Abstract
Background and Aims
Expected life history trade-offs associated with sex differences in reproductive investment are often undetected in seed plants, with the difficulty arising from logistical issues of conducting controlled experiments. By controlling genotype, age and resource status of individuals, a bryophyte was assessed for sex-specific and location-specific patterns of vegetative, asexual and sexual growth/reproduction across a regional scale.
Methods
Twelve genotypes (six male, six female) of the dioecious bryophyte Bryum argenteum were subcultured to remove environmental effects, regenerated asexually to replicate each genotype 16 times, and grown over a period of 92 d. Plants were assessed for growth rates, asexual and sexual reproductive traits, and allocation to above- and below-ground regenerative biomass.
Key Results
The degree of sexual versus asexual reproductive investment appears to be under genetic control, with three distinct ecotypes found in this study. Protonemal growth rate was positively correlated with asexual reproduction and sexual reproduction, whereas asexual reproduction was negatively correlated (appeared to trade-off) with vegetative growth (shoot production). No sex-specific trade-offs were detected. Female sex-expressing shoots were longer than males, but the sexes did not differ in growth traits, asexual traits, sexual induction times, or above- and below-ground biomass. Males, however, had much higher rates of inflorescence production than females, which translated into a significantly higher (24x) prezygotic investment for males relative to females.
Conclusions
Evidence for three distinct ecotypes is presented for a bryophyte based on regeneration traits. Prior to zygote production, the sexes of this bryophyte did not differ in vegetative growth traits but significantly differed in reproductive investment, with the latter differences potentially implicated in the strongly biased female sex ratio. The disparity between males and females for prezygotic reproductive investment is the highest known for bryophytes.
Keywords: Bryum argenteum, silver moss, bryophyte, sex ratio, reproductive investment, ecotype, trade-off, gender specific fitness, asexual reproduction, sexual reproduction, protonema, inflorescence
INTRODUCTION
In seed plants, studies explicitly demonstrating trade-offs between reproductive investment (RI) and life history traits or stress tolerance (and thereby indicating a cost of reproduction) are few (e.g. Sutherland and Vickery, 1988; Piquot et al., 1998), even though a large body of evidence indicates that current reproduction negatively affects vegetative propagation (Obeso, 2002). Reasons for the general absence of a trade-off between RI and life history traits are thought to reside in (a) the presence of storage organs such as roots that provide a reservoir of carbon that buffers carbon losses from the maternal plant into RI (Ehrlén and van Groenendael, 2001), (b) organ preformation (Geber et al., 1997), (c) a significant level of self-nutrition for fruits during maturation as well as resource resorption during senescence (Ashman, 1994), and (d) a positive ratio between maternal plant size and offspring size and quality (e.g. Sletvold, 2002). Finally, potentially confounding variables such as plant age, genotype and reproductive history are seldom controlled and may mask gender differences (Wheelwright and Logan, 2004).
Bryophytes might be excellent systems to address sex-specific RI and life history trade-offs because they show strong sex-specific RI, limited compensatory resource use, and can be easily manipulated experimentally (e.g. McLetchie, 1992). A fundamental disparity exists in sexual RI between the sexes of plant species with unisexual individuals (dioecy) that should hold for both vascular plants and bryophytes: males incur a higher prezygotic RI relative to females, while females incur a higher total RI (prezygotic + post-zygotic) (Delph, 1999). This disparity arises because, in the vast majority of plants, investment in offspring maturation (seeds, fruit, and, in bryophytes, the sporophyte) far exceeds investments in pollen, sperm and associated structures, and normally the nutrition of these structures is coupled to the maternal individual. A breakdown of pre- and post-zygotic RI is quantified for seed plants in numerous instances (e.g. Delph, 1990; Lovett Doust and LaPorte, 1991; Korpelainen, 1992; Obeso, 2002; Queenborough et al., 2007). However, only three studies have assessed pre- and post-zygotic RI differences in bryophytes (Stark et al., 2000; Laaka-Lindberg, 2001; Bisang et al., 2006) and experimental approaches to trade-offs in bryophytes are limited to three species (Ehrlén et al., 2000; Fuselier and McLetchie, 2002; Stark et al., 2009a). Sex differences in life histories coupled with disturbance regimes have been used to explain male rarity in the liverwort Marchantia inflexa (Garcia-Ramos et al., 2007). Recently in Hylocomium splendens, Rydgren et al. (2010) found that even a slightly lower fitness profile of males compared with nonsporophytic females (survival and vegetative offspring production) can explain female-biased sex ratios in this species, with the authors suggesting that the higher costs incurred by expressing males may drive evolution toward male rarity. It is therefore expected that the sexes would differ in vegetative fitness at or shortly after sex expression; data of this kind are sorely needed.
