Abstract
The persistence of the motivational salience of drug-related environmental cues and contexts is one of the most problematic obstacles to successful treatment of drug addiction. Behavioral approaches to extinguishing the salience of drug-associated cues, such as cue exposure therapy, have generally produced disappointing results which have been attributed to, among other things, the context specificity of extinction and inadequate consolidation of extinction learning. Extinction of any behavior or conditioned response is a process of new and active learning, and increasing evidence suggests that glutamatergic neurotransmission, a key component of the neural plasticity that underlies normal learning and memory, is also involved in extinction learning. This review will summarize findings from both animal and human studies that suggest that pharmacological enhancement of glutamatergic neurotransmission facilitates extinction learning in the context of drug addiction. Pharmacological agents that have shown potential efficacy include NMDA partial agonists, mGluR5 receptor positive allosteric modulators, inhibitors of the GlyT1 glycine transporter, AMPA receptor potentiators, and activators of the cystine-glutamate exchanger. These classes of cognition-enhancing compounds could potentially serve as novel pharmacological adjuncts to cue exposure therapy to increase success rates in attenuating cue-induced drug craving and relapse.
Keywords: Extinction, learning, glutamate, NMDA, AMPA, mGluR5, GlyT1 glycine transporter, receptor potentiator, allosteric modulator, cystine-glutamate exchanger.
INTRODUCTION
Drug addiction is a disorder of the nervous system characterized by compulsive drug intake despite negative consequences, numerous failed attempts at abstinence, and a narrowing of the behavioral repertoire towards drug-seeking and drug intake. Chronic, repeated drug use leads to the formation of powerful and lasting associations between the drug’s effects, drug-specific withdrawal symptoms, and the environmental cues and contexts that are present at the time these drug-related effects are experienced. As a result, these stimuli become overconditioned and overlearned [1], so that when experienced in the absence of the drug, they produce physiological responses (i.e., sympathetic nervous system activation) and maladaptive motivational states (i.e., drug craving). In other words, repeated drug intake causes drug-associated external stimuli to become motivationally “hypersalient” and exert significant control over the addict’s behavior [2, 3]. These drug-related stimuli are frequent triggers of drug craving, which leads to the resumption of drug-seeking behavior and ultimately relapse [2, 4-6]. Attempts at extinguishing the motivational salience of drug-associated cues and contexts by behavioral modification techniques such as cue exposure therapy have been met with limited success [7-9]. This general lack of success has been attributed to numerous factors including the high degree of context specificity of extinction [10-15] and inadequate consolidation of extinction learning [16]. Historically, most of what is known about the neural circuits and cellular and molecular mechanisms underlying extinction learning has been derived from the study of extinction of conditioned fear [17]. However, there has been a recent resurgence of interest in understanding the neural mechanisms underlying extinction of drug-related cue reactivity, and pharmacologically targeting specific components of extinction learning to reduce the ability of drug-related cues and memories to elicit drug craving and relapse [16, 18, 19]. Current evidence, although limited, suggests that many of the neural mechanisms that underlie extinction of conditioned fear and that of drug-related cue reactivity and drug-seeking behavior overlap to a significant degree [20].
Since extinction is a process of active learning, it is not surprising that many of the key neural underpinnings of extinction learning are mediated by glutamatergic neurotransmission, an established critical mediator of enduring changes in synaptic efficacy such as long-term potentiation (LTP) and long-term depression (LTD) that accompany learning [21-25]. It is therefore plausible to hypothesize that potentiation of glutamate-mediated neural plasticity might enhance various forms of learning, including extinction learning. However, since glutamatergic neurotransmission and homeostasis is tightly regulated [26, 27], and overstimulation of which can lead to neuronal hyperexcitability and excitotoxicity [27-29], pharmacological potentiation of glutamatergic transmission with the intent of enhancing extinction learning will therefore require subtle modulatory pharmacological mechanisms of action rather than full glutamate receptor agonism.
Following brief overviews of animal models of extinction of drug-seeking behavior, the neuroanatomical substrates of extinction, and glutamatergic synaptic transmission, the majority of this review will focus on pharmacological agents that enhance glutamate-mediated neurotransmission and have been shown to enhance extinction learning in animals as well as human in the context of the motivational salience of drug-related stimuli. The ultimate therapeutic goal of such pharmacological agents would be to serve as pharmacological adjuncts to cue exposure therapy to improve the meager success rates currently achieved by this technique alone. While not the focus of this review, it is worth mentioning that drugs of abuse produce lasting changes in the brain, including enduring synaptic plasticity [30-33], which have the net effect of tilting the motivation scale of addicts towards drug craving and drug-seeking behavior rather than abstinence or controlled drug intake. Therefore, therapeutic compounds that enhance extinction learning via glutamatergic mechanisms are likely to be acting on receptor, transporter, or exchanger proteins whose expression levels, subcellular distribution, or posttranslational modifications have been altered by chronic drug exposure, which may in turn increase or decrease the efficacy of a particular compound. While there is some evidence that certain therapeutic agents such as D-cycloserine (see below) facilitate extinction learning in drug addicts and patients with anxiety disorders, it is possible that other classes of compounds discussed in this review may only enhance extinction learning in subpopulations of drug addicts or only those with pathological anxiety.
Finally, a discussion of the topic of extinction would be incomplete if a brief summary of the phenomenon of reconsolidation were not provided. When the memory of a prior event is retrieved from long-term memory into working memory, the memory trace becomes labile, plastic, and susceptible to disruption or modification [34-37]. In order to maintain the memory trace, it must be reconsolidated back into long-term memory. The process of memory reconsolidation involves many of the cellular and molecular processes that underlie initial memory formation, including glutamatergic signaling, de novo protein synthesis, expression of immediate early genes, and intracellular signaling pathways related to glutamatergic transmission [16, 38]. From a therapeutic perspective, it has been hypothesized that disruption of the reconsolidation of drug-related memories may reduce their motivational influence on drug craving and relapse [16, 18, 19]. However, agents that disrupt reconsolidation are amnestic in nature, whereas some of the pharmacological strategies to enhance glutamatergic signaling in order to facilitate extinction discussed in the present are pro-mnemonic in nature. Thus, disruption of reconsolidation and enhancement of extinction learning represent two viable yet opposing strategies for reducing the influence of drug-associated stimuli and drug memories on drug-seeking and relapse. To underscore this point, Lee and colleagues [39] showed that infusions of the N-methyl-D-asparate (NMDA) glutamate receptor partial agonist D-cycloserine (DCS) into the basolateral amygdala potentiated the reconsolidation of a cocaine-related memory, where Botreau and colleagues [40] showed that the same manipulation facilitated the extinction of a cocaine conditioned place preference. Although these disparate results were likely a result of procedural differences in the timing of DCS administration (i.e., during the consolidation [40] or reconsolidation [39] time window), they highlight the importance of procedural variables, such as timing of drug administration during the targeted stage of learning and memory, that may be crucial determinants of the success of an amnestic or pro-mnemonic approach in decreasing the influence of drug-associated stimuli and drug memories on craving and relapse.
ANIMAL MODELS OF EXTINCTION OF DRUG-SEEKING BEHAVIOR
Cue exposure therapy in human drug addicts is designed to desensitize an individual’s conditioned physiological and psychological responses to drug-related stimuli as well as enhance cognitive and behavioral skills for coping with these responses. Early studies revealed that cue exposure therapy held significant potential for effective treatment of drug addiction [41-44]. However, recent meta-analyses of the effectiveness of cue exposure therapy for treatment of addiction have revealed that the success of this technique is only modest at best [7-9]. The general lack of success of cue exposure therapy alone on drug-related cue reactivity and drug craving has been attributed to numerous factors, namely the high degree of context specificity of extinction learning [7, 8, 10-12, 14, 15, 45] and inadequate consolidation of extinction learning [16]. While the context specificity of extinction learning can potentially be addressed by performing cue exposure therapy in multiple contexts [46], some investigators have shown this approach to be disappointingly ineffective [47]. With regards to increasing the consolidation of extinction learning to improve the success of cue exposure therapy, we hypothesize that this effort can be approached pharmacologically by modulating glutamatergic neurotransmission and using established animal models of addiction and extinction of drug-seeking behavior prior to moving the drug into clinical trials.
Two of the most widely used animal models of drug addiction are the conditioned place preference (CPP) and intravenous drug self-administration (IVSA) paradigm [48-52]. Both of these models are amenable to the study of extinction. In the CPP paradigm, an animal is passively administered a drug of abuse (such as cocaine) and then placed in a conditioning compartment with unique tactile, visual, and/or olfactory environmental cues for a set period of time. Drug conditioning sessions are alternated with saline conditioning, whereby physiological saline is passively administered and the animal is placed in an adjacent compartment with tactile, visual, and/or olfactory cues that are distinct from the drug conditioning compartment. With repetition of these conditioning procedures over a period of 1-2 weeks, the animal learns to associate the euphorigenic and physiological effects of the drug with the physical environment in which it is received. When given the opportunity to explore both compartments in a drug-free state, the animal will subsequently demonstrate an increased amount of time spent in the drug-paired compartment as compared to time spent in the saline-paired compartment (or as compared with the amount of time spent in the drug-paired compartment during a pre-test prior to drug conditioning). Extinction of an established CPP is accomplished either by repeatedly pairing the previously drug-paired compartment with saline, or by allowing the CPP to dissipate over a period of several weeks with repeated testing of place preference in the absence of further conditioning.
In the IVSA paradigm, an animal is trained to perform an operant response (such as pressing a lever) to obtain an infusion of a drug solution through an indwelling intravenous catheter. Drug infusions are usually accompanied by discrete auditory and/or visual stimuli, which allows for associations between discrete environmental cues and drug infusions to be formed. Following the stabilization and maintenance of drug self-administration behavior for a period of time, the behavior can be subject to extinction by replacing the drug solution with saline, or by changing the outcome of the operant response so that it no longer has any programmed consequences (i.e., drug infusion or cue presentation). During extinction training, new response-outcome contingencies are learned (i.e., the operant response no longer produces drug delivery), and since no drug reinforcer is delivered, a gradual decrease in operant responding is observed. Many researchers then proceed to administer a priming dose of the self-administered drug, expose the animal to drug-associated cues, or introduce a mild stressor, all of which can result in a resumption of responding on the lever that previously delivered the drug infusion. This is referred to as “reinstatement” of drug-seeking behavior, and is an established model of relapse. The reinstatement model has been widely used to elucidate the neural substrates of relapse-like behaviors as well as assess the potential efficacy of pharmacological compounds or behavioral manipulations that might be developed as anti-relapse treatments [53, 54]. However, relatively little attention has been given to examining the neural circuitry and neuronal adaptations underlying extinction process in the CPP and IVSA paradigms, nor has there been much attention dedicated to the study of pharmacological enhancement of extinction learning.
