Abstract
A key to the analysis of function after total hip replacement (THR) is the ability to identify gait adaptations specific to design features and surgical procedures. In a randomised controlled design, we evaluated the mechanics of gait after THR with a hip resurfacing system or conventional prosthesis. We also investigated whether gait adaptations returned to normal postoperatively. Similar improvements in mechanics of gait were found, except for peak abductor moments, which improved more in the conventional group. Gait speed increased significantly, but with no differences between groups. The increase in walking speed was reflected as significant improvement within groups in most kinematic and kinetic variables. Significant differences between the operated and non-operated hip were seen in all patients, but with no difference between groups. Mean curves of joint angle profiles and moments in all anatomical planes during a gait cycle revealed that gait impairment persisted with no differences between the conventional prosthesis and the resurfacing system.
Introduction
Modern total hip replacement (THR) designs and surgical approaches have shown excellent long-term results especially in older less active patients, whereas some concern exists that THR may provide less optimal outcomes in younger and more active patients. As a result, there has long been an interest in more bone-conserving replacement procedures, such as resurfacing hip replacement (RHR) [14].
The theoretical advantages of resurfacing include less inflammatory debris and osteolysis, minimal resection of the femoral head, improved joint stability, and improved biomechanics [6, 20]. Restoration of normal movement patterns of the hip after THR provides better clinical function and reduced wear [1, 6, 20]. A key to analysis of function after joint replacement is the ability to identify gait adaptations specific to design features and surgical procedures, and several studies have used gait analysis to study functional outcome after THR. Impairments of gait adaptation in the hip are believed to be caused by reduced muscle strength in the gluteal muscles and reduced range of hip extension especially in the late stance phase [9, 10]. Because the resurfacing surgical technique is more invasive than conventional THR, we postulated that range of motion and muscle strength would be more affected during the early phase of rehabilitation in patients receiving a resurfacing implant than in patients receiving a conventional prosthesis. Furthermore, we expected persisting gait impairments to be less in patients after resurfacing arthroplasty due to better joint stability and biomechanics.
In a prospective randomised controlled study, we evaluated mechanics of gait after THR in patients with two different types of implant and procedure to examine differences in gait pattern. Furthermore, we investigated to what extent gait adaptations had improved three months postoperatively.
Patients and methods
This study is a prospective randomised controlled trial. The study was approved by the local ethics committee and fulfilled the Helsinki Declaration. Patients between the ages of 50 and 65 years with osteoarthritis scheduled for elective primary unilateral THR were assessed for eligibility. Exclusion criteria were insufficient bone density; exposure to chrome, cobalt, and molybdenum; kidney disease; fracture sequelae; hip joint dysplasia; previous hip joint disorders in childhood; patients with rheumatoid arthritis; and patients with more than one joint affected by arthritis.
Patients were randomised by means of opening sealed envelopes. Block randomisation into blocks of six was used. The sequences were computer generated, and the randomisation was performed by a nurse who was not part of the research group.
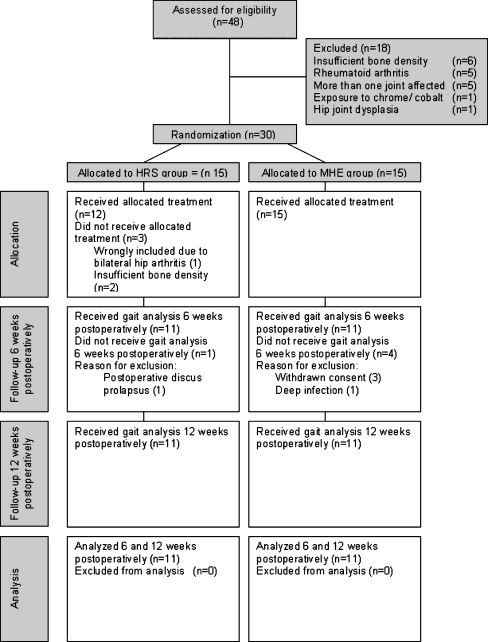
In the study period 48 patients were assessed for eligibility, and 18 patients did not meet the inclusion criteria. Thirty patients were included and randomised to receive the hip resurfacing system (HRS group) or the conventional hybrid prosthesis (MHE group).
