Abstract
The present review discusses the functional and molecular diversity of GABAρ receptors. These receptors were originally described in the mammalian retina, and their functional role in the visual pathway has been recently elucidated; however new studies on their distribution in the brain and spinal cord have revealed that they are more spread than originally thought, and thus it will be important to determine their physiological contribution to the GABAergic transmission in other areas of the central nervous system. In addition, molecular modeling has revealed peculiar traits of these receptors that have impacted on the interpretations of the latest pharmacolgical and biophysical findings. Finally, sequencing of several vertebrate genomes has permitted a comparative analysis of the organization of the GABAρ genes.
Keywords: GABA receptor gene organization, Ligand Gated Ion Channel, Receptor structure, TPMPA.
DIVERSITY OF GABA RECEPTORS
GABA is one of the most important inhibitory neurotransmitters in the vertebrate central nervous system (CNS) and is involved in manifold physiological and pathological processes. As a molecule, it was first described in the early 1900’s, whereas its presence in the CNS and its role as a neurotransmitter were recognized only in the 1950’s [1-4]. During the following two decades, numerous studies were done to determine the specific mechanism of action of GABA, and in the 1960’s firm evidence established its inhibitory role in the cerebral cortex [5]. It is estimated that GABA is the most abundant inhibitory neurotransmitter in the CNS; it is widely distributed in all major areas of the brain and participates in about 40% of the inhibitory synapses in adult vertebrates [6, 7].
GABA exerts its actions through two different types of plasma membrane receptors: GABAA and GABAB, which have different pharmacological, structural, and molecular characteristics [8-10]. In the 1990´s the existence of a new class of GABAA receptor was reported: the GABAρ, and in this review we will discuss the molecular properties, tissue distribution and the structural genes coding for these GABAρ receptors. We will also present the evidence for their expression in the brain, a comparative analysis of their genomic structures, data derived from molecular modeling, and insight on their intracellular trafficking.
During the mid 1970’s several isolated reports described the existence of a type of GABA receptor that was insensitive to bicuculline, which contrasted with the antagonistic effect of this alkaloid on classical GABAA receptors. This peculiar component was also found in several areas of the CNS, including the cat spinal cord [11], frog optic tectum [12, 13], guinea pig superior colliculus [14], cerebellum, and rat cerebral cortex [15]. However, it was not until 1991 when expression of retinal mRNA in X. laevis oocytes clearly demonstrated the presence of an ionotropic GABA receptor, insensitive to bicuculline and resistant to baclofen [16]. This new receptor was named GABAρ to denote its retinal origin.
The first subunit of this receptor family was cloned from a human retina cDNA library. The gene was named GABAρ1 [17], and it codes for a receptor that is abundant in the retina; when expressed in X. laevis oocytes it exhibited the same characteristics as the receptor found after injection of retinal mRNA. To denote the clear pharmacological and molecular properties of this receptor, a new family name was coined: the GABAC receptor, which comprised at that point the GABAρ1 subunit but eventually grew to a total of three subunits: GABAρ1, GABAρ2, and GABAρ3. Currently, the name GABAC is in disuse, and the three GABAρ genes are included in the GABAA receptor family (GABAA receptor, ρ subunits); they are part of the Cys-loop superfamily of neurotransmitter receptors, also called the ligand-gated ion-channel (LGIC), which includes the GABAA receptors, nicotinic acetylcholine receptors (nAChR), glycine receptors (GlyR), ionotropic 5-HT receptors (5HT3), and a Zn2+-activated ion channel [18].
BASIC STRUCTURE, BIOPHYSICS, AND PHARMACOLOGY
Based on studies performed with other ionotropic receptors and on electrophysiological evidence, it is thought that GABAρ subunits assemble into a pentamer that forms a Cl- channel in its center. The general structure of every subunit consists of an extracellular amino terminal domain, four transmembrane domains, and an extracellular carboxy terminus. The binding site of GABA is located in the extracellular amino-terminal domain that, upon activation, leads to the opening of the ion channel with the subsequent flux of Cl- through it.
There are three very distinctive functional characteristics that are unique to the GABAρ receptor: long mean opening time of the channel, low conductance, and low rate of desensitization and the mean open time of the channel ranges from 150 to 200 ms, which is more than five-fold longer than that of other GABAA subunits [19-23].
The conductance of the human GABAρ1 channel ranges between 0.6 and 1.6 pS, and is considerably smaller than other GABAA subunits [24]. Membrane noise analysis of the cloned GABAρ receptors of the white perch showed that the conductance of GABAρ1A is 0.2 pS, that of GABAρ2A is 3.2 pS, and that of GABAρ2B is 3.5 pS [25]. It has been shown in X. laevis oocytes that after activation, GABAρ1 keeps the channel conducting even after ten minutes or more of exposure to the agonist, and the magnitude of the response diminishes only 8 to 10% during this time. Furthermore, repetitive applications of the agonist do not lead to a diminished response to subsequent applications, in clear contrast with other types of ionotropic receptors [16, 26]. In the axon terminal of the retinal bipolar neurons of the goldfish, the conductance of the GABAρ receptor is 4 pS [27], and it is 8 pS in the bipolar neurons of the rat [20]. In summary, the channel of the homomeric GABAρ receptors conducts for a longer time with respect to different heteromeric combinations of the GABAA receptors, the main-state conductances are smaller, and have a lower rate of desensitization; however, we still have to characterize these properties for GABAρ3 receptors in the ganglion neurons of the retina and neurons in several other areas of the brain that express them.
One of the most relevant characteristics is the high sensitivity to GABA, with an EC50 in the range of 0.8 to 2.2 μM for homomeric GABAρ1 and GABAρ2 recombinant receptors [16, 21, 22, 28-30] and around 7.5 μM for homomeric GABAρ3 receptors [22], which is over five-fold more sensitive than other GABAA subunits. In addition, GABAA currents on bipolar cells are rapid and transient, whereas GABAρ currents are slower and desensitize very little; furthermore, GABAρ receptors also have much higher sensitivity to GABA than typical GABAA receptors in retinal bipolar cells [31].
Four agents have been of great use for the study of GABAρ receptors due to their high selectivity. Cis-4-aminocrotonic acid (CACA) and cis-2-aminomethyl cyclopropanocarboxylic acid (CAMP) are the most selective agonists for GABAρ receptors [32], whereas the 1, 2, 5, 6-tetrahydropyridine-4 methylphosphinic acid (TPMPA) and 3-aminocyclopentyl methylphosphinic acid [(±)-cis-3-ACPMPA] are selective antagonists [33, 34]. Other antagonists reported to be highly specific for GABAρ1 are guanidine-acetic acid, amino-cyclopent-1-enyl phosphinic acid, and 3-aminocyclobutane phosphinic acid [35, 33, 5], whereras cyclothiazide blocks GABAρ2 [36]. Cis-and trans-(3-aminocyclopentanyl) butylphosphinic acid are a new generation of conformationally restricted analogues that competitively block GABAρ receptors and prevent the development of experimental myopia [37, 38]. In addition, these compounds enhance learning and memory in rats, as assesed by the reduced time that the animals take to find the platform in the Morris water maze, which suggests a role for the receptor in cognitive processes.
The action of GABA on the GABAρ receptors is allosterically modulated by a wide variety of chemical entities which interact with distinct binding sites at the GABA receptor complex [39, 40], either acting as inhibitors (Zn2+, Ni2+, Cd2+) or potentiators (Ba2+, Sr2+) [41, 42]. The lanthanides potentiate the GABA receptors in the cloned GABAρ1 receptor [41, 43].
Finally, the insensitivity to the GABAA receptor antagonist bicuculline, its resistance to the GABAB-receptor agonist baclofen [32, 35, 44, 45], and the lack of response to the GABAA-receptor modulators, such as benzodiazepines [32, 46], barbiturates, and neurosteroids [32, 47] set apart this class of receptor.
MOLECULAR MODELING
The Cys-loop receptors, a gene superfamily to which the ionotropic GABAρ receptors belong, have two conserved cysteines that form a disulfide bond [48]. Current understanding of the structural basis of how ionotropic Cys-loop receptors work is relatively advanced compared to other areas of ion channel biophysics [48-55]. Nevertheless, many questions remain, not only about biophysical properties but also about the structural basis for how these receptors affect cellular and systems physiology.
The best information about the molecular structure of the Cys-loop family comes from the nicotinic acetylcholine receptor (nAChR). This receptor forms heteropentameric complexes [14, 56-60] that have dextrohelicoidal symmetry with respect to an axis at the midpoint of the ion pathway and a tilt angle of 10º [61]. The GABAρ receptors share three main domains with all the family members. The first one is the N-terminal extracellular domain, which consists of 10 β-sheets, 2 α-helices, and one 310-helix; it is very similar to the conformation of the immunoglobulin β-sandwich domain and contains the agonist binding site and the Cys-loop. This domain also forms an extracellular ionic-preselection vestibule by means of the electrostatic potential confered by the receptor surface that is accessible to the solvent [61].
The second main domain is the pore itself, composed of the second (M2) of four transmembrane α-helices (M1–M4), whereas the remaining three (M1, M3, M4) form a “hydrophobic shelter”, protecting and incorporating the pore into the plasma membrane. The pore contains subdomains of functional importance, such as the “gate” associated with hydrophobic residues in the middle of an α-helix which is structurally distorted when the receptor is activated, leading to global conformational changes, and the “selectivity filter” which is conferred by the electrostatic restriction of charged residues in the narrowest and most intracellular region of the channel [49, 61-63].
Each transmembrane α-helix is connected with the next one through a loop; the third loop has an intracellular disposition and a variable length according to the subunit that forms receptor. Functionally, the second loop is very important and confers several structural properties, such as key interactions with the cytoskeleton, anchoring and stabilizing the receptor, and determining its cellular location; it probably also affects the ion conductance. Finally, this loop forms another α-helix just before the M4 helix; this continued loop is called the MA helix and it forms an intracellular funnel-like structure which gives rise to a vestibule through which the ions are forced to enter the cell by way of the intersubunit window-like spaces [64].
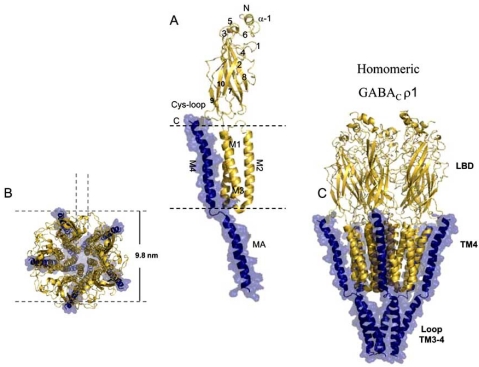
In constrast, the GABAρ receptor has proven to be difficult to overexpress and purify, and its structure has not been solved by high-resolution methods such as X-ray crystallography or nuclear magnetic resonance (NMR) [65, 66]. Thus, the homology-based modeling currently gives the most accurate and reliable structural model. It is based on the general observation that evolutionarily related (homologous) proteins are likely to have similar structures [67, 68]. We developed a model for the GABAρ receptor (Fig. 1), using the well-known structure of the close homologue, nAChR from the Torpedo marmorata, and prokaryotic homologues such as the Erwinia chrysanthemi and Gleobacter violaceus [69, 70]. These models has helped us to design experiments to determine the functional role of amino acid residues of the M4 domain [71], and it will be useful to understand the structural rearrangements generated by ligand binding which lead to the channel activation.
