Abstract
There is growing interest in the diagnosis of cognitive impairment among children with epilepsy. It is well known that status of seizures control has to be carefully investigated because it can be sufficient “per se” to cause progressive mental deterioration conditions. Subclinical electroencephalographic discharges may have subtle effects on cognition, learning and sleep patterns, even in the absence of clinical or sub-clinical seizures. In this respect, electroencephalographic monitoring (long-term and nocturnal recording) and in particular an all night video-polysomnography (V-NPSG) record can be crucial to detect the presence of unrecognized seizures and/or an inter-ictal nocturnal EEG discharge increasing. Epileptic encephalopathies (EE) are a group of conditions in which the higher cognitive functions are deteriorate as a consequence of epileptic activity, which, in fact, consists of frequent seizures and/or florid and prolonged interictal paroxysmal discharges, focal or generalized. AEDs represent the first line in opposing the burden of both, the poor seizures control and the poor interictal discharges control, in the cognitive deterioration of EE affected children. Thus, to improve the long-term cognitive/behavioural prognosis in these refractory epileptic children, it should be taken into account both a good seizures control and a strict sleep control, choosing carefully antiepileptic drugs which are able to control not only seizures clinically recognizable but even the EEG discharges onset and its increasing and spreading during sleep. Here, we review the efficacy and safety of the newer AEDs that, to date, are used in the treatment of EE in infancy and childhood.
Key words: Epileptic encephalopathy, refractory epilepsy, AED, anticonvulsant therapy, epilepsy of infancy, epilepsy of childhood, interictal epileptic discharges, subtle seizures.
INTRODUCTION
The ILAE Task Force on Classification and Terminology proposed the term “Epileptic Encephalopathies” (EE) to refer to a category of epilepsies in which the ictal and interictal epileptiform (clinical and EEG) abnormalities may contribute to progressive cerebral dysfunction. This category includes the following: Ohtahara syndrome, West Syndrome, Dravet Syndrome, Doose Syndrome, Lennox-Gastaut Syndrome, Landau-Kleffner Syndrome and Electrical Status Epilepticus during Sleep (ESES) or Continuous Spike-Wave during Slow Sleep (CSWS) [1]. Recently, the so-called “severe epilepsy with multiple independent spike foci” has been added among the EE [2]. However for some entities, such as Dravet syndrome, the relationship between seizures severity and psycomothor delay has not been clarified.
The main features of EEs include: (I) electrographic EEG paroxysmal activity that is often aggressive with an high “spikes rate” or “paroxysmal discharges density” (II) seizures that are usually multi-form and intractable, (III) cognitive, behavioral, and neurological deficits that may be relentless, and (IV) sometimes early death [3]. However the clinical spectrum of EEs is largely varied depending on the age at onset, epileptic acitvity, genetic and enviromental factors [4]. It include severe forms with cognitive and motor deterioration to slight mild form with better course [5]. Several reports suggest that in some children, the seizure control [6] results in an improvement of developmental outcome [7]. In the treatment of EE the first line consists of pharmacological intervention [8, 9]. However the response to anticonvulsants is often poor despite aggressive and often off-label use of a variety of drugs [10]. New AEDs [gabapentine (GBP), topiramate (TPM), levetiracetam (LEV), zonisamide (ZNS) and rufinamide (RUF)] (Table 1) have given physicians new options for the treatment of patients with epilepsy and, in particular, EE affected. However, 25-30% of children with epilepsy are still refractory to these wider treatment options. Therefore, the development of new AEDs should be continued especially those targeting pediatric epilepsies and developing brains [11].
Table 1.
Main Drugs used in Epileptic Encephalopathies. MHD: Oxcarbazepine Metabolite 10-Hydroxycarbazepine; Im: Intramuscularly
| DRUG | Seizure Type | Oral Dose (mg/Kg/Day) | N° of Daily Doses | Therapeutic Serum Level | Side Effects and Toxicity |
|---|---|---|---|---|---|
| Clobazam | -Add-on / wide spectrum | 0, 25-1 | 1-3 | nd | Drowsiness, dizziness, constipation, dry mouth, tremor. |
| Clonazepam | -Add on / wide spectrum | 0, 05 - 0, 15 | 2-3 | 20 - 80 µg/ml | Fatigue, drowsiness, hypotonia, behavior disturbances, salivary, bronchial hypersecretion, respiratory depression. |
| Felbamate | -Add-on / Lennox-Gastaut syndrome | 15 - 45 | 2-3 | 25 -100 µg / ml | Somnolence, anorexia, gastric discomfort, nervousness, aplastic anemia, hepatotoxicity. |
| Gabapentin | -Add-on/ focal and secondary generalized | 10 - 30 | 2-3 | 6-10 µg/ml | Somnolence, headache, tremor, nystagmus, fatigue and weight gain, rarely beahavioural disorders. |
| Lamotrigine |
- Add on/ idiopathic
general - Add on/ focal - Add on/ Lennox Gastaut syndrome |
1 - 15 | 2-3 | 3 -10 µg/ml | Dizziness, diplopia, ataxia, somnolence, rash, Stevens-Johnson syndrome, Lyell’s syndrome. |
| Levetiracetam | -Add-on/ focal | 10 - 60 | 2 | 5 - 45 µg /ml | Dizziness; irritability; anxiety, headache., weakness; nausea; psychotic events (rare) |
| Nitrazepam | - Monotherapy infantile spasms | 0, 25 - 2, 5 | 2 | 40 -180 ng/ml | Hypotonia, drowsiness, aspiration, pneumonia. |
| Oxcarbazepine |
-Add on / focal - newly diagnosed / focal |
10 - 45 | 2-3 | 12-35 ng/ml (MHD) | Somnolence, headache, ataxia, vomiting, hyponatriemia, rash. |
| Rufinamide | -Add-on / Lennox-Gastaut syndrome | 5-45 | 2 | nd | Dizziness, headache, nausea, somnolence, double vision, fatigue, ataxia, vomiting. |
| Stiripentol | -Add on / Dravet’s syndrome | 50 - 100 | 2-3 | nd | Drowsiness, loss of appetite. |
| Tiagabine | - Add on / focal | 0, 5 -2 | 2-3 | 20-100 µg/ml | Dizziness, abdominal pain, nervousness, difficulty with concentration, non convulsive SE. |
| Topiramate |
-Add-on / generalized Tonic clonic -Add-on/ focal -Add-on /Lennox Gastaut -Monotherapy in newly diagnosed focal |
1- 12 | 2-3 | 4 - 25 µg/ml | Weight loss, paresthesias, emotional lability, difficulty concentrating and word-finding, hypohidrosis, kidney stones. |
| Valproate | - Monotherapy /generalized | 15-60 | 2 | 40-100 µg/L | Hepatic failure, pancreatitis, thrombocytopenia, weight gain, tremor, menstrual irregularities. |
| Vigabatrin |
-Monotherapy/ infantile
spasms -monotherapy/newly diagnosed, focal |
40-100; 100-150 for infantile spasms |
2 | nd | Excitation, drowsiness, weight gain, psychosis (rare), visual field defects. |
| Zonisamide | - Add on / wide spectrum | 2-12 | 2 | 27-43 µg/ml | Somnolence, dizziness, ataxia, abdominal discomfort, decreased spontaneity, rash, hypohidrosis, psychiatric disorders. |
| ACTH | - Monotherapy/ infantile spasms | 1, 5-3 UI/Kg/day im for 1-2weeks | 2 | -- | Hyperglycemia, hypertension, electrolyte abnormalities, gastrointestinal disturbances, infections |
| Prednisone | -Monotherapy/ infantile spasms | 2mg/Kg/day | 2 | -- | Obesity, infections, hypertension, aggression, weight gain. |
| Hydrocortisone | -Monotherapy/ infantile spasms | 5-20 mg/Kg/day | 2 | -- | Cushing syndrome, irritation, sleep problems, weight gain, hypertension. |
Currently, beside classical anti-epileptic drugs, some less conventional therapies are utilized in the treatment of intractable seizures, as there are ketogenic diet, immunoglobulins, steroids and adrenocorticotrophic hormone [12, 13].
Here we review the more frequent EE clinical pictures (at the onset and during their natural course) and their pharmacological treatments, focusing on the more recent data about efficacy and safety of the newer AEDs nowdays available.
OHTAHARA SYNDROME
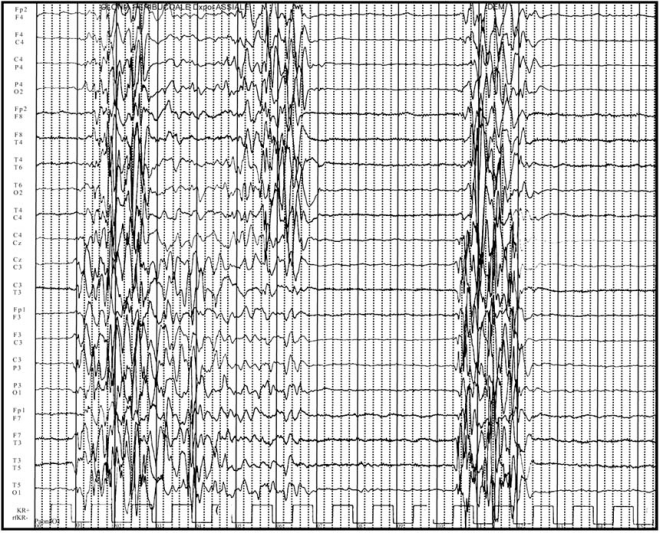
Ohtahara syndrome (OS), also known as Early-infantile EE with suppression-bursts (EIEE) was first described by Ohtahara et al. in 1976 as the earliest form of the age-dependent EE [4]. The syndrome is outwardly characterized by early onset (within a few months of life) of tonic spasms and partial seizures (with or without clustering), and receives its more elaborate name from the severe and continuous pattern of burst activity on the electroencephalogram, the so-called “suppression burst” (SB) [14]. This EEG pattern is characterized by high voltage bursts alternating with almost flat suppression phases at an approximately regular rate “Fig. (1)”. The peculiar feature of SB in OS is the consistent appearance in both waking and sleeping status and regular appearance of periodicity [15].
Fig. (1).
EEG pattern of burst suppression.
OS range widely from 0.2% to 4% of all childhood epileptic patients [6, 16]. The cardinal seizure type is the tonic spasm, lasting 1-10 s, which may be isolated or occur in clusters. The spasms occur both during waking and sleeping states; they may be generalized and symmetrical or lateralized [17]. Daily seizure frequency is very high, ranging from 10 to 300 spasms in 10 to 20 clusters [18]. In addition to tonic spasms, partial motor seizures, hemiconvulsions, or generalized tonic seizures can be recorded in about 30% of cases [19]. Myoclonic seizures are rather rarely observed [20]. Seizures are difficult to control and with rare exceptions, patients have profound mental retardation [3].