These problems (an inability to detect trade-offs in seed plants) are likely to be greatly lessened with bryophytes, owing to an absence of storage organs and a relatively large and dependent post-zygotic structure (the sporophyte) in relation to the gametophyte. Investment into the sporophyte ranges from 40–114 % of total annual vegetative biomass, compared with levels often below 20 % for fruits or cones in seed plants (Kimmerer, 1991; Ehrlén et al., 2000; Stark et al., 2000; Rydgren and Økland, 2003). Such large investments into sexual reproduction relative to vegetative growth suggest a higher likelihood of trade-offs among bryophytes. While capable of significant self-nutrition, maturation of the sporophyte is largely dependent upon resources imported from the maternal plant (Proctor, 1977; Ligrone and Gambardella, 1988). These factors in bryophyte populations should help unveil potential manifestations of RI disparities between sexes as sex-specific life history features or sex-specific trade-off patterns.
Bryum argenteum appears to conform to the pattern normally seen in bryophytes with unisexual individuals (dioicy), i.e. (strongly) female-biased sex ratios in the field and yet a primary sex ratio of close to unity (Stark et al., 2010). Pilot studies also confirmed that asexual and sexual reproduction can occur in the laboratory under typical growing conditions and thus an opportunity arose to detect any sex differences in vegetative growth rates, asexual RI and prezygotic RI under controlled conditions, as an investigatory step in understanding biased sex ratios in this wide-ranging species. Rarely have studies experimentally considered the potential sex differences that may exist among the primary axes of growth and asexual and sexual reproduction in a bryophyte.
The following five questions, with the null hypothesis in each case that no sex-specific patterns should be present, are asked of B. argenteum. (1) Are there sex-specific differences in vegetative growth traits (evaluating rates of protonemal and shoot proliferation, and shoot size at sex expression)? (2) Are there sex-specific patterns in asexual fitness (evaluating specialized asexual propagule production)? (3) Are there sex-specific patterns of prezygotic RI (evaluating the production and biomass of gametangial structures)? (4) Do trade-offs occur among growth, asexual and sexual traits? (5) Are these patterns similar across regions within the continental USA?
MATERIALS AND METHODS
Species description
Bryum argenteum Hedw. is one of the few species of bryophytes found on all continents, a cosmopolitan distribution consistent with patterns of spore germination following desiccation and freezing stress (van Zanten, 1978). Known to occupy disturbed and landscaped habitats, B. argenteum is considered as weedy or invasive (Crum and Anderson, 1981; Longton, 1981; Ochi, 1994) and, in addition to occurring on natural substrata, can grow in golf courses and city parks (Burnell et al., 2004). It is characterized by a silvery appearance, especially when dry, caused by the upper portion of the leaves being hyaline (achlorophyllose; Flowers, 1973; Fig. 1A). Bryum argenteum is dioecious (dioicous), producing perigonia terminal on male shoots and perichaetia terminal or proximal by innovation along female shoots (Fig. 1B, C). Stems can produce large amounts of deciduous bulbils (axillary or terminal) and, depending on their length, branchlets that detach and float, serving as a primary mode of local dispersal (Selkirk et al., 1998). In the present study these deciduous bulbils (asexual propagules) are referred to as ‘shootlets’ because of their resemblance to a compact shoot fragment (Fig. 1D). Gametangial induction (sex expression) in B. argenteum is day neutral, temperature independent (to 25 °C), pH independent (5·5–7·0), promoted by KNO3, and has a light preference of approx. 50–200 µmol m−2 s−1 (Chopra and Bhatla, 1981).
Fig. 1.