NEUROANATOMICAL BASIS OF EXTINCTION
As mentioned earlier, most of what is known about the neural substrates of extinction learning has been derived from the study of the extinction of conditioned fear. Intracranial neuronal recordings and microstimulation/microinjection studies in rodents, as well as brain imaging studies in humans, have revealed that prefrontal cortex (PFC), hippocampus, and amygdala are involved in the extinction of conditioned fear [17, 55-61]. More specifically, electrical stimulation of the ventromedial PFC (vmPFC, which contains the infralimbic cortex, ILC) in rodents facilitates the extinction of conditioned fear [62], and this region is activated when human subjects are exposed to fear-evoking stimuli following extinction of the fear response [59]. The vmPFC exerts inhibitory control over activity of the amygdala and its ability to generate fear responses [17]. Fear responses evoked by environmental contexts previously paired with noxious stimuli, as well as the extinction of these responses, are mediated by the hippocampus [63, 64]. Activity of the hippocampus is governed by numerous inputs including reciprocal connections with both the vmPFC and amygdala.
Emerging evidence suggests a similar regulatory role of the vmPFC in the extinction of drug-seeking behavior and drug cue reactivity [20], and glutamatergic mechanisms have also been implicated. For example, Hsu and Packard showed that blockade of NMDA receptors in the PFC inhibited the extinction of an amphetamine CPP [65], whereas Peters and colleagues demonstrated that activation of the ILC by local infusion of AMPA blocked cocaine-induced reinstatement of extinguished cocaine-seeking behavior [66]. In contrast, inactivation of the ILC actually induced the reinstatement of cocaine-seeking [66]. Similar observations were shown by Ovari and Leri, who demonstrated that inactivation of the vmPFC resulted in a re-emergence of a previously extinguished heroin CPP [67]. Since the vmPFC appears to have a facilitatory influence on extinction learning, it is possible that the dysfunction and underactivity of this and other regions of the PFC that are often observed in long-term drug users [1, 68-73] limits the ability of the addict to extinguish his or her own drug-seeking behavior.
The amygdala, particularly the basolateral region (BLA), plays a role in the extinction of the salience of drug cues. The BLA is highly implicated in the formation of associations between drug and natural rewards and discrete environmental cues [74-77], and exposure of current and former drug users to drug-related cues activates the amygdala [78-84]. As will be discussed below, infusions of glutamatergic ligands into the BLA can alter extinction of conditioned fear-related responses. It is also likely that the hippocampus is involved in the extinction of the salience of drug-related contextual cues, as inhibition of the dorsal hippocampus attenuates context-induced reinstatement of cocaine-seeking behavior [85, 86], likely via interactions with the PFC and BLA [87]. Although these studies offer a rudimentary neural circuitry of addiction-related extinction learning, clearly more studies are warranted to more accurately define a comprehensive neural circuitry that underlies the extinction of drug-seeking behavior and the hypersalience of drug-related cues.
THE GLUTAMATERGIC SYNAPSE
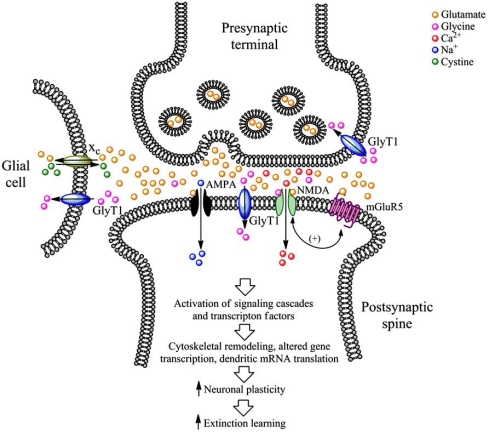
A diagram of a typical glutamatergic synapse is shown in Fig. (1). Following release into the synaptic cleft, glutamate can bind to one of three different types of ionotropic glutamate receptors (iGluRs) located on the head of the postsynaptic spine: the NMDA receptor, the α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor, and the kainic acid (kainate, KA) receptor.
Fig. (1).
The glutamatergic synapse and pharmacological targets for increasing synaptic plasticity and extinction learning. NMDA receptor function can by potentiated by activation of the glycine co-agonist binding site with the partial NMDA receptor agonist DCS or D-serine. Alternatively, NMDA receptor function can be also increased indirectly by inhibition of GlyT1 function, which elevates extracellular levels of the NMDA receptor co-agonist glycine, or by positive allosteric modulation of mGluR5, which indirectly increase NMDA receptor function. AMPA receptor activity can be enhanced with AMPA receptor potentiators. Finally, a nonspecific increase in extracellular glutamate can be obtained by stimulation of the glial cystine-glutamate exchanger (xc) with cystine derived from cystine prodrugs such as N-acetylcysteine.
NMDA receptors are heteromeric protein complexes that form ligand-gated ion channels composed of at least one NR1 subunit (for which there are at least 8 splice variants) and a combination of NR2A-D and NR3A or 3B subunits [88, 89]. In addition to being stimulated by glutamate, endogenous amino acids such as D-serine and glycine act as co-agonists at the NMDA receptor. The NR2 subunit contains the glutamate binding region, whereas the NR1 subunit contains the glycine-binding domain. Extracellular glycine levels are regulated by two subtypes of plasma membrane glycine transporters (designated GlyT1 and GlyT2). GlyT1 was originally thought to be located only on glial cells [90-91], but has recently been demonstrated to be found to be highly abundant at glutamatergic synapses, where it can be localized to postsynaptic membrane densities (see Fig. (1)) and physically interact with NMDA receptors [92]. NMDA receptors are primarily permeable to Ca2+ ions upon activation, but certain subunit combinations allow for the influx of K+ and Na+ ions as well. The NMDA receptor has been extensively implicated in mediating neural plasticity as well as learning and memory processes [25, 93, 94].
AMPA receptors are also heteromeric protein complexes that form ligand-gated ion channels. These receptors are composed of various subunits termed GluR1-4 (also termed GluRA-D) and GluRδ1 and 2 [95]. Once activated, AMPA receptors are permeable to various cations including Ca2+, Na+, and K+, although the majority of AMPA receptors in the brain contain GluR2 subunits, which render the channel impermeable to Ca2+. Both NMDA and AMPA receptors are necessary for the induction of many forms of synaptic plasticity such as LTP and LTD [96-99].
KA receptors (not shown in Fig. (1)) are ligand-gated ion channels composed of various subunits that can form homomeric tetramers composed of GluR5, 6 or 7 subunits, or heteromeric complexes containing GluR5 or KA1 or 2 subunits [95]. KA receptors are permeable to Na+ and K+ ions and, like NMDA and AMPA receptors, contribute to excitatory postsynaptic currents and synaptic plasticity [100, 101]. Although a role for KA receptors in the reinstatement of drug-seeking behavior has been suggested [26], we know of no studies published to date implicating a role for these receptors in extinction learning. However, given their role in regulating synaptic plasticity, studies examining the role of KA receptor in extinction are clearly warranted.
In addition to iGluRs, glutamate can also bind to metabotropic glutamate receptors (mGluRs), which are located either in the perisynaptic annulus or on presynaptic terminals. mGluRs are seven transmembrane domain-containing G-protein coupled receptors (GPCRs) that mediate slower, modulatory glutamatergic transmission. mGluRs are typically divided into three distinct groups, based on their pharmacological and signal transduction properties. Group I mGluR receptors (mGluR1 and mGluR5) activate the Gα q class of G-proteins [102-104]. Group I mGluRs, particularly mGluR5, are positively coupled to NMDA receptor function (see Fig. (1)) [105-107], such that activation of mGluR5 receptors increases NMDA-mediated excitatory postsynaptic activity. Group II (mGluR2 and mGluR3) and Group III (mGluR4, mGluR6, mGluR7, and mGluR8) mGluRs, activate the Gα i class of G-proteins and are negatively coupled to adenylyl cyclase (AC) activity,
Activation of iGluRs alone is sufficient for the propagation of the action potential by the postsynaptic neuron, and can activate various intracellular signaling molecules including protein kinase A (PKA), mitogen-activated protein kinase (MAPK), and extracellular signal-related kinase (ERK) [108] (see Fig. (1)). Activation of additional signaling molecules, such as protein kinase C (PKC) and release of Ca2+ ions from intracellular stores, is achieved by activation of Group I mGluRs. Together, the simultaneous activation of iGluRs and mGluRs initiates a host of intracellular signaling cascades that result in phosphorylation of ligand- and voltage-gated ion channels, activation of other protein kinases and transcription factors, which eventually lead to the molecular events underlying hallmarks of neural plasticity such as LTP and LTD [21, 23, 109-111]. Such downstream events include initiation and/or regulation of dendritic mRNA translation and localized de novo protein synthesis, changes in gene expression in the nucleus, and cytoskeletal remodeling (see Fig. (1)). Glutamate-mediated neural plasticity is also characterized by changes in glutamate receptor trafficking such as insertion of AMPA receptors into postsynaptic plasma membrane [22].
Extracellular glutamate levels are tightly regulated by a family of sodium-dependent excitatory amino acid transporters (EAATs). To date, five separate EAATs have been identified, designated EAAT1-5. EAAT1-3 are alternatively termed glutamate aspartate transporter (GLAST), glutamate transporter 1 (GLT-1), and excitatory amino acid carrier 1 (EAAC1), respectively, and are primarily (but not exclusively) localized to glial cells [112-113], whereas EAAT4 is primarily localized to neurons and EAAT5 is primarily found in the retina [114]. This family of EAATs provides numerous mechanisms to prevent an excessive accumulation of extracellular glutamate, which if high enough concentrations are reached, can result in excitotoxicity. Conversely, glutamate can be transported from within glial cells to the extracellular environment by the cystine-glutamate exchanger (xc) [115-118].
It should be noted that, in support of the notion that glutamatergic transmission is involved in extinction learning, animal studies have shown that extinction training following IVSA or CPP results in various neuroadaptive changes in numerous proteins found at glutamateric synapses. In the PFC, levels mRNA encoding the NR1 subunit of the NMDA receptor are initially elevated in the mPFC during the early phases of extinction, but later become decreased with extended extinction training [119]. Since NR1 subunits are present in all NMDA receptors, these findings suggest a general up-regulation of NMDA receptor expression occurs during the early phase of extinction, which may be a result of plastic events occurring during extinction learning. In the dorsal striatum and nucleus accumbens, which are dopaminergically innervated regions that are highly involved in mediating habit-learning, reward salience, and drug reinforcement, numerous extinction-induced neuroadaptive changes have been observed. Such changes include increases in levels of the NR1 subunit of the NMDA receptor [119-121], the GluR1 and GluR2/3 subunits of the AMPA receptor [120-122]. Conversely, extinction-induced reductions in expression of mGluR5 have been observed in these regions [123]. In the hippocampus, extinction of a morphine CPP results in an increase in phosphorylation of the serine 845 residue of the GluR1 subunit of the AMPA receptor [124]. Such findings indicate that a significant degree of plasticity in glutamatergic synaptic components in various brain regions occurs as a result of extinction training, which lends further support to the notion that extinction is an active learning process involving glutamatergic transmission.
