Abstract
Lesions containing abnormal aggregated tau protein are one of the diagnostic hallmarks of Alzheimer's disease (AD) and related tauopathy disorders. How aggregated tau leads to dementia remains enigmatic, although neuronal dysfunction and loss clearly contribute. We previously identified sut-2 as a gene required for tau neurotoxicity in a transgenic Caenorhabditis elegans model of tauopathy. Here, we further explore the role of sut-2 and show that overexpression of SUT-2 protein enhances tau-induced neuronal dysfunction, neurotoxicity and accumulation of insoluble tau. We also explore the relationship between sut-2 and its human homolog, mammalian SUT-2 (MSUT2) and find both proteins to be predominantly nuclear and localized to SC35-positive nuclear speckles. Using a cell culture model for the accumulation of pathological tau, we find that high tau levels lead to increased expression of MSUT2 protein. We analyzed MSUT2 protein in age-matched post-mortem brain samples from AD patients and observe a marked decrease in overall MSUT2 levels in the temporal lobe of AD patients. Analysis of post-mortem tissue from AD cases shows a clear reduction in neuronal MSUT2 levels in brain regions affected by tau pathology, but little change in regions lacking tau pathology. RNAi knockdown of MSUT2 in cultured human cells overexpressing tau causes a marked decrease in tau aggregation. Both cell culture and post-mortem tissue studies suggest that MSUT2 levels may influence neuronal vulnerability to tau toxicity and aggregation. Thus, neuroprotective strategies targeting MSUT2 may be of therapeutic interest for tauopathy disorders.
INTRODUCTION
Lesions containing abnormal aggregated tau protein are a hallmark of Alzheimer's disease (AD). Likewise, neuropathological examination reveals abnormal tau-containing lesions in virtually all cases of frontotemporal lobar degeneration tau (FTLD-tau), Guam amyotrophic lateral sclerosis/Parkinson's dementia complex, Pick's disease, progressive supranuclear palsy and corticobasal degeneration (1,2). Furthermore, abnormal tau findings are frequently present in chronic traumatic encephalopathy (3).
A variety of dominant mutations in the human tau gene (MAPT) causes FTLD-tau, demonstrating tau abnormalities can initiate neurodegeneration (4–6). Hence, modeling tauopathy by expressing human tau cDNAs in the neurons of model organisms has been a productive strategy for recapitulating many of the pathological features of AD and other tauopathies (reviewed in 7,8). However, the role played by accessory factors in pathological tau remains unclear and effective neuroprotective strategies remain elusive.
As a thoroughly studied model organism, Caenorhabditis elegans offers a number of distinct advantages for modeling human diseases including small size, short generation time, rapid transgenics, robust classical genetics, a simple well-characterized nervous system and well-studied behavior (reviewed in 9). We have used C. elegans to model human tauopathy. In this model, neuronal expression of human tau causes a progressive behavioral defect, aggregation of insoluble phosphorylated tau and neurodegeneration, all hallmarks of authentic human tauopathy disorders (10).
In the previous work, we conducted unbiased classical genetic screens and cloned two novel genes that when mutated ameliorate the tau phenotypes; the mutated genes are suppressor of tauopathy genes, sut-1 (11) and sut-2 (12). Subsequent characterization of the sut-2 gene has revealed an essential role for this protein in tau pathology in C. elegans and conservation of the encoded SUT-2 protein across animal phyla. Loss of function mutations in the worm sut-2 gene results in depletion of the SUT-2 protein and prevents tau neurotoxicity and the associated accumulation of insoluble tau. Furthermore, the physical interaction between SUT-2 proteins and HOOK proteins suggested a possible role in protein aggregation (12). Here, we further explore the role of SUT-2 and its human homolog mammalian SUT-2 (MSUT2) in the tau pathological cascade and assess the relationship between tau pathology and MSUT2 in AD post-mortem tissue.
RESULTS
SUT-2 protein drives tau neurotoxicity in C. elegans
We have previously shown that loss of sut-2 ameliorates the neurotoxic effects of human tau while decreasing accumulation of insoluble tau protein in a transgenic C. elegans model of tauopathy (12). To determine whether SUT-2 protein levels drive neurotoxicity, we overexpressed SUT-2 protein in tau-transgenic worms and observe robust enhancement of tau-related phenotypes (Fig. 1). We drove expression of a SUT-2 cDNA containing an in-frame green fluorescent protein (GFP) tag in order to monitor SUT-2 expression and localization in response to tau neurotoxicity. The SUT-2::GFP transgene expresses GFP in frame at the C-terminus of the normal SUT-2 coding sequence and transgene expression is controlled by the normal endogenous sut-2 promoter, 5′UTR (untranslated region) and 3′UTR sequences (Fig. 1A). This transgene yields high-level expression of a SUT-2::GFP fusion protein ∼7-fold higher than normal endogenous levels of SUT-2 protein in non-transgenic animals (Supplementary Material, Fig. S1). Overexpression of SUT-2 alone has little effect on worm locomotion (Supplementary Material, Movie S1). However, overexpression of SUT-2 in tau-transgenic animals results in a profound enhancement of tau neurotoxicity (Supplementary Material, Movie S2; Fig. 1B). In contrast, SUT-2 overexpression does not alter locomotion in a C. elegans polyglutamine model (13) of neurotoxicity (Supplementary Material, Fig. S2), suggesting that SUT-2 is not a generic modifier of neurodegenerative phenotypes, but rather is specific to neurotoxicity associated with tau pathology.
