Abstract
Lung cancer is the leading cause of cancer mortality. Bone metastases are a common complication in lung cancer. The therapeutic approach and the type of surgical treatment of these lesions have not been clearly defined. Outcome and prognosis of patients with bony metastases and a variety of surgical interventions were analysed retrospectively. In 58 patients we performed 62 surgeries. The most common locations of metastases were the spine (32 patients), the proximal femur (10) and the pelvis (11). Twenty-one patients had a singular and 20 had multiple osseous lesions; 17 showed additional visceral involvement. Nine patients had a local progression of their disease and 49 a systemic progression. Patients with local progression (n = 9) had a better prognosis than the patients with systemic progression (p = 0.0083). Fracture (p = 0.0017) worsened prognosis, whereas the number of bone lesions or the presence of a visceral lesion did not. Patients with small lesions showed a better survival than patients with large lesions (p = 0.02). Ten percent of the patients died within 30 days and 78% within one year after surgery. Fracture of bone due to metastatic lung cancer worsens the prognosis whereas the number of bone lesions, the presence of a visceral lesion and the surgical approach do not.
Introduction
Lung cancer is the leading cause of cancer death worldwide [1]. The American Cancer Society statistics indicate that within the United States there were 219,440 new cases of lung and bronchial cancer in 2009 with mortality in 159,390 patients [1]. The skeleton is the most common site affected by metastatic cancer [2] and metastatic lesions are the most common malignant tumours affecting the skeleton [3].
In many cases bone metastases are the first sign of a malignant disease [4]. Bone metastases are common in patients with lung cancer and 36% of all patients undergoing surgery for bone metastasis are patients with lung cancer [5–9].
There is still a lack of data regarding the role of surgery in the management of bone metastasis although the prognosis of the patients could be improved by applying multimodal therapies [10–12]. Since treatment of bone metastases is palliative, it must be weighed up to complications in terminally ill patients. We analysed retrospectively the prognosis and outcome of patients in our institute with metastatic bone disease as a result of lung cancer, which, to our knowledge, is the first report of a major series.
Material and methods
Between 1980 and 2005, 58 patients with lung cancer and bone metastases underwent surgical procedures. The patients’ age, gender, treatment of primary tumour and extraosseous metastases, time of appearance of bone and visceral metastases, clinical presentation, surgical treatment, complications, and survival were analysed. Statistical analyses were performed using Kaplan-Meier life table analyses and the log rank test for univariate analysis. A multivariate analysis was completed incorporating the variables fracture and local progression of disease. The mean age of the 48 men and ten women was 61.5 years (range, 16–89 years). Most of the patients were between 50 and 59 (18 patients) or 60 and 69 years old (16 patients). One patient was younger than 40, eight between 40 and 49, 12 between 70 and 79 years and three patients were aged 80 years or older.
The most common anatomic locations of surgery were the spine (32 patients), the proximal femur (ten patients), the shaft of the femur (three patients) and the pelvis (11 patients). Ten patients had combined lesions of the spine, for example, cervico-thoracal or thoracal-lumbal lesions. Lesions were also seen at the humerus, the ribs, the clavicle, the tibia and the patella.
All patients presented with pain; 26 (45%) reported pain for pathological fracture and 15 (26%) for neurological impairment. The mean duration of symptoms was 4.2 months (range 0.4–24.7; median 3.1 months).
In 18 (31%) patients the bone lesions were the first sign of the disease. At presentation, 21 (36%) patients had a mono-ostotic lesion. Twenty (34%) patients had polyostotic disease (more than one lesion), and 17 (29%) patients showed additional visceral involvement. The interval from diagnosis of lung cancer to surgical treatment of the osseous metastasis ranged from 0 to 122 months (mean 5.5 months; median 0.5 months).
In 32 (55%) cases the histology verified the existence of an adenocarcinoma, in eight (14%) cases a small cell carcinoma, in eight cases (14%) a squamous cell carcinoma, in six (10%) cases a large cell carcinoma, in two (3%) cases a poorly differentiated carcinoma was found and in two cases a non-classified carcinoma. Overall, 62 operations were performed; four patients were operated twice.
Surgical therapy varied due to different techniques, tumour sites and extent of the disease. Incisional biopsy was performed in 29 (47%) patients. From 19 patients with involvement of the spine, 11 were treated by laminectomy, three by dorsal procedure including instrumentation (Fig. 1) and three by ventral corporectomy. In two patients a vertebroplasty was done.