Bryum argenteum plants and structures: (A) male (left) and female (right) plants from field-collected patches in Kentucky; (B) an expressing male culture viewed from above (perigonium at arrow); (C) an expressing female culture viewed from the side (perichaetium at arrow); (D) shootlets (= bulbils, apical at ‘1’, lateral at ‘2’); (E) excised perigonium used in prezygotic reproductive-effort estimates showing detached perigonium (millimetre scale at right); (F) excised perichaetium used in prezygotic reproductive-effort estimates showing detached perichaetium (millimetre scale at left).
Genotype and shootlet selection
Genotypes of B. argenteum were selected with an eye toward balancing the two sexes with place of origin and time in continuous culture. From each of three localities in North America (Arizona, New Mexico and Kentucky), two males and two females were selected as follows: (1) Arizona, Santa Rita Mts, 1585 m a.s.l., shoots collected on 17 March 2008 and placed into culture on 21 March 2008 (AZ plants); New Mexico, Peloncillo Mts, 1353 m a.s.l., capsule collected on 30 March 2007, single spore isolates raised in the laboratory and placed into culture on 11 March 2008 (NM plants); Kentucky, Lexington, University of Kentucky campus, 300 m a.s.l., shoots collected on 12 October 2007 and placed into culture on 2 January 2008 (one pair) and 8 February 2008 (one pair) (KY plants). These 12 stock cultures were grown under experimental conditions described below to produce a sufficient number of replicate clones (16) for use in the experiment. With the exception of the capsule collection, when field collections were made, at least 5 m separated distinct patches, making it highly probable that these were distinct genotypes within a sex (Selkirk et al., 1998; Cronberg et al., 2003, but see Cronberg et al., 2006).
Shootlets (axillary bulbils) of B. argenteum were collected from stock cultures by gently passing a moistened sterile probe across the hydrated plants, collecting shootlets as they adhered to the probe, and placing the shootlets into a droplet of sterile water. Approximately 50 shootlets from each genotype were accumulated in this fashion in a droplet of water. Twenty (20) shootlets were randomly selected (16 + 4 extras) that measured between 0·5 and 1·0 mm in length. These 20 shootlets were transferred into a micropacket of weighing paper and placed into the growth chamber (where they desiccated slowly over a period of 2 weeks) until used on day 0 of the experiment. Thus the experiment consisted of a comparison of six genotypes of each sex replicated 16 times through shootlet cloning, yielding 192 cultures (96 males and 96 females). On day 0, the shootlets in each micropacket were hydrated with sterile water and a single shootlet was transferred to the centre of a prehydrated Petri dish, using a sterile probe, and the dish was lidded and placed into the growth chamber.
Growing conditions and experimental observations
Cultures were grown on sieved, dry-autoclaved, locally collected sand in plastic Petri dishes (inner diameter 35 mm), watered each week with sterile distilled water supplemented with 3 drops of a 30 % Hoagland's solution (Hoagland and Arnon, 1938) beginning on week 4, and grown in a Percival growth chamber (Percival model E30B, Boone, IA, USA). Photoperiod was set at 12 h, temperature at 20 °C in the light and 8 °C in the dark, relative humidity ranged from 60 to 70 %, and light levels ranged from 100 to 410 µmol m−2 s−1. Petri dishes were randomly repositioned on each of two growth chamber shelves (once per day during the first 7 d, on days 10 and 14, and once per week thereafter) and the shelves were rotated within the chamber (upper shelf to second shelf and second to upper) at these times, thus equalizing the light conditions (quality and quantity) especially during the shootlet germination time (the first 2 weeks).
Once plants began to produce shootlets, care was taken when watering the cultures so as to minimize shootlet movement within the cultures (shootlets will detach and float). On days 3, 4, 5, 6, 7, 10, 14, and once a week thereafter to day 92, the following observations were made using a dissecting microscope at up to ×60: growth traits – protonemal emergence (day), protonemal advance (reaching 8 mm and 16 mm from original shootlet), shoot production (appearance of first and tenth shoot); asexual traits – shootlet production (appearance of first and tenth shootlet); and prezygotic traits – sex expression date (appearance of first and fifth inflorescence). Sex expression was recorded when gametangia first became visible at ×60 (Fig. 1E, F).