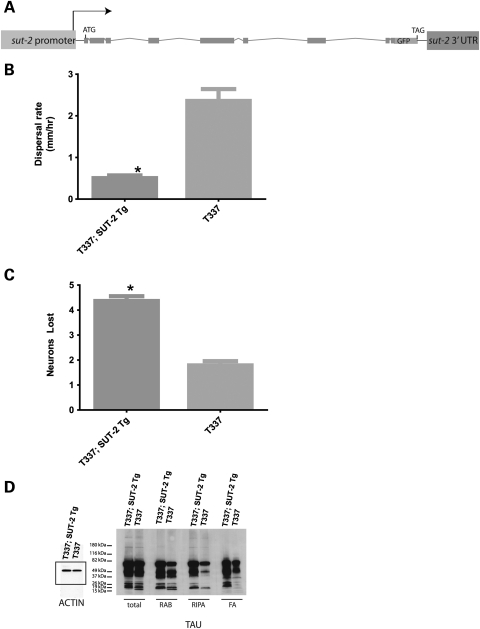
Figure 1.
SUT-2 overexpression exacerbates tau neurotoxicity. Overexpression of a SUT-2::GFP fusion protein alters tau-related phenotypes. (A) Diagram of the SUT-2::GFP transgene. (B) The SUT-2 transgene exacerbates tau induced neuronal dysfunction. T337 Tg has significantly higher dispersal rate than T337;SUT-2 double-transgenic animals (P < 0.0001). Non-transgenic control strains and the SUT-2::GFP transgene alone have similar locomotion with a dispersal rate of ∼15 mm/h. (C) SUT-2 overexpression exacerbates tau-mediated degeneration of GABAergic neurons. Comparison of T337;SUT-2::GFP double-transgenic animals with single T337 transgenic animals reveals a significant increase in neurodegenerative changes caused by SUT-2 overexpression (P < 0.0001). (D) SUT-2 protein overexpression drives accumulation of detergent-insoluble tau species as detected by extraction of tau protein using buffers of increasing solubilizing strength. Tau protein was sequentially extracted using high salt (RAB) and detergent-containing buffer (RIPA) to obtain detergent-insoluble tau protein from tau-transgenic animals with or without the SUT-2::GFP-expressing transgene.
To examine the interplay between SUT-2 overexpression and neurodegeneration, we placed the SUT-2 overexpressing transgene in the background of an unc-25::GFP marker transgene that expresses GFP in all 19 GABAergic motor neurons (14). We monitored tau-induced neurodegeneration in live animals by examining the structural integrity of GABAergic neurons and their processes in tau-transgenic animals with or without the SUT-2::GFP overexpression transgene. In the wild-type worms, both the dorsal and the ventral nerve cords are continuous and contain the normal complement of 19 inhibitory motor neurons (13 ventral D type and 6 dorsal D type GABAergic neurons). Previous work with tau-transgenic animals demonstrated age-dependent discontinuities in both dorsal and ventral cord axons of GABAergic neurons (10). Furthermore, loss of sut-2 reduced the tau-related neurodegenerative disruption of axons and loss of neurons (12). Overexpression of SUT-2 causes increased tau toxicity, enhanced degeneration of neurons and degeneration of axons, neuronal swelling and dimming of the GABAergic GFP-reporter output (Supplementary Material, Fig. S3). To measure the severity of these degenerative changes, GABAergic neuronal loss was scored as described previously (10–12). A significant increase in neurodegenerative loss of GABAergic neurons was observed in SUT-2::GFP/T337 double-transgenic worms relative to the T337 tau transgene alone (Fig. 1C).
SUT-2 overexpression promotes tau accumulation and aggregation
Accumulation of insoluble aggregates of tau protein characterizes human tauopathy disorders. To address whether SUT-2 overexpression-induced changes in neurodegeneration coincide with changes in tau protein levels or solubility, we analyzed tau protein levels and solubility in SUT-2 overexpressing worms. We sequentially extracted worm lysates using buffers of increasing solubilizing strength. We initially homogenized T337, and T337;SUT-2::GFP worm pellets in reassembly buffer (RAB), a high salt buffer, yielding the soluble tau fraction. We re-extracted material insoluble in RAB with radio immunoprecipitation assay buffer (RIPA), an ionic and non-ionic detergent-containing buffer, yielding the detergent-soluble fraction. Subsequently, we recovered a detergent-insoluble material by extraction with formic acid (FA; Fig. 1D). T337;SUT-2::GFP animals exhibit an increase in both total and insoluble tau levels. This is consistent with our previous observations that loss of sut-2 results in decreased detergent-insoluble tau protein (12). Hence, SUT-2 protein plays an important role in the accumulation of insoluble tau protein as increased sut-2 activity drives the tau protein aggregation in worm neurons.
Human MSUT2 is a multi-isoform protein expressed in the nucleus of neurons
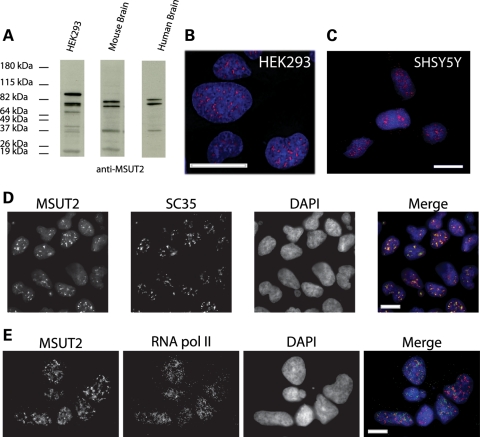
Our previous work (12) and Figure 1 above clearly implicate SUT-2 in neurotoxicity pathways in C. elegans. To address whether the human homolog of SUT-2, MSUT2, could play a role in authentic human neurotoxicity pathways, we raised antibodies against human MSUT2 protein and examined its expression pattern. Immunoblotting with MSUT2-specific antibodies revealed that MSUT2 is expressed in cultured human cells, mouse brain and human brain samples (Fig. 2A). Multiple isoforms are expressed in human brain corresponding to 76, 68 and 36 kDa consistent with approximate predicted sizes for MSUT2 isoforms (NCBI gene) (15). We investigated MSUT2 protein expression patterns in cultured cells by immunofluorescent staining and observe that MSUT2 is expressed in the nuclear compartment of both human embryonic kidney 293 (HEK293) and SH-SY5Y cell cultures (Fig. 2B and C). HEK293 is an embryonic kidney cell line with some neuronal features (16), SH-SY5Y is a neuroblastoma cell line with neuron-like properties. We see similar staining patters in both cell lines with a clearly punctate nuclear staining pattern reminiscent of nuclear speckles (Fig. 2B and C). Dual-label immunofluorescence experiments reveal co-localization between MSUT2 and nuclear speckle protein SC35 but not between MSUT2 and RNA polymerase II, a non-speckle nuclear resident protein (Fig. 2D and E). The nuclear speckle localization is also consistently observed in a variety of C. elegans cells (12).