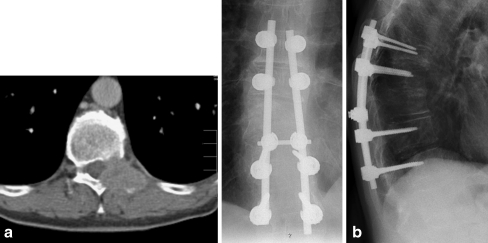
Fig. 1.
a A 58-year-old patient with a metastatic lesion of the thoracic spine (Th 10) with myelon compression in the CT scan as a first manifestation of an adenocarcinoma of the lung. b Radiograph of the thoracic spine of the same patient after tumour resection and instrumentation (Th8-Th12)
In eight (13%) patients, the tumour was resected and a tumour endoprosthesis (seven proximal femur, one proximal humerus) was implanted (Fig. 2). Three patients received a standard endoprosthesis. In one patient tumour resection, cementation, and instrumentation and in two patients tumour resection without reconstruction were performed.
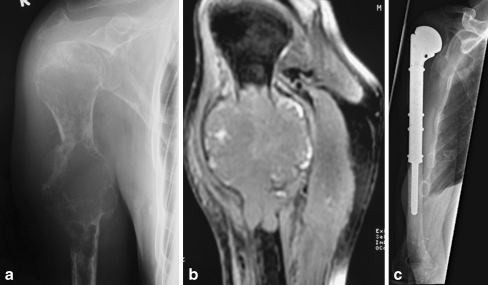
Fig. 2.
a Radiograph of a 56-year-old patient showing a large osteolytic lesion in the right humerus with a pathologic fracture as first manifestation of metastatic lung cancer. b MRI of the same patient indicates the infiltration of the bone metastasis in the surrounding soft tissues. c Radiograph of the patient showing the tumour prosthesis after resection of the metastasis
Radiation therapy was administered in 49 patients, in 11 patients before surgery. In the remaining nine patients radiotherapy was not administered due to the very bad clinical prognosis with a very short survival time. Chemotherapy was given to 20 patients, to six of these preoperatively.
Results
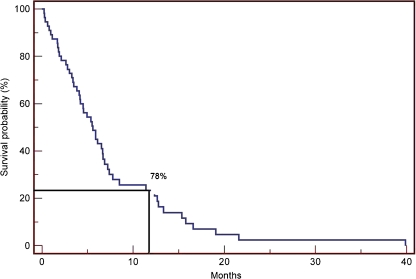
Six (10%) patients died within the first 30 days after surgery and 78% of the patients died within the first year after surgical treatment of the skeletal lesions. The mean and median survivals were 7.1 and 5.4 months, respectively (Fig. 3). Eight percent of the patients suffered complications associated with surgical treatment. These complications included pneumonia (1), cardiac arrest (1), severe thromboembolic event (1), fracture of the tibia (1) and rapid deterioration of general condition (1). The hair-line fracture of the tibia happened while a prosthetic reconstruction of the distal femur and was related to the surgical procedure itself. The thromboembolic event happened in the same patient who had a cardiac arrest and needed resuscitation after spinal surgery at T12 level. Nine (14.5%) patients showed local progression, which was defined as a progressive local disease of the surgical pre-treated lesion. Seven of these patients had no radiotherapy before the local progression. Four patients with local progression were treated with a second surgical procedure: one instrumentation of the thoracic spine, one endoprosthesis of the hip, one tumour resection with cementation and instrumentation of the femur and one amputation of the lower leg, each after a local biopsy. All the patients that had not had radiotherapy before the primary therapy (besides the one with the amputation) received a postoperative radiotherapy, one of them in combination with a second chemotherapy. Two of the patients were treated with radiotherapy alone (one after a dorsal decompression of the spine, one after a biopsy of the spine). Two of the patients that had a radiotherapy before the local progression were treated with a second chemotherapy only (one after a ventral resection of the spine, one after a partial resection of the clavicle). One patient with a prosthesis of the humerus was treated with radiation and a second chemotherapy. The patients with local progression had a significantly better prognosis than the patients without as they survived longer (p = 0.008; Table 1). The median of the survival rate of the patients without local progression was 4.5 months (range, 0.23–31 months); the median of those with a local progression was 16.5 months (range 4.1–39.9 months).
Fig. 3.
Survival probability after surgical therapy of 58 patients with bone metastases secondary to lung cancer
Table 1.