Post-experimental observations and analyses
On day 92, the last observations were made and then the Petri dishes were opened and plants allowed to dry in the growth chamber (this happened over approx. 48–72 h). Over the next few weeks, the following measurements were carried out on each dish: final shoot density, final shootlet density, final inflorescence density, above-ground (sex expressing) shoot length and biomass, and estimates of above-ground and below-ground regenerative biomass. For determination of densities of shoots, shootlets and inflorescences, each culture was rehydrated and an approx. 1/8th section of each culture (a ‘pie slice’ approx. 100 mm2) was randomly selected, the boundaries of this region trowelled using a sterile probe, and the number of shoots, shootlets and inflorescences counted (rehydration was necessary to discriminate by colour between vegetative shoot apices and shoot apices producing undetached shootlets). Following these counts, the culture was allowed to dry as before. Densities of shoots, shootlets, and inflorescences were estimated using the counts in conjunction with image analysis of the culture area (Spot, Diagnostic Instruments, Sterling Heights, MI, USA).
For a subset of randomly selected Petri dishes representing a quarter of each replicated genotype, above-ground regenerative biomass (including shoots, shootlets and inflorescences) was separated from below-ground regenerative biomass (including protonemata and rhizoids) as follows. The culture quarter section was rehydrated, excavated, cleaned by successive agitations in sterile water until plants were relatively free of sand, and then above- and below-ground structures were manually teased apart, allowed to air-dry at 40 % RH, and dried for 3 d at 40 °C. Biomass was determined to the nearest microgram. Both the counts of structures and the biomasses were converted to estimates of whole dish counts/biomass using image analysis.
For estimating sexual prezygotic RI as inflorescence biomass per unit area, five dishes of each genotype having at least five mature inflorescences visible at ×60 were randomly selected. Each perigonial shoot was hydrated on a microscope slide and after pulling back the smaller vegetative leaves, the perigonium (inclusive of perigonial leaves) was severed from the shoot using a razor blade (Fig. 1E). Each perichaetial shoot was cut at the level of the subtending innovation, and the perichaetium (inclusive of perichaetial leaves) was severed from the shoot using a razor blade (Fig. 1F). These inflorescences were air-dried for 2 weeks in the laboratory, placed into the oven at 40 °C for 4 d, and then weighed in groups of approx. 25 (five inflorescences from five dishes) to the nearest microgram. To estimate shoot length and mass, three expressing shoots from each dish expressing sex were randomly selected, removed at ground level, measured in length to the nearest 0·1 mm and then dried and weighed to the nearest microgram. Male shoots (n = 99) were represented from two localities (four genotypes), while female shoots (n = 78) were represented from one locality (two genotypes).
Statistics
Growth rates and asexual and sexual reproduction
The aim was to test for sex, location and genotype effects. However, genotypes are nested within sex and location (i.e. a genotype can only belong to a single sex and location). Thus the effects of sex and location were not determined in a single analysis; sex and location were tested in separate multivariate analyses of variance (MANOVAs). Multivariate analysis is favoured over multiple univariate analyses when response variables can interact with each other and group differences may be a feature of more than one response variable; performing multiple univariate tests can inflate the alpha value (Scheiner, 1993). The dependent variables were: for growth – growth rate of protonema and shoot density per square millimetre; for asexual reproduction – shootlet density per square millimetre; for sexual prezygotic reproduction – inflorescence production. The main effects of sex and location were tested using the mean squares of the genotype (sex) or the genotype (location) as the error term, respectively, in the separate MANOVAs. Growth rate was log transformed and shoot, shootlet and inflorescence density were square-root transformed.
Trade-offs
To detect overall trade-offs, the relationships among these traits were tested using the partial correlation coefficients. To detect sex-specific trade-offs, trait relationships having negative correlation coefficients were noted. An ANOVA was then used to determine if the slopes of the relationship between the negatively related traits differed between males and females.
Shoot length and mass
For plants that had expressed sex, differences in shoot length and shoot mass were tested with MANOVAs. The main effects of sex and location on shoot length and mass were tested using the mean squares of the genotype (sex) or the genotype (location) as the error term, respectively, in the separate MANOVAs. Length and mass were log transformed.
Above- and below-ground allocation patterns
To detect sex and location patterns in allocation to above- or below-ground biomass differences among these response variables were tested with MANOVAs. The main effects of sex and location on above- and below-ground biomass were tested using the mean squares of the genotype (sex) or the genotype (location) as the error term, respectively, in the separate MANOVAs. Biomass data were log transformed. The relationships among these traits were examined using partial correlation coefficients.