In all cases, an uncemented acetabular component and a cemented femur component were used.
The hip resurfacing system (HRS) from Biomet® was used in the HRS group, and in the conventional group, a Mallory-Head cup (Biomet) and an Exeter stem (Stryker®) were used. Compared to the conventional prosthesis (MHE), the articulating surface of the HRS is much larger. The resurfacing surgical procedure is more invasive than the conventional procedure and includes a loosening of the gluteus maximus fibres from the bursa and a release of the distal muscle insertion from the femoral bone. A posterior approach was used in all cases.
All operations were performed by one senior surgeon, and all patients followed the same standardised postoperative rehabilitation program with full weight bearing allowed from the day after the operation.
All patients were discharged with a home training exercise program, and no further rehabilitation was established.
In order to assess patients’ self-reported functional status preoperatively, The Western Ontario and McMasters University Osteoarthritis Index (WOMAC) were completed.
All patients underwent three-dimensional (3D) gait analysis six and 12 weeks postoperatively.
The laboratory gait evaluation included simultaneous recording of body kinematics, kinetics, and muscle activation patterns in patients walking unassisted at their natural cadence. Gait analyses were performed by one examiner (physiotherapist) in the Gait and Movement Laboratory at the Hammel Neurocenter. All staff members administrating gait analysis were blinded to the type of prosthesis.
The 3D gait analysis was carried out using a Vicon 612 eight-camera system (Vicon, Oxford, UK) operating at 100 Hz and using a Helen Hayes marker set-up [4, 11]. Ground reaction forces were recorded using one AMTI force plate located in the middle of a ten-metre walkway. The sampling rate of the force plate data was set at 2000 Hz. Data from the force-plate and data from the cameras (frame rate 60 Hz) were synchronised and captured in a data station (Vicon Workstation). Before each measurement session, a static and dynamic calibration was carried out to allow the system to define the capture volume and the relative position and orientation of each the cameras. A reconstruction process was carried out to create a virtual 3D motion, combining data from every camera by calculating the 3D position of each marker in each frame and linking these points into a trajectory. On this basis, a 3D model for each segment of the lower body could be constructed. The relative angles between coordinate systems of each segment in the lower limb, the absolute angles between a coordinate system of pelvis and the laboratory coordinate system, and the moment of force in each joint from the kinematics data and the ground reaction force could then be calculated. Reconstruction and calculations were carried out by the Vicon clinical manager software (Vicon workstation).
Three of five trials of each leg were selected on the criteria of speed similarity as recommended by Vardaxis et al. [22]. These trials were processed for further analysis with Vicon Plug-In-Gait software (Vicon, Oxford, UK). The beginning of a gait cycle was defined as the moment of heel strike, and the end of the cycle was defined as the next heel strike of the same leg. The gait cycle was normalised on a time basis of 100%.
Data from 22 age- and sex-matched healthy adults from the normal-material database of the Hammel Neurocenter Gait Laboratory were used as healthy controls (HC). The healthy controls followed the same protocol for gait analysis as described above.
Outcome measures
End-point outcome measures were changes over time, changes in the magnitude of the peak values of gait parameter variables of the operated hip and differences between operated hip and non-operated hip.
Temporal-spatial variables analysed were gait speed, cadence, stride length, step length, stance phase duration, and single support for both limbs.
Kinematic and kinetic variables analysed were range of motion (ROM) of the hip joint in all directions and the corresponding moments for both limbs. Positive, negative, and total work power during a gait cycle were calculated.