One of the most remarkable characteristics of OS is its age dependent evolution: approximately 75% of children with Ohtahara syndrome transform into West Syndrome (WS) during 3-6 months of age and further from WS to Lennox-Gastaut Syndrome (LGS) at 1-3 years of age, in some cases [14, 21].
Etiology is heterogeneous but development in neuroimaging technique, particularly MRI, disclosed structural abnormalities of the brain as the main cause of OS [19]. Most cases are associated with bilateral cortical dysplasia [22], but other causes have been reported, including focal cortical dysplasia, pachigyria, agyria, hemimegalencephaly, corpus callosum hypo- or agenesis, dysgeneses of the collicoli, and posterior fossa anomalies [2, 23]. Even cryptogenic cases may have undetectable microdysgenesis or migration disorders which cause progressive atrophy [22]. Even cytochrome oxidase deficiency [24], and non-ketotic hyperglycemia [25] have been reported.
Specific mutations in the ARX (aristaless-related homeobox) gene at Xp22.13 have been found in male subjects with EIEE [26]. Recently, heterozygous mutations in the gene encoding syntaxin-binding protein 1 (Stxbp1) (also known as MUNC18-1), an evolutionary conserved neuronal Sec1/Munc-18 (SM) protein (STXBP1) that is essential in synaptic vesicle release in several species, have been identified in four unrelated individuals with EIEE [27].
Seizures are extremely intractable and prognosis is severe with psychomotor retardation; there are many early deaths in early infancy [2]. Valproate (VPA) (20-30 mg/Kg/day), benzodiazepines (BDZ) [Clobazam (CLB) 0, 25-1mg/Kg/day; Clonazepam (CZP) 0, 1-0, 2 mg/Kg/day], adrenocorticotrophic hormone (ACTH) (1, 5-3 UI/Kg/day) and steroids ( 2mg/Kg/day) including liposteroid [28] are often tried, but their efficacy is limited [12]. Thyrotropin releasing hormone (TRH) or its analog [29] and ketogenic diet [30, 31] have a partial effect in some cases. Gamma-globulin treatment (100 - 400 mg/Kg/day) is reported to have considerable efficacy [32, 33]. Recently chloral hydrate therapy (58 mg/kg/day) has been revealed successful in some cases of OS [34]. ACTH has proven effective in OS and, more interestingly, in some cases it was effective after the transition to WS. In relation to the age at ACTH treatment, patients treated before 3 months of life seem to be less effective. Moreover ACTH treatment is worth trying particularly in cryptogenic cases and to try again after the transitional stage to WS in those without sufficient efficacy during OS stage [4]. In anecdotic cases CZP and acetazolamide (AZD) were effective in OS [94]. Vigabatrin (GVG) (starting dose of 40-50 mg/kg/day up to 100-250 mg/kg/day) [4] and ZNS ( at doses ranging from 2 to over 12 mg/Kg/day) [2, 35] have been reported to be of some value. The efficacy of ZNS is observed in some cases with complete seizure suppression [2, 35]. ZNS is reported to be effective for partial seizures and for various types of seizure disorders, including infantile spasms (IS) [36] as that observed in patients with OS. Its broad antiepileptic profile may be mediated by an effect on the voltage-sensitive Na+ channels [35] or on the voltage-dependent T-type calcium current [37].
Clinical and EEG features of OS can be secondary to inborn errors of vitamin metabolism such as pyridoxine-dependent seizures (PDS), folinic acid-responsive seizures, and pyridoxal phosphate- dependent seizures. It has become good clinical practice to test for pyridoxine (100 mg i.v. in a single dose, to be repeated and possibly increased to 500 mg in total), folinic acid (3-5 mg/kg per day for two to three days) and PLP (30 mg/kg/day in three doses for at least one day) dependency in every neonate and infant with ‘difficult-to treat seizures’ [38].
Surgical interventions demonstrated successful in selected infants with brain abnormalities (e.g., megalencephaly) has provided promising results [39, 40].
WEST SYNDROME AND INFANTILE SPASMS
One of the most known epileptic syndrome is the West syndrome (WS), also called Infantile Spasms (IS) or Salaam Spams/Tics. Its incidence is estimated about 0, 16-0, 42 per 1000 live births [4]. In the first description, it was characterized by a famous triad that consists of seizures (IS), characteristic abnormalities to the electroencephalography (hypsarrhytmia) and psychomotor retardation. The causes of this syndrome are heterogeneous and it can be divided in three principal group: symptomatic, cryptogenetic and idiopathic. In the symptomatic group the epileptic desease is associated with various brain damage due to prenatal, perinatal and postnatal causes. Cryptogenetic and idiopathic forms remain of unknown origin in the majority of the cases. In patients who does not meet all the triad criteria’s findings for WS (spasms, hypsarrhytmia and psychomotor delay) the term “infantile spasm” (IS) is preferred [41].
WS occurs in the first year of life, with a peak age at 5 months [42]. Seizures are heterogeneous and generally consist of sudden bilaterally and symmetrical spasms of the neck, trunk and extremities (flexor, extensor or mixed type), with loss of consciousness lasting few seconds or minutes. Usually spasms occur in clusters (from 20 at 100 spasm). In many infants the seizures occur at awakening and during crying [42]. This form of epilepsy is usually refractory; that’s why its prognosis is so poor. Early treatment at the onset, decreases the probability of negative prognosis or LGS later onset [41, 42].
Peculiar EEG findings are fundamental for WS diagnosis. In 1950 Gastaut and co-workers described the EEG features associated with WS and then Gibbs and others conied the term hypsarrhythmia to define an EEG characterized by “random high voltage slow waves and spikes, that vary form moment to moment in duration and in location: at the onset, they appear to be focal and then they seem to originate from multiple foci; in few cases the spike discharge becomes generalized” [43]. The third typical feature is the presence of psychomotor retardation. Early start of the therapy with specific anticonvulsant drugs is very important to control the seizures and to improve the outcome [44]. In 1958 Sorel and Dusaucy-Bauloye reported that adrenocorticotropic hormone (ACTH) controlled the spasms in a number of cases. After this discovery, various studies reported the efficacy of ACTH and other agents appeared. ACTH and corticosteroids would be efficacy due to suppress CRH synthesis. High level of CRH during the developmental stage in these patients would produce epileptogenic alterations in brainstem [45]. The optimum treatment for IS has yet to be proven with confidence, in part because of the different objectives of published studies [46].
Many new therapeutic options have been tried: GVG, ZNS, Nitrazepam (NZP), Methisergide, LEV, TPM, Lamotrigine (LTG), Pyridoxine, Ketogenic diet, immunoglobulin therapy, felbamate (FLB), and thyrotropin-releasing hormone [47, 48]. ACTH is generally estimated to be more effective than corticosteroids and it appears to positively alter long-term prognosis in the criptogenetic cases more than in the symptomatic ones [18]. A common schedule includes ACTH, 20 U/day intramuscularly (IM) for two weeks and if no response occurs, the dosage is increased to 30 and then 40 U/day IM for an additional four weeks, then ACTH is replaced with oral prednisone ( 2mg/Kg/day for two weeks). If the seizure persist, prednisone is given for un additional four weeks. The side effects of ACTH include hyperglycemia, hypertension, electrolyte abnormalities, gastrointestinal disturbances, infections ( including Pneumocystic carinii pneumonia) and transient brain shrinkage observed by CT scanning [50]. GVG has been considered the drugs of first choice for patients affected by IS associated with tuberous sclerosis and may also be a second-line agent for the treatment of other symptomatic IS. The efficacy of GVG is increased with high doses (100-150 mg/Kg/day) compared with the standard doses (40-100 mg/Kg/day) [50]. Unfortunately, rethinopathy GVG-related represents a limit for its use [51, 52]. Brief period and low dose to minimize the probability of visual field defect GVG related has been suggested [14]. In case of lack of improvement, GVG should be discontinued after 12 weeks [53]. GVG represents the only real achievement in the effective treatment of IS associated with Tuberous Sclerosis as well as with Focal Cortical Dysplasia (FCD) or Down Syndrome. In FCD, GVG treatment seems to be able to prevent diffusion of the paroxysmal activity outside of the dysplasia [14]. Pyridoxine has been reported to be efficacy for IS in Japanese patients, but there are no randomized controlled trials in literature [41]. ZNS, intravenous immunoglobulin, liposteroid, ketogenic diet, TRH, TPM, all have been administered for the treatment of IS, but there are insufficient data to determine whether these drugs or combination therapies are effective or not [43].
To date, ACTH and GVG are considered the most efficacy antiepileptic treatment for WS and IS. However for the potential risk of more severe adverse effects with ACTH, many clinicians in Canada prefer GVG as first line [54, 55]. There are no data on the long-term benefits of different therapies in WS and IS both, on seizure control and neuropsychological development.
SEVERE MYOCLONIC EPILEPSY OF INFANCY (SMEI) OR DRAVET SYNDROME
Severe myoclonic epilepsy of infancy (SMEI; OMIM 607208) was first described in 1982 by Dravet C et al. [56] and recognized as a specific syndrome in 1989 [57]. The term Dravet Syndrome (DS) was proposed in 2001 [58]. SMEI is an intractable epilepsy of infancy appearing during the first year of life in previously healthy children; generally the febrile seizures are the first clinical manifestations at around 6 months of age (often as “febrile status epilepticus”), followed later by afebrile seizures, often clonic and unilateral, of long duration and frequent status epilepticus; between 1 and 4 years of age other seizures can be observed such as myoclonic jerks, focal seizures and atypical absence [59, 60]. The seizures can be triggered by fever or warm water [59]. Development is normal in the first year of life, with a slowing of psychomotor development, starting in the second year of life, that flows into a severe mental retardation before the age of six years [60]. Ataxia, pyramidal signs and interictal myoclonus can occur; the seizures are drug-resistant, persisting into adulthood [59, 60]. Patients with similar clinical features of SMEI, but with not all the diagnostic guidelines complete, belong to a subgroup called Borderline SMEI (SMEB) [61, 62]. The EEG is normal in the first twelve months, then generalized spike and spike-waves complexes at multiple foci appear; photosensitivity can be observed in almost the half of patients [60]. Unexpected EEG in DS was recently described [63].
