Abstract
The organic nitrate pentaerithrityl tetranitrate treatment is devoid of nitrate tolerance, which has been attributed to the induction of the antioxidant enzyme heme oxygenase-1. With the present study we tested, whether chronic treatment with pentaerithrityl tetranitrate can improve angiotensin-II induced vascular oxidative stress and dysfunction. In contrast to isosorbide-5-mononitrate (75mg/kg/d/7d), treatment with pentaerithrityl tetranitrate (15mg/kg/d/7d) improved the impaired endothelial and smooth muscle function and normalized vascular and cardiac reactive oxygen species production (mitochondria, NADPH oxidase activity and uncoupled endothelial nitric oxide synthase) as assessed by dihydroethidine staining, lucigenin-enhanced chemiluminescence and quantification of dihydroethidine oxidation products in angiotensin-II (1mg/kg/d/7d) treated rats. The antioxidant features of pentaerithrityl tetranitrate were recapitulated in spontaneously hypertensive rats. In addition to increase in heme oxygenase-1 protein expression, pentaerithrityl tetranitrate but not isosorbide-5-mononitrate normalized vascular reactive oxygen species formation, augmented aortic protein levels of the tetrahydrobiopterin-synthesizing enzymes GTP-cyclohydrolase-I and dihydrofolate reductase in angiotensin-II treated rats, thereby preventing endothelial nitric oxide synthase uncoupling. Knockout of heme oxygenase-1 completely abolished the beneficial effects of pentaerithrityl tetranitrate in angiotensin-II treated mice, whereas heme oxygenase-1 induction by hemin (25mg/kg) mimicked the effect of pentaerithrityl tetranitrate. Improvement of vascular function in this particular model of arterial hypertension by pentaerithrityl tetranitrate largely depends on the induction of the antioxidant enzyme heme oxygenase-1 and identifies pentaerithrityl tetranitrate, in contrast to isosorbide-5-mononitrate, as an organic nitrate being able to improve rather than to worsen endothelial function.
Keywords: pentaerithrityl tetranitrate, isosorbide-5-mononitrate, angiotensin-II, SHR, endothelial dysfunction, vascular oxidative stress
Introduction
Both arterial hypertension and coronary artery disease are associated with an activation of the circulating and local renin-angiotensin-system and increased oxidative stress within the vascular wall1, 2. Angiotensin II (AT-II) treatment has been shown to cause endothelial dysfunction, which is at least in part mediated by increased vascular reactive oxygen species (ROS) levels3, 4. ROS sources involved may include the NADPH-oxidases3; an uncoupled endothelial nitric oxide synthase (eNOS)4 as well as mitochondrial superoxide sources5. The crucial role of the NADPH oxidase as an important superoxide source was further substantiated by the demonstration that Nox1 overexpression in transgenic mice potentiates AT-II-induced hypertension6 while blood pressure responses to AT-II were reduced in Nox1-deficient mice7. Increased vascular ROS production and endothelial dysfunction are also accompanied by increased eNOS expression but decreased vascular nitric oxide (NO) production8. Recently, we were able to demonstrate that pharmacological intervention with a statin or an AT1-receptor-blocker improved vascular dysfunction and reduced oxidative stress in an experimental model of diabetes mellitus9, 10 and identified the down-regulation of tetrahydrobiopterin (BH4) synthesizing enzymes, GTP-cyclohydrolase-I (GCH-I) and dihydrofolate reductase (DHFR), as key-events for the development of endothelial dysfunction8.
Organic nitrates act as endothelium-independent vasodilators of coronary arteries, venous capacity vessels and collaterals. Nitroglycerin (GTN) is one of the most widely used anti-ischemic drugs for more than a century. The chronic efficacy of nitrates, however, is blunted due to side effects such as the development nitrate tolerance and endothelial dysfunction. Recent data indicate that GTN-induced ROS formation accounts for both phenomena since ROS formed in response to GTN therapy since both phenomena can be corrected by treatment with antioxidants11, 12. Treatment with mono- and dinitrates also causes tolerance and endothelial dysfunction13, although both compounds are clearly not bioactivated by the mitochondrial aldehyde dehydrogenase (ALDH-2)14. These findings may explain results from a retrospective analysis indicating increased mortality in response to treatment of patients with myocardial infarction with mono- and dinitrates15. Among all organic nitrates, the most frequently used compounds in the treatment of coronary artery disease comprise GTN, pentaerythrityl tetranitrate (PETN; in Germany), isosorbide dinitrate (ISDN) and isosorbide-5-mononitrate (ISMN; USA).
More recently, we and others were able to demonstrate that different organic nitrates have distinct pharmacological effects with respect to their bioactivating process and their capacity to stimulate vascular superoxide production. For instance, we demonstrated that the nitrate pentaerythrityl tetranitate (PETN), while still undergoing mitochondrial activation by the ALDH-212, does not induce tolerance or stimulate vascular superoxide production,12. While GTN caused a strong oxidative stress mediated inhibition of the mitochondrial ALDH-2, PETN induced an upregulation of the antioxidant enzyme HO-1, which preserved ALDH-2 activity. These observations from experimental animal studies were substantiated by clinical data obtained in humans indicating that in contrast to GTN, PETN does not induce tolerance16 and endothelial dysfunction17.
Based on these considerations we investigated in the AT-II infusion model and in spontaneously hypertensive rats (SHR), to what extent different organic nitrates such as PETN and ISMN are able to modulate oxidative stress, endothelial dysfunction and also tolerance development.