To estimate to what extent normal gait adaptation was restored, the operated hip was compared with the non-operated hip 12 weeks postoperatively. To evaluate whether it was reasonable to assume that the non-operated hip was an appropriate reference, mean curves of kinematic and kinetic variables of the operated and non-operated hips were compared with hip values obtained from a matched healthy control population.
To compare patient’s self-reported physical functioning at baseline, WOMAC scores were calculated preoperatively.
Statistics
Data from previous studies were used to estimate sample size. The estimate of the observed ROM in the sagittal plane during one stride was 39.4 (SD 5.3). With a clinical relevant difference in ROM of 10%, a power of 80% and a significance level of 5%, sample sizes were estimated to consist of 28 patients. Normally distributed data are described by means and standard deviation (SD), and statistically tested using Student’s t test. Data not normally distributed are described by medians and ranges, and statistically tested using Mann-Whitney test. Frequency was compared using Fisher’s exact test.
Peak values of gait parameter variables are described by means and SD, and analysed by a repeated measurement model. Changes over time and score level were compared between the HRS and the MHE groups and tested for significant differences by a repeated measurement model (two-way ANOVA). Peak values of gait parameter variables of operated and non-operated hip in the HRS and MHE groups were compared and analysed for differences by a repeated measurement model (two-way ANOVA). Mean curves of kinematic and kinetic values during a gait cycle of HRS, MHE, and HC are described but not analysed statistically.
The significance level was set at 0.05. We used the Statistical Package for the Social Sciences (SPSS, Inc.) version 11.0 for Windows.
Results
In the study period 30 patients were included and randomised, and 22 patients fulfilled the study protocol. Progress through the phases of the study is illustrated in Fig. 1. We intended to continue randomisation until 14 patients in each group had fulfilled the study protocol. However, during the study period the resurfacing surgical procedure was transferred to another hospital in the region. To secure internal validity regarding one surgeon, postoperative pain management and postoperative rehabilitation procedures, we decided to violate the protocol regarding sample size.
Fig. 1.
Progress through the phases of the study
Patient characteristics and perioperative data (Table 1) were similar except for the HRS group having a significantly longer surgical time of 93 min (range 70–120) compared with 72 min (range 47–100) in the MHE group (P = 0.02). Between the sixth and twelfth postoperative week gait speed increased significantly by approximately 12%, but with no significant differences between groups. Increased speed was reflected as significant changes in most of the assessed kinematic and kinetic gait parameters within the patient groups (Table 2). No significant differences between groups in changes in peak values or in levels were seen between six and 12 weeks postoperatively except for peak abductor moments, which improved significantly more in the MHE group compared to the HRS group (P = 0.01) (Table 2). A comparison between operated and non-operated hips during a gait cycle revealed an asymmetric gait pattern in all patients 12 weeks postoperatively. Because no significant differences were seen between groups all THR patients were pooled and analysed. The mean differences between operated and non-operated hip in step length and stand phase duration were, respectively, 0.03 m (95% CI 0.1–0.4, P = <0.001) and 0.7% (95% CI 0.3–1.1, P = 0.003). Furthermore, significantly less power was produced by the muscles around the operated hip compared to the non-operated hip (11.9 W; 95% CI 8.6–15.2, P = <0.001). The reduced muscle power was reflected in differences in peak moments especially in relation to peak flexor (229.4 Nmm/kg; 95% CI 158.7–300.1, P = <0.001) and abductor moments (82.7 Nmm/kg; 95% CI 33.0–112).
Table 1.