GLUTAMATERGIC TARGETS FOR ENHANCING EXTINCTION LEARNING
Evidence reviewed so far that suggests that extinction of drug-seeking behavior and the hypersalience of drug-associated stimuli is an active learning process, and that glutamatergic transmission is a complex and critical mediator of synaptic plasticity and extinction learning. We therefore hypothesize that pharmacological enhancement of glutamatergic transmission, via subtle modulatory mechanisms that do not result in excitotoxicity, may facilitate extinction learning in drug addiction. Pharmacological compounds that increase glutamatergic transmission, many of which are either approved for use in humans by regulatory agencies or are in clinical trials for the treatment of various neurological conditions, may therefore serve as adjuncts to cue exposure therapy. We will now review five different pharmacological classes of compounds by which glutamatergic transmission can be enhanced, and associated findings from human and animal studies that support the notion that such compounds may enhance extinction learning. This review will focus primarily on compounds that are systemically active, and the chemical structures of these compounds are shown in Fig. (2). It should be noted that various compounds in several of these classes are currently in active preclinical or clinical development, but their chemical structure has not yet been revealed and are thus not shown in Fig. (2).
Fig. (2).
Chemical structures of various systemically active pharmacological agents, categorized by mechanism of action, that may increase extinction learning via subtle enhancement of glutamatergic transmission.
NMDA Receptor Partial Agonists
D-cycloserine (DCS, Fig. (2)) is a partial agonist at the N-methyl-D-aspartate (NMDA) glutamate receptor subtype that binds to the glycine co-agonist site located on the NR1 subunit, which is present in all NMDA receptors in the central nervous system. Numerous studies have shown that DCS facilitates the extinction of conditioned fear responses in animals and humans (see [125, 126] for reviews), and several recent animal studies have shown that DCS also facilitates the extinction of drug-seeking behavior. Botreau and colleagues [40] demonstrated in rats that had been administered cocaine (20 mg/kg i.p.) in the CPP paradigm, administration of DCS (15 mg/kg i.p.) immediately, but not 4 hours, following CPP extinction tests facilitated the extinction of the cocaine CPP (i.e., DCS reduced the number of test sessions required for the cocaine CPP to extinguish). The fact that the effects were observed when DCS was administered immediately following the extinction tests, and not 4 hours later, suggests that this compound facilitated the consolidation of extinction learning. These findings were independently replicated in mice by Thanos and colleagues [127].
Botreau and colleagues [40] also demonstrated that infusion of DCS directly into the BLA immediately following extinction sessions facilitated the extinction of a cocaine CPP. However, Lee and colleagues [39] found intra-BLA administration of DCS actually potentiated cue-induced reinstatement of cocaine-seeking behavior. Although Botreau and colleagues did not examine reinstatement of CPP, their findings of facilitatory effects of DCS on extinction may seem contradictory to the potentiating effects of DCS on reinstatement as observed by Lee and colleagues. These apparently discrepant findings are most likely attributable to the vastly different methodologies used in these studies. Botreau and colleagues administered DCS into the BLA immediately following extinction sessions in the CPP paradigm [40], while Lee and colleagues [39] employed an entirely different methodology. These investigators trained rats to intravenously self-administer cocaine for 10 days with standard response-contingent presentation of visual cues during drug infusions. Three days after the final self-administration session, animals were infused with DCS or vehicle into the BLA prior to a drug cue “reactivation” session, where animals were placed in the self-administration apparatus and exposed to 30 response-independent presentations of the cocaine-paired visual stimulus, which presumably reactivated the cocaine-cue “memory”. Three days following this session, animals were tested for cue-induced reinstatement of drug-seeking behavior (i.e., resumption of lever pressing that resulted in drug cue presentation but no drug delivery). The results of this experiment showed a potentiation of cue-induced reinstatement in animals receiving DCS into the BLA as compared with those receiving vehicle [39]. The authors attributed the ability of DCS to increase cue-induced cocaine-seeking to potentiation of the “reconsolidation” of the drug-cue memory. Thus, whether DCS facilitates the consolidation of extinction learning or promotes reconsolidation of drug-related memories are highly dependent on the experimental procedures used and timing of DCS administration.
DCS (15 mg/kg i.p.) was also shown to facilitate the extinction of a cocaine CPP when extinction tests were performed at intervals ranging from 3 to 14 days [128]. In addition, these investigators also demonstrated that acute administration of a priming dose of cocaine (5 mg/kg i.p.) did not reinstate cocaine CPP in animals treated with DCS during extinction, suggesting that increased consolidation of extinction learning may enable resistance to reinstatement and possibly reduce the incidence of relapse. However, Kelley and colleagues [129] found that DCS treatment failed to impair cocaine-primed reinstatement of a cocaine CPP. These discrepant findings can again be explained by methodological differences between these two studies. In the study by Kelley and colleagues [129], DCS was administered prior to CPP extinction tests (which would affect the acquisition of extinction learning), whereas in the study by Paolone and colleagues [128] the drug was administered following extinction sessions, which would affect the consolidation of extinction learning.
With regards to other drugs of abuse, DCS (30 mg/kg i.p.) administered prior to extinction testing following place conditioning with ethanol (2 g/kg i.p.) in mice was shown to be ineffective in facilitating the extinction of an ethanol CPP [130]. Combined with the findings of Kelley et al. [129] mentioned above, these data suggest that DCS, when used in CPP paradigms, may be more effective in facilitating the consolidation of extinction learning rather than its acquisition. However, an operant self-administration study where discriminative stimuli (i.e., distinct olfactory scents) were used to allow the animals to predict the availability of oral ethanol or water [131] showed that pre-extinction session administration of DCS (5 mg/kg i.p.) facilitated the extinction of lever pressing for alcohol but not water when the reinforcer was withheld. DCS treatment also reduced the magnitude of reinstatement of alcohol-seeking behavior produced by the combined presentation of the alcohol-associated discriminative stimulus and presentation of alcohol to serve a gustatory and olfactory cue. Finally, post-extinction session administration of DCS (30 mg/kg i.p.) has been shown to facilitate the extinction of operant food-seeking behavior in mice [132]. Thus, while DCS may selectively facilitate the consolidation of contextual extinction learning in CPP paradigms, there is evidence that DCS may facilitate both the acquisition and consolidation of instrumental extinction learning. Clearly more research in this area is needed to identify the ideal time points at which DCS administration produces the most robust facilitation of extinction learning.
In addition to facilitating the appetitive salience of drug-associated cues, DCS also facilitates the extinction of the conditioned aversive effects of drugs, such as drug withdrawal symptoms. It was recently demonstrated that administration of DCS (15 mg/kg i.p.) prior to naloxone-precipitated morphine withdrawal in morphine-dependent rats facilitated the extinction of a resulting conditioned place aversion [133]. While additional studies are needed to confirm that DCS facilitates the extinction of the conditioned aversive properties of abstinence-induced drug withdrawal, which is more akin to drug withdrawal experienced by human addicts, these findings are encouraging as they demonstrate that DCS can facilitate both appetitive and aversive conditioned effects of drugs of abuse. This is especially important in light of the fact that drug use in humans is motivated by negative reinforcement processes that alleviate both conditioned and unconditioned drug withdrawal symptoms [134].
While all of the aforementioned studies have been performed in rodents, there is recent evidence to suggest that DCS may not facilitate extinction in all species. A recently published study showed that, as expected, pre-extinction session administration of DCS (30 mg/kg) facilitated the extinction of operant responding for a cocaine-paired lever in rats, but had no effect no effect in monkeys [135]. Nonetheless, this pre-extinction administration of DCS was effective in reducing the reacquisition of cocaine self-administration in both species. These results suggest that relapse-related behaviors may be altered by therapeutic drug treatment during extinction despite a lack of observable effects during the extinction phase.
Similar to its cyclic analogue DCS, the endogenous NMDA receptor co-agonist D-serine (Fig. (2)) has recently been shown to affect extinction responding following drug self-administration. In rats with a history of intravenous cocaine self-administration, administration of D-serine at a dose of 100 mg/kg i.p. several hours prior to extinction training sessions facilitated the extinction of responding on the lever that previously delivered cocaine, but had no effect on the ability of a cocaine-associated cue to reinstate lever pressing [136]. This same group of investigators subsequently showed that the same dose of D-serine administered before or after extinction training in animals with a history of cocaine self-administration had no effect on responding during the first day of extinction (although only a single 90-minute extinction training session was allowed), but reduced the magnitude of reinstatement of responding induced by a priming dose of cocaine (0.5 mg/kg i.v.) [137]. Interestingly, these effects were not observed in animals that underwent one day of abstinence (i.e., no extinction training), suggesting an interaction between the experience of extinction training and the ability of D-serine to attenuate the reinstatement of cocaine-seeking behavior.
Only two reports to date have been published on the effectiveness of DCS in reducing reactivity to drug-associated cues in humans. In a study by Santa Ana and colleagues [138], 25 cigarette smokers were administered either placebo (n=13) or DCS (n=12, 50 mg orally) 1 hour prior to two smoking-related cue exposure tests. It was found that participants receiving DCS demonstrated significant reductions in physiological arousal (as measured by skin conductance) in response to presentation of smoking-related cues, as well as reductions in self-reported urges to smoke as compared with participants that received placebo. These encouraging preliminary findings suggest that DCS may be an effective adjunct to cue exposure therapy in facilitating extinction of conditioned responses to smoking-associated cues. However, another recent preliminary study conducted by Price and colleagues on the effects of DCS on cue reactivity in cocaine addicts found opposing results [139]. DCS (50 mg orally) or placebo (n=5 subjects per group) was administered 2 hours prior to cocaine-related cue reactivity tests. Surprisingly, subjects receiving DCS reported higher subjective ratings of cocaine craving during the first but not the second cue exposure test session. However, these preliminary results should be interpreted with caution, as only 5 subjects per group were used, yielding a statistical power value of 0.49, well below the standard accepted value of 0.80. In addition, statistical analysis of craving measures revealed only trends towards statistical significance, with p-values ranging from 0.06 to 0.10. Finally, in the Santa Ana study [138], smokers were required to refrain from smoking overnight prior to cue testing, whereas in the study by Price and co-workers, cocaine addicts who were also cigarette smokers were given nicotine patches to prevent nicotine craving, and the presence of nicotine in the bloodstream may have altered the subjective reports of cocaine craving. Clearly, more studies on the effects of DCS on cue reactivity with large sample sizes, subjects addicted to other drugs of abuse, different timings of DCS administration, and the use of more than two cue exposure sessions to allow a greater degree of extinction learning to occur, are needed to determine the effectiveness of DCS in reducing cue reactivity in drug addicts.
mGluR5 Positive Allosteric Modulators
While DCS and D-serine are direct partial agonists at the glycine binding site of the NMDA receptor, an alternative approach to increasing NMDA receptor function is by positive allosteric modulation of mGluR5 receptors. As shown in Fig. (1), these receptors are primarily located on the perisynaptic annulus of postsynaptic dendritic spines. mGluR5 receptors are positively coupled to NMDA receptor function, such that potentiation of mGluR5 function increases NMDA receptor-mediated postsynaptic currents. mGluR5 receptors are highly expressed in forebrain regions such as the frontal cortex, olfactory bulb, dorsal and ventral striatum, amygdala, and hippocampus [140]. These receptors are known to be critically involved in drug reward and reinforcement [141-146], synaptic plasticity [21, 23, 109-111], and learning and memory [147]. A role for mGluR5 receptors in extinction learning was recently demonstrated by Xu and colleagues [148], who demonstrated that mice lacking functional mGluR5 receptors failed to show evidence of extinction of contextual or cue conditioned fear. Thus, it is plausible (and now documented in the literature) that potentiation of mGluR5 function might actually enhance learning in certain paradigms, including extinction learning, as described below.