The neuroradiological studies are normal in the most of patients [64], although different brain abnormalities can occur [65]; differently, genetic factors play an important role: a familial history of febrile seizures or epilepsy is present in up to 64% of SMEI patients, and mutations of SCN1A (alpha-1 subunit of sodium channel) gene are considered responsible [60]. The numerous groups which described SCN1A mutations (truncations, splice site, deletion, missense mutations) in SMEI patients, report a frequency oscillating from 33 to 100% of cases and depending from the methods used for screening and diagnosis [65-70]; the real estimated percentage of mutations in classical SMEI patients is 80% [59]. Moreover the coexistence, in SMEI patients, of a family history of seizure disorders belonging to the Generalized Epilepsy with Febrile Seizures plus (GEFS+) spectrum, and the high percentage (95%) of de novo SCN1A mutations, suggested the concept that SMEI is the most severe clinical picture of GEFS+ phenotypes [59]. Recently it was established that de novo mutations have mainly a paternal origin [71]. Additionally, GABRG2 gene has been found as responsible for DS in two cases [59], and PCDH19 gene has been related to SMEI SCN1A negative patients [72].
An early diagnosis of DS or SMEI is important to start a treatment that could be able to reduce the number of seizures and, mainly, to prevent the episodes of status epilepticus, in order to improve developmental outcome; in fact it is well know that the developmental delay is related to the onset and the frequency of status epilepticus [62]. Moreover, a well-timed treatment could reduce the risk of sudden death, higher in SMEI than other epilepsies (15% versus 5%) [73].
VPA, phenobarbital (PB) and BDZ [CPZ, lorazepam (LPZ)] are the most useful drugs in DS, by means of a reduction of the frequency and duration of the seizures [74]; ethosuximide (ESM) and high doses of piracetam can decrease myoclonic seizures [74]. Several case reports of improvement with bromide, corticosteroids, immunoglobulins, phenytoin (PTH) are reported [74]. Also various studies have suggested the efficacy, rated according to seizure type and frequency, of several AEDs added to these baseline drugs; among these TPM (starting dose of 0.5-1 mg/kg/day, followed by a 2-week titration at increments of 1-3 mg/kg/day up to a maximum daily dose of 12 mg/kg) [75] and LEV (starting dose of 10 mg/kg/day up to 50 to 60 mg/kg/day in two doses) [76].
In addition, a CYP450 inhibitor, stiripentol (STP), is considered effective in patients with SMEI if associated with VPA and BDZ, by means of an increase of their plasma concentrations and a decrease of their metabolities [77, 78]. Also, STP seems to increase the GABAergic transmission, suggesting antiepileptic properties “per se” [78]. The advised dose of STP is 50 mg/kg/day with a maximum of 3500 mg/day, 2 or 3 times a day, preferably during meals [78]. It is important to notice that specific antiepileptic drugs, such as LTG and carbamazepine (CBZ), may exacerbate myoclonic seizures in Dravet Syndrome [62]. The utility of ketogenic diet in SMEI patients has been showed by a seizures reduction [79, 80]. Medications which act mainly as sodium channels blockers (LTG, CBZ, PTH) should not be used anymore: the SCN1A gain or loss of function observed in SMEI is available explanation of the inefficiency of these drugs [81]. More recently it has been reported the possible role of a voltage-gated calcium channel blocker (Vg-CCB), verapamil (VRP), as an add-on anticonvulsant medication in Dravet Syndrome [82].
DOOSE SYNDROME (MYOCLONIC-ATONIC EPILEPSY)
The term myoclonic-atonic (or astatic) epilepsy (MAE) was used to describe a primary generalized epilepsy of childhood characterized by myoclonic and/or astatic seizures as main clinical manifestations [83, 84].
Initially this term was applied by Doose to a large subgroup of idiophatic epilepsies with atonic and myoclonic seizures. After the ILAE Classification of epileptic syndromes [57], MAE was included in the “cryptogenetic and symptomatic epilepsy syndromes”, and then, subsequently, in the group of generalized epilepsy [58]. It onsets between 7 months and 8 years of age (peak between 1 and 5 years; boys more affected than girls) with seizures and previous normal development. A quick evolution towards generalized jerks and astatic falls, which can appear numerous times daily, is usually observed. Febrile seizures are observed in one-third of cases [59]. Myoclonic-astatic seizures are described as brief massive or axial symmetric jerk of neck, shoulders, arms and legs, lasting 2-3 seconds, that, if intense and associated with a sudden loss of muscle tone, can cause a fall [49]. Drops attack and falls, present in about 2/3 of the patients [85], are responsible for head and face trauma. Other types of seizures can occur such as generalized tonic-clonic and atypical absence [86]. The children can show also non-convulsive status, presenting as blurring of consciousness associated with ataxia or hypertonus, and face and distal muscles contractions; these episodes can arise slowly and persist for hours, days or weeks [86]. The ictal EEG shows spike waves and poli-spike waves complexes at a frequency of 2-4 Hz, while the interictal EEG could be initially normal; later, discharges of spike-waves at 3 Hz appear, particularly during sleep [86]. A rhythmic theta activity at 4-7 Hz in parietal and occipital regions and blocked by eye opening is frequent [85, 87]. Photosensitivity is not unusual [86]. The ictal EEG of non-convulsive status shows irregular slow waves and spike-waves complexes [86].
The outcome of MAE at diagnosis is unpredictable; some children may develop a severe course with a drug-resistant epilepsy, while others, with frequent and severe attacks, may have their seizures resolved after a period of 3 years approximately [85]. The cognitive outcome depends mainly on the clinical pattern [88, 89]. The number, frequency and type of seizures (falls, atypical absence, GTC, non-convulsive status) have been associated with a poor mental outcome [83, 85]. The neuropsychological findings suggest that electroclinical abnormalities can temporarily affect cognitive and behavioral functioning. Early effective AEDs could improve cognitive outcome [88], as usually happens in the most of intractable childhood epilepsy.
Neuroradiological studies are generally normal [86]. The genetic basis of MAE are unclear. The presence of a complex inheritance was suggested by previous studies on family members of MAE patients [83, 84]. In addition mutations of sodium channel subunit genes (SCN1A and SCN1B) and of GABAa receptor subunit gene (GABRG2) have been found in patients with myoclonic-astatic epilepsy belonging to GEFS+ family, suggesting the hypothesis that MAE could belong to the GEFS+ spectrum [107], while study of sporadic cases did not report any mutations [68].
AEDs used in MAE are primarily VPA and ESM [85, 87]. In particular the association between VPA and ESM can be advantageous expecially against myoclonic seizures; the individual doses are generally regular, although some children need of higher doses [90]. BDZ as CZP are helpful, although its use is limited by effects on the behavior [86]. LTG can be used to control generalized seizures in MAE [87, 91]. The use of CBZ and GVG is not recommended because they can raise myoclonic attacks as in others myoclonic epilepsy [86]. The efficacy of others drugs, such as TPM, LEV, AZD, ESM and sulhtiame (SLT) has been reported by anedoctal case reports [86, 89, 92, 93]. Some studies have reported the role of the ketogenic diet in patients with myoclonic-atonic epilepsy [85, 87, 94], by means of both a reduction in seizures and even a complete seizure control; it should be used early especially in drug resistant cases [87, 94].
LENNOX-GASTAUT SYNDROME
LGS is a rare epileptic encephalopathy described for the first time in 1960 by Lennox and his colleagues as a triad of symptoms comprising generalized slow spikes-waves (SSW), mental deficiency and early onset of multiple and different seizures types [95, 96].
As regards the etiology it is usually divided into symptomatic or criptogenetic group, based on the presence or absence of neurological abnormalities or specific causes. The most frequent type is the criptogenetic form. Symptomatic cases present different causes such as hypoxic-ischemic encephalopathy, vascular demage, perinatal meningoencephalitides, tuberous sclerosis, Down’s Syndrome, trauma, brain tumor and malformations [95]; in the 10-25 % of the patients a previous history of WS is reported [97].
Clinically the type of seizures are different depending on the phases of the syndrome; tonic seizures are the most frequent and peculiar type of seizures (tonic axial seizures, particularly during sleep), but are not necessarily present at the onset. Tonic refers to “a sustained increase in muscle contraction lasting a few seconds or minutes”. The patients can present flexor movement of head and trunk with apnoea and brief cry associated with abduction of the limbs, which usually involves the arms; in others patients the tonic seizures might involve most muscle (global tonic attacks) [98]. The second seizure mainly associated with LGS is atypical absence, characterized by a brief loss of consciousness. Atonic falls or drop attacks are hazardous and occur in 56% of patients who have slow spike-waves, but are not diagnostic of LGS [99].
Usually the onset of LGS is about 8 years of age and occurrence rates peak between 3 and 5 years. The first manifestation, especially in cryptogenetic form, is a drop attack, followed by other type of seizures; EEG abnormalities are not yet present and psychomotor/behavioral development at the time of first seizure seems normal. Later several seizures types occur as tonic, atonic, atypical absence, myoclonic, partial or generalized tonic-clonic seizures. At EEG the characteristic and peculiar abnormalities are burst of diffuse slow spike-waves at 2-2, 5 c/s during wakefulness, or burst or fast waves and slow polyspikes and generalized fast activity at about 10 c/s during sleep; this latter EEG finding is almost pathognomonic for LGS. Developmental delay increases with time and the patients lose cognitive and intellectual skills. The prognosis of these patients is very poor and often their epilepsy remains untreatable [97].
Old antiepileptic drugs for LGS treatment include BDZ [CLB, CZP, diazepam (DZP), LZP, NZP), PB, Primidone (PRM), PTH, VPA, GVG [98]. BZD are still prescribed with the specific risk in LGS of precipitating tonic status epileptic (TSE) [100]. Though they can control tonic-clonic seizures, PB and PRM should be avoided due to cognitive and sedative side effects [101]. PTH can control tonic-clonic seizures and reduce tonic seizures in LGS, but it can aggravate atypical absences and myoclonic seizures [96]. More recent therapeutic options in LGS are FBM, LTG, TPM and RUF [91, 102]. FBM was the first to be approved for adjunctive therapy in LGS and showed a significant effect on “major” seizures. Severe adverse effects appeared a few months after approval and so, since 1994, FBM was administered exclusively for LGS refractory to other AEDs. In the last studies it was demonstrated that the risk/benefit of FBM therapy supports its use as an important add-on option for patients with severe refractory epilepsies [103].