Figure 2.
MSUT2 is a multi-isoform protein expressed in the nucleus of brain and cultured cells. (A) Immunoblotting demonstrates specificity of MSUT2 antibody in HEK293, mouse brain and human brain extracts. Note that the predicted human MSUT2 isoforms are 82, 65, 64 and 25 kDa. (B) MSUT2 immunofluorescence staining on HEK293. (C) MSUT2 staining on SH-SY5Y. (D) Localization of MSUT2 to SC35-positive nuclear speckles. (E) Lack of co localization between RNApol II and MSUT2. Scale bars are 10 µm.
MSUT2 levels but not localization changes in response to tau aggregation
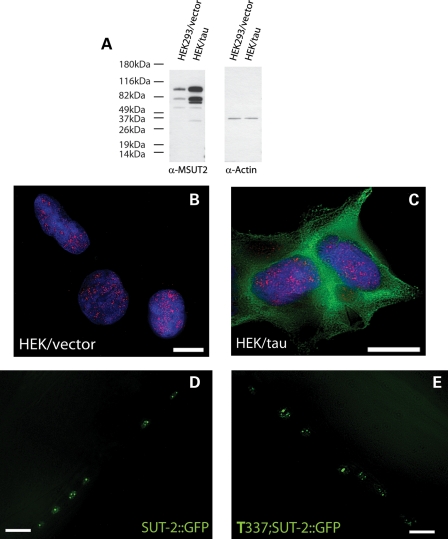
Since SUT-2 levels influence tau toxicity and aggregation, we explored whether MSUT2 protein expression is responsive to tau aggregation. We examined MSUT2 levels by analyzing its expression in HEK293 cells with or without transgenic tau expression. Immunoblotting of protein fractions from HEK or HEK/tau cells reveal that MSUT2 levels are increased ∼2-fold in cells that overexpress tau (Fig. 3A). A similar up-regulation of SUT-2 protein is also seen in tau-transgenic C. elegans (Supplementary Material, Fig. S1).
Figure 3.
Tau expression increases MSUT2 protein levels, but has no effect on cellular localization. (A) Immunoblotting reveals an increase in MSUT2 protein in response to tau expression. (B) Immunofluorescence microscopy of MSUT2 (red) in nucleus of HEK293 cells. (C) HEK/tau cells overexpressing human wild-type (4R1N) tau. (D) Live mount image of non-transgenic C. elegans overexpressing SUT-2::GFP shows a nuclear speckle pattern as does (E) T337;SUT-2::GFP double-transgenic C. elegans. Scale bars are 10 µm.
SUT-2 and MSUT2 contain sequences controlling both nuclear localization and nuclear export, suggesting that they may shuttle between nucleus and cytoplasm as part of their normal function. To investigate whether this localization changes in response to tau expression, we immunostained HEK/tau cells for MSUT2. We observe that MSUT2 expression in HEK/tau cells appears to be increased relative to the parental HEK293 cells and may be slightly more diffusely distributed in the nucleus, whereas some MSUT2 appears in the cytoplasm (Fig. 3B versus C). Similarly, SUT-2::GFP-reporter localization in non-transgenic animals is unchanged compared with animals carrying the T337 tau transgene. In both cases, the GFP signal in these live animals is localized to discrete domains in the nuclei of neurons resembling speckle staining (Fig. 3D versus E).
MSUT2 is decreased in neurons in AD
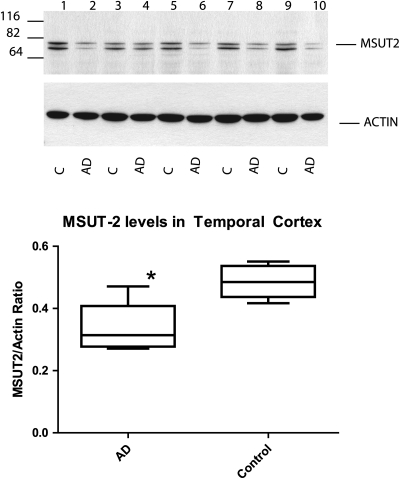
Having evidence that MSUT2 is expressed in the human brain, we sought to explore its expression in AD and any relationship with neurodegenerative changes. We analyzed total protein in samples of age-matched human temporal cortex by immunoblotting to compare MSUT2 levels between AD and control brain tissue. The temporal cortex was chosen for analysis because it is a brain region known to be profoundly impacted by neurodegenerative changes and tau pathology in AD. We see that each sample has similar levels of total MSUT2 protein; however, there is a marked difference in the amount of the prominent ∼76 kDa band corresponding to full-length MSUT2 in the AD samples when compared with the control samples (Fig. 4A). Densitometry analyses of the major MSUT2-specific bands demonstrate a ∼2-fold decrease of MSUT2 relative to actin in AD specimens when compared with normal control specimens (Fig. 2B). This difference in MSUT2 levels between AD and control temporal cortex samples is statistically significant (P = 0.0088, paired students t-test). Thus, MSUT2 protein is depleted in the AD temporal cortex relative to normal controls. Since we observed that MSUT2 changes in tau pathology affected brain regions, we wanted to see if MSUT2 was subject to expression changes in brain regions relatively spared from tau pathology in AD. Thus, we examined MSUT2 levels in post-mortem cerebellum samples taken from AD cases and age-matched controls. We observe low MSUT2 levels in cerebellum regardless of disease state and no change in MSUT2 levels between cerebellum samples from AD cases and controls (Supplementary Material, Fig. S5E).