Patient characteristics and surgical data in the hip resurfacing system (HRS) and conventional hybrid prosthesis (MHE) groups
| Variables | HRS group (N = 11) | MHE group (N = 11) | p values |
|---|---|---|---|
| Age in years (median ) | 60 (51–65) | 61 (56–65) | 0.7 a |
| Femaleb | 7/11 (0.6) | 8/11 (0.7) | 0.6 c |
| Male | 4/11 (0.4) | 3/11 (0.3) | |
| Weight, kg (median and range) | 77 (64.4–96.2) | 77 (60.8–94.8) | 0.7 a |
| Height, m (median and range) | 168.5 (156.5–178.5) | 167.5 (159.5–181.5) | 0.9 a |
| BMI (median and range) | 27 (22–34) | 27 (21–31) | 0.6 a |
| Surgical time, min (median and range) | 93 (70–120) | 72 (47–100) | 0.02 a |
| Harris hip score (median and range) | 53 (36–68) | 50 (26–65) | 0.5 a |
| WOMAC pain (median and range) | 28 (15.5–38.5) | 22 (8.5–44) | 0.6 a |
| WOMAC stiff (median and range) | 12.5 (3–17.5) | 11 (2–18.5) | 0.9 a |
| WOMAC function (median and range) | 72.5 (54–125.5) | 95 (37.5–149) | 0.3 a |
| WOMAC total (median and range) | 112 (95.5–170) | 128.5 (48–211.5) | 0.6 a |
BMI body mass index, WOMAC Western Ontario and McMaster Universities Arthritis Index
aMann-Whitney test
bProportions are given in parentheses
cFisher’s exact test
Table 2.
Temporal-spatial, kinematic, and kinetic gait parameter variables of operated hips in the HRS and MHE groups six and 12 weeks postoperatively. Data are described by means and standard deviation (SD). Differences within and between groups are analysed by a repeated measurement model where 1 indicates difference in changes over time between groups, 2 is the difference in level between groups, and 3 indicates changes over time within groups
| Variables | HRS group (N = 11) | MHE group (N = 11) | P values | ||||
|---|---|---|---|---|---|---|---|
| 6 weeks (mean/SD) | 12 weeks (mean/SD) | 6 weeks (mean/SD) | 12 weeks (mean/SD) | 1 | 2 | 3 | |
| Temporal-spatial variables | |||||||
| Gait speed (m/s) | 1.19 (0.3) | 1.32 (0.2) | 1.10 (0.3) | 1.25 (0.2) | 0.9 | 0.4 | 0.001 |
| Cadence (steps/min) | 113.2 (14.1) | 118.4 (8.4) | 110.0 (10.7) | 115.7 (4.6) | 0.9 | 0.5 | 0.01 |
| Stride (m) | 1.27 (0.2) | 1.33 (0.1) | 1.19 (0.2) | 129 (0.1) | 0.4 | 0.4 | 0.004 |
| Step length operated leg (m) | 0.64 (0.1) | 0.68 (0.1) | 0.60 (0.1) | 0.66 (0.1) | 0.6 | 0.4 | <0.001 |
| Stand phase operated leg (%) | 61.2 (1.6) | 60.6 (0.8) | 62.0 (2.9) | 61.2 (1.9) | 0.8 | 0.4 | 0.05 |