Factors influencing the median survival of the patients
| Factor | Factor present | Factor not present | p |
|---|---|---|---|
| Fracture, n = 26 | 3.0 (0.2–19.1) | 6.3 (0.6–39.9) | 0.0017 |
| Local progression, n = 9 | 16.5 (4.1–39.9) | 4.5 (0.23–31) | 0.0083 |
Data given as median survival in months (range)
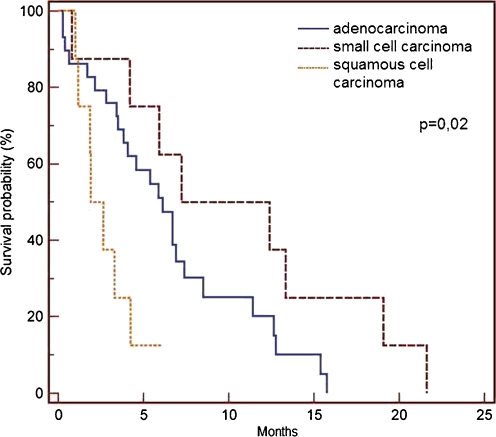
The patient's age and gender did not influence the survival time, nor did the extent of the surgical procedure. Patients with small cell lung cancer survived longer than patients with adenocarcinoma or squamous cell carcinoma (p = 0.02) (Fig. 4).
Fig. 4.
Overall survival probability dependent on the type of histology
The diagnosis of a fracture at the time of surgery worsened the survival time significantly (p = 0.0017) whereas number of lesions present at surgery had no influence on the survival time.
Discussion
Lung cancer is the leading cause of death for male and female cancer patients with a five-year overall survival rate of less than 15% [1, 13–15].
The development of bone metastasis worsens the prognosis of these patients [5, 6]. Metastatic bone lesions are treated in order to control pain, to prevent pathological fractures and neurological impairment, and to maintain optimal quality of life for as long as possible [12]. The median survival time for lung cancer patients with synchronous bone metastasis is 5.5 months; the two-year survival rate is 3% [16]. Our study confirms these data; 22% of our patients were alive more than one year after surgery of bone metastasis.
Treatment for metastatic lung cancer consists of chemotherapy and/or radiotherapy, while surgery is indicated only in a few selected cases [6]. However, when bone metastases are the first symptom, biopsy or resection is performed for histology [17]. In the literature only a few cases have been reported with patients being progression-free even a few years after therapy of bony metastasis [6, 18, 19]. The authors of these publications suggest that metastatic bone tumours of unknown origin should be surgically treated whenever possible, taking the possibility of stage IV lung cancer into account. The surgical treatment of both primary and metastatic lesions of stage IV lung cancer might be selectively performed, because it is possible that the survival of the patient may thereby be successfully prolonged and a good quality of life maintained [6].
This forms a strong contrast to our own findings strengthening the importance of a closer look at those previous assumptions or future data series of other authors. This also stands up to findings about patients with metastatic bone disease due to primaries other than kidney cancer, where resection strategies prove to be of great benefit [20].
In our study a strong bias is evident because only patients with surgically treated lesions are included. This overemphasizes the spine and the lower extremity due to common indications such as fractures and neurological impairment. Despite that, the distribution of locations in this study seems to be equivalent to those in relevant literature [5, 19].
The types of lung cancer which most frequently metastasize to the bone are small cell carcinoma and adenocarcinoma [5, 21]. In our study, 32 (55%) out of 58 patients with osseous metastases had an adenocarcinoma. Furthermore, in our group patients with small cell cancer lived longer than patients with adenocarcinoma and squamous cell carcinoma (p = 0.02).
The incidence of pathological fractures of long bones in patients with metastatic bone disease is about 10% [22] and the survival rate is lower compared to patients without fracture [11, 23]. Our study supports these data.
In other studies, patients with solitary bony metastases survived significantly longer than those with multiple lesions [11, 24]. These studies, however, did not focus on patients with osseous lung cancer metastases exclusively. Despite the short overall survival in this group, this finding could not be verified. Koizumi et al. have treated patients with lung cancer and 30% or less vertebral column invasion with preoperative and/or postoperative chemo radiotherapy [25]. De Meester et al. reviewed successful surgical treatment for patients with vertebral invasion who required en bloc resection of involved vertebra and surrounding tissue albeit their patients did not suffer from cortical involvement of the vertebra [26]. In cases where tumour invasion destroyed the vertebral cortical bone, the prognosis was poor [27]. It is considered essential, however, to manage severe pain despite the poor prognosis. There is a similar concept for patients with metastatic vertebral invasion [28]. Complete removal of vertebrae and multilevel laminectomy should be recommended in cases where tumour invasion extends to the spinal canal, transverse processes, facet joints or lamina [28].