Figure 4.
MSUT2 is decreased in AD brain samples from the temporal cortex. (A) MSUT2 levels in temporal cortex extracts were examined by immunoblotting with MSUT2-specific antibodies. Lysates from five age-matched controls and AD brains were compared by quantitative immunoblotting in triplicate. (B) Comparison of immunoblotting results shows a significant difference between control and AD brain samples (P = 0.0088).
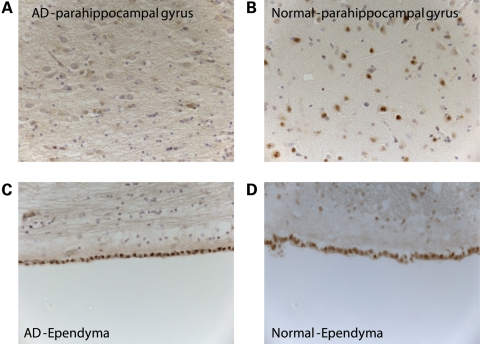
To investigate the relationship between MSUT-2 expression and neurodegenerative changes in AD, we conducted MSUT2 immunostaining on sections of post-mortem human brain tissue. We stained fixed, paraffin-embedded medial temporal lobe (MTL) sections from post-mortem non-demented control and AD brains with MSUT2 antibodies. These sections included hippocampal formation, parahippocampal and inferior temporal gyrus. We chose to examine the MTL because this is a site of profound and early AD-related neurodegenerative changes with robust tau pathology and it includes the temporal cortex, which was sampled for immunoblotting analysis above. Furthermore, MTL also plays an important role in memory encoding (17). Immunostaining with MSUT2-specific antibodies used for immunoblotting revealed a pronounced lack of MSUT2 staining in neuronal nuclei of AD cases relative to controls (Fig. 5A and B). However, MSUT2 immunostaining of ependymal cells from the same MTL sections was essentially identical in cases and controls (Fig. 5C and D). Thus, MSUT2 protein appears to be specifically depleted from neurons but not other cells in AD relative to controls. As was observed for staining in cultured cells (Fig. 2), MSUT2 appears to localize to nuclear speckles in human brain neurons (Fig. 5 and Supplementary Material, Fig. S4C). To demonstrate specificity of staining, we conducted immune-absorption studies. Pre-absorption of MSUT2 antibody with recombinant MSUT2 protein prior to immunostaining eliminates the nuclear staining in both neurons and ependymal cells shown above, demonstrating the specificity of the MSUT2 antibody preparation (Supplementary Material, Fig. S4). To examine the expression of MSUT2 in a brain region spared from tau pathology, we immunostained cerebellum sections for MSUT2. We observe only weak MSUT2 staining in neuronal nuclei of the cerebellum from both AD cases and controls with no obvious change in staining related to disease (Supplementary Material, Fig. S5A and B). Furthermore, prominent and specific immunostaining of cerebellar ependymal cells, in the same sections, is clearly consistent with the strong immunostaining of the ependyma from other brain regions and indicates that in contrast to cortical neurons, cerebellar neurons have innately low levels of MSUT2 (Supplementary Material, Fig. S5C and D).
Figure 5.
MSUT2 immunohistochemical staining is specifically reduced in hippocampal neurons but not ependymal cells of AD. Immunohistochemical staining reveals markedly reduced MSUT2 immunostaining of nuclei in hippocampal and parahippocampal neurons of AD patients (A) relative to controls (B). In contrast, nuclear staining is relatively unchanged in ependymal cells of AD (C) and controls (D).
Knockdown of MSUT2 decreases insoluble tau
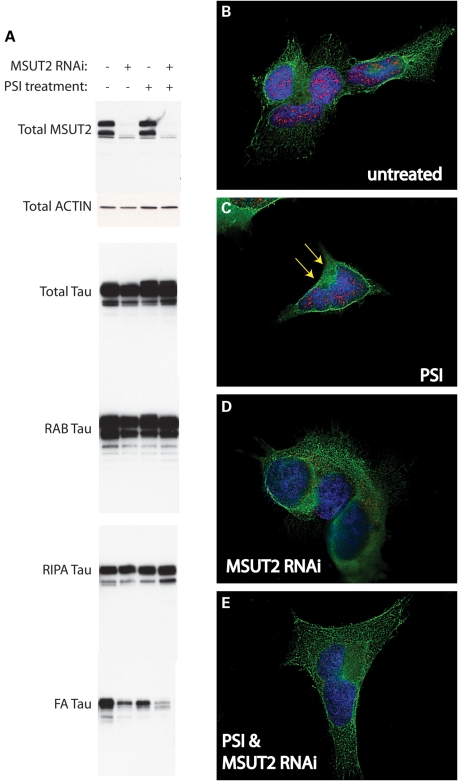
As shown in Figure 1D, SUT-2 plays a role in tau aggregation in C. elegans. Furthermore, MSUT2 binds to HOOK2 (12), a protein previously implicated in aggresome formation (18). To examine the role of human MSUT2 in tau aggresome formation, we utilized the HEK/tau cellular model where overexpressed wild-type human tau aggregates and then becomes recruited to aggresomes in response to proteasome inhibition (19). In order to evaluate the involvement of MUT2 in tau aggregation in human cells, we knocked down MSUT2 in the context of the HEK/tau model for aggresome formation and analyzed tau solubility. We conducted a sequential extraction of tau protein using buffers of increasing solubilizing strength as described previously (20). We observe that MSUT2 knockdown does not dramatically alter the total levels of soluble tau fractions, but does clearly decrease detergent-insoluble tau species in cells with aggresomes and to a lesser extent in cells without aggresome induction (Fig. 6A). However, in cells treated with a combination of MSUT2 RNAi and PSI, there is little change in bulk tau suggesting MSUT2 influences only insoluble tau species. We do notice a modest reduction in detergent-insoluble tau in PSI-treated cells when compared with untreated cells, possibly due to increased autophagic activity which is known to be stimulated by proteasome inhibition (21). Regardless, the decreased detergent-insoluble tau in HEK/tau cells under MSUT2 small interfering RNA (siRNA) treatment is consistent with increased SUT-2 levels exacerbating tau neurotoxicity in worms (Fig. 1).