Recent data have demonstrated efficacy of LTG against focal seizures, tonic-clonic seizures, tonic seizures, absence seizures and atonic seizures [104]. This drug is efficacy especially in major seizures, but worsening or no improvement of myoclonic jerks has been reported. If patients LGS affected are already being treated with an enzyme-inducing AED, addition of LTG has to introduce slowly and with caution. TPM showed efficacy in drop attacks associated with LGS in open and double bind randomized study confirming the efficacy of TPM in reducing the frequency of atonic seizures with improvement of quality of life [105].
RUF is a new AED that shows good tolerability and efficacy in reducing tonic and atonic seizures, associated with LGS [106, 107]. RUF is a triazole derivative and its mechanism of action is an inibition/modulation of NA+-dependent action potentials in neurons and inhibitory effect of the GluR5 subtypes at high concentrations. The protective index of RUF is higher than that of most common AEDs, like PHT, PB, VPA and ESM. Most common adverse events are fatigue, vomiting, loss of appetite, headache, somnolence and tremor [102].
Antiepileptic and other drugs used “off-label” in LGS are AZD, Allopurinol, Bromide, Flunarizine (FNZ), Pyridoxine, ZNS [30]. Future AEDs in development for the LGS are Carisbamate (CBM), Fluoro-felbamate (FFBM), Ganaxolone (GNX) derivates of LEV, Remacemide (RMC), STP, immune treatments, intravenous immunoglobulins, Ketogenic and modified Atkins diets [97].
The best treatment for LGS remains uncertain and no study to date has shown any one drug to be highly efficacious. Until further research has been undertaken, clinicians will need to continue to consider each patient individually, taking into account the potential benefit of each therapy weighed against the risk of adverse effects [102].
LANDAU-KLEFFNER SYNDROME
Landau Kleffner (LKS) is a rare syndrome of unknown etiology, that is more common in the children between 5 and 7 years af age. It is named for William Landau and Frank Kleffner, who in 1957 reported six affected children with different type of convulsive seizures and acquired aphasia [4]. This functional disorder consists of loss of language skills in children previously normal [44]. The regression of language may be sudden or prolonged period and aphasia can be primarily receptive or expressive; probably the language disorder can be defined as a verbal auditory agnosia, that consists of a loss of verbal comprehension, which may be confused as a acquired deafness [108]. The child can be unconscious of daily sounds and the hearing is normal, but behavioral problems as irritability and poor attention are very common. This aphasia is followed by gradual deterioration also in verbal production and, finally, mutism and failure to respond to non-verbal sounds [108]. Associated with language disorder the children with LKS in the majority of cases present different types of seizures, which include episodes of eye blinking or ocular deviation, head drop and minor automatisms with secondary generalization. In other cases, sizures are focal or toni-clonic generalized seizures, typical absences, partial complex ad occasionally myoclonic seizures [109].
EEG shows bilateral temporal spikes or spikes waves, increasing during sleep. In these patients an awake EEG usually demonstrates normal background activity and focal epileptiform abnormalities especially on the temporal lobes; rarely awake EEG can be normal, making video-NPSG mandatory in these subjects. During the sleep recording EEG it is observed an activation and diffusion of the epileptic discharges, whereas the pattern of electrical status epilepticus during sleep (ESES or CSWS) may often be observed [108]. ESES picture is characterized by a continuous diffuse spike-waves during SS [36] (see criteria definition for ESES in the dedicate paragraph). ESES or CSWS presence is not mandatory to make diagnosis of LKS, although it is frequently associated [110].
Early response to treatment and late outcome in LKS can be markedly different if the onset is in pre-linguistic phase (onset under the age of 3-4 years) and these children can be misdiagnosed for autistic due to impaired linguistic skills [111].
Paroxysmal activity of the temporal lobe in LKS children reinforces synaptic contacts and it is responsible for permanent language dysfunction. If present, ESES or CSWS is able to produce regression of the higher cognitive and executive functioning [112].
Clinical seizures control in LKS is easy to obtain much more than ESES picture remission. Sleep EEG increase discharges in the majority of patients can persist months or years causing sometimes severe cognitive impairment, which can be transient, fluctuant or, rarely, irreversible with permanent cognitive deficits [108].
Among available AEDs, CBZ may cause a worsening of seizures and it should be avoided. The treatment of choice in LSK appears to be VPA as mono-therapy or in combination with a benzodiazepine [4]. Some authors suggest that steroids and ACTH should be considered the treatment of choice especially in early onset of disease in young patient, because it seems to have favorable and long-lasting effects, even on ESES conditions [113]. The recommended dose of ACTH and steroids is high dose for a prolonged period ( 80 UI/d with a 3 month taper; prednisone 60 mg/d with a 3 month taper) [114]. It was noted that there may be relapses with steroids reduction and that some children may need to take steroids for months to years. More early the treatment is initiated and the shorter is the duration for which steroid is required and better is the outcome.
CLB, NZP, VPA, ESM and flunitrazepam have been used with benefits. PB, CBZ, PHT have been reported to be ineffective or harmful [4]. Patients treated with high-dose intravenous corticosteroids succesfully improve in speech abilities [114]. Also the successful use of intravenous immunoglobulin is known, with both language functions and EEG abnormalities influenced significantly by intravenous immunoglobulin administration [115].
The prognosis of LKS is benign in most cases, although the improvement of language depends on the age of the onset (pre and post-linguistic onset) of the disorder and the severity of the epileptic seizures. Early onset makes the prognosis poorer with persistent language difficulties even in the adult life [109].
ESES OR CSWS
ESES or CSWS is an EE responsible for less than 1% of the age-dependent childhood epilepsies, starting generally between the 5th and 7th year of life [7]. Even if these two terms are considered synonymous, ESES, firstly described by Tassinari [116], refers to the EEG pattern (continuous spike-wave complexes exclusively during non-REM sleep, with a spike-wave index accounting for at least 80-85% of SS), while CSWS indicates both EEG features and clinical neuropsychological characteristics of this EE [10, 117]. From a clinical point of view various seizures type are possible in CSWS affected patient: generalized tonic-clonic seizures during sleep, atypical absence, myoclonic and atonic seizures. Developmental delay and deterioration resulting in a IQ reduction, loss of speech, behavior and motor involvement (with ataxia, dystonia, dyspraxia) is often associated [7, 10]. In CSWS may be present a natural history consisting in three phases: initial period with seizures and no developmental involvement (I); intermediate period with seizures, neuropsychological regression and ESES (II); final period with only neuropsychological deficits (III). However this evolution could be absent, and patients with CSWS/ESES may present initially without seizures but “only” with developmental delay and/or behavior disturbance [10]. In these cases it could be useful to perform a video-NPSG investigation to deeper study the sleep EEG along the entire night. During puberty it is possible to observe a remission of seizures, an improvement of the behavioral and motor features and a normalization of EEG, although EEG anomalies and neuropsychological disturbances usually persist [39].
A well-known partial epilepsy strongly related to ESES or CSWS is the “Atypical Benign Partial Epilepsy of Infancy and Childhood” (ABPEI) also known as “Pseudo-Lennox Syndrome”, firstly described by Aicard’ e Chevrie [119]. It was often mistaken, in the past, for LGS because of the repeated atonic falls, absences and slow-wave activity at EEG. This form includes generalized seizures, atypical absences and atonic-astatic seizures. Axial tonic nocturnal seizures frequently observed in LGS are never observed in this type. ESES or CSWS can be associated with ABPEI or even, in rare cases, with “Rolandic Epilepsy” [119]. Actually, ABPEI, ESES or CSWS and Landau-Kleffner Syndromes are four epileptic conditions which clearly overlap and some discussion exist about whether they are complication (or particular evolution) of rolandic epilepsy, or if they instead represent different, although overlapping, epileptic conditions [119].
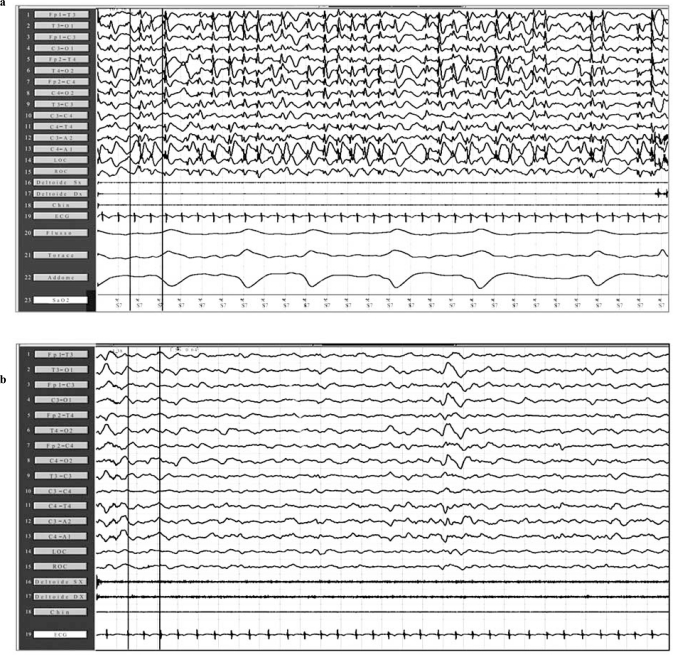
Numerous antiepileptic drugs have been used for the treatment of CSWS, such as LTG, LEV, VPA, steroids, BDZ; among the latter, high doses of DZP have been used with good results [10]. ESES or CSWS pictures may be sometimes particularly refractory to AEDs and corticosteroid therapy, in these cases, have shown sometimes good results “Fig. (2a and b)”.
Fig. (2).
Before (a) and after (b) oral administration of hydrocortisone for two weeks. Neuropolysomnographic showed both a complete remission of ESES activity and a clear improvement of sleep macrostructure.
ACTH or hydrocortisone are effective, but side-effects are well-known. High doses of BDZ are effective when given in by rapid venous infusion or rectally. However, the favorable effects observed after a single administration are short-lasting (few hours or days). On the other hand, prolonged remissions have been observed after continuous oral treatment with BZP (LZP, NZP). The rectal administration of DZP 1 mg/kg followed by an oral dose of 0.5 mg/kg/die for a period of 3 weeks gave positive results, with remissions lasting several months in 9/15 cases of ESES (60%) and in one typical case of LKF syndrome [110].
