Abstract
Background
Despite safety-related concerns, psychotropic medications are frequently prescribed to manage behavioural symptoms in older adults, particularly those with dementia. We assessed the comparative safety of different classes of psychotropic medications used in nursing home residents.
Methods
We identified a cohort of patients who were aged 65 years or older and had initiated treatment with psychotropics after admission to a nursing home in British Columbia between 1996 and 2006. We used proportional hazards models to compare rates of death and rates of hospital admissions for medical events within 180 days after treatment initiation. We used propensity-score adjustments to control for confounders.
Results
Of 10 900 patients admitted to nursing homes, atypical antipsychotics were initiated by 1942, conventional antipsychotics by 1902, antidepressants by 2169 and benzodiazepines by 4887. Compared with users of atypical antipsychotics, users of conventional antipsychotics and antidepressants had an increased risk of death (rate ratio [RR] 1.47, 95% confidence interval [CI] 1.14–1.91 for conventional antipsychotics and RR 1.20, 95% CI 0.96–1.50 for antidepressants), and an increased risk of femur fracture (RR 1.61, 95% CI 1.03–2.51 for conventional antipsychotics and RR 1.29, 95% CI 0.86–1.94 for antidepressants). Users of benzodiazepines had a higher risk of death (RR 1.28, 95% CI 1.04–1.58) compared with users of atypical antipsychotics. The RR for heart failure was 1.54 (95% CI 0.89–2.67), and for pneumonia it was 0.85 (95% CI 0.56–1.31).
Interpretation
Among older patients admitted to nursing homes, the risks of death and femur fracture associated with conventional antipsychotics, antidepressants and benzodiazepines are comparable to or greater than the risks associated with atypical antipsychotics. Clinicians should weigh these risks against the potential benefits when making prescribing decisions.
Despite concerns about their safety, psychotropic medications are used frequently to manage behavioural symptoms in older adults, particularly in those who have dementia. These medications tend to be used because the effectiveness of psychosocial and behavioural interventions remains unclear, and because implementation of those alternate interventions is often hampered by a lack of resources.1 In nursing homes, psychotropic agents are given to up to two-thirds of dementia patients.2–5
The safety of antipsychotic medications in older adults has been called into question. The United States Food and Drug Administration and Health Canada have issued advisories stating that certain atypical antipsychotics (risperidone, olanzapine and aripiprazole) have been associated with an increased risk of stroke and transient ischemic events, and both atypical and conventional antipsychotics have been associated with an increased risk of death.6–11 Given this problematic safety record, physicians may increasingly resort to alternative psychotropic agents for management of behavioural symptoms in older adults.1,12,13 However, comparative studies of the safety of other classes of psychotropic medications in such patients have not been conducted.
In the absence of randomized controlled trials, pharmacoepidemiologic studies using large databases are the best option available for defining the comparative safety of the psychopharmacologic treatment regimens used to manage behavioural symptoms in older adults with dementia. Rigorous methodologic approaches need to be applied to ensure that epidemiologic studies are unbiased by the selective prescribing that occurs in nonrandomized studies.1 We aimed to examine the association between various classes of psychotropic medications and a range of unintended health outcomes among older adults admitted to nursing homes. We focused on patients in nursing homes because use of psychotropic medication is known to be extensive in this setting,2–5 and medication safety is of particular concern given the complex array of medical illnesses among these patients.
Methods
Study design and data source
We conducted a population-based cohort study involving older adults newly admitted to a nursing home in the province of British Columbia (BC). We obtained ethics approval for our study from the Brigham and Women’s Hospital Institutional Review Board.
We used linked administrative data from the BC Ministry of Health. The data included longitudinal, person-specific information on health services received under the province’s universal insurance program, including physician services, admissions to hospital and long-term care, and prescription drugs recorded as dispensed by the province-wide PharmaNet database. Vital status information was linked from the BC Vital Statistics Agency.
Study cohort and medications
The study cohort consisted of all BC residents aged 65 years or older who were admitted to a nursing home between Jan. 1, 1996, and Mar. 31, 2006, and who were prescribed a psychotropic medication during the first 90 days after admission but not during the six months before admission. We employed a new-user design to avoid underascertainment of events occurring soon after therapy begins,14 and to ensure that baseline covariables at study entry were assessed before treatment initiation and not affected by the treatment itself.15
We classified patients into one of four treatment categories (atypical antipsychotics, conventional antipsychotics, antidepressants, or benzodiazepines and other hypnotic agents [referred to as benzodiazepines]) according to the first new prescription for a psychotropic agent that was filled within the 90-day enrollment window. The specific medications that comprised each category are listed in Appendix 1 (available at www.cmaj.ca/cgi/content/full/cmaj.101406/DC1).