Positive allosteric modulators (PAMs) of mGluR5 receptor function were originally designed to indirectly increase NMDA receptor function, and therefore possibly alleviate some of the cognitive deficits associated with schizophrenia in accordance with the NMDA receptor hypofunction model of this disorder [105-107, 149-151]. The development of mGluR5 PAMs was deemed to be advantageous over developing direct orthosteric agonists of the receptor for numerous reasons, including the poor selectivity offered by orthosteric agonists due to the high degree of homology of the glutamate binding site of the receptor across mGluR subtypes, poor brain penetrance of mGluR5 orthosteric agonists, and excessive receptor stimulation which causes rapid receptor desensitization. To circumvent these issues, mGluR5 PAMs were developed, which bind to the receptor at a site that is distinct from the orthosteric glutamate binding site and increase the functioning of the receptor upon the binding of endogenous glutamate. The first mGluR5 PAMs to be developed were (1E,2E)-1,2-bis(3-fluorobenzylidene)hydrazine (DFB) [152] and N-{5-chloro-2-[(-1,3-dioxoisoindolin-2-yl)methyl] phenyl}-2-hydroxybenzamide (CPPHA) [153, 154]. Another mGluR5 PAM that has been developed is 4-nitro-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (VU-29) [155], and it was recently demonstrated that this compound enhances LTP and LTD in the hippocampal CA1 region in vitro [156], suggesting that it may have cognition-enhancing properties. Unfortunately, these compounds exhibit poor brain penetrance which make them unsuitable for behavioral studies. Nonetheless, one study did demonstrate that intracerebroventricular infusion of DFB in rats improved performance in a spatial alternation task [157].
The first report of a systemically active mGluR5 PAM was published by Lindsley and colleagues [158], who characterized the compound 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB, see Fig. (2)), which displayed antipsychotic-like properties [159-160]. Another systemically active mGluR5 PAM, (S)-(4-fluorophenyl){3-[3-(4-fluorophenyl)-1,2,4-oxadiazol-5-yl]piperidin-1-yl} methanone (ADX47273, see Fig. (2)), was also characterized and demonstrated to have antipsychotic-like properties as well [161-162]. Interestingly, however, in normal unimpaired rodents, both CDPPB and ADX47273 administered s.c. or i.p. have been shown to have pro-cognitive and pro-mnemonic effects in behavioral tasks such as the novel object recognition task [161, 163], the five-choice serial reaction time test [161], and the Morris water maze [156].
Given the pro-cognitive and pro-mnemonic effects of CDPPB, the effects of this compound on extinction learning have recently been explored. It has been found that CDPPB (3-30 mg/kg s.c.) administered prior to extinction sessions facilitated the extinction of a previously established cocaine CPP [164], and these effects were blocked by the mGluR5 receptor antagonist 3-((2-methyl-4-thiazolyl)ethynyl)pyridine (MTEP) as well as an NMDA antagonist MK-801. Other studies show that CDPPB reduces extinction responding in rats with a history of intravenous cocaine or heroin self-administration [165]. Despite its ability to indirectly enhance NMDA receptor function, repeated administration of CDPPB (30 mg/kg s.c.) did not produce evidence of neurotoxicity [164]. Although mGluR5 PAMs are primarily being developed for the treatment of schizophrenia, these data suggest that mGluR5 PAMs may also be useful in facilitating the extinction of drug-seeking behavior. It remains to be determined if they also facilitate the extinction of drug-related cue reactivity.
Glycine Transporter Inhibitors
Another method for indirectly potentiating NMDA receptor function, and in turn enhancing synaptic plasticity and potentially facilitating extinction learning, is by inhibition of the activity of plasma membrane glycine transporters (GlyTs, with subtypes designated GlyT1 and GlyT2) [166-169]. These transporters, particularly the GlyT1 subtype, locally regulate extracellular levels of the endogenous NMDA receptor co-agonist glycine [170]. Early in situ hybridization and immunohistochemistry studies showed that GlyT1 transporters were primarily localized to glial cells [90, 91], but more recently it has been demonstrated by electron microscopy with improved antisera against GlyT1 that these transporters are highly concentrated at glutamatergic synapses on both the pre- and postsynaptic membranes, and physically interact with postsynaptic NMDA receptors [92]. GlyT1 transporters are widely expressed throughout the brain, although there is some regional variation in levels of protein expression, whereas GlyT2 tranporter expression is primarily confined to the spinal cord, brainstem, and cerebellum, where it is found on presynaptic terminals and glial cells.
One of the first systemically active and selective GlyT1 inhibitors to be characterized was N-[3-([1,1-biphenyl]-4-yloxy)-3-(4-fluorophenyl)propyl]sarcosine (NFPS, also known as ALX 5407) [171]. Many other selective and brain penetrant GlyT1 inhibitors have since been synthesized and characterized, including cis-N-methyl-N-(6-methoxy-1-phenyl-1,2,3,4-tetrahydronaphthalen-2-ylmethyl)aminomethyl carboxylic acid hydrochloride (Org 25935) [172], 2-chloro-N-[1-(ethylsulfonyl)-4-isobutylpiperidin-4-yl]methyl)}-4-(tri-fluoromethyl)benzamide (Compound 5) [173, 174], [2-(4-benzo[1,3]dioxol-5-yl-2-tert-butylphenoxy)ethyl]-methyl-amino}-acetic acid (LY2365109) [175], and 2-chloro-N-[(S)-phenyl[(2S)-piperidin-2-yl] methyl]-3-trifluoromethyl benzamide (SSR504734) [176]. The chemical structures of these compounds are shown in Fig. (2).
Inhibition of GlyT1 function by either pharmacological inhibition or targeted gene deletion has been shown to facilitate associative and spatial reversal learning, working and spatial memory, and object recognition memory [177-181]. Compound 5 has been shown to attenuate the ability of nicotine to reinstate sucrose-seeking [182], although the drug was given prior to reinstatement, and its effects on extinction learning were not determined in this study. To date only one study relevant to extinction has been published on GlyT1 inhibitors and extinction. In this study, local delivery of NFPS into the amygdala of rats facilitated the extinction of conditioned fear [183], which raises the possibility that GlyT1 function may also facilitate extinction learning in the context of drug addiction. While to our knowledge no such studies have been published to date on effects of GlyT1 inhibitors in the context of drug addiction, such studies are clearly warranted based on the aforementioned results.
AMPA Receptor Potentiators
Similar to NMDA receptors, AMPA receptors are critically involved in synaptic plasticity and learning and memory processes [21-25]. Substantial effort has been devoted to developing small molecule compounds that selectively increase AMPA receptor function without inducing neuronal hyperexcitability or excitotoxicity. Such compounds have been termed “AMPAkines”, AMPA receptor potentiators, or AMPA receptor positive allosteric modulators. These compounds facilitate LTP and improve performance in learning and memory tasks in rodents such as spatial navigation, odor discrimination, non-matching-to-sample short-term memory tests, and have yielded some positive results in psychological tests in humans [184, 185]. Numerous selective and systemically active AMPA receptor potentiators have been developed for potential use as cognition-enhancing agents in disorders such as schizophrenia and Alzheimer’s disease, including 4-[2-(phenylsulfonylamino)ethylthio]-2,6-difluoro-phenoxyacetamide (PEPA) [186], 6-(piperidin-1-ylcarbonyl) quinoxaline (CX-516) [187], 2,1,3-benzoxadiazol-6-yl-piperidin-1-ylmethanone (CX-691, also known as Farampator or Org 24448) [188], and 7-chloro-3-methyl-3-4-dihydro-2H-1,2,4 benzothiadiazine S,S-dioxide (IDRA-21) [189]. Recent studies have demonstrated that PEPA facilitates the extinction of conditioned fear responses [190-191], and relevant to addiction, LaLumiere and Kalivas recently demonstrated that infusions of PEPA into the infralimbic region of the prefrontal cortex enhances the retention of extinction learning following cocaine self-administration [192]. Further support for an important role of AMPA receptors in extinction learning comes from a study by Sutton and colleagues, who showed that increasing AMPA receptor function by virally-induced overexpression of the GluR1 and GluR2 subunits of the AMPA receptor in the nucleus accumbens facilitates the extinction of cocaine-seeking behavior [122]. Thus, potentiation of AMPA receptor function may be another possible pharmacological mechanism to facilitate extinction learning in addiction.
Cystine-Glutamate Exchanger Activators
Extracellular levels of glutamate are tightly regulated by various transporter proteins, including neuronal and glial glutamate transporters (as discussed previously) and other proteins such as the cystine-glutamate exchanger (often abbreviated xc) located on glial cells (see Fig. (1)). The cystine prodrug N-acetylcysteine (NAC, see Fig. (2)) is converted to cysteine following penetration into the brain and ultimately to cystine, which stimulates xc. The resulting effect is an increase in extracellular glutamate levels that arises from cytoplasmic pools inside glial cells expressing xc. NAC is particularly effective in elevating extracellular glutamate levels when they are below normal, such as during cocaine withdrawal [117, 193]. NAC has been shown in rodents to normalize deficits in extracellular levels of glutamate in forebrain regions such as the nucleus accumbens during cocaine withdrawal, and inhibit the ability of acute cocaine exposure to reinstate cocaine-seeking behavior [118, 193-195]. NAC also reverses the metaplasticity (an alteration in the ability to induce LTP or LTD) in the nucleus accumbens following cocaine self-administration [195]. In contrast, pharmacological blockade of xc with (S)-4-carboxyphenyl-glycine (CPG) actually promotes cocaine-seeking behavior [196]. Recently, NAC has been tested in clinical trials in human addicts and has been shown to reduce cue reactivity and craving in cocaine addicts [197, 198] and reduce cigarette smoking [199].