SEVERE EPILEPSY WITH MULTIPLE INDEPENDENT SPIKE FOCI
The term Severe Epilepsy with Multiple Indipendent Spike Foci (SE-MISF) was proposed by Othtahara et al. [22, 86] as epilepsy with “ three or more indipendent spike foci in both emispheres” and “generalized minor seizures”. This specific EEG pattern called Multiple Indipendent Spike Foci (MISF) had been previously defined by Noriega-Sanchez and Markand [120] as a specific electographic finding with “more then two independent foci”, related to clinical signs and occurring more frequently in children between the age of 4 and 7 years; the presence of multiple foci may be due to a diffuse cerebral pathology, usually involving both the hemispheres. The other peculiarities of SE-MISF are: presence of various types of high-frequency generalized minor seizures; usual association with mental retardation and neurological abnormalities; extensive cerebral pathology linked to nonspecific causes (pre-, peri- and post-natal factors such as neurocutaneous diseases, degenerative disease, infections, hypoxic ischemic encephalopathy, cerebral malformations, hydrocephalus); evolution between the age-dependent epileptic encephalopathy (WS and LGS); extreme seizures intractability [110].
The onset could be between 2 months and 13 years, thus indicating that its age-dependency is not identifiable such as in the others age-related EE; however, the catastrophic nature of SE-MISF is suggested by the cognitive deficits and deterioration observed after the recurrence of refractory seizures, even if it is not easy to establish if it could depends on the underlying brain pathology [110].
The treatment of SE-MISF is based on a combination of several antiepileptic drugs, such as VPA, BDZ, VGB, PTH, ZNS; in addition, only in few selected cases, there is indication for neurosurgical treatment [110].
CONCLUSIONS
The crucial role potentially acted by interictal electroencephalogram (EEG) discharges [121, 122], even without associated seizures [123], in order to cause more or less transitory cognitive impairment, can play a crucial role in the cognitive deterioration associated with EE, particularly in children with high rate of interictal spiking and frequent seizures. Cognitive functions, mainly under frontal lobe control, seemed to be particularly vulnerable to epileptic EEG activity during the period of brain development and maturation; this age-related disruption possibly interferes with the normal development of learning processes, showing, sometimes, a precise topographic correlation [122, 124-127]. From a physiopathological point of view, the most intriguing issue is represented by the relationship between ESES and the pattern of neuropsychological and/or motor derangement. Taking into consideration all above reported data, it become easy to understand how important is to look for new AEDs which will be able to achieve a better control of both, the seizures and interictal paroxysmal EEG abnormalities, particularly during sleep [128-130].
REFERENCES
- 1.Engel J ILAE Commission Report. A Proposed Diagnostic Scheme for People with Epileptic Seizures and with Epilepsy: Report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42(6):796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- 2.Yamatogi Y, Ohtahara S. Early-infantile epileptic encephalopathy with suppression-bursts, Ohtahara syndrome; its overview referring to our 16 cases. Brain Dev. 2002;24(1):13–23. doi: 10.1016/s0387-7604(01)00392-8. [DOI] [PubMed] [Google Scholar]
- 3.Panayiotopoulos CP. Epileptic encephalopathy in infancy and early childhood. In: Panayiotopoulos CP, editor. A clinical guide to epileptic syndromes and their treatment. 2nd. London: Sprinter; 2007. pp. 223–273. [Google Scholar]
- 4.Dulac O. Epileptic encephalopathies. Epilepsia. 2001;42(Suppl. 3):23–26. doi: 10.1046/j.1528-1157.2001.042suppl.3023.x. [DOI] [PubMed] [Google Scholar]
- 5.Nabbout R, Dulac O. Epileptic Encephalopathies: a brief overview. J. Clin. Neurophysiol. 2003;20(6):393–397. doi: 10.1097/00004691-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Oka E, Ishida S, Ohtsuka Y, Ohtahara S. Neuroepidemiological study of childhood epilepsy by application of international classification of epilepsies and epileptic syndromes (ILAE, 1989) Epilepsia. 1995;36:658–661. doi: 10.1111/j.1528-1157.1995.tb01042.x. [DOI] [PubMed] [Google Scholar]
- 7.MacAllister WS, Schaffer SG. Neuropsychological deficits in childhood epilepsy syndromes. Neuropsychol. Rev. 2007;17:427–444. doi: 10.1007/s11065-007-9048-4. [DOI] [PubMed] [Google Scholar]
- 8.Conry JA. Pharmacologic treatment of the catastrophic epilepsies. Epilepsia. 2004;45(Suppl 5):12–16. doi: 10.1111/j.0013-9580.2004.05004.x. [DOI] [PubMed] [Google Scholar]
- 9.Markand NK. Epileptic encephalopathies of childhood. J. Clin. Neurophysiol. 2003;20(6):391–392. [Google Scholar]
- 10.Holmes GL, Lenck-Santini PP. Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav. 2006;8:504–515. doi: 10.1016/j.yebeh.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Hwang H, Kim KJ. New antiepileptic drugs in pediatric epilepsy. Brain Dev. 2008;30:549–555. doi: 10.1016/j.braindev.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Nabbout R, Dulac O. Epileptic syndromes in infancy and childhood. Curr. Opin. Neurol. 2008;21:161–166. doi: 10.1097/WCO.0b013e3282f7007e. [DOI] [PubMed] [Google Scholar]
- 13.Wheless JW. Nonpharmacologic treatment of the catastrophic epilepsies of childhood. Epilepsia. 2004;45(Suppl. 5):17–22. doi: 10.1111/j.0013-9580.2004.05003.x. [DOI] [PubMed] [Google Scholar]
- 14.Parisi P, Bombardieri R, Curatolo P. Current role of Vigabatrin in infantile spasms. Eur. J. Paediatr. Neurol. 2007;11:331–336. doi: 10.1016/j.ejpn.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Fusco L, Pachatz C, Di Capua M, Vigevano F. Video/EEG aspects of early-infantile epileptic encephalopathy with suppression-bursts (Ohtahara syndrome) Brain Dev. 2001;23:708–714. doi: 10.1016/s0387-7604(01)00280-7. [DOI] [PubMed] [Google Scholar]
- 16.Kramer U, Nevo Y, Neufeld MY, Fatal A, Leitner Y, Harel S. Epidemiology of epilepsy in childhood: a cohort of 440 consecutive patients. Pediatr. Neurol. 1998;18:46–50. doi: 10.1016/s0887-8994(97)00154-9. [DOI] [PubMed] [Google Scholar]
- 17.Panayiotopoulos CP, Panayiotopoulos CP. A clinical guide to epileptic syndromes and their treatment. 2nd. London: Sprinter; 2007. pp. 223–273. [Google Scholar]
- 18.Ohtahara S, and Yamatogi Y. Epileptic Encephalopathies in Early Infancy With Suppression-Burst. J. Clin. Neurophysiol. 2003;20(6):398–407. doi: 10.1097/00004691-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Ohtahara S, and Yamatogi Y. Ohtahara syndrome: With special reference to its developmental aspects for differentiating from early myoclonic encephalopathy. Epilepsy Res. 2006;70S:S58–S67. doi: 10.1016/j.eplepsyres.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 20.Aicardi J, Ohtahara S. Severe neonatal epilepsies with suppression-burst pattern. In: Roger J, Bureau M, Dravet Ch, Genton P, Tassinari C, Wolf P, editors. Epileptic Syndromes in Infancy, Childhood and Adolescencce. 3rd. Eastleigh: John Libbey; 2002. pp. 33–44. [Google Scholar]
- 21.Ohtahara S, Ohtsuka Y, Yamatogi Y, Oka E. The early-infantile epileptic encephalopathy with suppression-burst: developmental aspects. Brain Dev. 1987;9:371–376. doi: 10.1016/s0387-7604(87)80110-9. [DOI] [PubMed] [Google Scholar]
- 22.Ohtsuka Y, Sato M, Sanada S, Yoshinaga H, Oka E. Suppression-burst patterns in intractable epilepsy with focal cortical dysplasia. Brain Dev. 2000;22:135–138. doi: 10.1016/s0387-7604(00)00090-5. [DOI] [PubMed] [Google Scholar]
- 23.Ruggieri M, Spalice A, Parisi P. Early Infantile Epileptic Encephalopathy with Burst-Suppression: Ohtahara syndrome. J. Pediatr. Neurol. 2010 In press. [Google Scholar]
- 24.Williams AN, Gray RG, Poulton K, Whitehouse WP. A case of Ohtahara syndrome with cytochrome oxydase deficiency. Dev. Med. Child. Neurol. 1998;40:568–570. doi: 10.1111/j.1469-8749.1998.tb15416.x. [DOI] [PubMed] [Google Scholar]
- 25.Gonzàlez de Dios J, Moya M, Pastore C, Izura V, Carratalà F. Early infantile epileptic encephalopathy and glycine encephalopathy. Rev. Neurol. 1997;25:1916–1918. [PubMed] [Google Scholar]
- 26.Kato M, Saitoh S, Kamei A, Shiraishi H, Ueda Y, Akasaka M, Tohyama J, Akasaka N, Hayasaka K. A longer polyalanine expansion mutation in the ARX gene causes early infantile epileptic encephalopathy with suppression-burst pattern (Ohtahara syndrome) Am. J. Hum. Genet. 2007;81:361–366. doi: 10.1086/518903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saitsu H, Kato M, Mizuguchi T, Hamada K, Osaka H, Tohyama J, Uruno K, Kumada S, Nishiyama K, Nishimura A, Okada I, Yoshimura Y, Hirai S, Kumada T, Hayasaka K, Fukuda A, Ogata K, Matsumoto N. De novo mutations in the gene encoding STXBP1 (MUNC18-1) cause early infantile epileptic encephalopathy. Nat. Genet. 2008;40:782–788. doi: 10.1038/ng.150. [DOI] [PubMed] [Google Scholar]
