doi: 10.1016/0167-4889(92)90078-P. [DOI] [PubMed] [Google Scholar]
- Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- Morohashi K, Yoshino A, Yoshimori A, Saito S, Tanuma S, Sakaguchi K, Sugawara F. Identification of a drug target motif: an anti-tumor drug NK109 interacts with a PNxxxxP. Biochem Pharmacol. 2005;70:37–46. doi: 10.1016/j.bcp.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Orru S, Caputo I, D’Amato A, Ruoppolo M, Esposito C. Proteomics identification of acyl-acceptor and acyl-donor substrates for transglutaminase in a human intestinal epithelial cell line. J Biol Chem. 2003;278:31766–31773. doi: 10.1074/jbc.M305080200. [DOI] [PubMed] [Google Scholar]
- Slaugenhaupt SA, Blumenfeld A, Gill SP, Leyne M, Mull J, Cuajungco MP, Liebert CB, Chadwick B, Idelson M, Reznik L, Robbins CM, Makalowska I, Brownstein M, Krappman D, Scheidereit C, Maayan C, Axelrod FB, Gusella JF. Tissue-specific expression of a splicing mutation in the IKABKAP gene causes familial dysautonomia. Am J Hum Genet. 2001;68:598–605. doi: 10.1086/318810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollid LM. Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol. 2002;2:647–655. doi: 10.1038/nri885. [DOI] [PubMed] [Google Scholar]
- Sugimura Y, Hosono M, Wada F, Yoshimura T, Maki M, Hitomi K. Screening for the preferred substrate sequence of transglutaminase using a phage-displayed peptide library: identification of peptide substrates for TGase 2 and factor XIIIa. J Biol Chem. 2006;281:17699–17706. doi: 10.1074/jbc.M513538200. [DOI] [PubMed] [Google Scholar]
- Sugimura Y, Hosono M, Kitamura M, Tsuda T, Yamanishi K, Maki M, Hitomi K. Identification of preferred substrate sequences for transglutaminase 1-development of a novel peptide that can efficiently detect cross-linking enzyme activity in the skin. FEBS J. 2008;275:5667–5677. doi: 10.1111/j.1742-4658.2008.06692.x. [DOI] [PubMed] [Google Scholar]
- Sugimura Y, Yokoyma K, Nio N, Maki M, Hitomi K. Identification of preferred substrate sequences of microbial transglutaminase from Streptomyces mobaraensis using a phage-displayed peptide library. Arch Biochem Biophys. 2008;477:379–383. doi: 10.1016/j.abb.2008.06.014. [DOI] [PubMed] [Google Scholar]