Methods and Materials
Materials
PETN was obtained from Actavis (Langenfeld, Germany). GTN was used from a Nitrolingual infusion solution (Pohl-Boskamp, Hohenlockstedt, Germany). All other chemicals were purchased from Sigma-Aldrich, Merck or Fluka.
Animal models, in vivo infusion of angiotensin-II and SHR
All animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the U.S. National Institutes of Health and was granted by the Ethics Committee of the University Hospital Mainz. Male Wistar rats (250g) were treated with either AT-II (1.0mg/kg/d) or solvent (0.9% NaCl) for 7d, as described previously18. Male SHR and Wistar-Kyoto control rats were obtained from Charles River Laboratories (Sulzfeld, Germany). Animals from both groups were treated with either PETN (15mg/kg/d), ISMN (75mg/kg/d) versus vehicle (DMSO). Male HO-1+/+ or HO-1+/− mice on a 129sv × BALB/c background were treated with either PETN (75mg/kg/d), (DMSO, 0.5μl/h) for 7d19, high dose AT-II (1.0mg/kg/d) versus solvent (0.9 % NaCl) for 7d with and without the HO-1 inducer hemin (25mg/kg single i.p. injection, 12h prior to sacrifice) or low dose AT-II (0.1mg/kg/d) versus solvent (0.9 % NaCl) with or without PETN (75mg/kg/d) for 7d. For details see “Extended Methods” in the online data supplement available at http://hyper.ahajournals.org.
Vascular reactivity studies
Vasodilator responses to the endothelium-dependent vasodilator acetylcholine (ACh) and the endothelium-independent vasodilator nitroglycerin (GTN) were determined in organ chambers by isometric tension studies using phenylephrine-preconstricted aortic ring segments, as previously described18. Murine aorta were preconstricted by prostaglandin F2α, as previously published19, 20.
Assessment of vascular and cardiac oxidative stress
Vascular and cardiac oxidative stress was assessed by L-012 (a luminol derivative)- or lucigenin-enhanced chemiluminescence (ECL) and dihydroethidine-dependent fluorescence as described elsewhere10, 14, 18, 21. For details see “Extended Methods” in the online data supplement available at http://hyper.ahajournals.org. For determination of cardiac ROS in mice, 2–3 hearts were pooled, mitochondria and membrane fractions were isolated as recently published11, 19.
Western Blot analysis and RT-PCR
Western blotting against eNOS, GCH-1, DHFR and HO-1 was performed as previously described10, 21. mRNA expression of HO-1 and ferritin (heavy-chain) was analyzed with quantitative real-time RT-PCR using an iCycler™ iQ system (Bio-Rad Laboratories, Munich, Germany). TaqMan® Gene Expression assays (Applied Biosystems, Foster City, CA) for HO-1 and GAPDH were purchased as probe and primer sets and gene expression was normalized to the endogenous control, GAPDH mRNA as described21. For details see “Extended Methods” in the online data supplement available at http://hyper.ahajournals.org.
Statistical Analysis
Results are expressed as means±SEM. pD2-values (potencies) were obtained by logit-transformation. One way ANOVA (with Bonferroni's or Dunn's correction for comparison of multiple means) was used for comparisons of vasodilator potency and efficacy; vascular and cardiac ROS formation as well as aortic protein and mRNA expression. P values <0.05 were considered significant.
Results
Effects of PETN and ISMN co-therapy on AT-II-induced vascular dysfunction
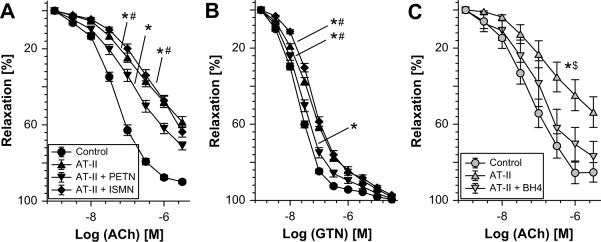
Weight gain in solvent-treated control animals was 50±6g in one week whereas AT-II treatment caused weight loss of 61±16g. Weight loss in AT-II treated animals was significantly improved in hypertensive rats by PETN co-therapy (3±17g), while ISMN treatment had no significant effect (−13±18g). In vivo treatment with PETN rather improved endothelial dysfunction in AT-II-treated animals (Figure 1A and Table S1 in the online data supplement available at http://hyper.ahajournals.org), while ISMN further impaired AT-II-induced endothelial dysfunction AT-II infusion-induced cross tolerance to GTN was corrected by PETN co-treatment, but not by ISMN (Figure 1B and Table S1). Treatment of vessels from AT-II-infused rats with the BH4 precursor sepiapterin normalized endothelial function (Figure 1C). Sensitivity of aorta to vasoconstriction by KCl and phenylephrine was not changed (Table S2 in the online data supplement available at http://hyper.ahajournals.org). Blood pressure data are presented in Figure S1 in the online data supplement available at http://hyper.ahajournals.org and show that AT-II-infused rats and SHR are valid models of experimental hypertension.
Figure 1.