Figure 6.
Knockdown of MSUT2 depletes aggregated and insoluble tau species. (A) Tau protein was sequentially extracted to obtain the detergent-insoluble tau protein from HEK/tau cells with or without PSI treatment and with or without MSUT2 RNAi knockdown. PSI treatment consisted of 2 μm PSI treatment for 18 h prior to harvest. MSUT2 RNAi involved treatment with chemical siRNA for 40 h prior to cell harvest. The total fraction was probed with MSUT2, actin and tau-specific antibodies. Three other fractions were generated by sequential extraction and probed using tau-specific antibodies. RAB contains tau solubilized by high salt; RIPA contains detergent-soluble tau, whereas FA contains the detergent-insoluble material FA-solubilized protein. (B–E) HEK/tau cells were subjected to siRNA treatment for 48 h (D and E) prior to 18 h treatment with 2 μm PSI (C and E). Fixed cells were double stained for tau (green) and MSUT2 (red) and examined with immunofluorescence microscopy.
siRNA knockdown of MSUT2 alters trafficking of pathological tau species
In HEK/tau cells stained for a normal tau protein with antibody T46, high levels of tau are distributed across the cytoplasm (Fig. 6B) (19), whereas MSUT2 remains nuclear. A previous study of SUT-2 suggests its normal function involves protein shuttling between the nucleus and the cytoplasm given the juxtaposition of nuclear localization and nuclear export signals (12). When HEK/tau cells are treated with PSI, tau-containing aggresomes form rapidly and reproducibly (Fig. 6C) (19), but there is no obvious change in MSUT2 protein. Treatment of HEK/tau cells with dicer-substrate RNA duplexes directed against MSUT2 (MSUT2 RNAi) causes a dramatic decrease in MSUT2 staining and a redistribution of the remaining MSUT2 out of the nucleus (Figs 3 and 6D) with little change in tau protein, suggesting MSUT2 may play an important but poorly understood role in the cytoplasm during the cellular response to toxic tau. In cells treated with both PSI and MSUT2 RNAi, fewer tau-containing aggresomes are formed and the intensity of tau immunofluorescence associated with aggresomes is diminished by ∼50% (Fig. 6E versus C). Thus, MSUT2 RNAi treatment yields reduced aggresomal tau (Fig. 6E) consistent with the sequential extraction data shown above (Fig. 6A).
DISCUSSION
We have investigated human MSUT2 in order to establish its relationship with pathological tau protein and neurodegeneration. The initial observation that loss of SUT-2 protein suppresses tauopathy in a transgenic C. elegans model led us to study human MSUT2 (12). Here, we expand upon the initial findings in worms in order to establish the functional role of SUT-2 in C. elegans neurodegeneration. We observe that not only is SUT-2 required for tau neurotoxicity, but that excess SUT-2 drives additional neuronal dysfunction, tau aggregation and neurodegeneration. To test the hypothesis that human MSUT2 plays a role in authentic tauopathy disorders, we explored MSUT2 protein expression. We showed that MSUT2 is a multi-isoform protein predominantly localized to nuclear speckles. We previously established a cellular model for tau aggregation and turnover (19). Using both cellular and worm models of tau pathology, we demonstrate that SUT-2 and MSUT2's localization does not change in response to accumulation of pathological tau. However, we observe a marked increase in MSUT2 in response to overexpression of tau. To further examine the connection between tau and MSUT2, we analyzed MSUT2 protein in post-mortem brain samples from AD patients. We observe a marked decrease in overall MSUT2 levels in AD relative to controls. In order to examine this relationship further, we conducted immunohistochemical staining of AD brain specimens for MSUT2 protein. We see a clear reduction in MSUT2 staining in neurons of AD brain cases compared with age-matched controls. To explore the functional consequences of decreased MSUT2 in human cells we knocked down MSUT2 in a cellular model of tauopathy and see a striking decrease in detergent-insoluble tau. When MSUT2 expression in HEK/tau cells is reduced with siRNA treatment, we observe changes in localization of both MSUT2 and tau. Knockdown of MSUT2 results in loss of MSUT2 in nuclear speckles and translocation of remaining MSUT2 to the cytoplasm, presumably via its endogenous nuclear export sequence. This observation suggests the cytoplasm could be an important site of MSUT2 activity. Likewise, tau is redistributed from the aggresome to other cytoplasmic regions upon MSUT2 knockdown, further supporting a cytoplasmic role for MSUT2 potentially in modulating clearance of tau aggregates. When MSUT2 is at normal levels, aggregated tau is moved to the aggresome for clearance in response to proteasome inhibition. However, when MSUT2 levels are reduced, aggresomal tau is either rapidly cleared or never deposited. Instead tau remains in a more soluble state upon a reduction in MSUT2 levels. These observations demonstrate a requirement for MSUT2 in the accumulation of aggregated tau species.
Tau aggregation and toxicity are coupled in worms and human cells
In both human cells and transgenic C. elegans, tau toxicity correlates with tau aggregation; this observation holds true in a wide variety of tauopathy models (reviewed in 22,23). Our work has demonstrated that loss of sut-2 in worms suppresses tau aggregation and neurotoxicity (24). Likewise, in human cells, loss of MSUT2 dramatically decreases insoluble tau suggesting that SUT-2 and MSUT2 proteins share a related function in the process of tau aggregation in both worms and humans. These findings also suggest that loss of MSUT2 or its inhibition in whole mammalian models may be protective against tau neurotoxicity—a hypothesis we aim to test in future studies.