The date of initiation of treatment was considered to be the index date. We excluded patients who, on the index date, filled prescriptions for multiple medications that belonged to different psychotropic classes. We also excluded patients with a diagnosis of cancer before the index date to avoid residual confounding introduced by selective prescribing of conventional antipsychotics as antiemetics for patients undergoing chemotherapy.10 Exposure to a class of psychotropic medication was considered to be discontinued if the prescription had not been refilled 14 days after the end of the last dispensed supply.
Baseline covariables were assessed during the six-month period before (and inclusive of) the index date. The period of follow-up began the day after the index date and extended up to 180 days.
Outcome measures
Selection of outcomes was informed by the safety concerns that have been raised for antipsychotics. All noncancer deaths occurring within 180 days after the index date were identified. Other outcomes included cardiac events (myocardial infarction, heart failure, cardiac arrest and ventricular arrhythmias), cerebrovascular events (ischemic stroke and transient ischemic attack), femur fracture, pneumonia and venous thromboembolism. Events were defined based on admission to hospital within 180 days after the index date with a relevant primary or secondary International Classification of Diseases (ICD) diagnostic or procedural code based on the 9th revision, clinical modification (ICD-9-CM) or the ICD-10 (Appendix 2, available at www.cmaj.ca/cgi/content/full/cmaj.101406/DC1).
Potential confounders
Potential confounding variables included age, sex, calendar year, level of care assigned at the time of admission to a nursing home, and clinical conditions that might affect the outcomes under consideration. Psychiatric morbidity was defined as dementia, depression, anxiety, delirium, a mood disorder, a psychotic disorder, a sleep disorder, alcohol or drug abuse, other psychiatric disorders, or prior use of anticholinergic and psychotropic drugs. Other comorbid conditions included hypertensive heart and kidney diseases, arrhythmias (a diagnosis plus use of antiarrhythmia medication), diabetes mellitus (a diagnosis plus use of antidiabetic medications), cerebrovascular disease (hemorrhagic and ischemic events), congestive heart failure, acute myocardial infarction, coronary artery disease, other evidence of ischemic heart disease (angina, percutaneous transluminal coronary angioplasty, coronary artery bypass graft or nitroglycerin use), other cardiovascular conditions (including valvular disease, aneurysm, peripheral vascular disease), HIV infection, epilepsy, Parkinson disease, osteoporosis, fracture history, pneumonia and chronic lung disease. We used the Charlson comorbidity index,16 number of physician visits for any reason, number of hospital admissions for any reason and of any length, number of distinct (nonpsychotropic) prescription drugs, and prior specialist care (i.e., psychiatrist, geriatrician or neurologist) as generic markers of comorbidity.17
Statistical analysis
We calculated the rates of the various outcomes with follow-up censored at the time of treatment discontinuation (plus a 30-day lag period) or treatment crossover, defined as a switch to or addition of a medication belonging to the comparator group (i.e., as-treated analysis).
We fit proportional hazards models for pairwise comparisons against atypical antipsychotics. We used propensity score adjustment to balance measured risk factors for the outcomes between medication user groups.18 Propensity scores were derived from predicted probabilities estimated in logistic regression models of conventional antipsychotic, antidepressant and benzodiazepine use versus atypical antipsychotic use. The propensity score models contained all of the potential confounders listed above, except those that were known to be strongly related to the exposure and thought to be unrelated to the outcomes. The latter were excluded to avoid unnecessarily increasing the variance of the estimated exposure effect19 and to reduce bias through adjustment for an instrumental variable in the presence of unmeasured confounders.20
We truncated 2.5% of patients on either extreme of the propensity score distribution, and divided patients into quintiles based on their propensity score value. The Cox models were adjusted for these quintiles. This approach ensured that the comparisons were made between patients that were homogeneous in terms of measured sociodemographic characteristics, health care utilization characteristics and medical history, including proxies for treatment indication (Appendix 3, available at www.cmaj.ca/cgi/content/full/cmaj.101406/DC1). Treatment with another psychotropic medication class after the index date was included as a time-varying covariable. Adjusted models were run separately in strata defined by dementia, and for patients with no prior use of either the treatment or comparator medication class. We also investigated the effect of restricting the group of patients initiating benzodiazepines to those who received medications used primarily to manage anxiety and agitation (as opposed to those used primarily to induce sleep).
In secondary analyses, we used high-dimensional propensity score adjustment in an effort to further reduce residual confounding.21 The high-dimensional propensity score algorithm evaluated thousands of diagnoses, procedures and pharmacy claim codes (referred to as data dimensions) to identify and prioritize covariables that served as proxies for unmeasured confounders, such as level of cognitive functioning, dependence in activities of daily living and severity of behavioural disturbances. Specifically, the 200 most prevalent codes in each data dimension were prioritized by calculating, for each covariable, the possible amount of confounding it could adjust for in a multiplicative model, given a binary exposure and outcome, after adjusting for demographic covariables. These covariables were then sorted in descending order of confounding potential, and the top 500 empirical covariables were selected. These empirically identified confounders were combined with investigator-identified covariables to improve confounding adjustment. We also conducted analyses that carried the first exposure forward throughout the 180 days (initially-treated analysis).