On the other hand, the limited survival rate of the patients included in this study does not promote such an extended approach. In our group of patients even those who had only a biopsy had the same prognosis as others with more extended interventions. Spaggiari et al. questioned the clinical outcome in patients who underwent hemivertebrectomy for apical chest tumours [29]. In cases where 50% or more of a vertebra was destroyed, total vertebrectomy seemed to be required. Hemi- and total vertebrectomy was performed by several surgeons to achieve complete resection. They emphasized the necessity of complete resection in order to gain control over local progression of vertebral invasion. However, the local progression rate is still high [30].
Böhm and Huber report of local progression in the proximal femur in two of two cases of non-small-cell lung cancer despite preoperative irradiation [30]. In this study a local progression rate was seen in nine of 62 (14.5%) cases. The patients with local progression had a significantly better prognosis than the patients without (p = 0.002). It is assumed that the progressions were at least in part due to the longer survival rate in those patients and not due to other factors. In these cases local progression had enough time to develop. Interestingly in nearly all the patients with a local progression (7), radiotherapy had not been part of the treatment before the development of local progression.
Conclusion
Patients with metastatic lung cancer have a poor survival. Histology of the tumour and incidence of pathologic fractures are risk factors for survival. Surgical therapy of bone lesions cannot prolong life but can achieve a higher quality of life as pain and neurologic symptoms are better controlled. Therefore we recommend the less invasive procedures.
Acknowledgment
We would like to thank Dr. Markus Schlemmer for his support during the linguistic revision.
Conflict of interest statement All authors disclose any financial or personal relationships with other people or organisations that could inappropriately influence (bias) their work.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 3.Dahlin CC, Unni KK. Bone tumours. 4. Springfield: Thomas; 1986. [Google Scholar]
- 4.Conroy T, Malissard L, Dartois D, Luporsi E, Stines J, Chardot C. Natural history and development of bone metastasis. Apropos of 429 cases. Bull Cancer. 1988;75:845–857. [PubMed] [Google Scholar]
- 5.Hanagiri T, Kodate M, Nagashima A, Sugaya M, Dobashi K, Ono M, et al. Bone metastasis after a resection of stage I and II primary lung cancer. Lung Cancer. 2000;27:199–204. doi: 10.1016/S0169-5002(99)00108-7. [DOI] [PubMed] [Google Scholar]
- 6.Higashiyama M, Kodama K, Takami K, Higaki N, Yamada T, Mano M, et al. Surgical treatment of bone metastasis followed by a primary lung cancer lesion: report of a case. Surg Today. 2004;34:600–605. doi: 10.1007/s00595-004-2758-9. [DOI] [PubMed] [Google Scholar]
- 7.Katagiri H, Takahashi M, Inagaki J, Sugiura H, Ito S, Iwata H. Determining the site of the primary cancer in patients with skeletal metastasis of unknown origin: a retrospective study. Cancer. 1999;86:533–537. doi: 10.1002/(SICI)1097-0142(19990801)86:3<533::AID-CNCR24>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Erturan S, Yaman M, Aydin G, Uzel I, Musellim B, Kaynak K. The role of whole-body bone scanning and clinical factors in detecting bone metastases in patients with non-small cell lung cancer. Chest. 2005;127:449–454. doi: 10.1378/chest.127.2.449. [DOI] [PubMed] [Google Scholar]
- 9.Salvatierra A, Baamonde C, Llamas JM, Cruz F, Lopez-Pujol J. Extrathoracic staging of bronchogenic carcinoma. Chest. 1990;97:1052–1058. doi: 10.1378/chest.97.5.1052. [DOI] [PubMed] [Google Scholar]
- 10.Lally BE, Urbanic JJ, Blackstock AW, Miller AA, Perry MC. Small cell lung cancer: have we made any progress over the last 25 years? Oncologist. 2007;12:1096–1104. doi: 10.1634/theoncologist.12-9-1096. [DOI] [PubMed] [Google Scholar]