Effects of in vivo PETN (15mg/kg/d) and ISMN (75mg/kg/d) treatment on the concentration response relationship to acetylcholine (ACh) (A) and nitroglycerin (GTN) (B) in aortic rings from angiotensin-II-infused (AT-II, 1mg/kg/d for 7d) rats. (C) The effect of sepiapterin (100 μM), a BH4 precursor, and PEG-SOD (100 U/ml) pretreatment of aortic rings from AT-II-infused rats for 1 h was determined in separate experiments. Data are the mean±SEM of n=36–57 aorta from 10–15 rats per group (A and B) and n=6–8 from 3 rats per group (C). P < 0.05: * vs. Ctr/DMSO; # vs. AT-II+PETN; $ vs. AT-II/BH4. The statistics were based on 1-way-ANOVA comparison of pD2-values and efficacies (see table S1) but also on comparisons of all concentrations in all groups by 2-way-ANOVA analysis (for sake of clarity significance is not shown for all data points).
The beneficial effects of PETN and sepiapterin on vascular function were almost absent in SHR but ISMN further impaired this parameter (Figure S2, in the online data supplement available at http://hyper.ahajournals.org).
Effects of PETN and ISMN co-treatment on vascular and cardiac ROS production as well as eNOS uncoupling in AT-II-induced hypertension
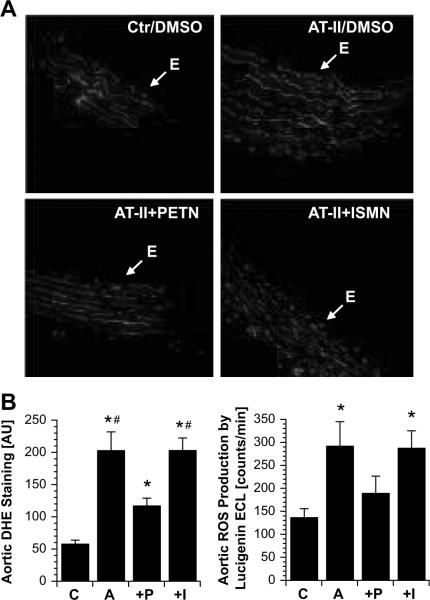
Dihydroethidine staining (fluorescent microtopography) demonstrated increased vascular superoxide throughout the vessel wall and the endothelium in vessel cryo-sections from AT-II-treated animals compared to controls (Figure 2A and B). While vascular superoxide in hypertensive rats was not modified by ISMN treatment, a marked reduction was observed under PETN therapy (Figure 2A and B). Lucigenin ECL in intact aortic ring segments yielded qualitatively similar results (Figure 2B). Angiotensin-II stimulated mitochondrial ROS formation and NADPH oxidase activity in tissue from the heart was normalized by PETN but not ISMN treatment (Figure 3A–C).
Figure 2.
Effects of in vivo PETN and ISMN treatment on vascular superoxide levels (A, B) in angiotensin-II-infused (AT-II) rats. (A) Transverse aortic cryo-sections were labeled with dihydroethidine (DHE, 1μM), which produces red fluorescence when oxidized by reactive oxygen species. “E” indicates the endothelium; lamina autofluorescence is green. Pictures shown are representative for at least 6 animals/group. E means endothelium. (B) Densitometric quantification of the dihydroethidine-derived reactive oxygen species signal throughout the vessel wall (left panel) and lucigenin (5μM) enhanced chemiluminescence (ECL) in intact aortic ring segments (right panel). The data are mean±SEM of n=18–19 experiments with tissue from at least 10 animals/group. P < 0.05: * vs. Ctr/DMSO; # vs. AT-II+PETN. C, control; A, angiotensin-II-treated; P, angiotensin-II- and PETN-treated; I, angiotensin-II- and ISMN-treated.
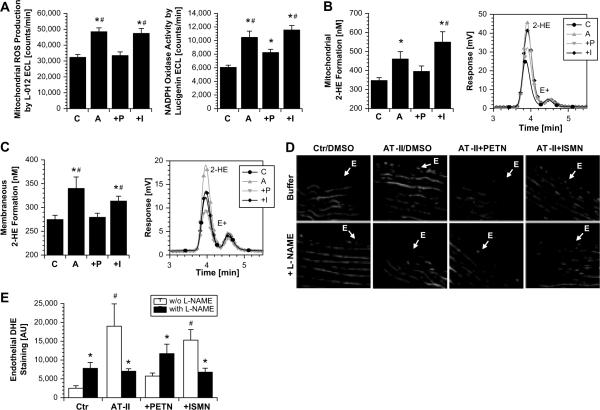
Figure 3.
Effects of in vivo PETN and ISMN treatment on mitochondrial reactive oxygen species formation (A, B), NADPH oxidase activity (A, C) and eNOS-dependent reactive oxygen species formation (uncoupling) (D, E) in angiotensin-II-infused (AT-II) rats. (A) Reactive oxygen species formation in isolated cardiac mitochondria was measured by L-012 (100μM) enhanced chemiluminescence (ECL, left panel) and reactive oxygen species production (NADPH oxidase activity) in membrane fractions from hearts was determined by lucigenin (5μM) enhanced chemiluminescence (ECL, right panel). (B, C) Reactive oxygen species formation in isolated cardiac mitochondria and NADPH oxidase activity in membraneous fractions was also quantified by 2-hydroxyethidium levels. The inserts show representative HPLC chromatograms. E+ means ethidium, 2-HE means 2-hydroxyethidium. The data are mean±SEM of n=31–43 (mitochondria and NADPH oxidase activity) experiments with tissue from at least 10 animals/group. The HPLC data are mean±SEM of n=9 (mitochondria), n=15 (NADPH oxidase activity) experiments with tissue from 3–5 animals/group. (D, E) Fluorescence microscopy revealed reactive oxygen species formation by red staining (upper column). To determine eNOS-dependent reactive oxygen species formation, vessels were pre-incubated with the NOS inhibitor L-NAME (lower column). Densitometric data are presented by bar graphs (solid, w/o L-NAME and open, with L-NAME). Pictures and data shown are representative for at least 4 animals/group. For methodological details see “Figure S4” in the online data supplement available at http://hyper.ahajournals.org. P < 0.05: * vs. Ctr/DMSO; # vs. AT-II+PETN. C, control; A, angiotensin-II-treated; P, angiotensin-II- and PETN-treated; I, angiotensin-II- and ISMN-treated.