Results
Of the 10 900 patients included in the study cohort, 1942 initiated an atypical antipsychotic, 1902 a conventional antipsychotic, 2169 an anti-depressant and 4887 a benzodiazepine. Most patients initiated treatment shortly after admission (median 1 day, interquartile range 0–13 days).
Overall, users of atypical antipsychotics had more diagnosed psychiatric comorbidities, used more medications indicated for dementia and psychotropic medications, and had more visits to psychiatrists (Table 1). Compared with users of atypical antipsychotics, users of conventional antipsychotics had fewer comorbidities and required less intensive care. Users of antidepressants were sicker than users of atypical antipsychotics, with considerably more pre-existing diseases of the circulatory system, diabetes, chronic lung disease and prior fractures, and with worse general indicators of comorbidity. Users of benzodiazepines had more pre-existing cardiovascular disease, diabetes, chronic lung disease and fractures than users of atypical antipsychotics, but general indicators of comorbidity indicated they were in slightly better health otherwise.
Table 1:
Characteristics of the study cohort, stratified by class of medication
| Characteristic | No. (%)* | |||
|---|---|---|---|---|
| Atypical antipsychotics n = 1942 | Conventional antipsychotics n = 1902 | Antidepressants n = 2169 | Benzodiazepines n = 4887 | |
| Sex, male | 811 (41.8) | 852 (44.8) | 832 (38.4) | 1876 (38.4) |
| Age, yr, mean (SD) | 84.0 (6.6) | 83.0 (6.8) | 83.6 (6.9) | 84.0 (7.0) |
| Year index prescription filled | ||||
| 1996–2000 | 515 (26.5) | 1275 (67.0) | 1085 (50.0) | 2714 (55.5) |
| 2000–2006 | 1427 (73.5) | 627 (33.0) | 1084 (50.0) | 2173 (44.5) |
| Nursing home care level† | ||||
| Personal care or intermediate care 1 | 5 (0.3) | 8 (0.5) | 23 (1.0) | 60 (1.2) |
| Intermediate care 2 | 334 (17.2) | 498 (26.2) | 681 (31.4) | 1608 (32.9) |
| Intermediate care 3 | 1241 (63.9) | 1115 (58.6) | 952 (43.9) | 2297 (47.0) |
| Extended care | 362 (18.6) | 281 (14.8) | 513 (23.7) | 922 (18.9) |
| Cardiovascular comorbidities | ||||
| Cardiac arrhythmia | 22 (1.1) | 17 (0.9) | 34 (1.6) | 73 (1.5) |
| Congestive heart failure | 219 (11.3) | 201 (10.6) | 328 (15.1) | 736 (15.1) |
| Hypertension | 384 (19.8) | 314 (16.5) | 516 (23.8) | 942 (19.3) |
| Ischemic heart disease | ||||
| Myocardial infarction | 66 (3.4) | 76 (4.0) | 113 (5.2) | 246 (5.0) |
| Coronary artery disease | 104 (5.4) | 89 (4.7) | 189 (8.7) | 326 (6.7) |
| Other ischemic heart disease‡ | 294 (15.1) | 253 (13.3) | 444 (20.5) | 948 (19.4) |
| Peripheral arterial disease | 38 (2.0) | 28 (1.5) | 81 (3.7) | 106 (2.2) |
| Valvular disease | 35 (1.8) | 29 (1.5) | 42 (1.9) | 85 (1.7) |
| Cerebrovascular disease | 296 (15.2) | 261 (13.7) | 456 (21.0) | 715 (14.6) |
| Psychiatric comorbidites | ||||
| Dementia | 1092 (56.2) | 947 (49.8) | 650 (30.0) | 1354 (27.7) |
| Depression | 218 (11.2) | 176 (9.3) | 459 (21.2) | 341 (7.0) |
| Delirium | 328 (16.9) | 228 (12.0) | 181 (8.3) | 277 (5.7) |
| Mood disorder | 60 (3.1) | 24 (1.3) | 80 (3.7) | 77 (1.6) |
| Psychotic disorder | 529 (27.2) | 368 (19.3) | 251 (11.6) | 537 (11.0) |
| Anxiety | 18 (0.9) | 13 (0.7) | 40 (1.8) | 42 (0.9) |
| Other comorbidities | ||||
| Diabetes | 160 (8.2) | 160 (8.4) | 297 (13.7) | 457 (9.4) |
| Parkinson disease | 85 (4.4) | 51 (2.7) | 118 (5.4) | 194 (4.0) |
| Epilepsy | 22 (1.1) | 28 (1.5) | 22 (1.0) | 49 (1.0) |
| Pneumonia | 158 (8.1) | 143 (7.5) | 197 (9.1) | 422 (8.6) |
| Chronic lung disease | 163 (8.4) | 139 (7.3) | 248 (11.4) | 498 (10.2) |
| Osteoporosis | 102 (5.3) | 77 (4.0) | 170 (7.8) | 264 (5.4) |
| Fracture | 251 (12.9) | 221 (11.6) | 293 (13.5) | 671 (13.7) |
| History of psychotropic medication use | ||||