- 28.Yoshikawa H, Ikeda S, Watanabe T. Liposteroid therapy in a case of early infantile epileptic encephalopathy with suppression burst. No To Hattatsu. 1998;30:551–554. [PubMed] [Google Scholar]
- 29.Ishii M, Tamai K, Sugita K, Tanabe Y. Effectiveness of TRH analog in a case of early infantile epileptic encephalopathy. No To Hattatsu. 1990;22:507–511. [PubMed] [Google Scholar]
- 30.Coppola G, Verrotti A, Ammendola E, Operto FF, Corte RD, Signoriello G, Pascotto A. Ketogenic diet for the treatment of catastrophic epileptic encephalopathies in childhood. Eur. J. Paediatr. Neurol. 2009 doi: 10.1016/j.ejpn.2009.06.006. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Takusa Y, Ito M, Kobayashi A, Sejima H, Kishi K, Shiraishi H. Effect of the ketogenic diet for West syndrome into which early infantile epileptic encephalopathy with suppression-burst was evolved. No To Hattatsu. 1995;27:383–387. [PubMed] [Google Scholar]
- 32.Billiau AD, Witters P, Ceulemans B, Kasran A, Wouters C, Lagae L. Intravenous immunoglobulins in refractory childhood-onset epilepsy: effects on seizure frequency, EEG activity, and cerebrospinal fluid cytokine profile. Epilepsia. 2007;48:1739–1749. doi: 10.1111/j.1528-1167.2007.01134.x. [DOI] [PubMed] [Google Scholar]
- 33.Miyake S, Yamashita S, Yamada M, Iwamoto H. Therapeutic effect of ACTH and gamma-globulin in 8 cases with the early-infantile epileptic encephalopathy with suppression-burst (EIEE) Shonika Rinsho. 1987;40:1681–1688. [Google Scholar]
- 34.Krsek P, Sebronová V, Procházka T, Maulisová A, Komárek V. Successful treatment of Ohtahara syndrome with chloral hydrate. Pediatr. Neurol. 2002;27:388–3891. doi: 10.1016/s0887-8994(02)00464-2. [DOI] [PubMed] [Google Scholar]
- 35.Ohno M, Shimotsuji Y, Abe J, Shimada M, Tamiya H. Zonisamide treatment of early infantile epileptic encephalopathy. Pediatr. Neurol. 2000;23:341–344. doi: 10.1016/s0887-8994(00)00197-1. [DOI] [PubMed] [Google Scholar]
- 36.Tsao CY. Current trends in the treatment of infantile spasms. Neuropsychiatr. Dis. Treat. 2009;5:289–299. doi: 10.2147/ndt.s4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matar N, Jin W, Wrubel H, Hescheler J, Schneider T, Weiergräber M. Zonisamide block of cloned human T-type voltage-gated calcium channels. Epilepsy Res. 2009;83:224–34. doi: 10.1016/j.eplepsyres.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Basura GJ, Hagland SP, Wiltse AM, Gospe SM. Clinical features and the management of pyridoxine-dependent and pyridoxine-responsive seizures: review of 63 North American cases submitted to a patient registry. Eur. J. Pediatr. 2009;168:697–704. doi: 10.1007/s00431-008-0823-x. [DOI] [PubMed] [Google Scholar]
- 39.Hmaimess G, Raftopoulos C, Kadhim H, Nassogne MC, Ghariani S, de Tourtchaninoff M, van Rijckevorsel K. Impact of early hemispherotomy in a case of Ohtahara syndrome with left parieto-occipital megalencephaly. Seizure. 2005;14:439–442. doi: 10.1016/j.seizure.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Komaki H, Sugai K, Maehara T, Shimizu H. Surgical treatment of early-infantile epileptic encephalopathy with suppression-bursts associated with focal cortical dysplasia. Brain Dev. 2001;23:727–731. doi: 10.1016/s0387-7604(01)00287-x. [DOI] [PubMed] [Google Scholar]
- 41.Hancock E, Osborne JP, Milnerc P. The treatment of West syndrome: a Cochrane review of the literature to December 2000. Brain Dev. 2001;23:624–634. doi: 10.1016/s0387-7604(01)00299-6. [DOI] [PubMed] [Google Scholar]
- 42.Karvelas G, Lortie A, Scantlebury MH, Duy PT, Cossette P, Carmant L. A retrospective study on aetiology based outcome of infantile spasms. Seizure. 2009;18(3):197–201. doi: 10.1016/j.seizure.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Mackay MT, Weiss SK, Adams-Webber T, Ashwal S, Stephens D, Ballaban-Gill K, Baram TZ, Duchowny M, Hirtz D, Pellock JM, Shields WD, Shinnar S, Wyllie E, Snead OC. Practice Parameter: Medical Treatment of Infantile Spasms Report of the American Academy of Neurology and the Child Neurology Society. Neurology. 2004;62:1668–1681. doi: 10.1212/01.wnl.0000127773.72699.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shield DW. Catastrophic epilepsy in childhood. Epilepsia. 2000;41(suppl. 2):S2–S6. doi: 10.1111/j.1528-1157.2000.tb01518.x. [DOI] [PubMed] [Google Scholar]
- 45.Hrachovy RA, Frost JD. Infantile epileptic encephalopathy with hypsarrhythmia (infantile spasms/West Syndrome) J. Clin. Neurophysiol. 2003;20(6):408–425. doi: 10.1097/00004691-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Hancock EC, Osborne JP, Edwards SW. Treatment of infantile spasms. Cochrane Database Syst. Rev. 2009;8(4) doi: 10.1002/14651858.CD001770.pub2. CD001770. [DOI] [PubMed] [Google Scholar]
- 47.Gumus H, Kumandas S, Per H. Levetiracetam monotherapy in newly diagnosed cryptogenic West Syndrome. Pediatr. Neurol. 2007;37(5):350–353. doi: 10.1016/j.pediatrneurol.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki Y. Zonisamide in West syndrome. Brain Dev. 2001;23:658–661. doi: 10.1016/s0387-7604(01)00301-1. [DOI] [PubMed] [Google Scholar]
- 49.Cohen-Sadana S, Kramera U, Ben-Zeevb B, Lahatc E, Sahard E, Nevoe Y, Eidlitzf T, Zehariaf A, Kivityg S, Goldberg-Sterng H. Multicenter long-term follow-up of children with idiopathic West syndrome: ACTH versus vigabatrin. Eur. J. Neurol. 2009;16:482–487. doi: 10.1111/j.1468-1331.2008.02498.x. [DOI] [PubMed] [Google Scholar]
- 50.Guerrini R. Epilepsy in children. Lancet. 2006;367(9509):499–524. doi: 10.1016/S0140-6736(06)68182-8. [DOI] [PubMed] [Google Scholar]
- 51.Fejerman N, Cersosimo R, Caraballo R, Grippo J, Corral S, Martino RH, Martino G, Aldao M, Caccia P, Retamero M, Macat MC, Di Blasi AM, Adi J. Vigabatrin as a first choice drug in the treatment of west syndrome. J. Child Neurol. 2000;15(3):161–165. doi: 10.1177/088307380001500304. [DOI] [PubMed] [Google Scholar]
- 52.Mikati AA, Lepejian GA, Holmes GL. Medical treatement of patients with infantile spasms. Clin. Neuropharmacol. 2002;25(2):61–70. doi: 10.1097/00002826-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 53.Willmore LJ, Abelson MB, Ben-Menachem E, Pellock JM, Shields DW. Vigabatrin: 2008 Update. Epilepsia. 2009;50(2):163–173. doi: 10.1111/j.1528-1167.2008.01988.x. [DOI] [PubMed] [Google Scholar]
- 54.Hee H, Ki Joong K. New antiepileptic drugs in pediatric epilepsy. Brain Dev. 2008;30:549–555. doi: 10.1016/j.braindev.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 55.Snead III OC, Donner EJ. A new generation of anticonvulsants for the treatment of epilepsy in children. Paediatr. Child Health. 2007;12(9):741–744. doi: 10.1093/pch/12.9.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dravet C, Roger J, Bureau M. Myoclonic epilepsies in childhood. In: Akimoto H, Kazamatsuri H, Seino M, Ward AA Jr, editors. Advances in epileptology. The XIIIth Epilepsy International Symposium. New York: Raven Press; 1982. pp. 135–140. [Google Scholar]
- 57.Commission on classification and terminology of the ILAE. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 58.Engel J. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42:1–8. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- 59.Scheffer IE, Harkin LA, Dibbens LM, Mulley JC, Berkovic SF. Neonatal epilepsy syndromes and generalized epilepsy with febrile seizures plus (GEFS+) Epilepsia. 2005;46(Suppl. 10):41–47. doi: 10.1111/j.1528-1167.2005.00358.x. [DOI] [PubMed] [Google Scholar]
- 60.Wolff M, Cassè-Perrot C, Dravet C. Severe Myoclonic Epilepsy of Infants (Dravet Syndrome): Natural History and Neuropsychological Findings. Epilepsia. 2006;47(Suppl. 2):45–48. doi: 10.1111/j.1528-1167.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- 61.Fukuma G, Oguni H, Shirasaka Y, Watanabe K, Miyajima T, Yasumoto S, Ohfu M, Inoue T, Watanachai A, Kira R, Matsuo M, Muranaka H, Sofue F, Zhang B, Kaneko S, Mitsudome A, Hirose S. Mutations of neuronal voltage-gated Na+ channel alpha 1 subunit gene SCN1A in core severe myoclonic epilepsy in infancy (SMEI) and in borderline SMEI (SMEB) Epilepsia. 2004;45:140–148. doi: 10.1111/j.0013-9580.2004.15103.x. [DOI] [PubMed] [Google Scholar]
- 62.Scheffer IE, Zhang YH, Jansen FE, Dibbens L. Dravet syndrome or genetic (generalized) epilepsy with febrile seizures plus? Brain Dev. 2009;5:391–400. doi: 10.1016/j.braindev.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 63.Nabbout R, Desguerre I, Sabbagh S, Depienne C, Plouin P, Dulac O, Chiron C. An unexpected EEG course in Dravet syndrome. Epilepsy Res. 2008;81:90–95. doi: 10.1016/j.eplepsyres.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 64.Dravet C, Bureau M, Guerrini R, Giraud N. Severe myoclonic epilepsy in infants. In: Roger J, Roger J, Dravet C, Bureau M, Dreifuss FE, Perret A, Wolf P, editors. Epileptic syndromes in infancy, childhood and adolescence. 2nd. London: John Libbey; 1992. pp. 75–88. [Google Scholar]