To assess the contribution of an uncoupled eNOS to superoxide formation due to eNOS uncoupling, rat aortic tissue was incubated with a NOS inhibitor, NG-nitro-L-arginine methylester (L-NAME). L-NAME increased dihydroethidine (DHE)-derived fluorescence within the endothelial monolayer from control rats (marked with E in Figure 3D) whereas the signal in the media was not changed. Inhibition of NOS in control aorta eliminates basal NO production leading to higher superoxide steady-state-levels (which is otherwise scavenged by NO). In contrast, NOS inhibition in vessels from AT-II treated rats with L-NAME decreased DHE-derived fluorescence exclusively within the endothelium, thereby identifying eNOS as a significant source of superoxide. L-NAME increased the endothelial signal in vessel sections from AT-II/PETN treated rats like in the control group), indicating that PETN treatment was able to prevent eNOS uncoupling (Figure 3D and E). The AT-II/ISMN group displayed no difference compared to the AT-II group, indicating that eNOS uncoupling was not prevented by ISMN co-therapy.
Similar beneficial effects of PETN but not ISMN were observed in SHR (Figure S3, in the online data supplement available at http://hyper.ahajournals.org).
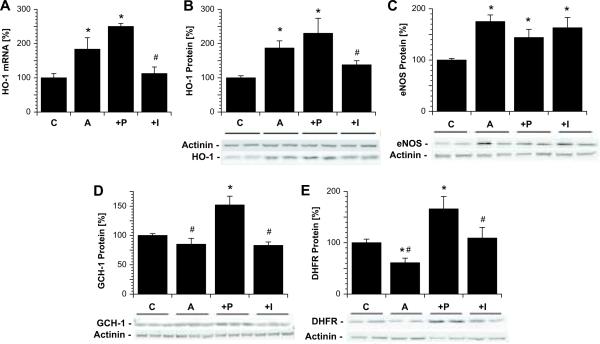
Effects of PETN and ISMN co-treatment on vascular expression of eNOS, tetrahydrobiopterin synthesizing enzymes and the antioxidative principle HO-1
Angiotensin-II treatment has been shown to increase aortic HO-1 gene expression at the mRNA and protein level22, which was further increased by in vivo PETN treatment, but decreased by ISMN co-therapy (Figure 4A and B). As before, we found a significant increases in the expression of eNOS in AT-II-treated rats, which was neither normalized by PETN nor ISMN (Figure 4C). It is important to note, however, that in response to PETN treatment, eNOS was recoipled by PETN while in response to ISMN, the expression of an uncoupled eNOS was increased. AT-II infusion tended to decrease the expression of GTP-cyclohydrolase-I (GCH-I) and significantly decreased the levels of dihydrofolate reductase (DHFR) another important enzyme for synthesis of BH4 (the so-called rescue pathway for BH2 recycling, Figure 4D and E). PETN co-treatment significantly increased expression of both BH4 synthesizing enzymes even to higher levels as compared to the control identifying another important property of PETN, how eNOS recoupling was achieved. The effects on BH4 synthase and BH2 reductase were not shared by ISMN.
Figure 4.
Effects of in vivo PETN and ISMN treatment on vascular heme oxygenase-1 (HO-1, mRNA and protein), eNOS, GTP-cyclohydrolase-I (GCH-I) and dihydrofolate reductase (DHFR) expression in aortic tissue from hypertensive rats. Expression of HO-1 mRNA (A) as well as HO-1 (B), eNOS (C), GCH-I (D) and DHFR (E) protein were assessed by RT-PCR and Western blot analysis, respectively. Representative blots are shown at the bottom of the densitometric bar graphs. The data are mean ± SEM of aortic rings from 6–8 animals/group. P < 0.05: * vs. Ctr/DMSO; # vs. AT-II+PETN. C, control; A, angiotensin-II-treated; P, angiotensin-II- and PETN-treated; I, angiotensin-II- and ISMN-treated.