- 11.Wedin R, Bauer HC, Wersall P. Failures after operation for skeletal metastatic lesions of long bones. Clin Orthop Relat Res. 1999;358:128–139. doi: 10.1097/00003086-199901000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Capanna R, Campanacci DA. The treatment of metastases in the appendicular skeleton. J Bone Joint Surg Br. 2001;83:471–481. doi: 10.1302/0301-620X.83B4.12202. [DOI] [PubMed] [Google Scholar]
- 13.Granville CA, Dennis PA. An overview of lung cancer genomics and proteomics. Am J Respir Cell Mol Biol. 2005;32:169–176. doi: 10.1165/rcmb.F290. [DOI] [PubMed] [Google Scholar]
- 14.Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. 1997;69:1–18. doi: 10.1016/S0304-3959(96)03267-8. [DOI] [PubMed] [Google Scholar]
- 15.Mundy GR. Mechanisms of bone metastasis. Cancer. 1997;80(8 Suppl):1546–1556. doi: 10.1002/(SICI)1097-0142(19971015)80:8+<1546::AID-CNCR4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 16.Stanley KE. Prognostic factors for survival in patients with inoperable lung cancer. J Natl Cancer Inst. 1980;65:25–32. [PubMed] [Google Scholar]
- 17.Shih WJ, Magoun S, Lahar B, Stipp V, Gross K. An unusual case of a tibial metastasis as the clinical presentation of bronchogenic adenocarcinoma. J Nucl Med Technol. 1998;26:91–93. [PubMed] [Google Scholar]
- 18.Hawighorst H, Gademann G. Cure in a patient with multiple osseus metastases in non-small cell lung cancer: a case report. Strahlenther Onkol. 1993;169:617–620. [PubMed] [Google Scholar]
- 19.Hirano Y, Oda M, Tsunezuka Y, Ishikawa N, Watanabe G. Long-term survival cases of lung cancer presented as solitary bone metastasis. Ann Thorac Cardiovasc Surg. 2005;11:401–404. [PubMed] [Google Scholar]
- 20.Durr HR, Maier M, Pfahler M, Baur A, Refior HJ. Surgical treatment of osseous metastases in patients with renal cell carcinoma. Clin Orthop Relat Res. 1999;367:283–290. [PubMed] [Google Scholar]
- 21.Mountain CF. The new international staging system for lung cancer. Surg Clin N Am. 1987;67:925–935. doi: 10.1016/s0039-6109(16)44330-6. [DOI] [PubMed] [Google Scholar]
- 22.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(Suppl):1588–1594. doi: 10.1002/(SICI)1097-0142(19971015)80:8+<1588::AID-CNCR9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 23.Hansen BH, Keller J, Laitinen M, Berg P, Skjeldal S, Trovik C, et al. The Scandinavian sarcoma group skeletal metastasis register. Survival after surgery for bone metastases in the pelvis and extremities. Acta Orthop Scand Suppl. 2004;75:11–15. doi: 10.1080/00016470410001708270. [DOI] [PubMed] [Google Scholar]
- 24.Chan D, Carter SR, Grimer RJ, Sneath RS. Endoprosthetic replacement for bony metastases. Ann R Coll Surg Engl. 1992;74:13–18. [PMC free article] [PubMed] [Google Scholar]
- 25.Koizumi K, Haraguchi S, Hirata T, Hirai K, Mikami I, Yamagishi S, et al. Surgical treatment of lung cancer with vertebral invasion. Ann Thorac Cardiovasc Surg. 2004;10:229–234. [PubMed] [Google Scholar]
- 26.DeMeester TR, Albertucci M, Dawson PJ, Montner SM. Management of tumor adherent to the vertebral column. J Thorac Cardiovasc Surg. 1989;97:373–378. [PubMed] [Google Scholar]
- 27.Ginsberg RJ, Martini N, Zaman M, Armstrong JG, Bains MS, Burt ME, et al. Influence of surgical resection and brachytherapy in the management of superior sulcus tumor. Ann Thorac Surg. 1994;57:1440–1445. doi: 10.1016/0003-4975(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 28.Gokaslan ZL, York JE, Walsh GL, McCutcheon IE, Lang FF, Putnam JB, Jr, et al. Transthoracic vertebrectomy for metastatic spinal tumors. J Neurosurg. 1998;89:599–609. doi: 10.3171/jns.1998.89.4.0599. [DOI] [PubMed] [Google Scholar]
- 29.Spaggiari L, Carbognani P, Solli P, Rusca M. Is it justified to ignore oncologic principles during VATS major lung resections? Ann Thorac Surg. 1998;66:303–304. doi: 10.1016/S0003-4975(98)00908-4. [DOI] [PubMed] [Google Scholar]
- 30.Bohm P, Huber J. The surgical treatment of bony metastases of the spine and limbs. J Bone Joint Surg Br. 2002;84:521–529. doi: 10.1302/0301-620X.84B4.12495. [DOI] [PubMed] [Google Scholar]