| Atypical antipsychotics | 0 (0.0) | 230 (12.1) | 151 (7.0) | 426 (8.7) |
| Conventional antipsychotics | 122 (6.3) | 0 (0.0) | 94 (4.3) | 199 (4.1) |
| Antidepressants | 414 (21.3) | 307 (16.1) | 0 (0.0) | 767 (15.7) |
| Benzodiazepines | 369 (19.0) | 332 (17.5) | 426 (19.6) | 0 (0.0) |
| Anticonvulsants | 74 (3.8) | 63 (3.3) | 68 (3.1) | 154 (3.2) |
| Medications for Alzheimer disease | 380 (19.6) | 181 (9.5) | 178 (8.2) | 395 (8.1) |
| General indicators of comorbidity§ | ||||
| Charlson comorbidity index score, mean (SD)** | 1.20 (1.28) | 1.12 (1.28) | 1.36 (1.56) | 1.06 (1.38) |
| Admitted to hospital | 1296 (66.7) | 1242 (65.3) | 1547 (71.3) | 3091 (63.2) |
| Visited a neurologist | 145 (7.5) | 152 (8.0) | 246 (11.3) | 407 (8.3) |
| Visited a psychiatrist | 643 (33.1) | 464 (24.4) | 485 (22.4) | 657 (13.4) |
| Visited a geriatrician | 232 (11.9) | 218 (11.5) | 198 (9.1) | 344 (7.0) |
| Number of physician visits, mean (SD) | 21.0 (17.2) | 16.7 (15.0) | 22.8 (18.7) | 18.2 (15.9) |
| Number of prescription drugs, mean (SD) | 9.0 (6.2) | 7.5 (5.7) | 10.5 (6.5) | 9.4 (6.5) |
Unless otherwise indicated.
Indicates the care level used to define a patient’s functional abilities. Personal care and intermediate care 1 were assigned to patients with low-care needs, whereas intermediate care 2, intermediate care 3 and extended care levels indicate increasingly higher care requirements.
Includes other acute and subacute forms of ischemic heart disease (International Classification of Diseases-9 [ICD-9] code 411) and angina pectoris (ICD-9 code 413).
Based on the period of 180 days before the index date.
Individual comorbidities were defined based on at least one hospital admission or at least two outpatient visits with the respective ICD codes.
Rates of noncancer-related mortality, femur fracture, heart failure and stroke were lower among patients who initiated atypical antipsychotics than among those who initiated other psychotropic medications. The rate of myocardial infarction was higher (Table 2). Cardiac arrest, ventricular arrhythmia and venous thromboembolism were uncommon events, with rates well below one per 100 person-years in all groups. Owing to the low number of events observed, these event types, as well as stroke and myocardial infarction, were not included in the final analysis.
Table 2:
Death and major medical events leading to hospital admission within 180 days after start of psychotropic medication, by class of medication
| Event | Events/person-years (rate per 100 person-years) | |||
|---|---|---|---|---|
| Atypical antipsychotics*n = 1942 | Conventional antipsychotics n = 1902 | Antidepressants n = 2169 | Benzodiazepines n = 4887 | |
| Death | 181/664.3 (27.3) | 170/438.8 (38.8) | 260/790.4 (32.9) | 420/1026.0 (40.9) |
| Myocardial infarction | 15/662.5 (2.3) | 2/438.6 (0.5) | 5/790.4 (0.6) | 21/1024.8 (2.1) |
| Heart failure | 23/661.1 (3.5) | 19/437.5 (4.3) | 56/783.3 (7.2) | 103/1017.0 (10.1) |
| Cardiac arrest | 1/664.3 (0.2) | 2/438.8 (0.5) | 2/790.4 (0.3) | 1/1026.0 (0.1) |
| Ventricular arrhythmia | 0/664.3 (0.0) | 0/438.8 (0.0) | 2/790.0 (0.3) | 1/1025.8 (0.1) |
| Stroke | 8/663.8 (1.2) | 9/438.2 (2.1) | 21/788.2 (2.7) | 31/1022.6 (3.0) |
| Venous thromboembolism | 4/663.6 (0.6) | 3/438.6 (0.7) | 1/790.2 (0.1) | 2/1025.6 (0.2) |
| Pneumonia | 50/657.2 (7.6) | 43/434.0 (9.9) | 75/780.9 (9.6) | 97/1017.9 (9.5) |
| Femur fracture | 56/656.0 (8.5) | 56/431.2 (13.0) | 83/776.0 (10.7) | 99/1014.7 (9.8) |
The events and person-years presented are those used in the comparison with conventional antipsychotics (i.e., patients were censored when they discontinued the index treatment or when they switched to or added treatment with a conventional antipsychotic).