Since NAC nonspecifically enhances glutamatergic transmission by increasing extracellular levels of this excitatory amino acid, it can potentially activate numerous postsynaptic glutamate receptors (i.e., AMPA, NMDA, mGluRs, etc.). It was recently demonstrated that the ability of NAC to restore LTP in the nucleus accumbens was due to actions of glutamate on presynaptic mGluR2/3 receptors, whereas the ability of NAC to restore LTD was due to stimulation of mGluR5 receptors [195], consistent with evidence that mGluR5 receptors mediate drug-induced alterations in synaptic plasticity [146]. Although studies on the effects of NAC on enhancement on normal learning processes are lacking, Zhou and Kalivas demonstrated that NAC reduced extinction responding following intravenous heroin self-administration in rats [200], and produced lasting reductions in the reinstatement of heroin-seeking behavior. Similar inhibitory effects of NAC on extinction responding have recently been reported in rats with a history of cocaine self-administration [201]. Thus, NAC may be a novel potential adjunct to cue exposure therapy to facilitate the extinction of cue-evoked cocaine craving as well as reducing cocaine and heroin-seeking behavior.
CONCLUSIONS
Drug-associated environmental stimuli induce drug craving, which in turn motivates drug-seeking behavior and often result in relapse. The combination of the prevalence of drug-associated cues in the addict’s day-to-day life, the context-specificity of extinction learning, and the enduring neuroadaptive changes in the brain produced by repeated drug use, create a substantial uphill battle in the development of successful treatments for drug addiction. As many common neural mechanisms underlie both normal learning and memory processes and drug addiction, targeting neural systems with either pro-cognitive or pro-mnemonic agents to facilitate extinction learning and improve cognitive abilities are potentially promising avenues for both basic and clinical research that may improve the long-term outcomes of the treatment of addiction [202]. We hypothesize that pharmacological agents that enhance glutamatergic transmission via subtle mechanisms, including NMDA receptor partial agonism, mGluR5 and AMPA receptor potentiation, GlyT1 inhibition, and cystine-glutamate exchanger activation, may be of potential benefit in enhancing synaptic plasticity and thereby facilitating extinction learning. Undoubtedly such pharmacological approaches, if devoid of toxicological or adverse side effects and approved by appropriate regulatory agencies, will have to be correctly incorporated into cue exposure therapy practices and cognitive-behavioral therapy in order to maximize their effectiveness.
ACKNOWLEDGEMENTS
RMC and MFO are supported by research grant DA024355 from the National Institute on Drug Abuse to MFO, JTG is supported by training grant AA007474 from the National Institute on Alcohol Abuse and Alcoholism, and JJW is supported by the College of Charleston. All authors have no conflicts of interest to declare.
REFERENCES
- 1.Redish AD, Jensen S, Johnson A. A unified framework for addiction: vulnerabilities in the decision process. Behav. Brain Sci. 2008;31:415–437. doi: 10.1017/S0140525X0800472X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J. Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien C, Childress A, McLellan A, Ehrman R, Ternes J. Progress in understanding the conditioning aspects of drug dependence. NIDA Res. Monogr. 1988;81:395–404. [PubMed] [Google Scholar]
- 4.Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O'Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res. Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- 5.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 6.Childress AR, McLellan AT, O'Brien CP. Role of conditioning factors in the development of drug dependence. Psychiatr. Clin. North Am. 1986;9:413–425. [PubMed] [Google Scholar]
- 7.Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- 8.Havermans RC, Jansen AT. Increasing the efficacy of cue exposure treatment in preventing relapse of addictive behavior. Addict. Behav. 2003;28:989–994. doi: 10.1016/s0306-4603(01)00289-1. [DOI] [PubMed] [Google Scholar]
- 9.Martin T, LaRowe SD, Malcolm R. Progress in cue extinction therapy for the treatment of addictive disorders: a review update. Open Addict. J. 2010;3:92–101. [Google Scholar]
- 10.Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol. Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- 11.Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol. Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 12.Bouton ME. Context and behavioral processes in extinction. Learn. Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- 13.Bouton ME, Swartzentruber D. Sources of relapse after extinction in Pavlovian and instrumental learning. Clin. Psychol. Rev. 1991;11:123–140. [Google Scholar]
- 14.Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol. Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Rescorla RA. Preservation of Pavlovian associations through extinction. Q. J. Exp. Psychol. 1996;49B:245–258. [Google Scholar]
- 16.Taylor JR, Olausson P, Quinn JJ, Torregrossa MM. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology. 2009;56(Suppl):186–195. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delgado MR, Nearing KI, LeDoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diergaarde L, Schoffelmeer AN, De Vries TJ. Pharmacological manipulation of memory reconsolidation: towards a novel treatment of pathogenic memories. Eur. J. Pharmacol. 2008;585:453–457. doi: 10.1016/j.ejphar.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Centonze D, Siracusano A, Calabresi P, Bernardi G. Removing pathogenic memories: a neurobiology of psychotherapy. Mol. Neurobiol. 2005;32:123–132. doi: 10.1385/MN:32:2:123. [DOI] [PubMed] [Google Scholar]
- 20.Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn. Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reis HJ, Guatimosim C, Paquet M, Santos M, Ribeiro FM, Kummer A, Schenatto G, Salgado JV, Vieira LB, Teixeira AL, Palotas A. Neuro-transmitters in the central nervous system & their implication in learning and memory processes. Curr. Med. Chem. 2009;16:796–840. doi: 10.2174/092986709787549271. [DOI] [PubMed] [Google Scholar]
- 22.Rao VR, Finkbeiner S. NMDA and AMPA receptors: old channels, new tricks. Trends Neurosci. 2007;30:284–291. doi: 10.1016/j.tins.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto E. Molecular mechanism of neuronal plasticity: induction and maintenance of long-term potentiation in the hippocampus. J. Pharmacol. Sci. 2006;100:433–442. doi: 10.1254/jphs.cpj06007x. [DOI] [PubMed] [Google Scholar]
- 24.Robbins TW, Murphy ER. Behavioural pharmacology: 40+ years of progress, with a focus on glutamate receptors and cognition. Trends Pharmacol. Sci. 2006;27:141–148. doi: 10.1016/j.tips.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riedel G, Platta B, Micheaub J. Glutamate receptor function in learning and memory. Behav. Brain Res. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- 26.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 27.Moulder KL, Meeks JP, Mennerick S. Homeostatic regulation of glutamate release in response to depolarization. Mol. Neurobiol. 2006;33:133–153. doi: 10.1385/MN:33:2:133. [DOI] [PubMed] [Google Scholar]
- 28.Dong XX, Wang Y, Qin ZH. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol. Sin. 2009;30:379–387. doi: 10.1038/aps.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi DW. Excitotoxic cell death. J. Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 30.Kauer JA. Learning mechanisms in addiction: synaptic plasticity in the ventral tegmental area as a result of exposure to drugs of abuse. Annu. Rev. Physiol. 2004;66:447–475. doi: 10.1146/annurev.physiol.66.032102.112534. [DOI] [PubMed] [Google Scholar]
- 31.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat. Rev. Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 32.Jones S, Bonci A. Synaptic plasticity and drug addiction. Curr. Opin. Pharmacol. 2005;5:20–25. doi: 10.1016/j.coph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Kalivas PW, O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacol. Rev. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- 34.Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat. Rev. Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- 35.Lee JL. Reconsolidation: maintaining memory relevance. Trends Neurosci. 2009;32:413–420. doi: 10.1016/j.tins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore JL, Roche RA. Reconsolidation revisited: a review and commentary on the phenomenon. Rev. Neurosci. 2007;18:365–382. doi: 10.1515/revneuro.2007.18.5.365. [DOI] [PubMed] [Google Scholar]
- 37.Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milton AL, Lee JL, Butler VJ, Gardner R, Everitt BJ. Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. J. Neurosci. 2008;28:8230–8237. doi: 10.1523/JNEUROSCI.1723-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JL, Gardner RJ, Butler VJ, Everitt BJ. D-cycloserine potentiates the reconsolidation of cocaine-associated memories. Learn. Mem. 2009;16:82–85. doi: 10.1101/lm.1186609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Botreau F, Paolone G, Stewart J. d-Cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behav. Brain Res. 2006;172:173–178. doi: 10.1016/j.bbr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Hammersley R. Cue exposure and learning theory. Addict. Behav. 1992;17:297–300. doi: 10.1016/0306-4603(92)90035-t. [DOI] [PubMed] [Google Scholar]
- 42.Heather N, Bradley BP. Cue exposure as a practical treatment for addictive disorders: why are we waiting? Addict. Behav. 1990;15:335–337. doi: 10.1016/0306-4603(90)90043-w. [DOI] [PubMed] [Google Scholar]
- 43.O'Brien CP, Childress AR, McLellan T, Ehrman R. Integrating systemic cue exposure with standard treatment in recovering drug dependent patients. Addict. Behav. 1990;15:355–365. doi: 10.1016/0306-4603(90)90045-y. [DOI] [PubMed] [Google Scholar]
- 44.Marlatt GA. Cue exposure and relapse prevention in the treatment of addictive behaviors. Addict. Behav. 1990;15:395–399. doi: 10.1016/0306-4603(90)90048-3. [DOI] [PubMed] [Google Scholar]
- 45.Tobena A, Fernandez-Teruel A, Escorihuela RM, Nunez JF, Zapata A, Ferre P, Sanchez R. Limits of habituation and extinction: implications for relapse prevention programs in addictions. Drug Alcohol Depend. 1993;32:209–217. doi: 10.1016/0376-8716(93)90085-5. [DOI] [PubMed] [Google Scholar]