| Single support operated leg (%) | 37.6 (2.4) | 38.9 (1.3) | 36.5 (3.7) | 38.9 (3.7) | 0.4 | 0.6 | 0.01 |
| Kinematic variables | |||||||
| Max hip flexion (angles in degrees) | 35.2 (5.0) | 34.5 (5.3) | 31.9 (8.0) | 31.5 (4.4) | 0.9 | 0.1 | 0.7 |
| Max hip extension (angles in degrees) | 5.5 (6.9) | 1.5 (6.2) | 3.0 (7.4) | −2.2 (4.2) | 0.6 | 0.2 | 0.001 |
| ROM in sagittal plan (angles in degrees) | 29.6 (7.2) | 33.6 (5.9) | 29.2 (5.6) | 33.6 (3.9) | 0.8 | 0.9 | >0.001 |
| Max adduction (angles in degrees) | 4.5 (2.8) | 4.2 (3.1) | 4.9 (5.8) | 6.0 (4.5) | 0.4 | 0.5 | 0.6 |
| Max abduction (angles in degrees) | −5.4 (4.5) | −7.3 (4.3) | −4.6 (4.3) | −5.9 (2.3) | 0.7 | 0.5 | 0.05 |
| ROM in frontal plan (angles in degrees) | 9.8 (3.2) | 11.5 (2.3) | 9.5 (3.2) | 11.9 (2.7) | 0.5 | 1.0 | 0.001 |
| Max internal rotation (angles in degrees) | 8.1 (5.7) | 7.3 (5.0) | 9.4 (6.2) | 10.0 (6.2) | 0.7 | 0.3 | 1.0 |
| Max external rotation (angles in degrees) | −5.7 (5.8) | −9.0 (4.4) | −5.2 (4.5) | −7.5 (10.3) | 0.8 | 0.7 | 0.2 |
| ROM in transverse plan (angles in degrees) | 13.7 (2.9) | 16.3 (6.0) | 13.6 (4.8) | 17.6 (5.8) | 0.6 | 0.8 | 0.01 |
| Kinetic variables | |||||||
| Max. positive moments sagittal plane (Nmm/kg) | 688.7(333.8) | 863.4 (347.7) | 664.8 (271.5) | 816.9 (214.5) | 0.8 | 0.8 | 0.004 |
| Max. negative moments sagittal plane (Nmm/kg) | −635.8 (209.5) | −748.6 (211.8) | −513 (171.3) | −675.4 (238.2) | 0.5 | 0.2 | 0.001 |
| Max. positive moments frontal plane (Nmm/kg) | 718.4 (78.3) | 733.3 (117.4) | 651.6 (127.4) | 773.5 (149.6) | 0.01 | 0.8 | 0.002 |
| Max. negative moments frontal plane (Nmm/kg) | −101.2 (101) | −71.3 (31.3) | −106.5 (87.1) | −117.1 (47.8) | 0.3 | 0.3 | 0.6 |
| Max. positive moments transverse plane (Nmm/kg) | 62.6 (26.8) | 65.9 (39.0) | 60.5 (26.6) | 77.6 (31.9) | 0.2 | 0.7 | 0.06 |
| Max. negative moments transverse plane (Nmm/kg) | −93.7 (36.8) | −124.8 (45.8) | −72.6 (51.1) | −97.9 (53.1) | 0.6 | 0.2 | <0.001 |
| Total work (power in watts) | 19.5 (9.1) | 27.0 (11.2) | 17.8 (8.6) | 25.0 (8.9) | 0.9 | 0.6 | <0.001 |
HRS hip resurfacing system, MHE conventional hybrid prosthesis
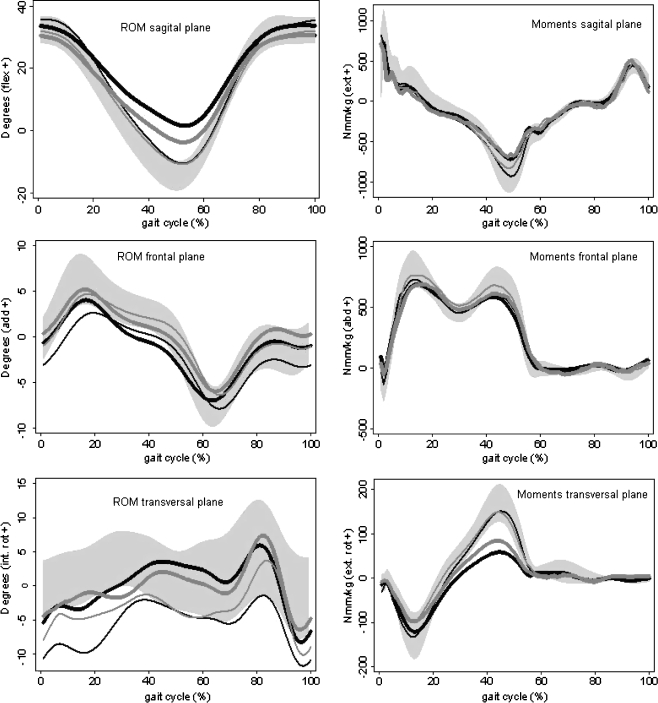
Mean curves of kinetic and kinematic variables during a gait cycle (Fig. 2) showed a reduction in dynamic ROM and in corresponding moments in all patients compared with the healthy controls.