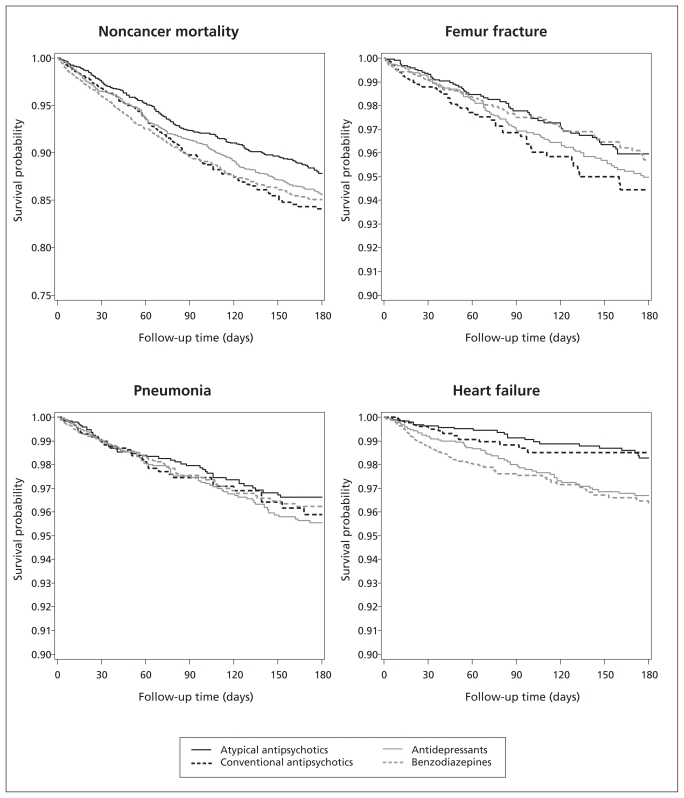
The time from treatment initiation to occurrence of events is shown in Figure 1. The corresponding rate ratios (RRs) are shown in Table 3. Patients who initiated conventional antipsychotics had a higher risk of noncancer-related death than users of atypical antipsychotics (RR 1.47, 95% CI 1.14–1.91), as well as an increased risk of femur fracture (1.61, 95% CI 1.03–2.51). The RR for pneumonia (1.03, 95% CI 0.62–1.69) and heart failure (0.91, 95% CI 0.41–2.01) were near null.
Figure 1:
Unadjusted Kaplan–Meier estimates of the probability of no events over time, by psychotropic medication class
Table 3:
Rate ratios for selected health outcomes within 180 days after initiation of therapy with psychotropic medication
| Variable | RR (95% CI) | |||
|---|---|---|---|---|
| Death | Femur fracture | Pneumonia | Heart failure | |
| Conventional v. atypical antipsychotics | ||||
| Unadjusted | 1.37 (1.11–1.69) | 1.47 (1.01–2.13) | 1.18 (0.78–1.78) | 1.18 (0.64–2.18) |
| Adjusted | ||||
| Age, sex and calendar year | 1.47 (1.13–1.90) | 1.58 (1.02–2.44) | 0.98 (0.60–1.59) | 1.32 (0.62–2.84) |
| Propensity score | 1.47 (1.14–1.91) | 1.61 (1.03–2.51) | 1.03 (0.62–1.69) | 0.91 (0.41–2.01) |
| High-dimensional propensity score | 1.52 (1.14–2.02) | 1.49 (0.93–2.41) | 0.94 (0.56–1.58) | 0.81 (0.36–1.85) |
| Propensity score adjusted by patient subgroup | ||||
| No history of either treatment* | 1.33 (0.99–1.77) | 1.67 (1.03–2.71) | 0.94 (0.55–1.63) | 0.95 (0.41–2.17) |
| Dementia† | 1.37 (0.96–1.95) | |||
| No dementia | 1.61 (1.10–2.36) | |||
| Antidepressants v. atypical antipsychotics | ||||
| Unadjusted | 1.25 (1.03–1.52) | 1.31 (0.92–1.85) | 1.31 (0.91–1.88) | 1.92 (1.19–3.07) |
| Adjusted | ||||
| Age, sex and year | 1.34 (1.10–1.64) | 1.26 (0.87– 1.81) | 1.29 (0.88–1.89) | 1.88 (1.15–3.08) |
| Propensity score | 1.20 (0.96–1.50) | 1.29 (0.86–1.94) | 1.09 (0.73–1.65) | 1.04 (0.60–1.80) |
| High-dimensional propensity score | 1.20 (0.95–1.51) | 1.24 (0.81–1.91) | 0.89 (0.58–1.39) | 0.80 (0.45–1.42) |
| Propensity score adjusted by patient subgroup | ||||
| No history of either treatment* | 1.24 (0.97–1.59) | 1.46 (0.91–2.34) | 1.24 (0.76–2.02) | 1.00 (0.53–1.88) |
| Dementia† | 1.22 (0.88–1.70) | |||
| No dementia | 1.19 (0.88–1.61) | |||
| Benzodiazepines v. atypical antipsychotics | ||||
| Unadjusted | 1.37 (1.14–1.64) | 1.13 (0.80–1.60) | 1.13 (0.79–1.61) | 2.59 (1.61–4.16) |
| Adjusted | ||||
| Age, sex and year | 1.52 (1.25–1.85) | 0.98 (0.68–1.42) | 0.99 (0.67–1.46) | 2.49 (1.51– 4.12) |
| Propensity score | 1.28 (1.04–1.58) | 0.99 (0.66–1.51) | 0.85 (0.56–1.31) | 1.54 (0.89– 2.67) |
| High-dimensional propensity score | 1.20 (0.96–1.50) | 1.13 (0.73–1.75) | 0.83 (0.54–1.28) | 1.29 (0.71–2.35) |
| Propensity score adjusted by patient subgroup | ||||
| No history of either treatment* | 1.17 (0.92–1.48) | 1.04 (0.64–1.70) | 0.78 (0.49–1.24) | 1.87 (0.98–3.57) |
| Dementia† | 1.21 (0.87–1.68) | |||