SUT-2/MSUT2 as determinants of tau toxicity
Clearly, SUT-2 and MSUT2 levels increase in response to tau in both the cellular and C. elegans tauopathy models. However, the localization of SUT-2/MSUT2 does not change from a distinctly nuclear speckle pattern in response to tau. Thus, the observed decrease in MSUT2 in the MTL neurons of AD patients is unlikely to result from a compensatory regulation of MSUT2 levels. Rather, we hypothesize that MSUT2 protein is a direct determinant of vulnerability to tau neurotoxicity. In other words, high SUT-2/MSUT2 protein levels increase tau neurotoxicity, whereas low MSUT2 levels decrease tau neurotoxicity. The data gathered thus far are consistent with this hypothesis. Loss of SUT-2 suppresses tau neurotoxicity and aggregation in tau-transgenic C. elegans (12), whereas high levels of SUT-2 exacerbate tau neurotoxicity and aggregation (Fig. 1). Similarly, MSUT2 knockdown promotes clearance of pathological tau in human cultured cells (Fig. 6). Consequently, we infer decreased MSUT2 levels observed in affected regions of AD brain result because neurons with high levels of MSUT2 are particularly vulnerable to tau aggregation and neurotoxicity resulting in their loss during the course of disease progression. Furthermore, low cerebellar MSUT2 levels are consistent with the relative sparing of cerebellum from tau pathology and neurodegeneration in AD. Regardless, these data demonstrating MSUT2 changes in AD neurons are consistent with MSUT2's involvement in tau-induced neurodegeneration and suggest that the participation of sut-2 in tau pathology in both our C. elegans and human cellular tauopathy models bears some correspondence with the authentic pathogenic process in tauopathies.
MSUT2 as a potential target for intervention in AD and tauopathies
Tau pathology has been demonstrated to be a central aspect of AD pathology but remains incompletely understood. Likewise, tau pathology is evident in a variety of other tauopathy disorders (reviewed in 25). In C. elegans and cultured cells, we have demonstrated that loss of SUT-2 is neuroprotective against tau-related insults, whereas excess SUT-2 makes C. elegans neurons more vulnerable to tau toxicity. We now have evidence of a link between MSUT2 and tauopathies such as AD. Although further study of MSUT2 modulation of tauopathy in mammalian models and humans is needed, small molecule inhibitors of MSUT2 are an attractive and novel candidate neuroprotective strategy for tauopathy disorders.
MATERIALS AND METHODS
MSUT2 antibody preparation
MSUT2 antibody Rbt9857 was prepared commercially using the Rockland Immunochemical polyclonal antibody service. Purified MSUT2 peptide derived from the C-terminal region of the protein was the immunogen injected into rabbit hosts. Anti-sera were affinity purified using an affinity column coupled with glutathione S-transferase (GST)-MSUT2. The GST-MSUT2 protein expression construct was prepared by inserting the Zinc finger domain coding fragment of the MSUT2 cDNA into the pGEX 6P-1 expression vector (Pharmacia) to generate a construct encoding a GST-MSUT2-ZF fusion protein. Recombinant protein was purified as described previously (26). Pure recombinant GST-MSUT2 was coupled to a solid matrix using the Aminolink plus immunoimmobilization reagents (Pierce).
Cell culture and drug treatments
HEK/tau cells were cultured under standard culture conditions [Dulbecco's modified Eagle medium, 10% defined fetal bovine serum, penicillin (50 IU/ml)–streptomycin (50 μg/ml) + Zeocin (10 μg/μl)] for 18 h. RNAi experiments were carried out as per protocol in the TriFECTa Dicer-Substrate RNAi manual (Integrated DNA Technologies).
Caenorhabditis elegans strains and transgenics
N2 (Bristol) was used as wild-type C. elegans and maintained as described previously (27). Line CK10–bkIs10[Paex-3::tau–V337M), Pmyo-2::GFP] carries a chromosomally integrated transgene encoding the 1N4R isoforms of human tau carrying the V337M FTDP-17 mutation with expression driven by the pan-neuronal promoter aex-3 and has a pronounced Unc phenotype (10). Strain CZ1200 has an integrated unc-25::GFP transgene expressed in GABAergic neurons (28). The polyQ neurotoxicity model was partially recapitulated using the PF25B5.3::Q86-YFP plasmid (13), a generous gift from Dr Richard Morimoto. Strain CK241 carries a Q86:YFP-expressing transgene and has a pronounced Unc phenotype as described previously (12).
Tau extraction and immunoblotting
Tau fractions were obtained as described (10). To analyze detergent-insoluble tau accumulation, worm or HEK293/Tau cell pellets were sequentially extracted using buffers of increasing solubilizing strengths. To generate the total fraction, cells or worms were resupsended in 1× sample buffer (10 mm Tris, pH 6.8, 1 mm ethylenediaminetetraacetic acid (EDTA), 40 mm dithiothreitol (DTT), 1% sodium dodecyl sulfate (SDS), 10% sucrose) and analyzed by immunoblotting. Cells or worms were homogenized in high salt RAB (0.1m 2-(N-morpholino)ethanesulfonic acid, 1 mm ethylene glycol tetraacetic acid, 0.5 mm MgSO4, 0.75 m NaCl, 0.02 m NaF, 0.5 mm phenylmethylsulfonyl fluoride (PMSF), 0.1% protease inhibitor cocktail, pH 7.0) and ultra-centrifuged at 50 000g yielding the soluble fraction (supernatant) and an insoluble pellet. The heat-stable protein was analyzed as the RAB fraction. Next, the RAB-insoluble material was re-extracted with an ionic and non-ionic detergent-containing RIPA buffer (50 mm Tris,150 mm NaCl, 1% NP40,5 mm EDTA, 0.5% deoxycholate, 0.1% SDS, 0.5 mm PMSF, 0.1% protease inhibitor cocktail, pH 8.0) and centrifuged as above yielding abnormal tau in the supernatant. Finally, the detergent-insoluble pellet was re-extracted with 70% FA to solubilize detergent-insoluble tau. The three fractions were analyzed by immunoblotting.