Fig. 2.
Averages of joint angle profiles (ROM) and moments in all anatomical planes during a gait cycle walking at a self-selected speed for the operated and non-operated hips in the hip resurfacing system (HRS) and conventional hybrid prosthesis (MHE) groups 12 weeks after surgery, and the average values of both hips in the control group. The HRS group is represented by the black lines (bold operated hip, narrow non-operated hip); the MHE group is represented by the grey lines (bold operated hip, narrow non-operated hip). The shaded areas represent equal boundaries of ±1 standard deviation (SD) for controls
Discussion
In this prospective randomised study, we found no evidence for the hypothesis that a more extensive procedure in the HRS group affected the early phase of rehabilitation, or that gait impairment in the later phase of rehabilitation were less in the HRS group due to better joint stability and biomechanics.
No significant differences between groups in kinetic, kinematic, and temporal-spatial gait parameter variables of the hip were found six and 12 weeks postoperatively with respect to changes in or levels of peak values, except for peak abductor moments which changed more in the MHE group. The reason for this finding could be a faster recovery of peak abductor moments due to the less invasive surgical procedure in the MHE group. A criticism of our study is that because of a short follow-up period, it might only be the impact of differences in surgical procedure that is compared and not the implants.
In a study by Mont et al. [17] gait adaptation in patients receiving hip resurfacing arthroplasty was compared between patients receiving a standard prosthesis and healthy controls one year after THR. In contrast to our study, Mont et al. showed that hip kinematics (abductor and extensor moments) and functionality (speed) returned to normal to a greater degree in patients receiving a resurfacing implant compared to patients receiving a conventional prosthesis. Because this study [17] used an uncontrolled retrospective design, and only one postoperative time point of evaluation, the study results could be biased because of a highly selected and motivated resurfacing group. Gore et al. [7] compared patients before and after resurfacing or conventional replacement and found that before surgery the group undergoing resurfacing was younger, had less pain, slightly more hip motion, greater muscle strength, walked faster, and used fewer assistive devices during walking than did the group receiving the conventional replacement. After surgery, the group with resurfacing maintained its advantage in muscle strength and walking velocity.
The argument for choosing a short follow-up period was that three months after surgery patients are expected to return to normal physical activities and work, and therefore after that period factors other than different types of implant could affect gait adaptations and bias results. Furthermore, it has previously been reported that the greatest improvements in mechanics of gait occurred within the early rehabilitation phase [18, 23], and our findings after three months are in accordance with the residual hip impairment reported in other studies examining gait adaptations before and after THR with a longer follow-up period [5, 13, 16].
Another criticism of our study is that due to a small sample size lost information could be considered. However, because we were able to detect significant differences in changes over time between groups in one of the assessed variables, and because all other comparisons between groups revealed P values far from being significantly different, we do not believe the study is under-powered.
A weakness of our study is that we did not perform preoperative gait analysis in order to estimate gait impairment at baseline. Because patients were included after fairly strict inclusion and exclusion criteria to assure a homogenous sample, and because the preoperative WOMAC and Harris hip scores did not differ between groups, we do not believe that the results of our study are biased by differences between groups in physical functioning.