Fig. (1).
Structural model of the GABAρ receptors. A) Ribbon diagram of a single GABAρ1 subunit viewed in the plane of the transmembrane domains, beginning at the N-terminus of the ribbon and finishing at the C-terminus. The extracellular α-helix is labeled α-1. The β-strands are labeled 1-10; the four transmembrane α-helices (M1-M4), MA domains, and the cys-loop are highlighted. Dashed lines indicate the position of the plasma membrane. B) Transverse, and C) longitudinal representations of the GABAρ1 homopentameric complex, with respect to the plane of the plasma membrane, highlighting the main domains: extracellular domain (ECD), transmembrane domain (MD), intracellular domain (MA), and the ion pathway.
Binding and gating are mutually coupled in an allosteric way in the GABAρ1 receptor, in such a way that the binding for GABA appears strongly potentiate its affinity becoming virtually infinite, locking the agonist in the binding pocket. Channel gating thus produces broad conformational changes from the agonist binding pocket to the gating of the channel [30, 72]. A recent study has pointed out that the increase in agonist binding affinity and the channel opening do not occur simultaneously [73], but instead in two sequential steps: 1) the distortion in the binding domain, and 2) the channel opening. There is an intermediate state, termed the “flip state”, with the receptor binding domain switching to a high affinity state before channel opening. This event is well described by the Monod-Wyman-Changeux model of allosteric activation (MWC), suggesting strong similarity to the process of enzymatic catalysis [74-76].
The structural model of the agonist binding pocket was further validated and extended when the high resolution structure of the acetylcholine binding protein (AchBP) was determined [77-81]. In addition, the functional connection between the agonist binding pocket and channel opening was defined by using as template the structures of bacterial homologues [69, 70, 82]. Thus, it has been determined that tilting of M2, dilation of the pore, and a quaternary twist of the five subuints, are the key events of channel gating [83]. There are five potential binding pockets on each of the five subunit interfaces of Cys-loop receptors, and the five tyrosine residues (Tyr102, Tyr198, Tyr200, Tyr241, and Tyr247) that play a central role in the binding site are conserved in GABAρ1 [84, 85]. It is stated that the GABA carboxylate group forms a salt bridge with Arg104, Ser168, Ser243, whereas its ammonium moiety is engaged in cationπ interactions or forms a hydrogen bond with Tyr198 in the so-called ‘‘loop C’’ that connects β-sheets 9 and 10 [44, 86, 87].
To achieve the long-range communication that is fundamental to the Cys-loop receptor function, other extracellular domain residues must be involved in communicating the binding event to the channel gate, playing a crucial step in the gating pathway [88]. Different regions and residues of the Cys-loop receptor subunits are essential in channel gating: loops β1-β2, β6-β7, and β8-β9 at the interface between the extracellular domain and transmembrane domains have been identified as constitutive transducers of the opening signal from the agonist binding site to the transmembrane domain [55].
However, in spite of these advances and understanding progressively more about the structural characteristics of the receptor, further experiments will be necessary to determine the precise, detailed mechanism of allosteric regulation, which may enable the design of drugs to palliate diseases involving GABAρ receptors.
DISTRIBUTION IN THE CNS AND BEYOND
GABAρ receptors have been extensively studied in the retina of several species, where they are mainly expressed in bipolar and horizontal cells [25, 38, 89-92, 95]; at least one report shows their expression in photoreceptors [96], and for many years it was believed that their expression was limited to this structure of the nervous system. However, over the last several years evidence has accumulated showing that GABAρ receptors are, in fact, widely expressed, not only in the retina or areas related to the visual system but also in the peripheral nervous systems and even in the gastrointestinal, where the motor function within the myenteric plexus could be influenced by GABAρ activation of the inhibitory motor neurons. In the cardiovascular system GABA acts directly on cardiac function through GABA receptors located within the electrical conduction system cells [97], and in sperm cells are involved in the acrosome reaction [98].
It is now known that GABAρ subunits are expressed in the brain cortex, thalamus, hippocampus, superior colliculus, cerebellum, and spinal cord [99-104], ventral and dorsal lateral geniculate nuclei [105, 106], thyrotropin secreting cells of the pituitary [99, 107], the dorsolateral geniculate gyrus of the thalamus [108], and pretectal nucleus of the optic tract [109]. However, there are other areas of the CNS where their expression of GABAρ subunits remains to be determined or is puzzling.
Retina
Numerous studies showed that GABAρ1 and GABAρ2 were specifically expressed in the bipolar and horizontal cells of the retina, whereas GABAρ3 was found in the ganglion neurons [38, 89, 90, 92, 93, 95, 103, 110-114]. Further insight into the physiological role of these receptors in the retina was gained through the study of knock out mice for the GABAρ1 subunit [115, 116]. This subunit plays no role in retinal development since there was no evidence of anatomical alterations in the retina when its expression was eliminated. However, its elimination leads to the total absence of GABAρ responses in the internal and external plexiform layers and a defect in the transmission of visual signals from bipolar neurons to third order neurons. This suggests that, at least in the retina, the expression of GABAρ1 is necessary for the appropriate functional modulation between bipolar and ganglionar cells [31, 117-119]. Furthermore, it was recently reported that two forms of GABAρ-mediated inhibition exist in the terminals of bipolar cells and that they could be evoked by vesicular and by non-vesicular GABA release. These forms of inhibition are independently activated and regulated, which suggests a diversity of mechanisms that control the output signal of the bipolar cell terminals [119].
Midbrain and Superior Colliculus
The superfical layers of the mammalian superior colliculus (SC) are involved in processing the visual information, and it is precisely in this area that GABAρ receptors are expressed [14, 120, 121]. GABAergic interneurons in the striatum gray superficiale (SGS) and pretectal nuclear complex (PNC) have been found to express the receptor and GABA transporter 1 (GAT 1) [102, 122-124]. In situ hybridization revealed GABAρ1 and GABAρ2 in the superficial gray layer of the rat SC [125, 126]. These findings have been confirmed by immunohistochemistry and electrophysiology [102, 109, 125, 127, 128]. Furthermore, a punctate pattern of expression was found in the superficial gray layer, which suggests the synaptic localization of these receptors. Interestingly, an increase in neuronal excitability was observed after the activation of GABAρ receptors in this structure [128], in contrast to their effects in the retinal bipolar neurons [112]. Finally, GABAρ2 seems to be of particular importance in the SC, because the knockout of GABAρ1 does not eliminate GABAρ receptor responses there [129], whereas GABAρ responses are completely abolished in the retina [38]. By comparison to previously published data, this could indicate a fundamental difference between the retina and the SC in the rules that govern the expression and subunit composition of the GABAρ receptors. In the visual cortex, only the GABAρ2 subunit mRNA is expressed [102]. Finally, it has also been shown by in situ hybridization that GABAρ1 and GABAρ2 are expressed in the Stratum opticum [121], optic nerve tract [99], and superficial gray layer [130].
Thalamus
Also considered to be related to the visual system [131], the thalamus expresses the GABAρ1 receptor [100], and they have been found in several nuclei. They were located in a subpopulation of cells in the dorsolateral geniculate nucleus; the GABAρ subtype mediate 25% or more of the total current of the GABAergic ionotropic receptors predominantly, if not exclusively, expressed by local GABAergic interneurons [132]. GABAρ receptors are also expressed in neurons of the tectothalamic projection [121] and nucleus habenularis medialis [127].
Brain Cortex
There is very little information about GABAρ receptors in this structure. While the expression of GABAA receptors is well known and in general, the GABAergic system plays a major role in the inhibitory functions of the cerebral cortex, the expression of GABAρ receptors in this vast area of intricate neuronal networks is not well understood. By RT-PCR it has been possible to identify the GABAρ2 subunit in the frontal, temporal, occipital, and parietal areas of the human cortex, whereas GABAρ1 was found only in the frontal and temporal cortex [100]. It has also been demonstrated by in-situ hybridization in the rat brain cortex that GABAρ subunits are localized in the visual area of the occipital cortex in layers I-III [104, 121]. However, there are no data to confirm these findings or give further insight into the cellular localization of these receptors in the cortex.
Basal Ganglia
Evidence about the expression of GABAρ receptors in this structure is still limited. So far, the expression of GABAρ3 mRNA has been shown in rat [94] and of GABAρ1 and GABAρ2 in bovine striatum, and potential alternatively spliced variants of the GABAρ1 subunit were identified in this species [133, 134]. The presence of GABAρ in the basal ganglia is an interesting result, since the GABAergic system in this region is well known to be involved in movement control, and GABAρ receptors were found in spiny pyramidal neurons of the dorsal caudate putamen [134]. However, the electrophysiological responses to GABA in this structure are consistent with the more common GABAA receptors [58]. Further research will be necessary to clarify the expression and precise cellular localization of GABAρ subunits and in addition, it would be necessary to explain the lack of GABAρ receptor-like currents. One possibility could be a co-assembly of GABAρ with GABAA subunits [8, 95, 135, 136], which would mask the typical GABAρ pharmacology, but such complexes have not yet been reported in this area of the brain.
Pituitary
Evidence of the expression of GABAρ1, GABAρ2, and GABAρ3 has been found in the pituitary of rat and guinea pig, including localization in somatrophs, gonadrops, and in an adenoma cell line (GH3) that was derived from this gland [99, 107, 133]. Interestingly, the GABA currents found in TSH cells are insensitive to bicuculline and resistant to baclofen, but they desensitize rapidly like a classic GABAA receptor [99]. Other evidence suggests a specific role of GABAρ receptors in the facilitation of prolactin secretion from anterior pituitary cells [137].
Cerebellum
One of the first clues to the existence of GABAρ receptors was obtained from binding studies with tritriated baclofen and GABA, which showed the presence of GABA and CACA sites that are insensitive to bicuculine and resistant to baclofen [138]. Later on, GABAρ1 and GABAρ2 were detected in human cerebellum with higher expression of GABAρ2 [127, 100]. Finally, GABAρ1-3 mRNAs subunits and GABAρ-like currents were reported in X. laevis oocytes injected with mRNA of cerebellar cortex [139], in agreement with previous evidence for the presence of mRNA in the Purkinje cell line and basket cells [103, 133], where a role for GABAρ receptors in the phasic inhibition at interneuron-Purkinje cell synapses has been suggested [140].
Brain Stem and Spinal Cord
There is clear evidence for the expression of GABAρ receptors in the spinal cord and brain stem as well as their co-assembly with other GABAA subunits [45, 99, 120, 133, 141, 142], which suggests the formation by GABAA and GABAρ of functional, heteromeric receptors [120, 135, 134]. These studies also indicate that the mRNA of GABAρ receptors is more abundant in the spinal cord than in the brainstem.
Corpus Callosum
There is little evidence supporting the expression of GABAρ receptors in the comissures, where the population of neurons is very low and the main cell types are neuroglia. The expression of GABAρ1 mRNA was demonstrated [133]; however, its function has not been proved, and GABA currents reported in several studies show only the classic GABAA component [143].