- 65.Striano P, Mancardi MM, Biancheri R, Madia F, Gennaro E, Paravidino R, Beccaria F, Capovilla G, Dalla Bernardina B, Darra F, Elia M, Giordano L, Gobbi G, Granata T, Ragona F, Guerrini R, Marini C, Mei D, Longaretti F, Romeo A, Siri L, Specchio N, Vigevano F, Striano S, Tortora F, Rossi A, Minetti C, Dravet C, Gaggero R, Zara F. Brain MRI findings in severe myoclonic epilepsy in infancy and genotype-phenotype correlations. Epilepsia. 2007;48(6):1092–1096. doi: 10.1111/j.1528-1167.2007.01020.x. [DOI] [PubMed] [Google Scholar]
- 66.Claes L, Ceulemans B, Audenaert D, Smets K, Löfgren A, Del-Favero J, Ala-Mello S, Basel-Vanagaite L, Plecko B, Raskin S, Thiry P, Wolf NI, Van Broeckhoven C, De Jonghe P. De novo SCN1A mutations are a major cause of severe myoclonic epilepsy of infancy. Hum. Mutat. 2003;21:615–21. doi: 10.1002/humu.10217. [DOI] [PubMed] [Google Scholar]
- 67.Gennaro E, Veggiotti P, Malacarne M, Madia F, Cecconi M, Cardinali S, Cassetti A, Cecconi I, Bertini E, Bianchi A, Gobbi G, Zara F. Familial severe myoclonic epilepsy of infancy: truncation of Nav1.1 and genetic heterogeneity. Epilep. Disord. 2003;5:21–25. [PubMed] [Google Scholar]
- 68.Nabbout R, Gennaro E, Dalla Bernardina B, Dulac O, Madia F, Bertini E, Capovilla G, Chiron C, Cristofori G, Elia M, Fontana E, Gaggero R, Granata T, Guerrini R, Loi M, La Selva L, Lispi ML, Matricardi A, Romeo A, Tzolas V, Valseriati D, Veggiotti P, Vigevano F, Vallée L, Dagna Bricarelli F, Bianchi A, Zara F. Spectrum of SCN1A mutations in severe myoclonic epilepsy of infancy. Neurology. 2003;60:1961–67. doi: 10.1212/01.wnl.0000069463.41870.2f. [DOI] [PubMed] [Google Scholar]
- 69.Sugawara T, Mazaki-Miyazaki E, Fukushima K, Shimomura J, Fujiwara T, Hamano S, Inoue Y, Yamakawa K. Frequent mutations of SCN1A in severe myoclonic epilepsy in infancy. Neurology. 2002;58:1122–24. doi: 10.1212/wnl.58.7.1122. [DOI] [PubMed] [Google Scholar]
- 70.Wallace RH, Hodgson BL, Grinton BE, Gardiner RM, Robinson R, Rodriguez-Casero V, Sadleir L, Morgan J, Harkin LA, Dibbens LM, Yamamoto T, Andermann E, Mulley JC, Berkovic SF, Scheffer IE. Sodium channel alpha-1 subunit mutations in severe myoclonic epilepsy of infancy and infantile spasms. Neurology. 2003;61:765–769. doi: 10.1212/01.wnl.0000086379.71183.78. [DOI] [PubMed] [Google Scholar]
- 71.Heron SE, Scheffer IE, Iona X, Zuberi SM, Birch R, McMahon JM, Bruce CM, Berkovic SF, Mulley JC. De novo SCN1A mutations in Dravet syndrome and related epileptic encephalopathies are largely of paternal origin. J. Med. Genet. 2009 doi: 10.1136/jmg.2008.065912. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 72.Depienne C, Bouteiller D, Keren B, Cheuret E, Poirier K, Trouillard O, Benyahia B, Quelin C, Carpentier W, Julia S, Afenjar A, Gautier A, Rivier F, Meyer S, Berquin P, Hélias M, Py I, Rivera S, Bahi-Buisson N, Gourfinkel-An I, Cazeneuve C, Ruberg M, Brice A, Nabbout R, Leguern E. Sporadic Infantile Epileptic Encephalopathy Caused by Mutations in PCDH19 Resembles Dravet Syndrome but Mainly Affects Females. Plos Genetic. 2009;5(2) doi: 10.1371/journal.pgen.1000381. e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kassaï B, Chiron C, Augier S, Cucherat M, Rey E, Gueyffier F, Guerrini R, Vincent J, Dulac O, Pons G. Severe myoclonic epilepsy in infancy: a systematic review and a meta-analysis of individual patient. Epilepsia. 2008;49(2):343–348. doi: 10.1111/j.1528-1167.2007.01423.x. [DOI] [PubMed] [Google Scholar]
- 74.Dravet C, Bureau M, Oguni H, Fukuyama Y, Cokar O. Severe myoclonic epilepsy in infancy (Dravet syndrome) In: Roger J, Bureau M, Dravet C, Genton P, Tassinari CA, Wolf P, editors. Epileptic syndromes in infancy, childhood and adolescence. 3rd. London: John Libbey; 2002. pp. 81–103. [Google Scholar]
- 75.Coppola G, Capovilla G, Montagnini A, Romeo A, Spano M, Tortorella G, Veggiotti P, Viri M, Pascotto A. Topiramate as add-on drug in severe myoclonic epilepsy in infancy: an Italian multicenter open trial. Epilepsy Res. 2002;49:45–48. doi: 10.1016/s0920-1211(02)00010-4. [DOI] [PubMed] [Google Scholar]
- 76.Striano P, Coppola A, Pezzella M, Ciampa C, Specchio N, Ragona F, Mancardi MM, Gennaro E, Beccaria F, Capovilla G, Rasmini P, Besana D, Coppola GG, Elia M, Granata T, Vecchi M, Vigevano F, Viri M, Gaggero R, Striano S, Zara F. An open-label trial of levetiracetam in severe myoclonic epilepsy of infancy. Neurology. 2007;69:250–254. doi: 10.1212/01.wnl.0000265222.24102.db. [DOI] [PubMed] [Google Scholar]
- 77.Chiron C. Stiripentol. Neurotherapeutics. 2007;4:123–125. doi: 10.1016/j.nurt.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chiron C, Marchand MC, Tran A, Rey E, d’Athis P, Vincent J, Dulac O, Pons G. Stiripentol in severe myoclonic epilepsy in infancy: a randomised placebo-controlled syndrome-dedicated trial. STICLO study group. Lancet. 2000;356:1638–42. doi: 10.1016/s0140-6736(00)03157-3. [DOI] [PubMed] [Google Scholar]
- 79.Caraballo RH, Fejerman N. Dravet syndrome: a study of 53 patients. Epilepsy Res. 2006;70(Suppl 1):S231–S238. doi: 10.1016/j.eplepsyres.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 80.Korff C, Laux L, Kelley K, Goldstein J, Koh S, Nordli D., Jr Dravet syndrome (severe myoclonic epilepsy in infancy): a retrospective study of 16 patients. J. Child. Neurol. 2007;22:185–94. doi: 10.1177/0883073807300294. [DOI] [PubMed] [Google Scholar]
- 81.Ceulemans B, Boel M, Claes L, Dom L, Willekens H, Thiry P, Lagae L. Severe myoclonic epilepsy of infancy: toward an optimal treatment. J. Child. Neurol. 2004;19:516–521. doi: 10.1177/08830738040190070701. [DOI] [PubMed] [Google Scholar]
- 82.Iannetti P, Parisi P, Spalice A, Ruggieri M, Zara F. Addition of verapamil in the treatment of severe myoclonic epilepsy of infancy. Epilepsy Res. 2009;85(1):89–95. doi: 10.1016/j.eplepsyres.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 83.Doose H. (1992) Myoclonic astatic epilepsy of early childhood. In: Roger J, Bureau M, Dravet C, Dreifuss FE, Perret A, Wolf P, editors. Epileptic syndromes in infancy, childhood and adolescence. 2nd. London: John Libbey; 1992. pp. 103–114. [Google Scholar]
- 84.Doose H, Baier WK. Genetic factors in epilepsies with primary generalized minor seizures. Neuropediatrics. 1997;18(suppl 1):1–64. [Google Scholar]
- 85.Oguni H, Tanaka T, Hayashi K, Funatsuka M, Sakauchi M, Shirakawa S, Osawa M. Treatment and long-term prognosis of myoclonic-astatic epilepsy of early childhood. Neuropediatrics. 2002;33:122–132. doi: 10.1055/s-2002-33675. [DOI] [PubMed] [Google Scholar]
- 86.Guerrini R, Aicardi J. Epileptic encephalopathies with myoclonic seizures in infant and children (severe myoclonic epilepsy and myoclonic-astatic epilepsy) J. Clin. Neurophysiol. 2003;20(6):449–461. doi: 10.1097/00004691-200311000-00007. [DOI] [PubMed] [Google Scholar]
- 87.Kilaru S, Bergqvist AGC. Current treatment of myoclonic astatic epilepsy:clinical experience at the children’s hospital of Philadelphia. Epilepsia. 2007;48(9):1703–07. doi: 10.1111/j.1528-1167.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- 88.Filippini M, Boni A, Dazzani G, Guerra A, Gobbi G. Neuropsychological findings: myoclonic astatic epilepsy (MAE) and Lennox-Gastaut syndrome (LGS) Epilepsia. 2006;47(Suppl 2):56–59. doi: 10.1111/j.1528-1167.2006.00691.x. [DOI] [PubMed] [Google Scholar]
- 89.Nabbout R, Gennaro E, Dalla Bernardina B, Dulac O, Madia F, Bertini E, Capovilla G, Chiron C, Cristofori G, Elia M, Fontana E, Gaggero R, Granata T, Guerrini R, Loi M, La Selva L, Lispi ML, Matricardi A, Romeo A, Tzolas V, Valseriati D, Veggiotti P, Vigevano F, Vallée L, Dagna Bricarelli F, Bianchi A, Zara F. Spectrum of SCN1A mutations in severe myoclonic epilepsy of infancy. Neurology. 2003;60:1961–67. doi: 10.1212/01.wnl.0000069463.41870.2f. [DOI] [PubMed] [Google Scholar]
- 90.Lin YP, Itomi K, Takada H, Kuboda T, Okumura T, Aso K, Negoro T, Watanabe K. Benign myoclonic epilepsy in infants: video-EEG features and long-term follow-up. Neuropediatrics. 1998;29:268–271. doi: 10.1055/s-2007-973573. [DOI] [PubMed] [Google Scholar]
- 91.Dulac O, Kaminska A. Use of lamotrigine in Lennox-Gastaut and related epilepsy syndromes. J. Child. Neurol. 1997;12(suppl 1):S23–28. doi: 10.1177/0883073897012001071. [DOI] [PubMed] [Google Scholar]
- 92.Mikaeloff Y, de Saint-Martin A, Mancini J, Peudenier S, Pedespan JM, Vallee L, Motte J, Bourgeois M, Arzimanoglou A, Dulac O, Chiron C. Topiramate: efficacy and tolerability in children according to epilepsy syndromes. Epilepsy Res. 2003;53:225–32. doi: 10.1016/s0920-1211(03)00028-7. [DOI] [PubMed] [Google Scholar]
- 93.Tennison MB, Greenwood RS, Miles MV. Methosuximide for intractable childhood seizures. Pediatrics. 1991;87:186–9. [PubMed] [Google Scholar]
- 94.Caraballo RH, Cersósimo RO, Sakr D, Cresta A, Escobal N, Fejerman N. Ketogenic diet in patients with myoclonic-astatic epilepsy. Epileptic Disord. 2006;8(2):151–55. [PubMed] [Google Scholar]