- 46.Stasiewicz PR, Brandon TH, Bradizza CM. Effects of extinction context and retrieval cues on renewal of alcohol-cue reactivity among alcohol-dependent outpatients. Psychol. Addict. Behav. 2007;21:244–248. doi: 10.1037/0893-164X.21.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacKillop J, Lisman SA. Effects of a context shift and multiple context extinction on reactivity to alcohol cues. Exp. Clin. Psychopharmacol. 2008;16:322–331. doi: 10.1037/a0012686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olmstead MC. Animal models of drug addiction: where do we go from here? Q. J. Exp. Psychol. 2006;59:625–653. doi: 10.1080/17470210500356308. [DOI] [PubMed] [Google Scholar]
- 49.Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict. Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- 50.Panlilio LV, Goldberg SR. Self-administration of drugs in animals and humans as a model and an investigative tool. Addiction. 2007;102:1863–1870. doi: 10.1111/j.1360-0443.2007.02011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gardner EL. Use of animal models to develop antiaddiction medications. Curr. Psychiatry Rep. 2008;10:377–384. doi: 10.1007/s11920-008-0061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacol. Ther. 2005;108:18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 53.Epstein DH, Preston KL. The reinstatement model and relapse prevention: a clinical perspective. Psychopharmacology (Berl) 2003;168:31–41. doi: 10.1007/s00213-003-1470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacol. Rev. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol. Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 57.Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 59.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol. Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 60.Barad M, Gean PW, Lutz B. The role of the amygdala in the extinction of conditioned fear. Biol. Psychiatry. 2006;60:322–328. doi: 10.1016/j.biopsych.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 61.Sotres-Bayon F, Cain CK, LeDoux JE. Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol. Psychiatry. 2006;60:329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 62.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn. Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J. Neurosci. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J. Neurosci. 2001;21:1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hsu E, Packard MG. Medial prefrontal cortex infusions of bupivacaine or AP-5 block extinction of amphetamine conditioned place preference. Neurobiol. Learn. Mem. 2007;89:504–512. doi: 10.1016/j.nlm.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 66.Peters J, Lalumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J. Neurosci. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ovari J, Leri F. Inactivation of the ventromedial prefrontal cortex mimics re-emergence of heroin seeking caused by heroin reconditioning. Neurosci. Lett. 2008;444:52–55. doi: 10.1016/j.neulet.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 68.Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 69.Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol. Biochem. Behav. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kalivas PW. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox. Res. 2008;14:185–189. doi: 10.1007/BF03033809. [DOI] [PubMed] [Google Scholar]
- 71.Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch. Neurol. 2007;64:1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- 72.Everitt BJ, Hutcheson DM, Ersche KD, Pelloux Y, Dalley JW, Robbins TW. The orbital prefrontal cortex and drug addiction in laboratory animals and humans. Ann. N. Y. Acad. Sci. 2007;1121:576–597. doi: 10.1196/annals.1401.022. [DOI] [PubMed] [Google Scholar]
- 73.Schoenbaum G, Shaham Y. The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biol. Psychiatry. 2008;63:256–262. doi: 10.1016/j.biopsych.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Everitt BJ, Morris KA, O'Brien A, Robbins TW. The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience. 1991;42:1–18. doi: 10.1016/0306-4522(91)90145-e. [DOI] [PubMed] [Google Scholar]
- 75.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum and prefrontal cortex. Neurosci. Biobehav. Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 76.Baxter MG, Murray EA. The amygdala and reward. Nat. Rev. Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 77.Olive MF. Role of the amygdala in drug-related memories. Cell Sci. 2009;6:87–103. [Google Scholar]
- 78.Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- 79.Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am. J. Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc. Natl. Acad. Sci. USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kilts CD. Imaging the roles of the amygdala in drug addiction. Psychopharmacol. Bull. 2001;35:84–94. [PubMed] [Google Scholar]
- 82.Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch. Gen. Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- 83.Brieter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 84.Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, Detre JA, Lerman C. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J. Neurosci. 2007;27:14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- 86.Ramirez DR, Bell GH, Lasseter HC, Xie X, Traina SA, Fuchs RA. Dorsal hippocampal regulation of memory reconsolidation processes that facilitate drug context-induced cocaine-seeking behavior in rats. Eur. J. Neurosci. 2009;30:901–912. doi: 10.1111/j.1460-9568.2009.06889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur. J. Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- 88.Stephenson FA. Structure and trafficking of NMDA and GABAA receptors. Biochem. Soc. Trans. 2006;34:877–881. doi: 10.1042/BST0340877. [DOI] [PubMed] [Google Scholar]
- 89.Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr. Opin. Pharmacol. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 90.Zafra F, Aragon C, Olivares L, Danbolt NC, Gimenez C, Storm-Mathisen J. Glycine transporters are differentially expressed among CNS cells. J. Neurosci. 1995;15:3952–3969. doi: 10.1523/JNEUROSCI.15-05-03952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zafra F, Gomeza J, Olivares L, Aragon C, Gimenez C. Regional distribution and developmental variation of the glycine transporters GLYT1 and GLYT2 in the rat CNS. Eur. J. Neurosci. 1995;7:1342–1352. doi: 10.1111/j.1460-9568.1995.tb01125.x. [DOI] [PubMed] [Google Scholar]
- 92.Cubelos B, Gimenez C, Zafra F. Localization of the GLYT1 glycine transporter at glutamatergic synapses in the rat brain. Cereb. Cortex. 2005;15:448–459. doi: 10.1093/cercor/bhh147. [DOI] [PubMed] [Google Scholar]
- 93.Castellano C, Cestari V, Ciamei A. NMDA receptors and learning and memory processes. Curr. Drug Targets. 2001;2:273–283. doi: 10.2174/1389450013348515. [DOI] [PubMed] [Google Scholar]
- 94.Perez-Otano I, Ehlers MD. Homeostatic plasticity and NMDA receptor trafficking. Trends Neurosci. 2005;28:229–238. doi: 10.1016/j.tins.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 95.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol. Rev. 1999;51:7–62. [PubMed] [Google Scholar]
- 96.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 97.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 98.Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30:126–134. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 99.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat. Rev. Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 100.Bortolotto ZA, Nistico R, More JC, Jane DE, Collingridge GL. Kainate receptors and mossy fiber LTP. Neurotoxicology. 2005;26:769–777. doi: 10.1016/j.neuro.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 101.Jane DE, Lodge D, Collingridge GL. Kainate receptors: pharmacology, function and therapeutic potential. Neuropharmacology. 2009;56:90–113. doi: 10.1016/j.neuropharm.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 102.Conn PJ, Pin J-P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 103.Hermans E, Challiss RA. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochem. J. 2001;359:465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Coutinho V, Knopfel T. Metabotropic glutamate receptors: electrical and chemical signaling properties. Neuroscientist. 2002;8:551–561. doi: 10.1177/1073858402238514. [DOI] [PubMed] [Google Scholar]
- 105.Conn PJ, Lindsley CW, Jones CK. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends. Pharmacol. Sci. 2009;30:25–31. doi: 10.1016/j.tips.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marino MJ, Conn PJ. Direct and indirect modulation of the N-methyl D-aspartate receptor: potential for the development of novel antipsychotic therapies. Curr. Drug Targets CNS Neurol. Disord. 2002;1:1–16. doi: 10.2174/1568007023339544. [DOI] [PubMed] [Google Scholar]
- 107.Marino MJ, Conn PJ. Glutamate-based therapeutic approaches: allosteric modulators of metabotropic glutamate receptors. Curr. Opin. Pharmacol. 2006;6:98–102. doi: 10.1016/j.coph.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 108.Ron D, Jurd R. The "ups and downs" of signaling cascades in addiction. Sci. STKE. 2005;2005 doi: 10.1126/stke.3092005re14. re14. [DOI] [PubMed] [Google Scholar]
- 109.Bellone C, Luscher C, Mameli M. Mechanisms of synaptic depression triggered by metabotropic glutamate receptors. Cell. Mol. Life Sci. 2008;65:2913–2923. doi: 10.1007/s00018-008-8263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gladding CM, Fitzjohn SM, Molnar E. Metabotropic glutamate receptor-mediated long-term depression: molecular mechanisms. Pharmacol. Rev. 2009;61:395–412. doi: 10.1124/pr.109.001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Anwyl R. Metabotropic glutamate receptor-dependent long-term potentiation. Neuropharmacology. 2009;56:735–740. doi: 10.1016/j.neuropharm.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 112.Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- 113.Shigeri Y, Seal RP, Shimamoto K. Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Res. Rev. 2004;45:250–265. doi: 10.1016/j.brainresrev.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 114.Danbolt NC. Glutamate uptake. Prog. Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 115.Melendez RI, Vuthiganon J, Kalivas PW. Regulation of extracellular glutamate in the prefrontal cortex: focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. J. Pharmacol. Exp. Ther. 2005;314:139–147. doi: 10.1124/jpet.104.081521. [DOI] [PubMed] [Google Scholar]
- 116.McBean GJ. Cerebral cystine uptake: a tale of two transporters. Trends Pharmacol. Sci. 2002;23:299–302. doi: 10.1016/s0165-6147(02)02060-6. [DOI] [PubMed] [Google Scholar]
- 117.Baker DA, Shen H, Kalivas PW. Cystine/glutamate exchange serves as the source for extracellular glutamate: modifications by repeated cocaine administration. Amino Acids. 2002;23:161–162. doi: 10.1007/s00726-001-0122-6. [DOI] [PubMed] [Google Scholar]