Hippocampus
Initially, the expression of GABAρ receptors in this area was suggested by the transient expression of a bicuculline-insensitive, GABA-gated Cl- channel during early development [144]. Later on, several studies reported the localization and electrophysiological expression of GABAρ receptors from both neurons in culture and slices of pyramidal and granule cells of hippocampus [99, 101, 106, 145-148]. Recent reports have suggested that activated GABAρ receptors help to protect against neurotoxicity in hippocampal cultures [149].
Amygdala
GABAρ receptors have been detected in several areas of the amygdala, including the lateral, basolateral, and central nuclei [150, 151]. Likewise, it has been reported postsynaptic GABAρ receptors in this area [152]. Neurons in the lateral division of the central amygdala have two types of fast inhibitory synapses with either GABAA or GABAρ receptors but targeted to spatially and functionally distinct synapses [152]. Recordings in the lateral division of central amygdala showed miniature inhibitory postsynaptic currents (mIPSC) with small amplitude and slow rise-time whose frequency indicated the presence of GABAρ receptors [153].
Invertebrates
Ionotropic GABA receptors described in invertebrates have characteristics that do not fit completely with those of vertebrate GABAρ receptors. The GABA-gated cation channel EXP-1, cloned from C. elegans [154], belongs to the LGIC superfamily and exhibits sequence homology to the Drosophila LCCH3 and GRD subunits. Interestingly, the Rdl (resistant to dieldrin) subunit of Drosophila displays 43 % homology at the amino acid level to the rat GABAρ1 subunit [155], but the functional and pharmacological characteristics are quite different. Analysis of the Drosophila gene sequences predicts one additional candidate, AAF48539 [156], and one more was predicted in the genome of one cnidarian Hydra magnipapillata, Gene ID 100201086 [157]. However, to date, no invertebrate receptor subunit has been characterized as a clear homolog of vertebrate GABAρ.
GENOMIC STRUCTURE OF THE GABAρ GENES AND INSIGHT INTO THEIR EVOLUTION
Completion of the sequence of several vertebrate genomes, including human and chimpanzee as well as other organisms such as the tunicate Ciona intestinalis, allowed defining the distribution and the intron-exon structure of the genes coding for ionotropic GABA-receptor subunits. Mapping and sequencing of GABAA subunit genes showed that they are organized in gene clusters composed of α, β, and γ subunit genes [68, 158-161]. It is generally considered that the evolution of the myriad of genes encoding for GABAA receptors comprised a number of events in the early evolution of the chordate lineage: first, a whole-genome duplication gave rise to the loci in at least two chromosomes; this was followed by a tandem duplication of an α-subunit gene, after which a second whole-genome duplication occurred [162]. These events could account both for the multiple isoforms of α, β, and γ subunits as well as for their localization in different chromosomes [158, 161].
The GABAρ coding-genes are considered to be very closely related to the GABAA- and Glycine-receptor genes (about 40% nucleotide sequence homology), and it has been proposed that the two families share a common ancestor [162]. However, the evolutionary history and structural organization of the genes encoding the three GABAρ subunits has been little explored [159, 163].
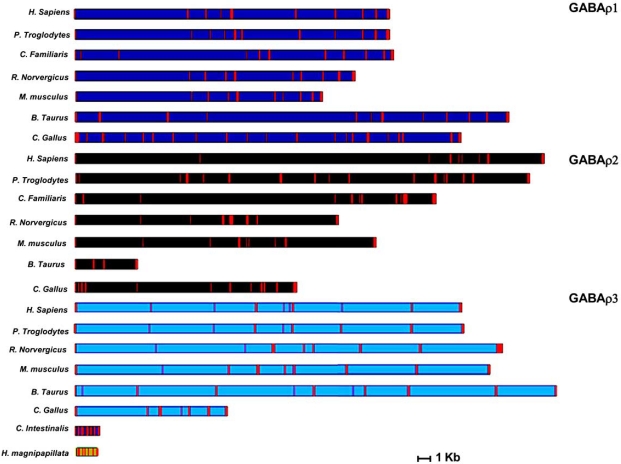
Two of the three genes encoding for GABAρ subunits (GABAρ1 and GABAρ2) are located on chromosome 6q14-q21 of the human genome [163], whereas GABAρ3 is found on chromosome 3q11-q13 [159]. DNA sequence comparisons among several completed genome sequences allowed us to determine the localization of GABAρ genes and several other GABAρ-like loci. The exon-intron composition of these genes, as predicted by computer analysis of intron-exon boundaries, is shown in Fig. (2). The DNA coding sequence homology between human GABAρ subunits and other vertebrates ranges from 82% for the fish Morone americana to 99% for chimpanzee Pan troglodytes.
Fig. (2).
Comparison of the exon and intron structure of GABAρ genes. Relative positions of the coding and non-coding sequences are shown to scale. Exons are illustrated in red for all the genes. Notice the compact structure of the C. intestinalis GABAρ- like gene.
The chromosome localization of the GABAρ genes within the genomes of higher vertebrates is clearly syntenic and matches their human and murine distribution: GABAρ1 and GABAρ2 are found as a single cluster, whereas GABAρ3 is located on a different chromosome.
Analysis of the genomic sequence of the tunicate C. intestinalis [164], revealed a GABAρ-like gene on chromosome 4. Although a full functional analysis of the structural gene is necessary, as well as of the receptor itself, it is clear that this gene is closely related to the GABAρ subunits [165]. We could not identify a second GABAρ-like gene, and an exhaustive search is needed in this and other tunicates in order to reconcile this observation with the hypothesis of the timing of the first vertebrate genome duplication that is thought to have occurred after the divergence of the tunicates and prior to the appearance of the jawed fish [166, 167].
Another interesting feature about the comparison of GABAρ genes is their structural variation among different phylogenetic lineages. The number of exons for the three genes is generally between 8 and 10; however, two interesting exceptions occur: 1) the predicted number of exons for GABAρ2 of Pan troglodytes is 16, whereas the human gene has only 8. Nevertheless, the nucleotide sequences of the coding regions are 99% identical for the two species. The unique distribution and number of exons in the chimpanzee GABAρ2 raises the possible existence of splicing isoforms of its mRNAs. 2) An extreme example of a radically different gene structure is that of Gallus gallus. In this species, the number of exons is 21 for GABAρ1, whereas GABAρ2 and GABAρ3 have 12 and 7, respectively.
Despite all the differences in the gene structure of GABAρ receptors, it is worth mentioning that the amino acid sequence of the three receptors is highly conserved among the different taxa evaluated here. With its ancient origin, C. intestinalis represents the most divergent amino acid sequence of all chordates, about 40% homologous to the human GABAρ1.
The cluster-organization of genes may be of developmental and physiological relevance, contributing to their time- and cell-specific expression. Support for this idea is provided by the coordinated expression of GABAρ1 and GABAρ2 during early retinal development, where both subunit genes are transcriptionally active in the bipolar neurons at postnatal day 9, whereas GABAρ3 is expressed in a population of ganglion neurons [111, 141]. Based on the genomic arrangement of GABAρ genes, future experimental approaches will define the role of the cluster organization within the transcription regulatory context as well as the possible implications of the exon/intron composition of the genes in generating mRNA variants. Furthermore, sequence analysis of the upstream regions of each gene could suggest conserved motifs important for their expression in the retina.
SUBCELLULAR DISTRIBUTION
The precise location of the receptor channels in the synaptic cleft is a determinant for them to play their essential role in transmitting the synaptic impulse. The GABAρ receptors have been detected in the synaptic axon terminal of the bipolar neuron of the retina [90, 31, 93, 27, 114, 168]. Here, they modulate the surround and lateral inhibition [169]. We do not yet know how the receptor reaches this terminal end, and we have ignored the cellular and molecular components that dictate the trafficking of the receptor from its point of translation and towards the axon terminal.
Numerous interactions within the synapse organization regulate protein localization [170, 171], mediating the proper clustering and anchoring of receptors to the cytoskeleton. For some years, it has been proposed that GABAρ receptors associate with the Microtubule-Associated Protein 1B (MAP1B) through the interaction with the second intracellular loop of the GABAρ1 subunit [172]. In contrast, Meixner [173] showed that disruption of MAP1B is not a crucial element in the expression and localization of the receptor. Controversy emerged once again when Billups [174] demonstrated the association of MAP-1B with two key residues (KY) in the large intracellular loop of GABAρ1, and showed that this interaction modulates the mean open-time and kinetics of the channel.
Those contrasting observations are not definitive and the saga will continue until precise experiments define the association between GABAρ receptors and cytoskeleton. On the other hand, an interaction proposed between GABAρ1 and the cellular retinoic acid receptor could serve as a link between the GABA signaling pathway and the control of gene expression in neurons [175]; however, this has not been proven experimentally.
Receptors tagged with fluorescent labels will certainly be major tools to determine associations between the GABAρ and molecular and cellular components and to follow the trafficking of receptors in the neuron [176-178]. Likewise, the fact that the trafficking of the receptor may be actually visualized by using real time fluorescence microscopy from the site of protein translation to its final destination at the plasma membrane could shed some light on the dynamics and mechanisms that control receptor recycling. Many of these processes are currently being explored for GABAA receptors [179, 180].
Since GABAρ receptor trafficking may be an important control point for regulating cell and network activity in the SNC and retina, new experimental approaches will help to answer emerging questions such as: What are the mechanisms involved in the liberation and/or selective retention that contribute to maintaining the dynamics and polarized distribution of the GABAρ receptors in neurons? Is the endocytic system a station of direct distribution versus liberation and/or is there a necessary step for the accumulation of the receptors, as well as of a new population of receptors expressed de novo? Is the distribution of receptors mediated by the differential distribution of proteins of membrane and/or cytoskeleton in the different neuronal compartments?
PERSPECTIVES
More studies are necessary to determine the precise distribution of GABAρ receptors in the central and peripheral nervous systems as well as in other organs and systems where they are expressed, such as the heart, the liver and the gastrointestinal tract [52, 99, 181]. We already know the location of the receptor in bipolar neurons of the retina, but future studies will disclose the cellular localization and function of the GABAρ receptors in other areas of the CNS such as the caudate nucleus, bulb, cerebellum, pons, and corpus callosum. The possible expression and role of alternatively spliced variants of these receptors in different areas of the CNS must also be examined [26, 99, 109, 133].
All of the evidence regarding the widespread distribution of GABAρ receptors suggests that they may be involved in more functions than previously thought, and we still have to determine their physiological relevance. For example, it has been shown that TPMPA, the selective antagonist of GABAρ receptors, increases both quiet and active waking, decreases total slow-wave sleep essentially by decreasing the slow-wave stage, and also decreases paradoxical sleep [9]. In the hippocampus, these receptors could also have a role in memory processes, as already suggested by some experiments [107]. According to the findings of several studies that located GABAρ receptors in areas such as spinal cord motoneurons, Purkinje cells of the cerebellar cortex, bulb, pons and caudate nucleus, they could also play an important role in movement control [103, 133]. Furthermore, the expression of GABAρ receptors in the amygdala [73, 182] and its possible correlation with anxiety should be considered. Alterations of GABA-signaling have been associated with alcohol dependence, and a recent study revealed single nucleotide polymorphisms in GABAρ2 that is associated with this dependance [183].