| No dementia | 1.35 (1.02–1.80) | |||
Note: CI = confidence interval, RR = incidence rate ratio.
Indicates patients without a history of use of either the referent (atypical antipsychotics) or comparator medication (conventional antipsychotics, antidepressants or benzodiazepines).
Because of the small number of events, subgroup analyses could not be run for nonmortality outcomes.
Among patients who initiated antidepressants, the associations for noncancer-related death (1.20, 95% CI 0.96–1.50) and femur fracture (1.29, 95% CI 0.86–1.94) were considerably weaker, and the associations for pneumonia (1.09, 95% CI 0.73–1.65) and heart failure (1.04, 95% CI 0.60–1.80) were near null.
Patients who initiated benzodiazepines were at increased risk for noncancer-related death (1.28, 95% CI 1.04–1.58). Virtually no difference was observed in the risk of femur fracture (0.99, 95% CI 0.66–1.51). The RR for pneumonia was 0.85 (95% CI 0.56–1.31). Users of benzodiazepines had an increased risk of admission to hospital for heart failure in the unadjusted analysis (RR 2.59). This association was reduced to 1.54 (95% CI 0.89–2.67) after adjustment for differences in pre-existing cardiovascular disease. When restricting the analyses to users of benzodiazepine medications primarily prescribed for anxiety, we confirmed the findings for noncancer-related death (1.42, 95% CI 1.12–1.81) and heart failure (1.93, 95% CI 1.03–3.60). The result for pneumonia was essentially unchanged; for femur fracture, the RR was 1.23 (95% CI 0.78–1.94).
Results from the initially-treated analysis were consistent with the findings from the as-treated analyses, although the effects tended to be attenuated (Appendix 4, available at www.cmaj.ca/cgi/content/full/cmaj.101406/DC1). The RR for non-cancer-related death did not differ meaningfully for those with and without a diagnosis of dementia. Restrictions to the subgroup of patients with no prior use of either the treatment or comparator class of medication did not meaningfully affect the associations for any of the outcomes. Likewise, in most cases, analyses using high-dimensional propensity score adjustments yielded no substantive changes. They tended to move the estimates toward the null (Table 3). Finally, by way of a sensitivity analysis, we evaluated deaths not expected to be causally related to the exposures. The distribution of causes of death is shown in Appendix 5 (available at www.cmaj.ca/cgi/content/full/cmaj.101406/DC1). Such causes included certain infectious and parasitic diseases; diseases of the blood and blood-forming organs; diseases of the digestive system; diseases of the genitourinary system; diseases of the skin and subcutaneous tissue; diseases of the musculoskeletal system and connective tissue; and symptoms, signs and abnormal clinical and laboratory findings not classified elsewhere. As expected, we observed effect estimates consistent with a null association for all three comparisons.