- 118.Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J. Neurosci. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Crespo JA, Oliva JM, Ghasemzadeh MB, Kalivas PW, Ambrosio E. Neuroadaptive changes in NMDAR1 gene expression after extinction of cocaine self-administration. Ann. N. Y. Acad. Sci. 2002;965:78–91. doi: 10.1111/j.1749-6632.2002.tb04153.x. [DOI] [PubMed] [Google Scholar]
- 120.Ghasemzadeh MB, Vasudevan P, Mueller C, Seubert C, Mantsch JR. Region specific alterations in glutamate receptor expression and subcellular distribution following extinction of cocaine self-administration. Brain Res. 2009;1267:89–102. doi: 10.1016/j.brainres.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 121.Self DW, Choi KH, Simmons D, Walker JR, Smagula CS. Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learn. Mem. 2004;11:648–657. doi: 10.1101/lm.81404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sutton MA, Schmidt EF, Choi K-H, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- 123.Ghasemzadeh MB, Vasudevan P, Mueller C, Seubert C, Mantsch JR. Neuroadaptations in the cellular and postsynaptic group 1 metabotropic glutamate receptor mGluR5 and Homer proteins following extinction of cocaine self-administration. Neurosci. Lett. 2009;452:167–171. doi: 10.1016/j.neulet.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Billa SK, Sinha N, Rudrabhatla SR, Moron JA. Extinction of morphine-dependent conditioned behavior is associated with increased phosphorylation of the GluR1 subunit of AMPA receptors at hippocampal synapses. Eur. J. Neurosci. 2008;29:55–64. doi: 10.1111/j.1460-9568.2008.06560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol. Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 126.Vervliet B. Learning and memory in conditioned fear extinction: effects of d-cycloserine. Acta Psychol. (Amst) 2008;127:601–613. doi: 10.1016/j.actpsy.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 127.Thanos PK, Bermeo C, Wang GJ, Volkow ND. D-Cycloserine accelerates the extinction of cocaine-induced conditioned place preference in C57BL/c mice. Behav. Brain Res. 2009;199:345–349. doi: 10.1016/j.bbr.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Paolone G, Botreau F, Stewart J. The facilitative effects of D-cycloserine on extinction of a cocaine-induced conditioned place preference can be long lasting and resistant to reinstatement. Psychopharmacology (Berl) 2008;202:403–409. doi: 10.1007/s00213-008-1280-y. [DOI] [PubMed] [Google Scholar]
- 129.Kelley JB, Anderson KL, Itzhak Y. Long-term memory of cocaine-associated context: disruption and reinstatement. Neuroreport. 2007;18:777–780. doi: 10.1097/WNR.0b013e3280c1e2e7. [DOI] [PubMed] [Google Scholar]
- 130.Groblewski PA, Lattal KM, Cunningham CL. Effects of D-cycloserine on extinction and reconditioning of ethanol-seeking behavior in mice. Alcohol. Clin. Exp. Res. 2009;33:772–782. doi: 10.1111/j.1530-0277.2009.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vengeliene V, Kiefer F, Spanagel R. D-cycloserine facilitates the extinction of conditioned alcohol-seeking behaviour in rats. Alcohol Alcohol. 2008;43:626–629. doi: 10.1093/alcalc/agn067. [DOI] [PubMed] [Google Scholar]
- 132.Shaw D, Norwood K, Sharp K, Quigley L, McGovern SF, Leslie JC. Facilitation of extinction of operant behaviour in mice by D-cycloserine. Psychopharmacology (Berl) 2008;202:397–402. doi: 10.1007/s00213-008-1312-7. [DOI] [PubMed] [Google Scholar]
- 133.Myers KM, Carlezon WA., Jr D-cycloserine facilitates extinction of naloxone-induced conditioned place aversion in morphine-dependent rats. Biol. Psychiatry. 2010;67:85–87. doi: 10.1016/j.biopsych.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol. Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- 135.Nic Dhonnchadha BA, Szalay JJ, Achat-Mendes C, Platt DM, Otto MW, Spealman RD, Kantak KM. D-cycloserine deters reacquisition of cocaine self-administration by augmenting extinction learning. Neuropsychopharmacology. 2010;35:357–367. doi: 10.1038/npp.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kelamangalath L, Swant J, Stramiello M, Wagner JJ. The effects of extinction training in reducing the reinstatement of drug-seeking behavior: involvement of NMDA receptors. Behav. Brain Res. 2007;185:119–128. doi: 10.1016/j.bbr.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kelamangalath L, Seymour CM, Wagner JJ. D-serine facilitates the effects of extinction to reduce cocaine-primed reinstatement of drug-seeking behavior. Neurobiol. Learn. Mem. 2009;92:544–551. doi: 10.1016/j.nlm.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Santa Ana EJ, Rounsaville BJ, Frankforter TL, Nich C, Babuscio T, Poling J, Gonsai K, Hill KP, Carroll KM. D-Cycloserine attenuates reactivity to smoking cues in nicotine dependent smokers: a pilot investigation. Drug Alcohol Depend. 2009;104:220–227. doi: 10.1016/j.drugalcdep.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Price KL, McRae-Clark AL, Saladin ME, Maria MM, DeSantis SM, Back SE, Brady KT. D-cycloserine and cocaine cue reactivity: preliminary findings. Am. J. Drug Alcohol Abuse. 2009;35:434–438. doi: 10.3109/00952990903384332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci. Lett. 1993;163:53–57. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- 141.Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat. Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- 142.Kenny PJ, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol. Sci. 2004;25:265–272. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 143.Olive MF. mGlu5 receptors: neuroanatomy, pharmacology, and role in drug addiction. Curr. Psychiatry Rev. 2005;1:197–214. [Google Scholar]
- 144.Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for drug addiction. Curr. Drug Abuse Rev. 2009;2:83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bird MK, Lawrence AJ. The promiscuous mGlu5 receptor - a range of partners for therapeutic possibilities? Trends Pharmacol. Sci. 2009;30:617–623. doi: 10.1016/j.tips.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 146.Bird MK, Lawrence AJ. Group I metabotropic glutamate receptors: involvement in drug-seeking and drug-induced plasticity. Curr. Mol. Pharmacol. 2009;2:83–94. doi: 10.2174/1874467210902010083. [DOI] [PubMed] [Google Scholar]
- 147.Simonyi A, Schachtman TR, Christoffersen GR. The role of metabotropic glutamate receptor 5 in learning and memory processes. Drug News Perspect. 2005;18:353–361. doi: 10.1358/dnp.2005.18.6.927927. [DOI] [PubMed] [Google Scholar]
- 148.Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning. J. Neurosci. 2009;29:3676–3684. doi: 10.1523/JNEUROSCI.5716-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat. Rev. Drug Discov. 2009;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Conn PJ, Tamminga C, Schoepp DD, Lindsley C. Schizophrenia: moving beyond monoamine antagonists. Mol. Interv. 2008;8:99–107. doi: 10.1124/mi.8.2.7. [DOI] [PubMed] [Google Scholar]
- 151.Chen Y, Conn PJ. mGluR5 positive allosteric modulators. Drugs Fut. 2008;33:355–360. [Google Scholar]
- 152.O'Brien JA, Lemaire W, Chen TB, Chang RS, Jacobson MA, Ha SN, Lindsley CW, Schaffhauser HJ, Sur C, Pettibone DJ, Conn PJ, Williams DL., Jr A family of highly selective allosteric modulators of the metabotropic glutamate receptor subtype 5. Mol. Pharmacol. 2003;64:731–740. doi: 10.1124/mol.64.3.731. [DOI] [PubMed] [Google Scholar]
- 153.O'Brien JA, Lemaire W, Wittmann M, Jacobson MA, Ha SN, Wisnoski DD, Lindsley CW, Schaffhauser HJ, Rowe B, Sur C, Duggan ME, Pettibone DJ, Conn PJ, Williams DL. A novel selective allosteric modulator potentiates the activity of native metabotropic glutamate receptor subtype 5 (mGluR5) in rat forebrain. J. Pharmacol. Exp. Ther. 2004;309:568–577. doi: 10.1124/jpet.103.061747. [DOI] [PubMed] [Google Scholar]
- 154.Zhao Z, Wisnoski DD, O'Brien JA, Lemaire W, Williams DL, Jr, Jacobson MA, Wittman M, Ha SN, Schaffhauser H, Sur C, Pettibone DJ, Duggan ME, Conn PJ, Hartman GD, Lindsley CW. Challenges in the development of mGluR5 positive allosteric modulators: the discovery of CPPHA. Bioorg. Med. Chem. Lett. 2007;17:1386–1391. doi: 10.1016/j.bmcl.2006.11.081. [DOI] [PubMed] [Google Scholar]
- 155.de Paulis T, Hemstapat K, Chen Y, Zhang Y, Saleh S, Alagille D, Baldwin RM, Tamagnan GD, Conn PJ. Substituent effects of N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamides on positive allosteric modulation of the metabotropic glutamate-5 receptor in rat cortical astrocytes. J. Med. Chem. 2006;49:3332–3344. doi: 10.1021/jm051252j. [DOI] [PubMed] [Google Scholar]
- 156.Ayala JE, Chen Y, Banko JL, Sheffler DJ, Williams R, Telk AN, Watson NL, Xiang Z, Zhang Y, Jones PJ, Lindsley CW, Olive MF, Conn PJ. mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology. 2009;34:2057–2071. doi: 10.1038/npp.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Balschun D, Zuschratter W, Wetzel W. Allosteric enhancement of metabotropic glutamate receptor 5 function promotes spatial memory. Neuroscience. 2006;142:691–702. doi: 10.1016/j.neuroscience.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 158.Lindsley CW, Wisnoski DD, Leister WH, O'Brien J A, Lemaire W, Williams DL, Jr, Burno M, Sur C, Kinney GG, Pettibone DJ, Tiller PR, Smith S, Duggan ME, Hartman GD, Conn PJ, Huff JR. Discovery of positive allosteric modulators for the metabotropic glutamate receptor subtype 5 from a series of N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamides that potentiate receptor function in vivo. J. Med. Chem. 2004;47:5825–5828. doi: 10.1021/jm049400d. [DOI] [PubMed] [Google Scholar]
- 159.Kinney GG, O'Brien JA, Lemaire W, Burno M, Bickel DJ, Clements MK, Chen TB, Wisnoski DD, Lindsley CW, Tiller PR, Smith S, Jacobson MA, Sur C, Duggan ME, Pettibone DJ, Conn PJ, Williams DL., Jr A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic-like effects in rat behavioral models. J. Pharmacol. Exp. Ther. 2005;313:199–206. doi: 10.1124/jpet.104.079244. [DOI] [PubMed] [Google Scholar]
- 160.Homayoun H, Moghaddam B. Orbitofrontal cortex neurons as a common target for classic and glutamatergic antipsychotic drugs. Proc. Natl. Acad. Sci. USA. 2008;105:18041–18046. doi: 10.1073/pnas.0806669105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu F, Grauer S, Kelley C, Navarra R, Graf R, Zhang G, Atkinson PJ, Wantuch C, Popiolek M, Day M, Khawaja X, Smith D, Olsen M, Kouranova E, Gilbert A, Lai M, Pausch MH, Pruthi F, Pulicicchio C, Brandon NJ, Comery TA, Beyer CE, Logue S, Rosenzweig-Lipson S, Marquis KL. ADX47273: a novel metabotropic glutamate receptor 5 selective positive allosteric modulator with preclinical antipsychotic-like and pro-cognitive activities. J. Pharmacol. Exp. Ther. 2008;327:827–839. doi: 10.1124/jpet.108.136580. [DOI] [PubMed] [Google Scholar]