One of the most interesting developments on GABAρ receptors research is the possible therapeutic applications that these receptors may have. In a model of hepatic encephalopathy that affects hippocampal neurons, the stimulation of GABAρ receptors reduces ammonia-induced accumulation of Cl-, leading to decreased hyperexcitability [147]. More recently, it has been described an association of polymorphisms in the GABAρ2 gene with serum creatinine levels, an important biomarker for assessment of kidney function [184], thus proposing a potential role of GABAρ receptors in the regulation of renal function. These are only a few of many potential regulatory roles of GABAρ receptors and its participation in different physiological processes [10, 58, 83, 97, 98, 132, 152, 181, 183-188]. On the other hand, expression mediated by adenovirus of GABAρ receptors suppressed neuronal hyperexcitability and the associated neuronal death [189] and therefore, virus-mediated neuronal GABAρ receptor expression offers a potential therapeutic tool for directly inhibiting hyperexcited neurons responsible for clinical problems, thereby avoiding the generalized nervous system depression associated with pharmacological therapy [189]. Thus, it will be important to know the precise regulatory mechanism that activates the expression of the receptor in the proper cell types. Furthermore, the potential of adenovirus to express tagged receptors in living cells may allow the tracking of the localization and dynamics [189, 190].
ACKNOWLEDGMENTS
GM-D and AE-M thank support from CONACYT-MEXICO. AMT and RM acknowledge support from PAPIIT-UNAM and CONACYT (101851). We want to thank to D. Pless for editing the manuscript.
REFERENCES
- 1.Awapara J, Landua AJ, Fuerst R, Seale B. Free gamma-aminobutyric acid in brain. J. Biol. Chem. 1950;187:35–9. [PubMed] [Google Scholar]
- 2.Florey E. An inhibitory and an excitatory factor of mammalian central nervous system, and their action of a single sensory neuron. Arch. Int. Physiol. 1954;62:33–53. doi: 10.3109/13813455409145367. [DOI] [PubMed] [Google Scholar]
- 3.Krnjevic´ K. Chemical nature of synaptic tTransmission in Vertebrates. Physiol. Rev. 1974;54:418–540. [Google Scholar]
- 4.Roberts E, Frankel S. gamma-aminobutyric acid in brain: its formation from glutamic acid. J. Biol. Chem. 1950;187:55–63. [PubMed] [Google Scholar]
- 5.Krnjevic´ K, Phillis JW. Iontophoretic studies of neurons in the mammalian cerebral cortex. J. Physiol. 1963;165:274–304. doi: 10.1113/jphysiol.1963.sp007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kandel E.R, Schwartz J, Jessel T. Principles of Neural Science. 3USA, McGraw-Hill Companies Inc; 2000. [Google Scholar]
- 7.Kumar R.J, Chebib M, Hibbs D.E, Kim H.L, Johnston G.A, Salam N.K, Hanrahan J.R. Novel gamma-aminobutyric acid rho1 receptor antagonists; synthesis, pharmacological activity and structure-activity relationships. J. Med. Chem. 2008;51:3825–40. doi: 10.1021/jm7015842. [DOI] [PubMed] [Google Scholar]
- 8.Bormann J. The 'ABC' of GABA receptors. Trends Pharmacol. Sci. 2000;21:16–19. doi: 10.1016/s0165-6147(99)01413-3. [DOI] [PubMed] [Google Scholar]
- 9.Enz R. GABA(C) receptors: a molecular view. Biol. Chem. 2001;382:1111–22. doi: 10.1515/BC.2001.141. [DOI] [PubMed] [Google Scholar]
- 10.Martin D.L, Olsen R.W. GABA in the nervous system: the view at fifty years. Philadelphia, PA: Lippincott W & Wilkins; 2000. [Google Scholar]
- 11.Johnston G.A, Curtis D.R, Beart P.M, Game C.J, McCulloch R.M, Twitchin B. Cis- and trans-4-aminocrotonic acid as GABA analogues of restricted conformation. J. Neurochem. 1975;24:157–160. doi: 10.1111/j.1471-4159.1975.tb07642.x. [DOI] [PubMed] [Google Scholar]
- 12.Nistri A, Sivilotti L. An unusual effect of gamma-aminobutyric acid on synaptic transmission of frog tectal neurones in vitro. Br. J. Pharmacol. 1985;85:917–921. doi: 10.1111/j.1476-5381.1985.tb11092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sivilotti L, Nistri A. Pharmacology of a novel effect of ã-aminobutyric acid on the frog optic tectum in vitro. Eur. J. Pharmacol. 1989;164:205–212. doi: 10.1016/0014-2999(89)90460-3. [DOI] [PubMed] [Google Scholar]
- 14.Arakawa T, Okada Y. Excitatory and inhibitory action of GABA on synaptic transmission in slices of guinea pig superior colliculus. Eur. J. Pharmacol. 1988;158:217–224. doi: 10.1016/0014-2999(88)90070-2. [DOI] [PubMed] [Google Scholar]
- 15.Drew C.A, Johnston G.A. Bicuculline- and baclofen-insensitive gamma-aminobutyric acid binding to rat cerebellar membranes. J. Neurochem. 1992;58:1087–1092. doi: 10.1111/j.1471-4159.1992.tb09366.x. [DOI] [PubMed] [Google Scholar]
- 16.Polenzani L, Woodward R.M, Miledi R. Expression of mammalian gamma-aminobutyric acid receptors with distinct pharmacology in Xenopus oocytes. Proc. Natl. Acad. Sci. 1991;88:4318–4322. doi: 10.1073/pnas.88.10.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cutting G.R, Lu L, O'Hara B.F, Kasch L.M, Montrose-Rafizadeh C, Donovan D.M, Shimada S, Antonarakis S.E, Guggino W.B, Uhl G.R, Uhl G.H. Jr. K. Cloning of the gamma-aminobutyric acid (GABA) rho 1 cDNA: a GABA receptor subunit highly expressed in the retina. Proc. Natl. Acad. Sci. 1991;88:2673–2677. doi: 10.1073/pnas.88.7.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsen R.W, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol. Rev. 2008;60:243–60. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chebib M. GABAc receptor ion channels. Clin. Exp. Pharmacol. Physiol. 2004;31:800–804. doi: 10.1111/j.1440-1681.2004.04083.x. [DOI] [PubMed] [Google Scholar]
- 20.Feigenspan A, Bormann J. Differential pharmacology of GABAA and GABAC receptors on rat retinal bipolar cells. Eur. J. Pharmacol. 1994;288:97–104. doi: 10.1016/0922-4106(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 21.Zhang D, Pan Z.H, Zhang X, Brideau A.D, Lipton S.A. Cloning of a gamma-aminobutyric acid type C receptor subunit in rat retina with a methionine residue critical for picrotoxinin channel block. Proc. Natl. Acad. Sci. 1995;92:11756–60. doi: 10.1073/pnas.92.25.11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang D, Pan Z.H, Awobuluyi M, Lipton S.A. Structure and function of GABA(C) receptors: a comparison of native versus recombinant receptors. Trends Pharmacol. Sci. 2001;22:121–32. doi: 10.1016/s0165-6147(00)01625-4. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Y, Ripps H, Qian H. A single amino acid in the second transmembrane domain of GABA rho receptors regulates channel conductance. Neurosci. Lett. 2007;418:205–9. doi: 10.1016/j.neulet.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wotring V.E, Chang Y, Weiss D.S. Permeability and single channel conductance of human homomeric rho1 GABAC receptors. J. Physiol. 1999;521:327–36. doi: 10.1111/j.1469-7793.1999.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian H, Pan Y. Co-assembly of GABA rho subunits with the GABA(A) receptor gamma(2) subunit cloned from white perch retina. Brain Res. Mol. Brain Res. 2002;103:62–70. doi: 10.1016/s0169-328x(02)00140-7. [DOI] [PubMed] [Google Scholar]
- 26.Martínez-Torres A, Vazquez A.E, Panicker M.M, Miledi R. Cloning and functional expression of alternative spliced variants of the rho1 gamma-aminobutyrate receptor. Proc. Natl. Acad. Sci. 1998;95:4019–22. doi: 10.1073/pnas.95.7.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer M.J. Functional segregation of synaptic GABAA and GABAC receptors in goldfish bipolar cell terminals. J. Physiol. 2006;577:45–53. doi: 10.1113/jphysiol.2006.119560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alakuijala A, Talvi-Oja K, Pasternack A, Pasternack M. Functional characterization of rat rho2 subunits expressed in HEK 293 cells. Eur. J. Neurosci. 2005;21:692–700. doi: 10.1111/j.1460-9568.2005.03880.x. [DOI] [PubMed] [Google Scholar]
- 29.Chang Y, Covey D.F, Weiss D.S. Correlation of the apparent affinities and efficacies of gamma-aminobutyric acid(C) receptor agonists. Mol. Pharmacol. 2000;58:1375–80. doi: 10.1124/mol.58.6.1375. [DOI] [PubMed] [Google Scholar]
- 30.Chang Y, Weiss D.S. Channel opening locks agonist onto the GABAc receptor. Nat. Neurosci. 1999;2:219–25. doi: 10.1038/6313. [DOI] [PubMed] [Google Scholar]
- 31.Jones S.M, Palmer M.J. Activation of the Tonic GABAC Receptor Current in Retinal Bipolar Cell Terminals by Non-Vesicular GABA Release. J. Neurophysiol. 2009;102:691–9. doi: 10.1152/jn.00285.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodward R.M, Polenzani L, Miledi R. Characterization of bicuculline/baclofen (rho-like) gamma-aminobutyric acid receptors expressed in Xenopus oocytes. II. Pharmacology of gamma-aminobutyric acid A and gamma-aminobutyric acid B receptor agonists and antagonists. Mol. Pharm. 1993;43:609–625. [PubMed] [Google Scholar]
- 33.Chebib M, Hanrahan J.R, Kumar R.J, Mewett K.N, Morriss G, Wooller S, Johnston G.A. (3-Aminocyclopentyl) methylphosphinic acids: novel GABA(C) receptor antagonists. Neuropharmacology. 2007;52:779–87. doi: 10.1016/j.neuropharm.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Ragozzino D, Woodward R.M, Murata Y, Eusebi F, Overman L.E, Miledi R. Design and in vitro pharmacology of a selective gamma-aminobutyric acidC receptor antagonist. Mol. Pharmacol. 1996;50:1024–30. [PubMed] [Google Scholar]
- 35.Chebib M, Johnston G.A. GABA-Activated ligand gated ion channels: medicinal chemistry and molecular biology. J. Med. Chem. 2000;43:1427–47. doi: 10.1021/jm9904349. [DOI] [PubMed] [Google Scholar]