- 95.Markand ON. Lennox-Gastaut Syndrome (childhood epileptic encephalopathy) J. Clin. Neurophysiol. 2003;20(6):426–441. doi: 10.1097/00004691-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 96.Sarzgar M, Bourgeois BF. Aggravation of epilepsy by antiepileptic drugs. Pediatric Neurol. 2005;33:227–234. doi: 10.1016/j.pediatrneurol.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 97.Arzimanoglou A, French J, Blume WT, Cross JH, Ernst JP, Feucht M, Genton P, Guerrini R, Kluger G, Pellock JM, Perucca E, Wheless JW. Lennox-Gastaut syndrome: a consensus approach on diagnosis, assessment, management, and trial methodology. Lancet Neurol. 2009;8:82–93. doi: 10.1016/S1474-4422(08)70292-8. [DOI] [PubMed] [Google Scholar]
- 98.Abu Saleh T, Stephen L. Lennox gastaut syndrome, review of the literature and a case report. Head Face Med. 2008;9:4–9. doi: 10.1186/1746-160X-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chevrie JJ, Aicardi J. Childhood epileptic encephalopathy with slow spike-wave. A statistical study of 80 cases. Epilepsia. 1972;13:259–71. doi: 10.1111/j.1528-1157.1972.tb05260.x. [DOI] [PubMed] [Google Scholar]
- 100.Dimario FJ, Clancy RR. Paradoxical precipitation of tonic seizures by lorazepam in child with atypical absence seizures. Pediatr. Neurol. 1988;4:249–251. doi: 10.1016/0887-8994(88)90040-9. [DOI] [PubMed] [Google Scholar]
- 101.Delanty N, French J. Treatment of Lennox-Gastaut syndrome: current recommendations. CNS Drugs. 1998;10:181–187. [Google Scholar]
- 102.Van Rijckevorsel K. Treatment of Lennox-Gastaut syndrome: overview and recent findings. Neuropsychiatr. Dis. Treat. 2008;4(6):1001–1019. doi: 10.2147/ndt.s1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pellock JM, Faught E, Leppik IE, Shinnar S, Zupanc ML. Felbamate: consensus of current clinical experience. Epilepsy Res. 2006;71:89–101. doi: 10.1016/j.eplepsyres.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 104.Donaldson JA, Glauser TA, Olberding LS. Lamotrigine adjunctive therapy in childhood epileptic encephalopathy (the Lennox Gastaut syndrome) Epilepsia. 1997;38(1):68–73. doi: 10.1111/j.1528-1157.1997.tb01079.x. [DOI] [PubMed] [Google Scholar]
- 105.Ailouni S, Shorman A, Daoud AS. The efficacy and side effects of topiramate on refractory epilepsy in infants and young children: a multicenter clinical trial. Seizures. 2005;14:459–463. doi: 10.1016/j.seizure.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 106.Glauser T, Kluger G, Sachdeo R, Krauss G, Perdomo C, Arroyo S. Efficacy and safety of rufinamide adjunctive therapy in patients with Lennox-Gastaut syndrome (LGS): a multicenter, randomized, double-blind, placebo-controlled, parallel trial. Neurology. 2005;70(21):1950–1958. doi: 10.1212/01.wnl.0000303813.95800.0d. [DOI] [PubMed] [Google Scholar]
- 107.Hakimian S, Cheng- Hakimian A, Anderson GD, Miller JW. Rufinamide: a new-antiepileptic medication. Exp. Opin. Pharmacother. 2007;8:1931–1940. doi: 10.1517/14656566.8.12.1931. [DOI] [PubMed] [Google Scholar]
- 108.Smith MC, Hoeppner TJ. Epileptic encephalopathy of late childhood. Landau-Kleffner syndrome and the syndrome of continuous spikes and waves during slow-waves sleep. J. Clin. Neurophysiol. 2003;20(6):462–472. doi: 10.1097/00004691-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 109.Duran MHC, Guimaraes CA, Medeiros LL, Guerreiro MM. Landau-Kleffner syndrome: long-term follow-up. Brain Dev. 2009;31:58–63. doi: 10.1016/j.braindev.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 110.Yamatogi Y, Ohtahara S. Multiple independent spike foci and epilepsy, with special reference to a new epileptic syndrome of "severe epilepsy with multiple independent spike foci". Epilepsy Res. 2006;70(Suppl 1):S96–104. doi: 10.1016/j.eplepsyres.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 111.Deonna T, Roulet E. Autistic spectrum disorder: evaluating a possible contributing or causal role of epilepsy. Epilesia. 2006;47(Suppl 2):79–82. doi: 10.1111/j.1528-1167.2006.00697.x. [DOI] [PubMed] [Google Scholar]
- 112.Saltik S, Uluduz D, Cokar O, Demirbilek V, Dervent AA. Clinical and EEG study on idiopathic partial epilepsies with evolution into ESES spectrum disorders. Epilepsia. 2005;46(4):524–533. doi: 10.1111/j.0013-9580.2005.45004.x. [DOI] [PubMed] [Google Scholar]
- 113.Gallagher S, Weiss S, Oram Cardy J, Humphries T, Harman KE, Menascu S. Efficacy of very high dose steroid treatment in a case of Landau-Kleffner syndrome. Dev. Med. Child Neurol. 2006;48(9):766–769. doi: 10.1017/S0012162206001630. [DOI] [PubMed] [Google Scholar]
- 114.Lerman P, Lerman-Sagie T, Kivity S. Effect of early corticosteroid therapy for Landau-Kleffner syndrome: case report. Dev. Med. Child Neurol. 1991;33:257–266. doi: 10.1111/j.1469-8749.1991.tb05115.x. [DOI] [PubMed] [Google Scholar]
- 115.Arts WF, Aarsen FK, Scheltens-de Boer M, Catsman-Berrevoets CE. Landau-Kleffner syndrome and CSWS syndrome: treatment with intravenous immunoglobulins. Epilepsia. 2009;50(Suppl 7):55–58. doi: 10.1111/j.1528-1167.2009.02221.x. [DOI] [PubMed] [Google Scholar]
- 116.Tassinari CA, Daniele O, Gambarelli F, Bureau-Paillas M, Robaglia L, Cicirata F. Excessive 7-14-sec positive spikes during REM sleep in monozygotic non-epileptic twins with speech retardation. Rev. Electroencephalogr. Neurophysiol. Clin. 1977;7(2):192–193. doi: 10.1016/s0370-4475(77)80079-8. [DOI] [PubMed] [Google Scholar]
- 117.Galanopoulou AS, Bojko A, Lado F, Moshé SL. The spectrum of neuropsychiatric abnormalities associated with electrical status epilepticus in sleep. Brain Dev. 2000;22(5):279–295. doi: 10.1016/s0387-7604(00)00127-3. [DOI] [PubMed] [Google Scholar]
- 118.Aicardi J, Chevrie JJ. Atypical benign partial epilepsy of childhood. Dev. Med. Child Neurol. 1982;24(3):281–292. doi: 10.1111/j.1469-8749.1982.tb13620.x. [DOI] [PubMed] [Google Scholar]
- 119.Fejerman N, Caraballo R, Tenembaum SN. Atypical evolutions of benign localization-related epilepsies in children: are they predictable? Epilepsia. 2000;41(4):380–390. doi: 10.1111/j.1528-1157.2000.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 120.Nabbout R, Kozlovski A, Gennaro E, Bahi-Buisson N, Zara F, Chiron C, Bianchi A, Brice A, Leguern E, Dulac O. Absence of mutations in major GEFS+ genes in myoclonic astatic epilepsy. Epilepsy Res. 2003;56(2-3):127–133. doi: 10.1016/j.eplepsyres.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 121.Sherman EM, Slick DJ, Connolly MB, Eyrl KL. ADHD, neurological correlates and health-related quality of life in severe pediatric epilepsy. Epilepsia. 2007;48(6):1083–1091. doi: 10.1111/j.1528-1167.2007.01028.x. [DOI] [PubMed] [Google Scholar]
- 122.Tassinari CA, Rubboli G. Cognition and paroxysmal EEG activities: from a single spike to electrical status epilepticus during sleep. Epilepsia. 2006;47(Suppl 2):40–43. doi: 10.1111/j.1528-1167.2006.00686.x. [DOI] [PubMed] [Google Scholar]
- 123.Leporte N, Sèbire G, Gillerot Y, Guerrini R, Ghariani S. Cognitive epilepsy: ADHD related to focal EEG discharges. Pediatr. Neurol. 2002;27(4):307–311. doi: 10.1016/s0887-8994(02)00441-1. [DOI] [PubMed] [Google Scholar]
- 124.Binnie CD, Kasteleijn-Nolst Trenité DG, Smit AM, Wilkins AJ. Interactions of epileptiform EEG discharges and cognition. Epilepsy Res. 1987;1(4):239–245. doi: 10.1016/0920-1211(87)90031-3. [DOI] [PubMed] [Google Scholar]
- 125.Deonna T. Rolandic epilepsy: neuropsychology of the active epilepsy phase. Epilep. Disord. 2000;2(Suppl 1):S59–61. [PubMed] [Google Scholar]
- 126.Metz-Lutz MN, Kleitz C, de Saint Martin A, Massa R, Hirsch E, Marescaux C. Cognitive development in benign focal epilepsies of childhood. Dev. Neurosci. 1999;21(3-5):182–190. doi: 10.1159/000017397. [DOI] [PubMed] [Google Scholar]
- 127.Seri S, Cerquiglini A, Pisani F. Spike-induced interference in auditory sensory processing in Landau-Kleffner syndrome. Electroencephalogr. Clin. Neurophysiol. 1998;108(5):506–510. doi: 10.1016/s0168-5597(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 128.Tassinari CA, Cantalupo G, Rios-Pohl L, Giustina ED, Rubboli G. Encephalopathy with status epilepticus during slow sleep: "The Penelope syndrome". Epilepsia. 2009;50(Suppl 7):4–8. doi: 10.1111/j.1528-1167.2009.02209.x. [DOI] [PubMed] [Google Scholar]
- 129.Parisi P, Bruni O, Villa MP, Verrotti A, Miano S, Luchetti A, Curatolo P. The relationship between sleep and epilepsy: the effect on cognitive functioning in children. Dev. Med. Child. Neurol. 2010 doi: 10.1111/j.1469-8749.2010.03662.x. DOI: 10.1111/j.1469-8749.2010.03662.x. In press. [DOI] [PubMed] [Google Scholar]
- 130.Parisi P. News on the horizon is not good: Interictal epileptic discharges continue to be unaffected by the therapeutic level of AEDs. Epilepsia. 2010;51(5):933–934. doi: 10.1111/j.1528-1167.2009.02418.x. [DOI] [PubMed] [Google Scholar]