Interpretation
In 10 900 older adults newly admitted to nursing homes in BC who began taking psychotropic medications, we observed risks of death that were higher among those who initiated conventional antipsychotics, antidepressants and benzodiazepines. We also observed risks of femur fracture that were higher with conventional antipsychotics, antidepressants and benzodiazepines used for anxiety, all compared with atypical antipsychotics. No clinically meaningful differences were observed for risk of pneumonia or heart failure, except possibly a lower risk of pneumonia and a higher risk of heart failure with benzodiazepines.
The increased risk of noncancer-related death observed for conventional antipsychotics is consistent with earlier findings.10,11,22,23 We did not confirm the finding from an earlier study involving community-dwelling patients24 in which users of atypical agents showed a greatly increased risk of pneumonia compared with users of conventional agents. Our finding of a higher risk of femur fracture among users of conventional antipsychotics than among users of atypical agents is in line with observations made by Rochon and colleagues25 regarding admissions to hospital for falls and fractures during the first 30 days after initiation of antipsychotic treatment among nursing home residents. In contrast, similar relative increases in risk among users of atypical and conventional antipsychotics compared with nonusers were found in a case–control study.26 The risk of heart failure among users of antipsychotics and the comparative safety of anti-depressants and benzodiazepines versus atypical antipsychotics have not been examined previously.
Limitations
As with any nonrandomized study, there is potential for residual confounding. We controlled for calendar year, sociodemographic characteristics, clinical factors and health care utilization factors that were likely to be independent predictors of adverse health outcomes. Nondifferential misclassification of confounders typically leads to incomplete adjustment of confounding bias.27 No information was available on cognitive and functional impairment, or on severity of behavioural disturbances. High-dimensional proxy adjustment based on propensity score techniques was used in an effort to improve control of confounding compared with adjustment limited to predefined covariables.28
Except for mortality, there is likely to be under-ascertainment of the outcomes. Because we implemented strict disease definitions to maximize specificity, we expect the relative measures of effect to be unbiased.29 Lack of consumption of filled prescriptions could lead to misclassification of exposure status. Compliance should be high in a population of nursing home patients that is closely monitored, but occasional use might be an important source of misclassification. Despite the population-based nature of this study, its limited cohort size resulted in imprecisely estimated associations for some outcomes. Our interpretation took this into account along with competing explanations, such as uncontrolled confounding or selection biases, separating the interpretation of precision from effect size.30–32 Moreover, the finding that some of these outcomes were rare is of clinical relevance in itself. Owing to the limited cohort size, we were unable to assess the effect of individual drugs, relevant subclasses, or dose. Potential mechanisms for the observed associations remain speculative (Appendix 6, available at www.cmaj.ca/cgi/content/full/cmaj.101406/DC1).
Conclusion
Our exploratory study adds to the growing evidence that conventional antipsychotics may be no safer for vulnerable older adults than atypical antipsychotics. In addition, our findings suggest that some of the other classes of psychotropic medications may carry similar risks. While awaiting confirmation of these initial findings — ideally in the context of a large randomized trial — clinicians considering these medications for their older nursing home patients should weigh these increased risks against potential benefits when making prescribing decisions.
Supplementary Material
See related commentary by Pollock and Mulsant at www.cmaj.ca/cgi/doi/10.1503/cmaj.110348
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: All of the authors contributed substantially to the conception and design of the study and to the analysis and interpretation of the data. Krista F. Huybrechts drafted the manuscript. All of the authors revised the manuscript critically for important intellectual content and approved the final version submitted for publication.
Funding: No external funding was received for this research.