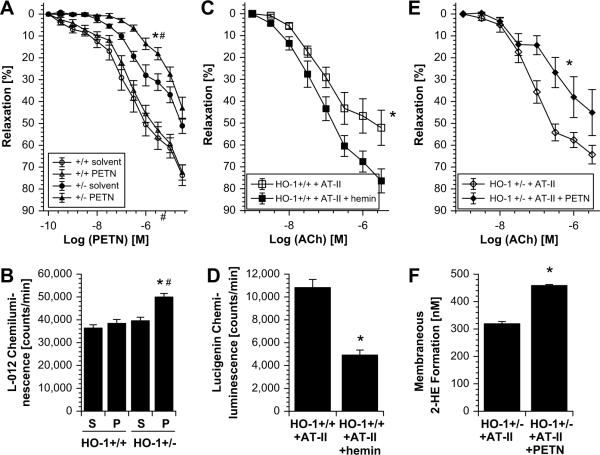
The role of HO-1 as the antioxidative principle of PETN was elucidated by three key experiments aiming to prove this hypothesis: The first experimental setup consisted of the treatment of control (HO-1+/+) and partially deficient (HO-1+/−) mice with PETN. In HO-1+/− but not HO-1+/+ mice PETN infusion induced tolerance against itself, envisaged by impaired vasodilator potency of the drug and increased mitochondrial ROS formation (Figure 5A and B). The second approach was based on HO-1 induction by the known inducer of this enzyme, hemin which improved AT-II-dependent endothelial dysfunction and prevented activation of NADPH oxidase (Figure 5C and D). The third experiment demonstrated that PETN did not improve endothelial dysfunction and cardiac oxidative stress in AT-II-treated HO-1+/− mice but rather further impaired vascular function and increased ROS formation in this setting (Figure 5E and F).
Figure 5.
Effects of HO-1 deficiency versus HO-1 induction on vascular improvement by pentaerithrityl tetranitrate (PETN). (A, B) PETN treatment (75mg/kg/d for 4d) had no effect on PETN potency (PETN-induced relaxation) in aorta from control mice (HO-1+/+) but caused nitrate tolerance in aorta from mice with partial HO-1 deficiency (HO-1+/−). In accordance, cardiac mitochondrial ROS formation (L-012 ECL) was increased in PETN-treated HO-1+/− mice. P < 0.05: * vs. HO-1+/+/DMSO; # vs. HO-1+/−/DMSO. S, solvent; P, PETN-treated. (C, D) Hemin (25mg/kg i.p.)-triggered HO-1 induction improved high dose AT-II (1mg/kg/d for 7d)-induced endothelial dysfunction (ACh-response) in aorta and NADPH oxidase activity in heart (lucigenin ECL) from control mice (HO-1+/+). P < 0.05: * vs. AT-II-treated HO-1+/+/DMSO. (E, F) PETN (75mg/kg/d for 7d) failed to prevent endothelial dysfunction (ACh-response) induced by low dose AT-II (0.1mg/kg/d for 7d) in aorta from HO-1+/− mice. In accordance, PETN did not improve NADPH oxidase activity (2-HE formation by HPLC analysis) in cardiac samples from AT-II-treated HO-1+/− mice. P < 0.05: * vs. AT-II-treated HO-1+/−/DMSO. All data are mean ± SEM of aortic rings and hearts from 4–5 animals/group.
Discussion
The present studies demonstrate that the organic nitrate PETN but not ISMN improves vascular function and reduces oxidative stress via inhibition of vascular superoxide production in mitochondria and by inhibition of the vascular NADPH oxidase in an experimental model of AT-II-induced hypertension. In almost all animal models where endothelial dysfunction is encountered such as atherosclerosis23, chronic congestive heart failure24, AT-II-induced hypertension4 and diabetes mellitus25 we established, that increased production of reactive oxygen species via activation of the vascular NADPH oxidase and xanthine oxidase contributed considerably to this phenomenon. Interestingly, in all models eNOS expression was up rather than down-regulated suggesting that eNOS might be dysfunctional, uncoupled under these circumstances4, 23–25.
Previously, it was conceptualized that treatment with an exogenous source of NO (e.g. GTN) could compensate for the diminished endothelial NO availability in atherosclerosis, thereby preventing the consequences of endothelial dysfunction such as enhanced constriction and increased platelet aggregation. Theoreticallay, however, nitric oxide, rapidly reacts with superoxide to produce the highly reactive intermediate, peroxynitrite a potent oxidant, which has been demonstrated to cause vascular (endothelial) dysfunction by inhibiting prostacyclin activity26 and by causing eNOS uncoupling via oxidation of the important eNOS cofactor tetrahydrobiopterin (BH4)27. Indeed, treatment of atherosclerotic animals with GTN worsened rather than improved endothelial function, caused consumption of plasma antioxidants such as alpha and beta carotene, decreased extra-cellular superoxide dismutase activity and lead to a dramatic increase in vascular protein tyrosine nitration as a footprint of peroxynitrite formation under GTN therapy28. In contrast to GTN PETN is capable to upregulate the important antioxidant enzyme heme oxygenase I, which has been shown tom play a key role in response to treatment with various organic nitrates, whether these animals develop tolerance end endothelial dysfunction21.
It remains to be established, however, whether PETN, a nitrate with antioxidant properties is able to improve endothelial dysfunction in an animal of endothelial dysfunction and oxidative stress. To address this issue we used the model of AT-II infusion where the superoxide producing enzymes are well charactrized. For comparison, AT-II treated animals were treated with the mononitrate ISMN. As before, infusion of AT-II lead to a marked degree of endothelial dysfunction as well as an attenuation of the endothelium-independent nitrovasodilator GTN associated with increased oxidative stress in vascular tissue. As superoxide sources the NADPH oxidase, the mitochondria and an uncoupled NO synthase were identified.