Sex expression
To test if sex expression differences existed between males and females or among locations, the proportion of each genotype expressing sex was estimated and these data were analysed with a two-way ANOVA (sex and location). Proportions were arcsine transformed.
SAS (SAS/STAT® software Ver. 9.1.3, SAS Institute Inc., Cary, NC, USA) was used to perform the statistical tests.
RESULTS
Growth rates and asexual and sexual reproduction
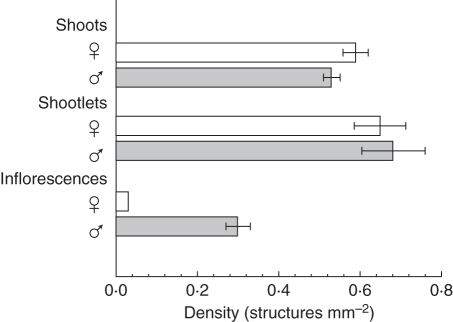
The MANOVAs were significant. In subsequent univariate analyses, genotypes were different for three of the dependent variables: shoot density (d.f. = 8, F = 12·54, P < 0·0001); shootlet density (d.f. = 8, F = 30·81, P < 0·0001); and inflorescence production (d.f. = 8, F = 58·01, P < 0·0001), but not protonemal growth rate (analysis not shown). Males had more inflorescences than females (d.f. = 1, F = 13·42, P < 0·01; Fig. 2). There were no other sex differences (analyses not shown). The timelines of protonemal and shoot production, including final shoot density, did not differ by sex (Table 1), with protonemal growth rate approx. 0·5 mm d−1, and shoot production rate approx. 1·7 shoots d−1. The timeline of both shootlet and inflorescence production was nearly identical between the sexes (Table 2 and Fig. 2). Locations were different in protonemal growth rate (d.f. = 2, F = 13·39, P < 0·01), shoot density (d.f. = 2, F = 5·47, P < 0·05), shootlet density (d.f. = 2, F = 4·93, P < 0·05) and inflorescence production (d.f. = 2, F = 5·97, P < 0·05; Table 3). Further analyses (Tukey's test, significant at P < 0·05) indicated that (a) protonemal growth rate was fastest in NM plants relative to both AZ and KY plants but did not differ between AZ and KY plants, (b) shoot density was higher in NM plants relative to KY plants but did not differ between NM and AZ nor between AZ and KY plants, (c) shootlet density was higher in KY plants compared with NM plants, but did not differ between KY and AZ nor between AZ and NM, and (d) NM plants had more inflorescences than KY plants but inflorescence production did not differ between NM and AZ nor between AZ and KY (Table 3).
Fig. 2.
Final densities of shoots, shootlets (bulbils) and inflorescences after 92 d in culture from individual shootlets, shown by gender for Bryum argenteum. Twelve cultures that did not produce shoots (four males, eight females) were excluded from the analysis. Values are means ± s.e. (the s.e. for female inflorescence density is zero).
Table 1.
Protonemal growth rates, a timeline of shoot production, and final shoot density from experimental cultures regenerated from individual shootlets of Bryum argenteum
| Protonemal emergence (d) | Protonemal advance of 16 mm (d) | First shoot production (d) | Ten shoots produced (d) | Final shoot density (shoots mm−2) | |
|---|---|---|---|---|---|
| Male | 11·59 ± 1·02 | 45·98 ±1·03 | 31·11 ± 1·21 | 37·28 ± .23 | 0·53 ± 0·02 |
| Female | 10·69 ± 0·83 | 47·32 ±0·87 | 30·72 ± 0·82 | 37·23 ± 0·86 | 0·59 ± 0·03 |
Data from 12 genotypes (six male, six female) collected from three different localities in the USA.
Values represent means ± s.e.
Shoots recognized were all produced from protonema regenerated from the shootlet.
Table 2.