Protein samples were brought (10 mm Tris, pH 6.8, 1 mm EDTA, 40 mm DTT, 1% SDS, 10% sucrose) by addition of 5× sample buffer boiled 5 min and loaded onto 4–15% pre-cast criterion SDS–PAGE gradient gels (Bio-Rad). For immunoblotting, we detected human tau using antibody 17025 at a dilution of 1:6000 (a generous gift from Virginia Lee) as described previously (12). We used anti-tubulin antibody at a dilution of 1:1000 (Developmental Studies Hybridoma Bank). MSUT2 antibody Rbt9857 was prepared as described above and used at a dilution of 1:1000. SUT-2 antibody was described previously (12). Secondary goat anti-mouse or goat anti-rabbit IgG were the secondary antibody reagents used at a dilution of 1:1000 (GE Lifesciences). Signals were measured by densitometry using Adobe Photoshop.
Immunofluorescence
HEK/tau cells grown on poly-d-lysine coated 12 mm round coverslips were fixed in 4% formaldehyde solution. Cells were washed 3 × 5 min in phosphate buffered saline (PBS)/Ca2+/Mg2+, then blocked in antibody buffer (PBS, 0.5% Triton X-100, 1 mm EDTA, 0.1% bovine serum albumin, 0.05% NaN2) + 10% normal goat serum. Primary antibodies were applied and incubated for 1 h at room temperature. Cells were washed 3 × 5 min in PBS/Ca2+/Mg2+, then re-blocked for 10 min. Appropriate secondary antibodies were applied and incubated for 20 min at room temperature. Cells were again washed 3 × 5 min in PBS/Ca2+/Mg2+, counterstained with 300 nm 4′,6-diamidino-2-phenylindole and mounted with ProLong Gold antifade.
Microscopy, image acquisition and processing
Microscopy was performed on a Nikon Eclipse TE300 epifluorescent microscope. Images were acquired using a Photometrics SenSys™ cooled CCD camera and IPLab image acquisition software (BD Biosciences Bioimaging). Images were deconvolved using MicroTome™ deconvolution software (BD Biosciences Bioimaging). Image processing with Adobe Photoshop consisted of histogram stretch, noise removal with Despeckle filter and sharpening using Unsharp Mask (50%, 3 pixel radius).
Immunohistochemistry
Immunohistochemistry for MSUT2 was performed on 10 μm sections that were pretreated by autoclaving in citrate buffer for 20 min exposed to 3% hydrogen peroxide, blocked in 5% milk, incubated with primary antibody anti-MSUT2 (1:100) for 1 h at room temperature, and then detected with avidin–biotin complex using 3,3′-diaminobenzidine as chromogenic substrate, followed by counterstaining with hematoxylin.
Human tissue
We obtained samples of post-mortem tissue from the University of Washington Alzheimer's Disease Research Center (ADRC) Neuropathology Core (PI, Dr. Thomas Montine) after receiving human subjects approval (University of Washington human subjects division approval: HSD# 06-0492-E/A 01: Molecular Regulators of Tauopathy, Kraemer PI).
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by grants from the Department of Veterans Affairs (Merit Review Grant #1147891 to B.C.K.) and National Institutes of Health (R01NS064131 to B.C.K., 2P50AG005136-27 and 5P50NS2062684-02 to J.B.L.).
Supplementary Material
ACKNOWLEDGEMENTS
We thank the reviewers for helpful comments and suggestions. We thank Elaine Loomis and Susan Danner for outstanding technical assistance. We thank Virginia Lee for tau antibodies and the Developmental Studies Hybridoma Bank (NICHD) for the β-tubulin antibody E7. We would like to thank the University of Washington Alzheimer's Disease Research Center Neuropathology Core for providing coded tissue samples (supported by NIA P50AG005136).
Conflict of Interest statement. None declared.