Experiments with isolated mitochondria and membrane fractions from the heart revealed that PETN and not ISMN treatment was able to reduce significantly mitochondrial ROS production and to inhibit NADPH oxidase activity. Previously we have proposed that superoxide production by the vascular NADPH oxidase and mitochondria might represent so called “kindling radicals”, which may react with NO to form peroxynitrite25. This intermediate in turn oxidizes BH4 to the BH3 radical thereby causing superoxide production by eNOS, the so called “bonfire” radicals27. Thus all measures which successfully reduce vascular superoxide production via inhibition of the NADPH oxidase should lead to a prevention of eNOS uncoupling. Indeed, as indicated by the DHE experiments with L-NAME PETN, but not ISMN prevented eNOS uncoupling in this model of oxidative stress. The observed reduction of mitochondrial ROS formation could be a direct consequence of decreased NADPH oxidase activity since it was recently demonstrated that GTN induced increases in NADPH oxidase-activity can trigger mitochondrial ROS formation via KATP channels11.
Downregulation of the BH4 synthesizing enzyme DHFR has been proposed to contribute substantially to endothelial dysfunction and eNOS uncoupling in the AT-II infusion model29. In two recent studies with diabetic rats we were able to demonstrate that the prevention of eNOS uncoupling in response to atorvastatin or the AT1-receptor blocker telmisartan was at least in part secondary to a upregulation of BH4 synthesizing enzyme such as the GCH-I and the DHFR9, 10. With the present studies we established that PETN and not ISMN was able to substantially upregulate not only DHFR but also GCH-I, which may also contribute considerably to the recoupling of the enzyme.
As mentioned before, eNOS protein was up rather than down-regulated in this animal model of hypertension. It is important to note that this is likely due to increased H2O2 production, which has been shown previously to increase eNOS expression at the transcriptional and translational level30. Thus, a reduction in vascular oxidative stress should always results in a normalization of eNOS under these circumstances. With the present studies we were able to show that AT-II upregulated eNOS was not modified by PETN or ISMN treatment. The fact, however, that in response to PETN but not ISMN treatment, eNOS was recoupled, explains why PETN treatment and not ISMN treatment improved endothelial dysfunction.
In one of our recent manuscripts we identified the antioxidant enzyme HO-1 as the key player determining whether an organic nitrate causes endothelial dysfunction and nitrate tolerance21. HO-1 exerts its beneficial effects on vascular function via formation of the sGC stimulator carbon monoxide, the antioxidant bilirubin and the chelator protein of free iron, ferritin31. Likewise, with the present studies we can show that AT-II upregulated HO-1 expression at the mRNA and protein levels, that was further stimulated by PETN but not by ISMN treatment. The results by using HO-1 deficient mice (HO-1+/−) clearly demonstrate that HO-1 largely contributes to the pleiotropic protective properties of PETN, since the beneficial effects of PETN on endothelial dysfunction in the AT-II hypertension model were almost completely abolished by heterozygous HO-1 deficiency. Otherwise, induction of HO-1 by hemin was able to prevent vascular dysfunction by high dose AT-II treatment of control mice (HO-1+/+).
To address whether similar effects can be observed in a genetically determined model of hypertension SHR were studied. The results affirm with those obtained from the AT-II infusion model. PETN, but not ISMN treatment markedly reduced vascular superoxide production as quantified by DHE staining and by lucigenin-enhanced chemiluminescence. The effects of PETN on vascular function in SHR were significantly different from those of ISMN, although less pronounced as compared to the effects in ATII-triggered hypertension. For detailed consideration of these differences see “Extended Results” in the online data supplement available at http://hyper.ahajournals.org. Therefore, the observations on the beneficial effects of PETN in both animal models of arterial hypertension are in accordance with previous studies indicating that PETN improves experimental atherosclerosis in rabbits32, 33. It should be noted that these authors have also reported on anti-atherosclerotic effects of ISMN and improvement of endothelial dysfunction by this drug34, 35 which, however, is at variance with our present observations and a human study indicating detrimental effects of ISMN on endothelial function in healthy volunteers13.
Perspectives
The results of the present studies show for the first time that in an animal model of oxidative stress, the organic nitrate PETN but not the mononitrate ISMN is able to improve endothelial dysfunction. PETN substantially inhibited NADPH oxidase activity, inhibited mitochondrial superoxide production and prevented eNOS uncoupling. The recoupling of eNOS may be a consequence of a reduction of oxidative stress secondary to upregulated HO-1 protein but may also be due to PETN mediated upregulation of the key enzymes for BH4 synthesis such as the GCH-I and the DHFR. These preclinical studies contribute to the understanding why PETN treatment does not cause tolerance or endothelial dysfunction. The spectrum of antioxidant features of this compound indicates that PETN may not be used only for the treatment of symptomatic coronary artery disease, but also to beneficially influence the progression of the atherosclerotic process.
Supplementary Material
Acknowledgments
We appreciate the expert technical support by Jörg Schreiner and Nicole Schramm. This study contains parts of the thesis work of Jens Kamuf.
Sources of Funding The study was supported by a vascular research grant from Actavis Deutschland GmbH (to T.M. and A.D.) and the German Heart Foundation/German Foundation of Heart Research (to S.S. and P.W.).