Timeline of shootlet (bulbil) production, expression of sex, and prezygotic reproductive investment (RI) from experimental cultures regenerated from individual shootlets of Bryum argenteum
| First shootlet production (d) | Ten shootlets produced (d) | First sex expression (d) | Five inflorescences produced (d) | Prezygotic sexual RI (μg mm−2) | |
|---|---|---|---|---|---|
| Male | 55·37 ± 1·19 (86) | 60·27 ± 0·85 (74) | 68·66 ± 1·28 (74) | 69·33 ± 1·12 (63) | 0·32 ± 0·05 |
| Female | 53·84 ± 0·75 (88) | 61·86 ± 0·75 (88) | 68·11 ± 1·66 (38) | 70·55 ± 1·15 (33) | 7·76 ± 0·69 |
Data from 12 genotypes (six male, six female) collected from three different localities in the USA.
Values represent means ± s.e. with n values in parenthesis.
Table 3.
Variation in reproductive and vegetative growth rates by site of origin in Bryum argenteum clones
| New Mexico | Arizona | Kentucky | |
|---|---|---|---|
| Protonemal growth rate (mm d−1) | 0·38 ± 0·00a | 0·34 ± 0·01b | 0·33 ± 0·01b |
| Shoot density (shoots mm−2) | 0·73 ± 0·09a | 0·47 ± 0·02ab | 0·46 ± 0·06b |
| Shootlet density (shootlets mm−2) | 0·13 ± 0·07a | 0·81 ± 0·28ab | 1·08 ± 0·27b |
| Inflorescence density (infl. mm−2) | 0·32 ±0·14a | 0·16 ± 0·09ab | 0·01 ± 0·00b |
Data from 12 genotypes (six male, six female) collected from three different localities in the USA.
Values represent means ± s.e. with superscript letters indicating significant differences at P < 0·05 (Tukey's test).
Trade-offs
Protonemal growth was positively correlated with asexual (as shootlet density) and prezygotic sexual reproduction (as inflorescence production). Asexual reproduction was negatively correlated with shoot density (Table 4 and Fig. 3). To detect sex-specific trade-offs, the slope for the negative relationship for males and females was compared by testing the effect of the interaction between sex and shoot density on shootlet density. Shootlets are produced on shoots and are considered here to depend on shoots. Location was used as a blocking factor. The interaction was tested using the genotype within sex as the error term to adjust for the number of unique individuals. The slope was not significant (analysis not shown). The possibility was also explored that the positively related traits (protonemal growth with asexual and prezygotic sexual reproduction) were sex specific by using the same methodology in testing for sex-specific trade-offs. Neither relationship was sex specific (analysis not shown).
Table 4.
Partial correlation coefficients using the residuals from the multivariate analysis of variance
| Protonemal growth rate | Shoot density | Inflorescence production | |
|---|---|---|---|
| Protonemal growth rate | 0·05 | 0·19* | |
| Shootlet (bulbil) density | 0·41** | −0·19* | −0·05 |
| Shoot density | −0·04 |
*, P < 0·05; **, P < 0·001.
Fig. 3.
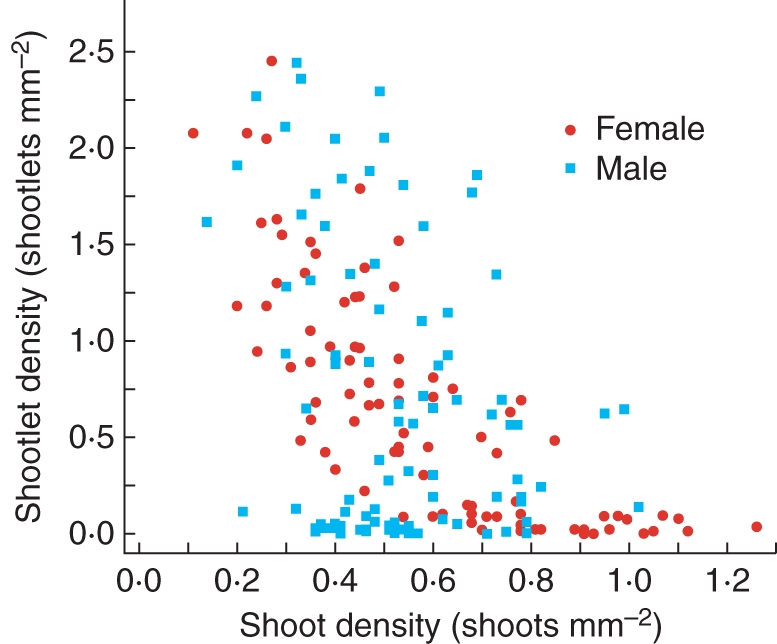
The relationship between shoot density and shootlet (bulbil) density by gender in Bryum argenteum clones cultured for 92 d from individual shootlets. Blue squares = male; red circles = female.