References
- 1.Wang PS, Brookhart MA, Setoguchi S, et al. Psychotropic medication use for behavioral symptoms of dementia. Curr Neurol Neurosci Rep 2006;6:490–5 [DOI] [PubMed] [Google Scholar]
- 2.Giron MST, Forsell Y, Bernsten C, et al. Psychotropic drug use in elderly people with and without dementia. Int J Geriatr Psychiatry 2001;16:900–6 [DOI] [PubMed] [Google Scholar]
- 3.Briesacher BA, Limcangco MR, Simoni-Wastila L, et al. The quality of antipsychotic drug prescribing in nursing homes. Arch Intern Med 2005;165:1280–5 [DOI] [PubMed] [Google Scholar]
- 4.Rapoport M, Mamdani M, Shulman K, et al. Antipsychotic use in the elderly: shifting trends and increasing costs. Int J Geriatr Psychiatry 2005;20:749–53 [DOI] [PubMed] [Google Scholar]
- 5.Jeste D, Sable J, Salzman C. Treatment of late-life disordered behavior, agitation, and psychosis. In: Salzman C, editor. Clinical geriatric psychopharmacology. 4th ed Philadelphia (PA): Lippincott, Williams & Wilkins; 2005. p.129–95 [Google Scholar]
- 6.Jeste DV, Blazer D, Casey D, et al. ACNP white paper: Update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology 2008;33:957–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. Silver Spring (MD): US Food and Drug Administration; 2005. Available: www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/UCM053171 (accessed 2011 Feb. 15) [Google Scholar]
- 8.Information for healthcare professionals: conventional antipsychotics. Silver Spring (MD): US Food and Drug Administration; 2008. Available: www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124830.htm (accessed 2010 May 3) [Google Scholar]
- 9.Health Canada advises consumers about important safety information on atypical antipsychotic drugs and dementia. Ottawa (ON): Health Canada; 2005. Available: www.hc-sc.gc.ca/ahc-asc/media/advisories-avis/_2005/2005_63-eng.php (accessed 2010 May 3) [Google Scholar]
- 10.Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ 2007;176:627–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gill SS, Bronskill SE, Normand S-LT, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 2007;146:775–86 [DOI] [PubMed] [Google Scholar]
- 12.Schneider LS, Tariot PN, Dagerman KS, et al. ;the CATIE-AD Study Group Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006;355:1525–38 [DOI] [PubMed] [Google Scholar]
- 13.Pollock BG, Mulsant BH, Rosen J, et al. Comparison of citalopram, perphenazine, and placebo for the acute treatment of psychosis and behavioral disturbances in hospitalized, demented patients. Am J Psychiatry 2002;159:460–5 [DOI] [PubMed] [Google Scholar]
- 14.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 2003;158:915–20 [DOI] [PubMed] [Google Scholar]
- 15.Schneeweiss S. A basic study design for expedited safety signal evaluation based on electronic healthcare data. Pharmacoepidemiol Drug Saf 2010;19:858–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol 1993;46:1075–9 [DOI] [PubMed] [Google Scholar]
- 17.Schneeweiss S, Seeger JD, Maclure M, et al. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol 2001;154:854–64 [DOI] [PubMed] [Google Scholar]
- 18.Braitman LE, Rosenbaum PR. Rare outcomes, common treatments: analytic strategies using propensity scores. Ann Intern Med 2002; 137:693–5 [DOI] [PubMed] [Google Scholar]
- 19.Brookhart MA, Schneeweiss S, Rothman KJ, et al. Variable selection for propensity score models. Am J Epidemiol 2006;163:1149–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brookhart MA, Stürmer T, Glynn RJ, Rassen J, Schneeweiss S. Confounding control in healthcare database research: challenges and potential approaches. Med Care 2010;48(6 Suppl):S114–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneeweiss S, Rassen J, Glynn R, et al. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 2009;20:512–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liperoti R, Onder G, Landi F, et al. All-cause mortality associated with atypical and conventional antipsychotics among nursing home residents with dementia: a retrospective cohort study. J Clin Psychiatry 2009;70:1340–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353:2335–41 [DOI] [PubMed] [Google Scholar]
- 24.Knol W, van Marum R, Jansen P, et al. Antipsychotic drug use and risk of pneumonia in elderly people. J Am Geriatr Soc 2008; 56:661–6 [DOI] [PubMed] [Google Scholar]
- 25.Rochon PA, Normand S-L, Gomes T, et al. Antipsychotic therapy and short-term serious events in older adults with dementia. Arch Intern Med 2008;168:1090–6 [DOI] [PubMed] [Google Scholar]
- 26.Liperoti R, Onder G, Lapane K, et al. Conventional or atypical antipsychotics and the risk of femur fracture among elderly patients: results of a case–control study. J Clin Psychiatry 2007;68:929–34 [DOI] [PubMed] [Google Scholar]
- 27.Greenland S, Robins J. Confounding and misclassification. Am J Epidemiol 1985;122:495–506 [DOI] [PubMed] [Google Scholar]
- 28.Rassen J, Glynn R, Brookhart M, Schneeweiss S. Covariate selection in high-dimensional propensity score analyses of treatment effects in small samples. Am J Epidemiol In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothman K, Greenland S, Lash T, editors. Validity in epidemiologic studies. In: Modern epidemiology. 3rd ed Philadelphia (PA): Lippincott Williams & Wilkins; 2008. p. 128–47 [Google Scholar]
- 30.Rothman KJ, Greenland S, Lash T, editors. Precision and statistics in epidemiologic studies. In: Modern epidemiology. 3rd ed Philadelphia (PA): Lippincott Williams & Wilkins; 2008. p. 148–67 [Google Scholar]
- 31.Lang JM, Rothman K, Cann C. That confounded P-value. Epidemiology 1998;9:7–8 [DOI] [PubMed] [Google Scholar]
- 32.Poole C. Beyond the confidence interval. Am J Public Health 1987;77:195–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.