Disclosures Statement A.D. and T.M. received a vascular research grant from Actavis Deutschland GmbH (Langenfeld, Germany).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hamilton C. Nitric oxide, oxidative stress and hypertension: A complex equation. J Hypertens. 2002;20:1055–1056. doi: 10.1097/00004872-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Touyz RM. Reactive oxygen species in vascular biology: Role in arterial hypertension. Expert Rev Cardiovasc Ther. 2003;1:91–106. doi: 10.1586/14779072.1.1.91. [DOI] [PubMed] [Google Scholar]
- 3.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin ii-mediated hypertension in the rat increases vascular superoxide production via membrane nadh/nadph oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, Munzel T. Effects of angiotensin ii infusion on the expression and function of nad(p)h oxidase and components of nitric oxide/cgmp signaling. Circ Res. 2002;90:E58–65. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- 5.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin ii-mediated mitochondrial dysfunction: Linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 6.Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, Griendling KK. Nox1 overexpression potentiates angiotensin ii-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 7.Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H, Yabe-Nishimura C. Nox1 is involved in angiotensin ii-mediated hypertension: A study in nox1-deficient mice. Circulation. 2005;112:2677–2685. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- 8.Schulz E, Jansen T, Wenzel P, Daiber A, Munzel T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal. 2008;10:1115–1126. doi: 10.1089/ars.2007.1989. [DOI] [PubMed] [Google Scholar]
- 9.Wenzel P, Daiber A, Oelze M, Brandt M, Closs E, Xu J, Thum T, Bauersachs J, Ertl G, Zou MH, Forstermann U, Munzel T. Mechanisms underlying recoupling of enos by hmg-coa reductase inhibition in a rat model of streptozotocin-induced diabetes mellitus. Atherosclerosis. 2008;198:65–76. doi: 10.1016/j.atherosclerosis.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wenzel P, Schulz E, Oelze M, Muller J, Schuhmacher S, Alhamdani MS, Debrezion J, Hortmann M, Reifenberg K, Fleming I, Munzel T, Daiber A. At1-receptor blockade by telmisartan upregulates gtp-cyclohydrolase i and protects enos in diabetic rats. Free Radic Biol Med. 2008;45:619–626. doi: 10.1016/j.freeradbiomed.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Wenzel P, Mollnau H, Oelze M, Schulz E, Wickramanayake JM, Muller J, Schuhmacher S, Hortmann M, Baldus S, Gori T, Brandes RP, Munzel T, Daiber A. First evidence for a crosstalk between mitochondrial and nadph oxidase-derived reactive oxygen species in nitroglycerin-triggered vascular dysfunction. Antioxid Redox Signal. 2008;10:1435–1447. doi: 10.1089/ars.2007.1969. [DOI] [PubMed] [Google Scholar]
- 12.Wenzel P, Hink U, Oelze M, Schuppan S, Schaeuble K, Schildknecht S, Ho KK, Weiner H, Bachschmid M, Munzel T, Daiber A. Role of reduced lipoic acid in the redox regulation of mitochondrial aldehyde dehydrogenase (aldh-2) activity. Implications for mitochondrial oxidative stress and nitrate tolerance. J Biol Chem. 2007;282:792–799. doi: 10.1074/jbc.M606477200. [DOI] [PubMed] [Google Scholar]
- 13.Thomas GR, DiFabio JM, Gori T, Parker JD. Once daily therapy with isosorbide-5-mononitrate causes endothelial dysfunction in humans: Evidence of a free-radical-mediated mechanism. J Am Coll Cardiol. 2007;49:1289–1295. doi: 10.1016/j.jacc.2006.10.074. [DOI] [PubMed] [Google Scholar]
- 14.Daiber A, Oelze M, Coldewey M, Bachschmid M, Wenzel P, Sydow K, Wendt M, Kleschyov AL, Stalleicken D, Ullrich V, Mulsch A, Munzel T. Oxidative stress and mitochondrial aldehyde dehydrogenase activity: A comparison of pentaerythritol tetranitrate with other organic nitrates. Mol Pharmacol. 2004;66:1372–1382. doi: 10.1124/mol.104.002600. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura Y, Moss AJ, Brown MW, Kinoshita M, Kawai C. Long-term nitrate use may be deleterious in ischemic heart disease: A study using the databases from two large-scale postinfarction studies. Multicenter myocardial ischemia research group. Am Heart J. 1999;138:577–585. doi: 10.1016/s0002-8703(99)70163-8. [DOI] [PubMed] [Google Scholar]
- 16.Jurt U, Gori T, Ravandi A, Babaei S, Zeman P, Parker JD. Differential effects of pentaerythritol tetranitrate and nitroglycerin on the development of tolerance and evidence of lipid peroxidation: A human in vivo study. J Am Coll Cardiol. 2001;38:854–859. doi: 10.1016/s0735-1097(01)01414-0. [DOI] [PubMed] [Google Scholar]
- 17.Gori T, Al-Hesayen A, Jolliffe C, Parker JD. Comparison of the effects of pentaerythritol tetranitrate and nitroglycerin on endothelium-dependent vasorelaxation in male volunteers. Am J Cardiol. 2003;91:1392–1394. doi: 10.1016/s0002-9149(03)00342-4. [DOI] [PubMed] [Google Scholar]