- 36.Xie A, Song X, Ripps H, Qian H. Cyclothiazide: a subunit-specific inhibitor of GABAC receptors. J. Physiol. 2008;586:2743–52. doi: 10.1113/jphysiol.2008.153346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chebib M, Gavande N, Wong K.Y, Park A, Premoli I, Mewett K.N, Allan R.D, Duke R.K, Johnston G.A, Hanrahan J.R. Guanidino Acids Act as ρ1 GABAC Receptor Antagonists. Neurochem. Res. 2009;34:1704–11. doi: 10.1007/s11064-009-9968-x. [DOI] [PubMed] [Google Scholar]
- 38.McCall M.A, Lukasiewicz P.D, Gregg R.G, Peachey N.S. Elimination of the rho1 subunit abolishes GABA(C) receptor expression and alters visual processing in the mouse retina. J.Neurosci. 2002;22:4163–74. doi: 10.1523/JNEUROSCI.22-10-04163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hansen S.L, Fjalland B, Jackson M.B. Differential modulation of the gamma-aminobutyric acid type C receptor by neuroactive steroids. Mol. Pharmacol. 1999;56:752–9. [PubMed] [Google Scholar]
- 40.Kaneda M, Mochizuki M, Aoki K, Kaneko A. Modulation of GABAC response by Ca2+ and other divalent cations in horizontal cells of the catfish retina. J. Gen. Physiol. 1997;110:741–7. doi: 10.1085/jgp.110.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calvo D.J, Vazquez A.E, Miledi R. Cationic modulation of rho-1 type gamma-aminobutyrate receptors expressed in Xenopus oocytes. Proc. Natl. Acad. Sci. 1994;91:2725–12729. doi: 10.1073/pnas.91.26.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaneda M, Andrasfalvy B, Kaneko A. Modulation by Zn2+ of GABA responses in bipolar cells of the mouse retina. Vis. Neurosci. 2000;17:273–81. doi: 10.1017/s0952523800172098. [DOI] [PubMed] [Google Scholar]
- 43.Goutman J.D, Escobar A.L, Calvo D.J. Analysis of macroscopic ionic currents mediated by GABAρ1 receptors during lanthanide modulation predicts novel states controlling channel gating. Br. J. Pharmacol. 2005:1–10. doi: 10.1038/sj.bjp.0706411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdel-Halim H, Hanrahan J.R, Hibbs D.E, Johnston G.A, Chebib M. A molecular basis for agonist and antagonist actions at GABA(C) receptors. Chem. Biol. Drug Des. 2008;71:306–27. doi: 10.1111/j.1747-0285.2008.00642.x. [DOI] [PubMed] [Google Scholar]
- 45.Park J.S, Higashi H, Nagata K, Yoshimura M. Bicuculline-resistant, Cl- dependent GABA response in the rat spinal dorsal horn. Neurosci. Res. 1999;33:261–8. doi: 10.1016/s0168-0102(99)00016-4. [DOI] [PubMed] [Google Scholar]
- 46.Amin J. A single hydrophobic residue confers barbiturate sensitivity to gamma-aminobutyric acid type C receptor. Mol. Pharmacol. 1999;3:411–23. [PubMed] [Google Scholar]
- 47.Morris K.D, Moorefield C.N, Amin J. Differential modulation of the gamma-aminobutyric acid type C receptor by neuroactive steroids. Mol. Pharmacol. 1999;56:752–9. [PubMed] [Google Scholar]
- 48.Connolly C.N, Wafford K.A. The Cys-loop superfamily of ligand-gated ion channels: the impact of receptor structure on function. Biochem. Soc. Trans. 2004;32:529–534. doi: 10.1042/BST0320529. [DOI] [PubMed] [Google Scholar]
- 49.Absalom N.L, Lewis T.M, Schofield P.R. Mechanisms of channel gating of the ligand-gated ion channel superfamily inferred from protein structure. Exp. Physiol. 2004;89:145–153. doi: 10.1113/expphysiol.2003.026815. [DOI] [PubMed] [Google Scholar]
- 50.Halliwell R.F. A short history of the rise of the molecular pharmacology of ionotropic drug receptors. Trends Pharmacol. Sci. 2007;28:214–219. doi: 10.1016/j.tips.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 51.Hogg R.C, Raggenbass M, Bertrand D. Nicotinic acetylcholine receptors: from structure to brain function. Rev. Physiol. Biochem. Pharmacol. 2003;147:1–46. doi: 10.1007/s10254-003-0005-1. [DOI] [PubMed] [Google Scholar]
- 52.Jensen A.A, Frølund B, Liljefors T, Krogsgaard-Larsen P. Neuronal nicotinic acetylcholine receptors: structural revelations, target identifications, and therapeutic inspirations. J. Med. Chem. 2005;48:4705–4745. doi: 10.1021/jm040219e. [DOI] [PubMed] [Google Scholar]
- 53.Lester H.A, Dibas M.I, Dahan D.S, Leite J.F, Dougherty D.A. Cys-loop receptors: new twists and turns. Trends Neurosci. 2004;27:329–336. doi: 10.1016/j.tins.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Sine S.M, Engel A.G. Recent advances in Cys-loop receptor structure and function. Nature. 2006;440:448–455. doi: 10.1038/nature04708. [DOI] [PubMed] [Google Scholar]
- 55.Wells G.B. Structural answers and persistent questions about how nicotinic receptors work. Front Biosci. 2008;13:5479–510. doi: 10.2741/3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng X, Ivanov I, Wang H, Sine S.M, McCammon J.A. Molecular-dynamics simulations of ELIC-a prokaryotic homologue of the nicotinic acetylcholine receptor. Biophys. J. 2009;96:4502–13. doi: 10.1016/j.bpj.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Enz R, Cutting GR. Molecular composition of GABAC receptors. Vision Res. 1998;38:1431–41. doi: 10.1016/s0042-6989(97)00277-0. [DOI] [PubMed] [Google Scholar]
- 58.Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nat. Rev. Neurosci. 2002;3:102–114. doi: 10.1038/nrn731. [DOI] [PubMed] [Google Scholar]
- 59.Ortells M.O, Lunt G.G. Evolutionary history of the ligand-gated ion-channel superfamily of receptors. Trends Neurosci. 1995;18:121–127. doi: 10.1016/0166-2236(95)93887-4. [DOI] [PubMed] [Google Scholar]
- 60.Sivilotti L, Colquhoun D. Acetylcholine receptors: too many channels, too few functions. Science. 1995;269:1681–1682. doi: 10.1126/science.7569892. [DOI] [PubMed] [Google Scholar]
- 61.Unwin N. Structure of the acetylcholine-gated channel. Novartis Found. Symp. 2002;245:5–15. [PubMed] [Google Scholar]
- 62.Corringer P.J, Le Novère N, Changeux J.P. Nicotinic receptors at the amino acid level. Annu. Rev. Pharmacol. Toxicol. 2000;40:431–458. doi: 10.1146/annurev.pharmtox.40.1.431. [DOI] [PubMed] [Google Scholar]
- 63.Song C, Corry B. Computational study of the transmembrane domain of the acetylcholine receptor. Eur. Biophys. J. 2009 doi: 10.1007/s00249-009-0476-3. [ahead in print] [DOI] [PubMed] [Google Scholar]
- 64.Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J. Mol. Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 65.Berman H, Henrick K, Nakamura H. Announcing the worldwide protein data bank. Nat. Struct. Biol. 2003;10:980. doi: 10.1038/nsb1203-980. [DOI] [PubMed] [Google Scholar]
- 66.Tai K, Fowler P, Mokrab Y, Stansfeld P, Sansom M.S. Molecular modeling and simulation studies of ion channel structures, dynamics and mechanisms. Methods Cell Biol. 2009;90:233–265. doi: 10.1016/S0091-679X(08)00812-1. [DOI] [PubMed] [Google Scholar]
- 67.Chothia C, Lesk A.M. The relation between the divergence of sequence and structure in proteins. EMBO J. 1986;5:823–6. doi: 10.1002/j.1460-2075.1986.tb04288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simon J, Wakimoto H, Fujita N, Lalande M, Barnard E. Analysis of the Set of GABAa receptor genes in the human genome. J. Biol. Chem. 2004;279:41422–41435. doi: 10.1074/jbc.M401354200. [DOI] [PubMed] [Google Scholar]
- 69.Bocquet N, Nury H, Baaden M, Le Poupon C, Changeux J.P, Delarue M, Corringer P.J. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature. 2009;457:111–4. doi: 10.1038/nature07462. [DOI] [PubMed] [Google Scholar]
- 70.Hilf R.J, Dutzler R. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature. 2008;452:375–9. doi: 10.1038/nature06717. [DOI] [PubMed] [Google Scholar]
- 71.Reyes-Ruiz J.M, Ochoa-de la Paz L.D, Martínez-Torres A, Miledi R. Functional impact of serial deletions at the C-terminus of the human GABArho1 receptor. Biochim. Biophys. Acta. 2010;5:1002–7. doi: 10.1016/j.bbamem.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 72.Colquhoun D. GABA and the single oocyte: relating binding to gating. Nat. Neurosci. 1999;2:201–2. doi: 10.1038/6298. [DOI] [PubMed] [Google Scholar]
- 73.Lape R, Colquhoun D, Sivilotti L.G. On the nature of partial agonism in the nicotinic receptor superfamily. Nature. 2008;454:722–7. doi: 10.1038/nature07139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Changeux J.P, Edelstein S.J. Allosteric receptors after 30 years. Neuron. 1998;21:959–80. doi: 10.1016/s0896-6273(00)80616-9. [DOI] [PubMed] [Google Scholar]
- 75.Edelstein S.J, Changeux J.P. Allosteric proteins after thirty years: the binding and state functions of the neuronal α7 nicotinic acetylcholine receptors. Experientia. 1996;52:1083–90. doi: 10.1007/BF01952106. [DOI] [PubMed] [Google Scholar]
- 76.Monod J, Wyman J, Changeux J.P. On the nature of allosteric proteins: a plausible model. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 77.Brejc K, van Dijk W.J, Klaassen R.V, Schuurmans M, van Der Oost J, Smit A.B, Sixma T.K. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–76. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- 78.Celie P.H, Klaassen R.V, van Rossum-Fikkert S.E, van Elk R, van Nierop P, Smit A.B, Sixma T.K. Crystal structure of acetylcholine-binding protein from Bulinus truncatus reveals the conserved structural scaffold and sites of variation in nicotinic acetylcholine receptors. J. Biol. Chem. 2005;280:26457–66. doi: 10.1074/jbc.M414476200. [DOI] [PubMed] [Google Scholar]
- 79.Celie P.H, van Rossum-Fikkert S.E, van Dijk W.J, Brejc K, Smit A.B, Sixma T.K. Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron. 2004;41:907–14. doi: 10.1016/s0896-6273(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 80.Chang Y.C, Wu W, Zhang J.L, Huang Y. Allosteric activation mechanism of the cys-loop receptors. Acta Pharmacol. Sin. 2009;30:663–72. doi: 10.1038/aps.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hansen S.B, Sulzenbacher G, Huxford T, Marchot P, Taylor P, Bourne Y. Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. EMBO J. 2005;24:3635–46. doi: 10.1038/sj.emboj.7600828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hilf R.J, Dutzler R. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature. 2009;457:115–8. doi: 10.1038/nature07461. [DOI] [PubMed] [Google Scholar]