An increased peak contact force of the hip joint has previously been shown in patients with disturbed gait patterns [2, 8, 15]. Information about loading of the hip joint can be achieved from actual gait moments. External moments provide a reflection of net agonist and antagonist muscle activity, and they can indicate which muscles are compromised during surgery. It is assumed that dysfunction of one muscle increases the joint contact force because a part of the required joint moments is taken over by other muscles with unfavourably short lever arms and therefore higher forces [3]. Studies have reported postoperative extensor and abductor muscle weakness and have called for increased muscle strengthening regimes after THR surgery [12, 21]. The results of our study support this need; but furthermore, our results showed a weakness in the flexor muscles in the late stance phase, which may be the reason for the reduced ROM in extension during late stance phase and a loading imbalance. Failure to correct loading imbalances could be a factor in the development of implant failures in THR patients. It has been shown that hip loading or ground reaction force can be altered through gait retraining in subjects with THR [24]. However, it is unknown what the goals of gait training should be in order to obtain the best loading parameters for patient function and implant longevity. Current levels of function achieved by THR patients may have been sufficient in the past, but younger and more physically active patients may place greater demands on the implant [19, 25]. Gait retraining in conjunction with intensive muscle strengthening could prove beneficial for the function and longevity of the implant especially among young patients.
Acknowledgement
The project was supported by grants from Forskningsinitiativet Århus Amt, The Society of Danish Physiotherapists and SAHVA.
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Asayama I, Chamnongkich S, Simpson KJ, Kinsey TL, Mahoney OM. Reconstructed hip joint position and abductor muscle strength after total hip arthroplasty. J Arthroplasty. 2005;20(4):414–420. doi: 10.1016/j.arth.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Bergmann G, Deuretzbacher G, Heller M, Graichen F, Rohlmann A, Strauss J, Duda GN. Hip contact forces and gait patterns from routine activities. J Biomech. 2001;34(7):859–871. doi: 10.1016/S0021-9290(01)00040-9. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann G, Graichen F, Rohlmann A, Westerhoff P, Bender A, Gabel U, Heinlein B. Loads acting on orthopaedic implants. Measurements and practical applications. Orthopade. 2007;36(3):195–202. doi: 10.1007/s00132-007-1055-x. [DOI] [PubMed] [Google Scholar]
- 4.Davis R, Ounpuu S, Tyburski D, Gage J. A gait analysis data collection and reduction technique. Hum Mov Sci. 1991;10:575–587. doi: 10.1016/0167-9457(91)90046-Z. [DOI] [Google Scholar]
- 5.Foucher KC, Hurwitz DE, Wimmer MA. Preoperative gait adaptations persist one year after surgery in clinically well-functioning total hip replacement patients. J Biomech. 2007;40(15):3432–3437. doi: 10.1016/j.jbiomech.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 6.Girard J, Lavigne M, Vendittoli PA, Roy AG. Biomechanical reconstruction of the hip: a randomised study comparing total hip resurfacing and total hip arthroplasty. J Bone Joint Surg Br. 2006;88(6):721–726. doi: 10.1302/0301-620X.88B6.17447. [DOI] [PubMed] [Google Scholar]
- 7.Gore DR, Murray MP, Gardner GM, Sepic SB. Hip function after total vs. surface replacement. Acta Orthop Scand. 1985;56(5):386–390. doi: 10.3109/17453678508994353. [DOI] [PubMed] [Google Scholar]