- 18.Oelze M, Daiber A, Brandes RP, Hortmann M, Wenzel P, Hink U, Schulz E, Mollnau H, von Sandersleben A, Kleschyov AL, Mulsch A, Li H, Forstermann U, Munzel T. Nebivolol inhibits superoxide formation by nadph oxidase and endothelial dysfunction in angiotensin ii-treated rats. Hypertension. 2006;48:677–684. doi: 10.1161/01.HYP.0000239207.82326.29. [DOI] [PubMed] [Google Scholar]
- 19.Mollnau H, Wenzel P, Oelze M, Treiber N, Pautz A, Schulz E, Schuhmacher S, Reifenberg K, Stalleicken D, Scharffetter-Kochanek K, Kleinert H, Munzel T, Daiber A. Mitochondrial oxidative stress and nitrate tolerance--comparison of nitroglycerin and pentaerithrityl tetranitrate in mn-sod+/− mice. BMC Cardiovasc Disord. 2006;6:44. doi: 10.1186/1471-2261-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oelze M, Warnholtz A, Faulhaber J, Wenzel P, Kleschyov AL, Coldewey M, Hink U, Pongs O, Fleming I, Wassmann S, Meinertz T, Ehmke H, Daiber A, Munzel T. Nadph oxidase accounts for enhanced superoxide production and impaired endothelium-dependent smooth muscle relaxation in bkbeta1−/− mice. Arterioscler Thromb Vasc Biol. 2006;26:1753–1759. doi: 10.1161/01.ATV.0000231511.26860.50. [DOI] [PubMed] [Google Scholar]
- 21.Wenzel P, Oelze M, Coldewey M, Hortmann M, Seeling A, Hink U, Mollnau H, Stalleicken D, Weiner H, Lehmann J, Li H, Forstermann U, Munzel T, Daiber A. Heme oxygenase-1: A novel key player in the development of tolerance in response to organic nitrates. Arterioscler Thromb Vasc Biol. 2007;27:1729–1735. doi: 10.1161/ATVBAHA.107.143909. [DOI] [PubMed] [Google Scholar]
- 22.Ishizaka N, Griendling KK. Heme oxygenase-1 is regulated by angiotensin ii in rat vascular smooth muscle cells. Hypertension. 1997;29:790–795. doi: 10.1161/01.hyp.29.3.790. [DOI] [PubMed] [Google Scholar]
- 23.Oelze M, Mollnau H, Hoffmann N, Warnholtz A, Bodenschatz M, Smolenski A, Walter U, Skatchkov M, Meinertz T, Munzel T. Vasodilator-stimulated phosphoprotein serine 239 phosphorylation as a sensitive monitor of defective nitric oxide/cgmp signaling and endothelial dysfunction. Circ Res. 2000;87:999–1005. doi: 10.1161/01.res.87.11.999. [DOI] [PubMed] [Google Scholar]
- 24.Mollnau H, Oelze M, August M, Wendt M, Daiber A, Schulz E, Baldus S, Kleschyov AL, Materne A, Wenzel P, Hink U, Nickenig G, Fleming I, Munzel T. Mechanisms of increased vascular superoxide production in an experimental model of idiopathic dilated cardiomyopathy. Arterioscler Thromb Vasc Biol. 2005;25:2554–2559. doi: 10.1161/01.ATV.0000190673.41925.9B. [DOI] [PubMed] [Google Scholar]
- 25.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–E22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- 26.Hink U, Oelze M, Kolb P, Bachschmid M, Zou MH, Daiber A, Mollnau H, August M, Baldus S, Tsilimingas N, Walter U, Ullrich V, Munzel T. Role for peroxynitrite in the inhibition of prostacyclin synthase in nitrate tolerance. J Am Coll Cardiol. 2003;42:1826–1834. doi: 10.1016/j.jacc.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: Implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 28.Warnholtz A, Mollnau H, Heitzer T, Kontush A, Moller-Bertram T, Lavall D, Giaid A, Beisiegel U, Marklund SL, Walter U, Meinertz T, Munzel T. Adverse effects of nitroglycerin treatment on endothelial function, vascular nitrotyrosine levels and cgmp-dependent protein kinase activity in hyperlipidemic watanabe rabbits. J Am Coll Cardiol. 2002;40:1356–1363. doi: 10.1016/s0735-1097(02)02133-2. [DOI] [PubMed] [Google Scholar]
- 29.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: Critical for nitric oxide bioavailability and role in angiotensin ii uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2005;102:9056–9061. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drummond GR, Cai H, Davis ME, Ramasamy S, Harrison DG. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ Res. 2000;86:347–354. doi: 10.1161/01.res.86.3.347. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi T, Morita K, Akagi R, Sassa S. Heme oxygenase-1: A novel therapeutic target in oxidative tissue injuries. Current medicinal chemistry. 2004;11:1545–1561. doi: 10.2174/0929867043365080. [DOI] [PubMed] [Google Scholar]
- 32.Mullenheim J, Muller S, Laber U, Thamer V, Meyer W, Bassenge E, Fink B, Kojda G. The effect of high-dose pentaerythritol tetranitrate on the development of nitrate tolerance in rabbits. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:269–275. doi: 10.1007/s002100100464. [DOI] [PubMed] [Google Scholar]
- 33.Hacker A, Muller S, Meyer W, Kojda G. The nitric oxide donor pentaerythritol tetranitrate can preserve endothelial function in established atherosclerosis. Br J Pharmacol. 2001;132:1707–1714. doi: 10.1038/sj.bjp.0704021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller S, Konig I, Meyer W, Kojda G. Inhibition of vascular oxidative stress in hypercholesterolemia by eccentric isosorbide mononitrate. J Am Coll Cardiol. 2004;44:624–631. doi: 10.1016/j.jacc.2004.02.062. [DOI] [PubMed] [Google Scholar]
- 35.Muller S, Laber U, Mullenheim J, Meyer W, Kojda G. Preserved endothelial function after long-term eccentric isosorbide mononitrate despite moderate nitrate tolerance. J Am Coll Cardiol. 2003;41:1994–2000. doi: 10.1016/s0735-1097(03)00392-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.