- 83.Chebib M, Hinton T, Schmid K.L, Brinkworth D, Qian H, Matos S, Kim H.L, Abdel-Halim H, Kumar R.J, Johnston G.A, Hanrahan J.R. Novel, potent, and selective GABAC antagonists inhibit myopia development and facilitate learning and memory. J. Pharmacol. Exp. Ther. 2009;328:448–57. doi: 10.1124/jpet.108.146464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harrison N.J, Lummis S.C. Molecular modeling of the GABAC receptor ligand binding domain. J. Mol. Model. 2005;12:317–324. doi: 10.1007/s00894-005-0034-6. [DOI] [PubMed] [Google Scholar]
- 85.Sedelnikova A, Smith C.D, Zakharkin S.O, Davis D, Weiss D.S, Chang Y. Mapping the r1 GABAC receptor agonist binding pocket. J. Biol. Chem. 2005;280:1535–1542. doi: 10.1074/jbc.M409908200. [DOI] [PubMed] [Google Scholar]
- 86.Lummis S.C., L, Beene D, Harrison N.J, Lester H.A, Dougherty D.A. A cation-pi binding interaction with a tyrosine in the binding site of the GABAC receptor. Chem. Biol. 2005;12:993–7. doi: 10.1016/j.chembiol.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 87.Osolodkin D.I, Chupakhin V.I, Palyulin V.A, Zefirov N.S. Molecular modeling of ligand-receptor interactions in GABA C receptor. J. Mol. Graph. Model. 2009;27:813–21. doi: 10.1016/j.jmgm.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 88.Gleitsman K.R, Shanata J.A, Frazier S.J, Lester H.A, Dougherty D.A. Long-range coupling in an allosteric receptor revealed by mutant cycle analysis. Biophys. J. 2009;96:3168–78. doi: 10.1016/j.bpj.2008.12.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feigenspan A, Bormann J. GABA-gated Cl- channels in the rat retina. Prog. Retin. Eye Res. 1998;17:99–126. doi: 10.1016/s1350-9462(97)00008-6. [DOI] [PubMed] [Google Scholar]
- 90.Fletcher E.L, Koulen P, Wässle H. GABAA and GABAC receptors on mammalian rod bipolar cells. J. Comp. Neurol. 1998;396:351–65. doi: 10.1002/(sici)1096-9861(19980706)396:3<351::aid-cne6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 91.Koulen P, Brandstätter J.H, Kröger S, Enz R, Bormann J, Wässle H. Immunocytochemical localization of the GABA(C) receptor rho subunits in the cat, goldfish, and chicken retina. J. Comp. Neurol. 1997;380:520–32. doi: 10.1002/(sici)1096-9861(19970421)380:4<520::aid-cne8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 92.Lukasiewicz P.D. GABAC receptors in the vertebrate retina. Mol. Neurobiol. 1996;12:181–94. doi: 10.1007/BF02755587. [DOI] [PubMed] [Google Scholar]
- 93.McGillem G.S, Rotolo T.C, Dacheux R.F. GABA responses of rod bipolar cells in rabbit retinal slices. Vis. Neurosci. 2000;17:381–9. doi: 10.1017/s0952523800173067. [DOI] [PubMed] [Google Scholar]
- 94.Ogurusu T, Yanagi K, Watanabe M, Fukaya M, Shingai R. Localization of GABA receptor rho 2 and rho 3 subunits in rat brain and functional expression of homooligomeric rho 3 receptors and heterooligomeric rho 2 rho 3 receptors. Recept. Channels. 1999;6:463–75. [PubMed] [Google Scholar]
- 95.Qian H, Dowling J.E. Novel GABA responses from rod-driven retinal horizontal cells. Nature. 1993;361:162–4. doi: 10.1038/361162a0. [DOI] [PubMed] [Google Scholar]
- 96.Picaud S, Pattnaik B, Hicks D, Forster V, Fontaine V, Sahel J, Dreyfus H. GABAA and GABAC receptors in adult porcine cones: evidence from a photoreceptor-glia co-culture model. J. Physiol. 1998;513:33–42. doi: 10.1111/j.1469-7793.1998.033by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jansen A, Hoepfner M, Herzig K.H, Riecken E.O, Scherübl H. GABA(C) receptors in neuroendocrine gut cells: a new GABA-binding site in the gut. Pflugers Arch. 2000;441:294–300. doi: 10.1007/s004240000412. [DOI] [PubMed] [Google Scholar]
- 98.Li S, Zhang Y, Liu H, Yan Y, Li Y. Identification and expression of GABAC receptor in rat testis and spermatozoa. Acta Biochim. Biophys. Sin (Shanghai) 2008;40:761–7. [PubMed] [Google Scholar]
- 99.Boue-Grabot E, Roudbaraki M, Bascles L, Tramu G, Bloch B, Garret M. Expression of GABA receptor rho subunits in rat brain. J. Neurochem. 1998;70:899–907. doi: 10.1046/j.1471-4159.1998.70030899.x. [DOI] [PubMed] [Google Scholar]
- 100.Enz R, Cutting G.R. GABAC receptor rho subunits are heterogeneously expressed in the human CNS and form homo- and heterooligomers with distinct physical properties. Eur. J. Neurosci. 1999;11:41–50. doi: 10.1046/j.1460-9568.1999.00423.x. [DOI] [PubMed] [Google Scholar]
- 101.Didelon F, Sciancalepore M, Savic' N, Mladinic' M, Bradbury A, Cherubini E. Gamma-aminobutyric acid-A rho receptor subunits in the developing rat hippocampus. J. Neurosci. Res. 2002;67:739–744. doi: 10.1002/jnr.10178. [DOI] [PubMed] [Google Scholar]
- 102.Jost B, Grabert J, Patz S, Schmidt M, Wahle P. GABAC receptor subunit mRNA expression in the rat superior colliculus is regulated by calcium channels, neurotrophins, and GABAC receptor activity. Brain Cell Biol. 2006;35:251–66. doi: 10.1007/s11068-008-9020-0. [DOI] [PubMed] [Google Scholar]
- 103.Rozzo A, Armellin M, Franzot J, Chiaruttini C, Nistri A, Tongiorgi E. Expression and dendritic mRNA localization of GABAc receptor rho1 and rho2 subunits in developing rat brain and spinal cord. Eur. J. Neurosci. 2002;15:1747–1758. doi: 10.1046/j.1460-9568.2002.02013.x. [DOI] [PubMed] [Google Scholar]
- 104.Wegelius K, Pasternack M, Hiltunen J.O, Rivera C, Kaila K, Saarma M, Reeben M. Distribution of GABA receptor rho subunit transcripts in the rat brain. Eur. J. Neurosci. 1998;10:350–357. doi: 10.1046/j.1460-9568.1998.00023.x. [DOI] [PubMed] [Google Scholar]
- 105.Born G, Schmidt M. A reciprocal connection between the ventral lateral geniculate nucleus and the pretectal nuclear complex and the superior colliculus: An in vitro characterization in the rat. Vis. Neurosci. 2007;25:39–51. doi: 10.1017/S0952523808080048. [DOI] [PubMed] [Google Scholar]
- 106.Saransaari P, Oja S.S. Taurine release modified by GABAergic agents in hippocampal slices from adult and developing mice. Amino Acids. 2000;18:17–30. doi: 10.1007/s007260050002. [DOI] [PubMed] [Google Scholar]
- 107.Gamel-Didelon K, Kunz L, Fohr K.J, Gratzl M, Mayerhofer A. Molecular and physiological evidence for functional gamma-aminobutyric acid (GABA)-C receptors in growth hormone-secreting cells. J. Biol. Chem. 2003;278:20192–5. doi: 10.1074/jbc.M301729200. [DOI] [PubMed] [Google Scholar]
- 108.Zhu J.J, Lo F.S. Three GABA receptor-mediated postsynaptic potentials in interneurons in the rat lateral geniculate nucleus. J. Neurosci. 1999;19:5721–5730. doi: 10.1523/JNEUROSCI.19-14-05721.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boller M, Schmidt M. Postnatal maturation of GABA(A) and GABA(C) receptor function in the mammalian superior colliculus. Eur. J. Neurosci. 2001;14:1185–93. doi: 10.1046/j.0953-816x.2001.01746.x. [DOI] [PubMed] [Google Scholar]
- 110.Dong C.J, Picaud S.A, Werblin F.S. GABA transporters and GABAC-like receptors on catfish cone- but not rod-driven horizontal cells. J. Neurosci. 1994;14:2648–58. doi: 10.1523/JNEUROSCI.14-05-02648.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Greka A, Lipton S.A, Zhang D. Expression of GABA(C) receptor rho1 and rho2 subunits during development of the mouse retina. Eur. J. Neurosci. 2000;12:3575–82. doi: 10.1046/j.1460-9568.2000.00247.x. [DOI] [PubMed] [Google Scholar]
- 112.Koulen P, Brandstätter J.H, Enz R, Bormann J, Wässle H. Synaptic clustering of GABA(C) receptor rho-subunits in the rat retina. Eur. J. Neurosci. 1998;10:115–27. doi: 10.1046/j.1460-9568.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- 113.Ogurusu T, Eguchi G, Shingai R. Localization of gamma-aminobutyric acid (GABA) receptor rho 3 subunit in rat retina. Neuroreport. 1999;3:925–7. doi: 10.1097/00001756-199703030-00022. [DOI] [PubMed] [Google Scholar]
- 114.Pan Z.H, Lipton S.A. Multiple GABA receptor subtypes mediate inhibition of calcium influx at rat retinal bipolar cell terminals. J. Neurosci. 1995;15:2668–79. doi: 10.1523/JNEUROSCI.15-04-02668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen Y, Zhou D, Zhou K, Ren Y, Dai W, Xu M, Lu L, Lu Z. Study on olfactory function in GABAC receptor/channel rho1 subunit knockout mice. Neurosci. Lett. 2009;427:10–5. doi: 10.1016/j.neulet.2007.06.059. [DOI] [PubMed] [Google Scholar]
- 116.Lummis S.C., L, Beene D, Harrison N.J, Lester H.A, Dougherty D.A. A cation-pi binding interaction with a tyrosine in the binding site of the GABAC receptor. Chem. Biol. 2005;12:993–7. doi: 10.1016/j.chembiol.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 117.Eggers E.D, McCall M.A, Lukasiewicz P.D. Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. J. Physiol. 2007;5:569–82. doi: 10.1113/jphysiol.2007.131763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ichinose T, Lukasiewicz P.D. GABA transporters regulate inhibition in the retina by limiting GABA(C) receptor activation. J. Neurosci. 2002;22:3285–92. doi: 10.1523/JNEUROSCI.22-08-03285.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kapousta-Bruneau N.V. Opposite effects of GABA(A) and GABA(C) receptor antagonists on the b-wave of ERG recorded from the isolated rat retina. Vis. Res. 2000;40:1653–65. doi: 10.1016/s0042-6989(00)00028-6. [DOI] [PubMed] [Google Scholar]