- 162.Schlumberger C, Pietraszek M, Gravius A, Klein KU, Greco S, More L, Danysz W. Comparison of the mGlu5 receptor positive allosteric modulator ADX47273 and the mGlu2/3 receptor agonist LY354740 in tests for antipsychotic-like activity. Eur. J. Pharmacol. 2009;623:73–83. doi: 10.1016/j.ejphar.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 163.Uslaner JM, Parmentier-Batteur S, Flick RB, Surles NO, Lam JS, McNaughton CH, Jacobson MA, Hutson PH. Dose-dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition memory is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology. 2009;57:531–538. doi: 10.1016/j.neuropharm.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 164.Gass JT, Olive MF. Positive allosteric modulation of mGluR5 receptors facilitates extinction of a cocaine contextual memory. Biol. Psychiatry. 2009;65:717–720. doi: 10.1016/j.biopsych.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Olive MF, Wischerath KC, Cleva RM, Gass JT. Positive allosteric modulation of mGluR5 receptors decreases extinction responding following intravenous heroin and cocaine self-administration: Correlation with hippocampal neurogenesis. Society for Neuroscience Meeting Planner, Chicago, IL, 2009, Program No. 348.11. Online
- 166.Gomeza J, Ohno K, Betz H. Glycine transporter isoforms in the mammalian central nervous system: structures, functions and therapeutic promises. Curr. Opin. Drug Discov. Dev. 2003;6:675–682. [PubMed] [Google Scholar]
- 167.Sur C, Kinney GG. Glycine transporter 1 inhibitors and modulation of NMDA receptor-mediated excitatory neurotransmission. Curr. Drug Targets. 2007;8:643–649. doi: 10.2174/138945007780618535. [DOI] [PubMed] [Google Scholar]
- 168.Sur C, Kinney GG. The therapeutic potential of glycine transporter-1 inhibitors. Expert. Opin. Investig. Drugs. 2004;13:515–521. doi: 10.1517/13543784.13.5.515. [DOI] [PubMed] [Google Scholar]
- 169.Yang CR, Svensson KA. Allosteric modulation of NMDA receptor via elevation of brain glycine and D-serine: the therapeutic potentials for schizophrenia. Pharmacol. Ther. 2008;120:317–332. doi: 10.1016/j.pharmthera.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 170.Betz H, Gomeza J, Armsen W, Scholze P, Eulenburg V. Glycine transporters: essential regulators of synaptic transmission. Biochem. Soc. Trans. 2006;34:55–58. doi: 10.1042/BST0340055. [DOI] [PubMed] [Google Scholar]
- 171.Aubrey KR, Vandenberg RJ. N[3-(4'-fluorophenyl)-3-(4'-phenylphenoxy)propyl]sarcosine (NFPS) is a selective persistent inhibitor of glycine transport. Br. J. Pharmacol. 2001;134:1429–1436. doi: 10.1038/sj.bjp.0704381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Walker GB, Hamilton W, Ge J, Bruin J, Shahid M, Hill D. ORG 25935: a selective glycine uptake inhibitor. Schizophrenia Res. 2001;49 (Suppl 1):97. [Google Scholar]
- 173.Wolkenberg SE, Zhao Z, Wisnoski DD, Leister WH, O'Brien J, Lemaire W, Williams DL, Jr, Jacobson MA, Sur C, Kinney GG, Pettibone DJ, Tiller PR, Smith S, Gibson C, Ma BK, Polsky-Fisher SL, Lindsley CW, Hartman GD. Discovery of GlyT1 inhibitors with improved pharmacokinetic properties. Bioorg. Med. Chem. Lett. 2009;19:1492–1495. doi: 10.1016/j.bmcl.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 174.Zhao Z, Leister WH, O'Brien JA, Lemaire W, Williams DL, Jr, Jacobson MA, Sur C, Kinney GG, Pettibone DJ, Tiller PR, Smith S, Hartman GD, Lindsley CW, Wolkenberg SE. Discovery of N-{[1-(propylsulfonyl)-4-pyridin-2-ylpiperidin-4-yl]methyl}benzamides as novel, selective and potent GlyT1 inhibitors. Bioorg. Med. Chem. Lett. 2009;19:1488–1491. doi: 10.1016/j.bmcl.2008.12.115. [DOI] [PubMed] [Google Scholar]
- 175.Perry KW, Falcone JF, Fell MJ, Ryder JW, Yu H, Love PL, Katner J, Gordon KD, Wade MR, Man T, Nomikos GG, Phebus LA, Cauvin AJ, Johnson KW, Jones CK, Hoffmann BJ, Sandusky GE, Walter MW, Porter WJ, Yang L, Merchant KM, Shannon HE, Svensson KA. Neurochemical and behavioral profiling of the selective GlyT1 inhibitors ALX5407 and LY2365109 indicate a preferential action in caudal vs. cortical brain areas. Neuropharmacology. 2008;55:743–754. doi: 10.1016/j.neuropharm.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 176.Depoortere R, Dargazanli G, Estenne-Bouhtou G, Coste A, Lanneau C, Desvignes C, Poncelet M, Heaulme M, Santucci V, Decobert M, Cudennec A, Voltz C, Boulay D, Terranova JP, Stemmelin J, Roger P, Marabout B, Sevrin M, Vige X, Biton B, Steinberg R, Francon D, Alonso R, Avenet P, Oury-Donat F, Perrault G, Griebel G, George P, Soubrie P, Scatton B. Neurochemical, electrophysiological and pharmacological profiles of the selective inhibitor of the glycine transporter-1 SSR504734, a potential new type of antipsychotic. Neuropsychopharmacology. 2005;30:1963–1985. doi: 10.1038/sj.npp.1300772. [DOI] [PubMed] [Google Scholar]
- 177.Yee BK, Balic E, Singer P, Schwerdel C, Grampp T, Gabernet L, Knuesel I, Benke D, Feldon J, Mohler H, Boison D. Disruption of glycine transporter 1 restricted to forebrain neurons is associated with a procognitive and antipsychotic phenotypic profile. J. Neurosci. 2006;26:3169–3181. doi: 10.1523/JNEUROSCI.5120-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Singer P, Feldon J, Yee BK. The glycine transporter 1 inhibitor SSR504734 enhances working memory performance in a continuous delayed alternation task in C57BL/6 mice. Psychopharmacology (Berl) 2009;202:371–384. doi: 10.1007/s00213-008-1286-5. [DOI] [PubMed] [Google Scholar]
- 179.Singer P, Boison D, Mohler H, Feldon J, Yee BK. Enhanced recognition memory following glycine transporter 1 deletion in forebrain neurons. Behav. Neurosci. 2007;121:815–825. doi: 10.1037/0735-7044.121.5.815. [DOI] [PubMed] [Google Scholar]
- 180.Singer P, Boison D, Mohler H, Feldon J, Yee BK. Deletion of glycine transporter 1 (GlyT1) in forebrain neurons facilitates reversal learning: enhanced cognitive adaptability? Behav. Neurosci. 2009;123:1012–1027. doi: 10.1037/a0016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tsai G, Ralph-Williams RJ, Martina M, Bergeron R, Berger-Sweeney J, Dunham KS, Jiang Z, Caine SB, Coyle JT. Gene knockout of glycine transporter 1: characterization of the behavioral phenotype. Proc. Natl. Acad. Sci. USA. 2004;101:8485–8490. doi: 10.1073/pnas.0402662101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Uslaner JM, Drott JT, Sharik SS, Theberge CR, Sur C, Zeng Z, Williams DL, Hutson PH. Inhibition of glycine transporter 1 attenuates nicotine- but not food-induced cue-potentiated reinstatement for a response previously paired with sucrose. Behav. Brain Res. 2010;207:37–43. doi: 10.1016/j.bbr.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 183.Mao SC, Lin HC, Gean PW. Augmentation of fear extinction by infusion of glycine transporter blockers into the amygdala. Mol. Pharmacol. 2009;76:369–378. doi: 10.1124/mol.108.053728. [DOI] [PubMed] [Google Scholar]
- 184.Arai AC, Kessler M. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr. Drug Targets. 2007;8:583–602. doi: 10.2174/138945007780618490. [DOI] [PubMed] [Google Scholar]
- 185.Morrow JA, Maclean JK, Jamieson C. Recent advances in positive allosteric modulators of the AMPA receptor. Curr. Opin. Drug Discov. Dev. 2006;9:571–579. [PubMed] [Google Scholar]
- 186.Sekiguchi M, Fleck MW, Mayer ML, Takeo J, Chiba Y, Yamashita S, Wada K. A novel allosteric potentiator of AMPA receptors: 4--2-(phenylsulfonylamino)ethylthio--2,6-difluorophenoxyaceta mide. J. Neurosci. 1997;17:5760–5771. doi: 10.1523/JNEUROSCI.17-15-05760.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Danysz W. CX-516 Cortex pharmaceuticals. Curr. Opin. Investig. Drugs. 2002;3:1081–1088. [PubMed] [Google Scholar]
- 188.Woolley ML, Waters KA, Gartlon JE, Lacroix LP, Jennings C, Shaughnessy F, Ong A, Pemberton DJ, Harries MH, Southam E, Jones DN, Dawson LA. Evaluation of the pro-cognitive effects of the AMPA receptor positive modulator, 5-(1-piperidinylcarbonyl)-2,1,3-benzoxadiazole (CX691), in the rat. Psychopharmacology (Berl) 2009;202:343–354. doi: 10.1007/s00213-008-1325-2. [DOI] [PubMed] [Google Scholar]
- 189.Zivkovic I, Thompson DM, Bertolino M, Uzunov D, DiBella M, Costa E, Guidotti A. 7-Chloro-3-methyl-3-4-dihydro-2H-1,2,4 benzothiadiazine S,S-dioxide (IDRA 21): a benzothiadiazine derivative that enhances cognition by attenuating DL-alpha-amino-2,3-dihydro-5-methyl-3-oxo-4-isoxazolepropanoic acid (AMPA) receptor desensitization. J. Pharmacol. Exp. Ther. 1995;272:300–309. [PubMed] [Google Scholar]
- 190.Yamada D, Zushida K, Wada K, Sekiguchi M. Pharmacological discrimination of extinction and reconsolidation of contextual fear memory by a potentiator of AMPA receptors. Neuropsychopharmacology. 2009;34:2574–2584. doi: 10.1038/npp.2009.86. [DOI] [PubMed] [Google Scholar]
- 191.Zushida K, Sakurai M, Wada K, Sekiguchi M. Facilitation of extinction learning for contextual fear memory by PEPA: a potentiator of AMPA receptors. J. Neurosci. 2007;27:158–166. doi: 10.1523/JNEUROSCI.3842-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn. Mem. 2010;17:168–175. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat. Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 194.Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, Grier MD, Baker DA. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J. Neurosci. 2007;27:13968–13976. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat. Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kau KS, Madayag A, Mantsch JR, Grier MD, Abdulhameed O, Baker DA. Blunted cystine-glutamate antiporter function in the nucleus accumbens promotes cocaine-induced drug seeking. Neuroscience. 2008;155:530–537. doi: 10.1016/j.neuroscience.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.LaRowe SD, Mardikian P, Malcolm R, Myrick H, Kalivas PW, McFarland K, Saladin M, McRae A, Brady K. Safety and tolerability of N-acetylcysteine in cocaine-dependent individuals. Am. J. Addict. 2006;15:105–110. doi: 10.1080/10550490500419169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.LaRowe SD, Myrick H, Hedden S, Mardikian P, Saladin M, McRae A, Brady K, Kalivas PW, Malcolm R. Is cocaine desire reduced by N-acetylcysteine? Am. J. Psychiatry. 2007;164:1115–1117. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- 199.Knackstedt LA, Larowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol. Psychiatry. 2009;65:841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhou W, Kalivas PW. N-Acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol. Psychiatry. 2008;63:338–340. doi: 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.LaRowe SD, Kalivas PW. The role of N-acetylcysteine in inhibiting responding during extinction in rats trained to self-administer cocaine. Open Addict. J. 2010;3:88–91. doi: 10.2174/1874941001003010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sofuoglu M. Cognitive enhancement as a pharmacotherapy target for stimulant addiction. Addiction. 2010;105:38–48. doi: 10.1111/j.1360-0443.2009.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]