- 8.Heller MO, Bergmann G, Kassi JP, Claes L, Haas NP, Duda GN. Determination of muscle loading at the hip joint for use in pre-clinical testing. J Biomech. 2005;38(5):1155–1163. doi: 10.1016/j.jbiomech.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 9.Hulet C, Hurwitz DE, Andriacchi TP, Galante JO, Vielpeau C. Functional gait adaptations in patients with painful hip. Rev Chir Orthop Reparatrice Appar Mot. 2000;86(6):581–589. [PubMed] [Google Scholar]
- 10.Hurwitz DE, Hulet CH, Andriacchi TP, Rosenberg AG, Galante JO. Gait compensations in patients with osteoarthritis of the hip and their relationship to pain and passive hip motion. J Orthop Res. 1997;15(4):629–635. doi: 10.1002/jor.1100150421. [DOI] [PubMed] [Google Scholar]
- 11.Kadaba MP, Ramakrishnan HK, Wootten ME. Measurement of lower extremity kinematics during level walking. J Orthop Res. 1990;8(3):383–392. doi: 10.1002/jor.1100080310. [DOI] [PubMed] [Google Scholar]
- 12.Long WT, Dorr LD, Healy B, Perry J (1993) Functional recovery of noncemented total hip arthroplasty. Clin Orthop Relat Res 288:73–77 [PubMed]
- 13.Madsen MS, Ritter MA, Morris HH, Meding JB, Berend ME, Faris PM, Vardaxis VG. The effect of total hip arthroplasty surgical approach on gait. J Orthop Res. 2004;22(1):44–50. doi: 10.1016/S0736-0266(03)00151-7. [DOI] [PubMed] [Google Scholar]
- 14.Marker DR, Strimbu K, McGrath MS, Zywiel MG, Mont MA. Resurfacing versus conventional total hip arthroplasty—review of comparative clinical and basic science studies. Bull NYU Hosp Jt Dis. 2009;67(2):120–127. [PubMed] [Google Scholar]
- 15.McGrory BJ, Morrey BF, Cahalan TD, An KN, Cabanela ME. Effect of femoral offset on range of motion and abductor muscle strength after total hip arthroplasty. J Bone Joint Surg Br. 1995;77(6):865–869. [PubMed] [Google Scholar]
- 16.Miki H, Sugano N, Hagio K, Nishii T, Kawakami H, Kakimoto A, Nakamura N, Yoshikawa H. Recovery of walking speed and symmetrical movement of the pelvis and lower extremity joints after unilateral THA. J Biomech. 2004;37(4):443–455. doi: 10.1016/j.jbiomech.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Mont MA, Seyler TM, Ragland PS, Starr R, Erhart J, Bhave A. Gait analysis of patients with resurfacing hip arthroplasty compared with hip osteoarthritis and standard total hip arthroplasty. J Arthroplasty. 2007;22(1):100–108. doi: 10.1016/j.arth.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Murray MP, Gore DR, Brewer BJ, Mollinger LA, Sepic SB (1981) Joint function after total hip arthroplasty: a four-year follow-up of 72 cases with Charnley and Muller replacements. Clin Orthop Relat Res 157:119–124 [PubMed]
- 19.Naal FD, Maffiuletti NA, Munzinger U, Hersche O. Sports after hip resurfacing arthroplasty. Am J Sports Med. 2007;35(5):705–711. doi: 10.1177/0363546506296606. [DOI] [PubMed] [Google Scholar]
- 20.Ong KL, Kurtz SM, Manley MT, Rushton N, Mohammed NA, Field RE. Biomechanics of the Birmingham hip resurfacing arthroplasty. J Bone Joint Surg Br. 2006;88(8):1110–1115. doi: 10.1302/0301-620X.88B8.17567. [DOI] [PubMed] [Google Scholar]
- 21.Shih CH, Du YK, Lin YH, Wu CC (1994) Muscular recovery around the hip joint after total hip arthroplasty. Clin Orthop Relat Res 302:115–120 [PubMed]
- 22.Vardaxis VG, Allard P, Lachance R, Duhaime M. Classification of able-bodied gait using 3-D muscle powers. Hum Mov Sci. 1998;17(1):121–136. doi: 10.1016/S0167-9457(97)00024-9. [DOI] [Google Scholar]
- 23.Wall JC, Ashburn A, Klenerman L. Gait analysis in the assessment of functional performance before and after total hip replacement. J Biomed Eng. 1981;3(2):121–127. doi: 10.1016/0141-5425(81)90004-2. [DOI] [PubMed] [Google Scholar]
- 24.White SC, Lifeso RM. Altering asymmetric limb loading after hip arthroplasty using real-time dynamic feedback when walking. Arch Phys Med Rehabil. 2005;86(10):1958–1963. doi: 10.1016/j.apmr.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Yun AG. Sports after total hip replacement. Clin Sports Med. 2006;25(2):359–364. doi: 10.1016/j.csm.2006.01.002. [DOI] [PubMed] [Google Scholar]