- 120.Frazao R, Nogueira M.I, Wässle H. Colocalization of synaptic GABAC-receptors with GABAA-receptors and glycine-receptors in the rodent central nervous system. Cell Tissue Res. 2007;330:1–15. doi: 10.1007/s00441-007-0446-y. [DOI] [PubMed] [Google Scholar]
- 121.Wahle P, Schmidt M. GABAC receptors are expressed in GABAergic and non-GABAergic neurons of the rat superior colliculus and visual cortex. Exp. Brain Res. 2009;199:3–4. doi: 10.1007/s00221-009-1710-z. [DOI] [PubMed] [Google Scholar]
- 122.Boller M, Schmidt M. GABAc receptors in the rat superior colliculus and pretectum participate in synaptic transmission. J. Neurophysiol. 2003;89:2035–045. doi: 10.1152/jn.00824.2002. [DOI] [PubMed] [Google Scholar]
- 123.Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J. Neurosci. 1993;11:4908–23. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yasumi M, Sato K, Shimada S, Nishimura M, Tohyama M. Regional distribution of GABA transporter 1 (GAT1) mRNA in the rat brain: Comparison with glutamic acid decarboxylase67 (GAD67) mRNA localization. Mol. Brain Res. 1997;44:205–218. doi: 10.1016/s0169-328x(96)00200-8. [DOI] [PubMed] [Google Scholar]
- 125.Clark S.E, Garret M, Platt B. Postnatal alterations of GABA receptor profiles in the rat superior colliculus. Neuroscience. 2001;104:441–54. doi: 10.1016/s0306-4522(01)00087-2. [DOI] [PubMed] [Google Scholar]
- 126.Wall M.J. Cis-4-amino-crotonic acid activates alpha 6 subunit-containing GABA(A) but not GABA(C) receptors in granule cells of adult rat cerebellar slices. Neurosci. Lett. 2001;316:37–40. doi: 10.1016/s0304-3940(01)02363-1. [DOI] [PubMed] [Google Scholar]
- 127.Albrecht B.E, Breitenbach U, Stühmer T, Harvey R.J, Darlison M.G. In situ hybridization and reverse transcription--polymerase chain reaction studies on the expression of the GABA(C) receptor rho1- and rho2-subunit genes in avian and rat brain. Eur. J. Neurosci. 1997;9:2414–22. doi: 10.1111/j.1460-9568.1997.tb01658.x. [DOI] [PubMed] [Google Scholar]
- 128.Pasternack M, Boller M, Pau B, Schmidt M. GABA(A) and GABA(C) receptors have contrasting effects on excitability in superior colliculus. J. Neurophysiol. 1999;82:2020–3. doi: 10.1152/jn.1999.82.4.2020. [DOI] [PubMed] [Google Scholar]
- 129.Schlicker K, McCall M, Schmidt M. GABAC receptor-mediated inhibition is altered, but not eliminated in the superior colliculus of GABAC ρ1 knockout mice. Vis. Res. 2009;44:3289–96. doi: 10.1152/jn.91001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Alakuijala A, Palgi M, Wegelius K, Schmidt M, Enz R, Paulin L, Saarma M, Pasternack M. GABA receptor rho subunit expression in the developing rat brain. Brain Res. Dev. Brain Res. 2005;154:15–23. doi: 10.1016/j.devbrainres.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 131.Temel Y, Visser-Vandewalle V, Ackermans L, Beuls E.A. Thalamus and penile erection. Int. J. Impot. Res. 2004;16:505–11. doi: 10.1038/sj.ijir.3901233. [DOI] [PubMed] [Google Scholar]
- 132.Schlicker K, Boller M, Schmidt M. GABAC receptor mediated inhibition in acutely isolated neurons of the rat dorsal lateral geniculate nucleus. Brain Res. Bull. 2004;63:91–7. doi: 10.1016/j.brainresbull.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 133.López-Chávez A, Miledi R, Martínez-Torres A. Cloning and functional expression of the bovine GABAC rho2 subunit. Molecular evidence of a widespread distribution in the CNS. Neurosci. Res. 2005;53:421–7. doi: 10.1016/j.neures.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 134.Rosas-Arellano A, Ochoa-de la Paz L.D, Miledi R, Martínez-Torres A. Brain distribution and molecular cloning of the bovine GABA rho1 receptor. Neurosci. Res. 2007;57:347–53. doi: 10.1016/j.neures.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 135.Milligan C.J, Buckley N.J, Garret M, Deuchars J, Deuchars S.A. Evidence for inhibition mediated by coassembly of GABAA and GABAC receptor subunits in native central neurons. J. Neurosci. 2004;24:7241–50. doi: 10.1523/JNEUROSCI.1979-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pan Z.H, Zhang D, Zhang X, Lipton S.A. Evidence for coassembly of mutant GABAC rho1 with GABAA gamma2S, glycine alpha1 and glycine alpha2 receptor subunits in vitro. Eur. J. Neurosci. 2000;12:3137–45. doi: 10.1046/j.1460-9568.2000.00198.x. [DOI] [PubMed] [Google Scholar]
- 137.Nakayama Y, Hattori N, Otani H, Inagaki C. γ-aminobutyric acid (GABA)-C receptor stimulation increases prolactin (PRL) secretion in cultured rat anterior pituitary cells. Biochem. Pharmacol. 2006;71:1705–10. doi: 10.1016/j.bcp.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 138.Drew C.A, Johnston G.A, Weatherby R.P. Bicuculline-insensitive GABA receptors: studies on the binding of (-)-baclofen to rat cerebellar membranes. Neurosci. Lett. 1984;52:317–321. doi: 10.1016/0304-3940(84)90181-2. [DOI] [PubMed] [Google Scholar]
- 139.Mejía C, García-Alcocer G, Berumen L.C, Rosas-Arellano A, Miledi R, Martínez-Torres A. Expression of GABArho subunits during rat cerebellum development. Neurosci. Lett. 2008;432:1–6. doi: 10.1016/j.neulet.2007.11.062. [DOI] [PubMed] [Google Scholar]
- 140.Harvey V.L, Duguid I.C, Krasel C, Stephens G.J. Evidence that GABA rho subunits contribute to functional ionotropic GABA receptors in mouse cerebellar Purkinje cells. J. Physiol. 2006;577:127–139. doi: 10.1113/jphysiol.2006.112482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rozzo A, Ballerini L, Nistri A. Antagonism by (1, 2, 5, 6-tetrahydropyridine-4-yl) methylphosphinic acid of synaptic transmission in the neonatal rat spinal cord in vitro: an electrophysiological study. Neuroscience. 1999;90:1085–92. doi: 10.1016/s0306-4522(98)00476-x. [DOI] [PubMed] [Google Scholar]
- 142.Zheng W, Xie W, Zhang J, Strong J.A, Wang L, Yu L, Xu M, Lu L. Function of gamma-aminobutyric acid receptor/channel rho 1 subunits in spinal cord. J. Biol. Chem. 2003;278:48321–9. doi: 10.1074/jbc.M307930200. [DOI] [PubMed] [Google Scholar]
- 143.Berger T, Walz W, Schnitzer J, Kettenmann H. GABA- and glutamate-activated currents in glial cells of the mouse corpus callosum slice. J. Neurosci. Res. 1992;31:21–7. doi: 10.1002/jnr.490310104. [DOI] [PubMed] [Google Scholar]
- 144.Strata F, Cherubini E. Transient expression of a novel type of GABA response in rat CA3 hippocampal neurones during development. J. Physiol. 1994;480:493–503. doi: 10.1113/jphysiol.1994.sp020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Alakuijala A, Alakuijala J, Pasternack M. Evidence for a functional role of GABA receptors in the rat mature hippocampus. Eur. J. Neurosci. 2006;23:514–20. doi: 10.1111/j.1460-9568.2005.04572.x. [DOI] [PubMed] [Google Scholar]
- 146.Filippova N, Sedelnikova A, Tyler W.J, Whitworth T.L, Fortinberry H, Weiss D.S. Recombinant GABA(C) receptors expressed in rat hippocampal neurons after infection with an adenovirus containing the human rho1 subunit. J. Physiol. 2001;535:145–53. doi: 10.1111/j.1469-7793.2001.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Irie T, Miyamoto E, Kitagawa K, Maruyama Y, Inoue K, Inagaki C. An anxiolytic agent, dihydrohonokiol-B, inhibits ammonia-induced increases in the intracellular Cl(-) of cultured rat hippocampal neurons via GABA(c) receptors. Neurosci. Lett. 2001;312:121–3. doi: 10.1016/s0304-3940(01)02201-7. [DOI] [PubMed] [Google Scholar]
- 148.Liu B, Hattori N, Jiang B, Nakayama Y, Zhang N.Y, Wu B, Kitagawa K, Taketo M, Matsuda H, Inagaki C. Single cell RT-PCR demonstrates differential expression of GABAC receptor ρ subunits in rat hippocampal pyramidal and granule cells. Mol. Brain Res. 2004;123:1–6. doi: 10.1016/j.molbrainres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 149.Yang L, Nakayama Y, Hattori N, Liu B, Inagaki C. GABAC-receptor stimulation activates cAMP-dependent protein kinase via A-kinase anchoring protein 220. J. Pharmacol. Sci. 2008;106:578–84. doi: 10.1254/jphs.fp0071362. [DOI] [PubMed] [Google Scholar]
- 150.Fujimura J, Nagano M, Suzuki H. Differential expression of GABA(A) receptor subunits in the distinct nuclei of the rat amygdala. Brain Res. Mol. Brain Res. 2005;138:17–23. doi: 10.1016/j.molbrainres.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 151.LeDoux J. Emotion circuits in the brain. Annu. Rev. Psychol. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 152.Delaney A.J, Sah P. GABA receptors inhibited by benzodiazepines mediate fast inhibitory transmission in the central amygdala. J. Neurosci. 1999;19:9698–9704. doi: 10.1523/JNEUROSCI.19-22-09698.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Delaney A.J, Sah P. Pathway-specific targeting of GABA(A) receptor subtypes to somatic and dendritic synapses in the central amygdale. J. Neurophysiol. 2001;86:717–23. doi: 10.1152/jn.2001.86.2.717. [DOI] [PubMed] [Google Scholar]
- 154.Beg A.A, Jorgensen E.M. EXP-1 is an excitatory GABA gated cation channel. Nat. Neurosci. 2003;6:1145–1152. doi: 10.1038/nn1136. [DOI] [PubMed] [Google Scholar]
- 155.Hosie A.M, Aronstein K, Sattelle D.B, Ffrenchconstant R.H. Molecular biology of insect neuronal GABA receptors. Trends Neurosci. 1997;20:578–583. doi: 10.1016/s0166-2236(97)01127-2. [DOI] [PubMed] [Google Scholar]
- 156.Witte I, Kreienkamp H.J, Gewecke M, Roeder T. Putative histamine-gated chloride channel subunits of the insect visual system and thoracic ganglion. J. Neurochem. 2002;83:504–514. doi: 10.1046/j.1471-4159.2002.01076.x. [DOI] [PubMed] [Google Scholar]