REFERENCES
- 1.Schneider A., Mandelkow E. Tau-based treatment strategies in neurodegenerative diseases. Neurotherapeutics. 2008;5:443–457. doi: 10.1016/j.nurt.2008.05.006. doi:10.1016/j.nurt.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kertesz A. Clinical features and diagnosis of frontotemporal dementia. Front. Neurol. Neurosci. 2009;24:140–148. doi: 10.1159/000197893. doi:10.1159/000197893. [DOI] [PubMed] [Google Scholar]
- 3.McKee A.C., Cantu R.C., Nowinski C.J., Hedley-Whyte E.T., Gavett B.E., Budson A.E., Santini V.E., Lee H.S., Kubilus C.A., Stern R.A. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. doi:10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spillantini M.G., Murrell J.R., Goedert M., Farlow M.R., Klug A., Ghetti B. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc. Natl Acad. Sci. USA. 1998;95:7737–7741. doi: 10.1073/pnas.95.13.7737. doi:10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark L.N., Poorkaj P., Wszolek Z., Geschwind D.H., Nasreddine Z.S., Miller B., Li D., Payami H., Awert F., Markopoulou K., et al. Pathogenic implications of mutations in the tau gene in pallido-ponto-nigral degeneration and related neurodegenerative disorders linked to chromosome 17. Proc. Natl Acad. Sci. USA. 1998;95:13103–13107. doi: 10.1073/pnas.95.22.13103. doi:10.1073/pnas.95.22.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutton M., Lendon C.L., Rizzu P., Baker M., Froelich S., Houlden H., Pickering-Brown S., Chakraverty S., Isaacs A., Grover A., et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. doi:10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 7.Brandt R., Hundelt M., Shahani N. Tau alteration and neuronal degeneration in tauopathies: mechanisms and models. Biochim. Biophys. Acta. 2005;1739:331–354. doi: 10.1016/j.bbadis.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Gotz J., Ittner L.M. Animal models of Alzheimer's disease and frontotemporal dementia. Nat. Rev. Neurosci. 2008;9:532–544. doi: 10.1038/nrn2420. doi:10.1038/nrn2420. [DOI] [PubMed] [Google Scholar]
- 9.Kraemer B., Schellenberg G.D. Using Caenorhabditis elegans models of neurodegenerative disease to identify neuroprotective strategies. Int. Rev. Neurobiol. 2007;77:219–246. doi: 10.1016/S0074-7742(06)77007-6. doi:10.1016/S0074-7742(06)77007-6. [DOI] [PubMed] [Google Scholar]
- 10.Kraemer B.C., Zhang B., Leverenz J.B., Thomas J.H., Trojanowski J.Q., Schellenberg G.D. Neurodegeneration and defective neurotransmission in a Caenorhabditis elegans model of tauopathy. Proc. Natl Acad. Sci. USA. 2003;100:9980–9985. doi: 10.1073/pnas.1533448100. doi:10.1073/pnas.1533448100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kraemer B.C., Schellenberg G.D. SUT-1 enables tau-induced neurotoxicity in C. elegans. Hum. Mol. Genet. 2007;16:1959–1971. doi: 10.1093/hmg/ddm143. doi:10.1093/hmg/ddm143. [DOI] [PubMed] [Google Scholar]
- 12.Guthrie C.R., Schellenberg G.D., Kraemer B.C. SUT-2 potentiates tau-induced neurotoxicity in Caenorhabditis elegans. Hum. Mol. Genet. 2009;18:1825–1838. doi: 10.1093/hmg/ddp099. doi:10.1093/hmg/ddp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brignull H.R., Moore F.E., Tang S.J., Morimoto R.I. Polyglutamine proteins at the pathogenic threshold display neuron-specific aggregation in a pan-neuronal Caenorhabditis elegans model. J. Neurosci. 2006;26:7597–7606. doi: 10.1523/JNEUROSCI.0990-06.2006. doi:10.1523/JNEUROSCI.0990-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin Y., Jorgensen E., Hartwieg E., Horvitz H.R. The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. J. Neurosci. 1999;19:539–548. doi: 10.1523/JNEUROSCI.19-02-00539.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Z.L., Dracheva S., Jang W., Maglott D., Bastiaansen J., Rothschild M.F., Reecy J.M. A QTL resource and comparison tool for pigs: PigQTLDB. Mamm. Genome. 2005;16:792–800. doi: 10.1007/s00335-005-0060-9. doi:10.1007/s00335-005-0060-9. [DOI] [PubMed] [Google Scholar]
- 16.Shaw G., Morse S., Ararat M., Graham F.L. Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J. 2002;16:869–871. doi: 10.1096/fj.01-0995fje. [DOI] [PubMed] [Google Scholar]
- 17.Markesbery W.R. Neuropathologic alterations in mild cognitive impairment: a review. J. Alzheimers Dis. 2010;19:221–228. doi: 10.3233/JAD-2010-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szebenyi G., Wigley W.C., Hall B., Didier A., Yu M., Thomas P., Kramer H. Hook2 contributes to aggresome formation. BMC Cell Biol. 2007;8:19. doi: 10.1186/1471-2121-8-19. doi:10.1186/1471-2121-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guthrie C.R., Kraemer B.C. Proteasome inhibition drives HDAC6 dependent recruitment of tau to aggresomes. J. Mol. Neurosci. 2010 doi: 10.1007/s12031-011-9502-x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishihara T., Hong M., Zhang B., Nakagawa Y., Lee M.K., Trojanowski J.Q., Lee V.M. Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron. 1999;24:751–762. doi: 10.1016/s0896-6273(00)81127-7. doi:10.1016/S0896-6273(00)81127-7. [DOI] [PubMed] [Google Scholar]
- 21.Zhu K., Dunner K., Jr, McConkey D.J. Proteasome inhibitors activate autophagy as a cytoprotective response in human prostate cancer cells. Oncogene. 2010;29:451–462. doi: 10.1038/onc.2009.343. doi:10.1038/onc.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee V.M., Kenyon T.K., Trojanowski J.Q. Transgenic animal models of tauopathies. Biochim. Biophys. Acta. 2005;1739:251–259. doi: 10.1016/j.bbadis.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Wentzell J., Kretzschmar D. Alzheimer's disease and tauopathy studies in flies and worms. Neurobiol. Dis. 2010;40:21–28. doi: 10.1016/j.nbd.2010.03.007. doi:10.1016/j.nbd.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liachko N.F., Guthrie C.R., Kraemer B.C. Phosphorylation promotes neurotoxicity in a Caenorhabditis elegans model of TDP-43 proteinopathy. J. Neurosci. 2010;30:16208–16219. doi: 10.1523/JNEUROSCI.2911-10.2010. doi:10.1523/JNEUROSCI.2911-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rankin C.A., Gamblin T.C. Assessing the toxicity of tau aggregation. J. Alzheimers Dis. 2008;14:411–416. doi: 10.3233/jad-2008-14408. [DOI] [PubMed] [Google Scholar]
- 26.Frangioni J.V., Neel B.G. Solubilization and purification of enzymatically active glutathione S-transferase (pGEX) fusion proteins. Anal. Biochem. 1993;210:179–187. doi: 10.1006/abio.1993.1170. doi:10.1006/abio.1993.1170. [DOI] [PubMed] [Google Scholar]
- 27.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cinar H., Keles S., Jin Y. Expression profiling of GABAergic motor neurons in Caenorhabditis elegans. Curr. Biol. 2005;15:340–346. doi: 10.1016/j.cub.2005.02.025. doi:10.1016/j.cub.2005.02.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.