- 157.Chapman J.A, Kirkness E.F, Simakov O, Hampson S.E, Mitros T, Weinmaier T, Rattei T, Balasubramanian P.G, Borman J, Busam D, Disbennett K, Pfannkoch C, Sumin N, Sutton G.G, Viswanathan L.D, Walenz B, Goodstein D.M, Hellsten U, Kawashima T, Prochnik S.E, Putnam N.H, Shu S, Blumberg B, Dana C.E, Gee L, Kibler D.F, Law L, Lindgens D, Martinez D.E, Peng J, Wigge P.A, Bertulat B, Guder C, Nakamura Y, Ozbek S, Watanabe H, Khalturin K, Hemmrich G, Franke A, Augustin R, Fraune S, Hayakawa E, Hayakawa S, Hirose M, Hwang J.S, Ikeo K, Nishimiya-Fujisawa C, Ogura A, Takahashi T, Steinmetz P.R, Zhang X, Aufschnaiter R, Eder M.K, Gorny A.K, Salvenmoser W, Heimberg A.M, Wheeler B.M, Peterson K.J, Böttger A, Tischler P, Wolf A, Gojobori T, Remington K.A, Strausberg R.L, Venter J.C, Technau U, Hobmayer B, Bosch T.C, Holstein T.W, Fujisawa T, Bode H.R, David C.N, Rokhsar D.S, Steele R.E. The dynamic genome of Hydra. Nature. 2010;7288:592–6. doi: 10.1038/nature08830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bailey M.E, Albrecht B.E, Johnson K.J, Darlison M.G. Genetic linkage and radiation hybrid mapping of the three human GABAC receptor rho subunit genes: GABRR1, GABRR2 and GABRR3. Biochim. Biophys. Acta. 1999;1447:307–312. doi: 10.1016/s0167-4781(99)00167-0. [DOI] [PubMed] [Google Scholar]
- 159.Bailey M.E, Matthews D.A, Riley B.P, Albrecht B.E, Kostrzewa M, Hicks A.A, Harris R, Müller U, Darlison M.G, Johnson K.J. Genomic mapping and evolution of human GABA(A) receptor subunit gene clusters. Mamm. Genome. 1999;10:839–43. doi: 10.1007/s003359901101. [DOI] [PubMed] [Google Scholar]
- 160.Russek S.J, Farb D.H. Mapping of the beta-2 subunit gene (GABRB2) to microdissected human chromosome 5q34-q35 defines a gene cluster for the most abundant GABA-A receptor isoform. Genomics. 1994;23:528–533. doi: 10.1006/geno.1994.1539. [DOI] [PubMed] [Google Scholar]
- 161.Russek S.J. Evolution of GABA(A) receptor diversity in the human genome. Gene. 1999;227:213–22. doi: 10.1016/s0378-1119(98)00594-0. [DOI] [PubMed] [Google Scholar]
- 162.Darlison M.G, Pahal I, Thode C. Consequences of the evolution of the GABA(A) receptor gene family. Cell Mol. Neurobiol. 2005;25:607–24. doi: 10.1007/s10571-005-4004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cutting G.R, Curristin S, Zoghbi H, O'Hara B, Seldin M.F, Uhl G.R. Identification of a putative gamma-aminobutyric acid (GABA) receptor subunit rho2 cDNA and colocalization of the genes encoding rho2 (GABRR2) and rho1 (GABRR1) to human chromosome 6q14-q21 and mouse chromosome 4. Genomics. 1992;12:801–6. doi: 10.1016/0888-7543(92)90312-g. [DOI] [PubMed] [Google Scholar]
- 164.Satou Y, Kawashima T, Shoguchi E, Nakayama A, Satoh N. An integrated data base of the ascidian, Ciona intestinalis: Towards functional genomics. Zool. Sci. 2005;22:837– 843. doi: 10.2108/zsj.22.837. [DOI] [PubMed] [Google Scholar]
- 165.Martyniuk C.J, Aris-Brosou S, Drouin G, Cahn J, Trudeau V.L. Early evolution of ionotropic GABA receptors and selective regimes acting on the mammalian-specific theta and epsilon subunits. PLoS One. 2007;2:e894. doi: 10.1371/journal.pone.0000894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Holland PW, Garcia-Fernández J, Williams NA, Sidow A. Gene duplications and the origins of vertebrate development. Dev. Suppl. 1994;00:125–133. [PubMed] [Google Scholar]
- 167.Zhu M, Zhao W, Jia L, Lu J, Qiao T, Qu Q. The oldest articulated osteichthyan reveals mosaic gnathostome characters. Nature. 2009;7237:469–74. doi: 10.1038/nature07855. [DOI] [PubMed] [Google Scholar]
- 168.Vaquero CF, de la Villa P. Localisation of the GABA(C) receptors at the axon terminal of the rod bipolar cells of the mouse retina. Neurosci. Res. 1999;35:1–7. doi: 10.1016/s0168-0102(99)00050-4. [DOI] [PubMed] [Google Scholar]
- 169.Shields CR, Lukasiewicz PD. Spike-dependent GABA inputs to bipolar cell axon terminals contribute to lateral inhibition of retinal ganglion cells. J. Neurophysiol. 2002;89:2449–58. doi: 10.1152/jn.00916.2002. [DOI] [PubMed] [Google Scholar]
- 170.Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat. Rev. Neurosci. 2008;9:331–43. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Giuditta A, Kaplan BB, van Minnen J, Alvarez J, Koenig E. Axonal and presynaptic protein synthesis: new insights into the biology of the neuron. Trends Neurosci. 2002;25:400–4. doi: 10.1016/s0166-2236(02)02188-4. [DOI] [PubMed] [Google Scholar]
- 172.Hanley J, Jones E, Moss S. GABA Receptor rho 1 subunit Interacts with a novel splice variant of the glycine transporter, GLYT-1. J. Biol. Chem. 2000;276:840–846. doi: 10.1074/jbc.275.2.840. [DOI] [PubMed] [Google Scholar]
- 173.Meixner A, Haverkamp S, Wässle H, Führer S, Thalhammer J, Kropf N, Bittner R, Lassmann H, Wiche G, Propst F. MAP1B Is Required for Axon Guidance and Is Involved in the Development of the Central and Peripherial Nervous System. J. Cell Biol. 2000;151:1169–1178. doi: 10.1083/jcb.151.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Billups D, Hanley JG, Orme M, Attwell D, Moss SJ. GABAC Receptor Sensitivity Is Modulated by Interaction with MAP-1b. J. Neurosci. 2001;20:8643–8650. doi: 10.1523/JNEUROSCI.20-23-08643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Song XQ, Meng F, Ramsey DJ, Ripps H, Qian H. The GABA rho1 subunit interacts with a cellular retinoic acid binding protein in mammalian retina. Neuroscience. 2005;136:467–75. doi: 10.1016/j.neuroscience.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 176.Gussin HA, Tomlinson ID, Little DM, Warnement MR, Qian H, Rosenthal SJ, Pepperberg DR. Binding of muscimol- conjugated quantum dot to GABAC receptors. J. Am. Chem. Soc. 2006;49:15701–13. doi: 10.1021/ja064324k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Martinez-Torres A, Miledi R. Expression of gamma-aminobutyric acid rho1 and rho1 delta 450 as gene fusions with the green fluorescent protein. Proc. Natl. Acad. Sci. 1999;98:1947–1951. doi: 10.1073/pnas.031584898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tomlinson ID, Gussin HA, Little DM, Warnement MR, Qian H, Pepperberg DR, Rosenthal SJ. Imaging GABA(c) Receptors with Ligand-Conjugated Quantum Dots. J. Biomed. Biotechnol. 2007;00: 76514. doi: 10.1155/2007/76514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Filippova N, Dudley R, Weissm DS. (1). Evidence for phosphorylation-dependent internalization of recombinant human ρ1 GABAC. J. Physiol. 1999;518:385–99. doi: 10.1111/j.1469-7793.1999.0385p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Michels G, Moss SJ. GABAA receptors: properties and trafficking. Crit. Rev. Biochem. Mol. Biol. 2007;1:3–14. doi: 10.1080/10409230601146219. [DOI] [PubMed] [Google Scholar]
- 181.Fletcher EL, Clark MJ, Senior P, Furness JB. Gene expression and localization of GABA(C) receptors in neurons of the rat gastrointestinal tract. Neuroscience. 2001;107:181–9. doi: 10.1016/s0306-4522(01)00339-6. [DOI] [PubMed] [Google Scholar]
- 182.Cunha C, Monfils MH, Ledoux JE. GABA(C) Receptors in the Lateral Amygdala: A Possible Novel Target for the Treatment of Fear and Anxiety Disorders? Front. Behav. Neurosci. 2010;12:4–6. doi: 10.3389/neuro.08.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Xuei X, Flury-Wetherill L, Dick D, Goate A, Tischfield J, Nurnberger J, Schuckit M, Kramer J, Kuperman S, Hesselbrock V, Porjesz B, Foroud T, Edenberg HJ. GABRR1 and GABRR2, encoding the GABA-A receptor subunits rho1 and rho2, are associated with alcohol dependence. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009;2:418–27. doi: 10.1002/ajmg.b.30995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pattaro C, De Grandi A, Vitart V, Hayward C, Franke A, Aulchenko YS, Johansson A, Wild SH, Melville SA, Isaacs A, Polasek O, Ellinghaus D, Kolcic I, Nöthlings U, Zgaga L, Zemunik T, Gnewuch C, Schreiber S, Campbell S, Hastie N, Boban M, Meitinger T, Oostra BA, Riegler P, Minelli C, Wright AF, Campbell H, van Duijn CM, Gyllensten U, Wilson JF, Krawczak M, Rudan I, Pramstaller PP. A meta-analysis of genome-wide data from five European isolates reveals an association of COL22A1, SYT1, and GABRR2 with serum creatinine level. BMC Med. Genet. 2010;11:41. doi: 10.1186/1471-2350-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Arnaud C, Gauthier P, Gottesmann C. Study of a GABAc receptor antagonist on sleep-waking behavior in rats. Psychopharmacology, (Berl) 2001;154:415–9. doi: 10.1007/s002130000653. [DOI] [PubMed] [Google Scholar]
- 186.Boue-Grabot E, Taupignon A, Tramu G, Garret M. Molecular and electrophysiological evidence for a GABAc receptor in thyrotropin-secreting cells. Endocrinology. 2000;141:1627–32. doi: 10.1210/endo.141.5.7476. [DOI] [PubMed] [Google Scholar]
- 187.Gibbs ME, Johnston GA. Opposing roles for GABAA and GABAC receptors in short-term memory formation in young chicks. Neuroscience. 2005;131:567–76. doi: 10.1016/j.neuroscience.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 188.Kang JQ, Shen W, Macdonald RL. The GABRG2 mutation, Q351X, associated with generalized epilepsy with febrile seizures plus, has both loss of function and dominant-negative suppression. J. Neurosci. 2009;29:2845–56. doi: 10.1523/JNEUROSCI.4772-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cheng Q, Kulli JC, Yang J. Suppression of neuronal hyperexcitability and associated delayed neuronal death by adenoviral expression of GABA(C) receptors. J. Neurosci. 2001;21:3419–28. doi: 10.1523/JNEUROSCI.21-10-03419.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ivic L, Zhang C, Zhang X, Yoon SO, Firestein S. Intracellular trafficking of a tagged and functional mammalian olfactory receptor. J. Neurobiol. 2002;50:56–68. doi: 10.1002/neu.10016. [DOI] [PubMed] [Google Scholar]