Shoot length and mass (sex expressing shoots)
Ninety-nine male shoots were assayed, representing four of the genotypes (two each from New Mexico and Arizona). Only females from New Mexico were assayed (n = 78, from two genotypes). Due to the sex expression patterns in the genotypes, it was possible only to examine sex differences in shoot length and shoot mass from the NM plants. Location effects could only be tested within males between the Arizona and New Mexico locations. Only the genotype within sex and genotype within location were significant in the MANOVAs. Subsequent univariate analyses found that genotypes differed among the NM plants across both sexes, and among the AZ and NM male plants for both shoot length and shoot mass (P < 0·001). The univariate test for shoot length suggests that males were shorter than females (d.f. = 1, F = 19·66, P = 0·047; Fig. 4) but this should be taken with caution as the MANOVA for a sex difference between the two male and two female genotypes was not significant.
Fig. 4.

Photograph of two typical females (on left) and two typical males (on right) of Bryum argenteum plants originating from New Mexico and grown from shootlets (bulbils) in culture for 92 d (with a millimetre scale).
Above- and below-ground allocation patterns
The MANOVAs were significant. In subsequent univariate analyses, genotypes were different for both above- and below-ground biomass (d.f. = 8, F = 2·58, P = 0·02 and d.f. = 8, F = 3·92, P = 0·002, respectively). Males and females did not differ and the locations did not differ in above- or below-ground biomass (analysis not shown). Above- and below-ground biomass tended to be positively associated (partial correlation coefficient = 0·307, P = 0·064; Table 5).
Table 5.
Final biomass production from experimental cultures regenerated from individual shootlets (bulbils) of Bryum argenteum
| Above-ground final biomass (μg mm−2) | Below-ground final biomass (μg mm−2) | Total biomass (μg mm−2) | |
|---|---|---|---|
| Male | 23·68 ± 2·44 | 18·86 ± 3·79 | 42·55 ± 3·62 |
| Female | 20·94 ± 1·83 | 26·28 ± 5·26 | 47·23 ± 4·79 |
Data from 12 genotypes (six male, six female) collected from three different localities in the USA.
Values represent means ± s.e.
Biomass reflects measurements from 48 of the 192 cultures balanced among sex and genotype; below-ground biomass includes protonemata and rhizoids; above-ground biomass includes shoots, shootlets and inflorescences.
Sex expression
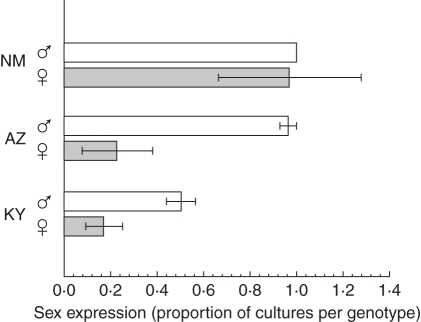
Among genotypes the percentage sex expression ranged from 7·7 % (an AZ female) to 100 % (a NM female and two NM males; Fig. 5). Males were more likely to express sex than females (82·1 % ±10·3 vs. 45·5 % ±16·9, d.f. = 1, F = 24·36, P = 0·0026). There was an overall location effect (d.f. = 2, F = 28·84, P = 0·0008) with NM plants more likely to express sex (98·4 % ±1·6) than AZ (59·4 % ±22·1) and KY (33·5 % ±10·4) plants, and sex expression between AZ and KY plants did not differ (Tukey's test, P > 0·05). There was an effect on sex expression due to the interaction between sex and location (d.f. = 2, F = 6·33, P = 0·33), indicating that the sex effect depended on location. In NM, both sexes had over 93 % sex expression but in AZ and KY males were more likely to express sex than their female counterparts from AZ and KY. Of those cultures that expressed sex, males and females did not differ in the timeline to first expression (day 68) or the time to produce five inflorescences (days 69–71; Table 2). However, twice as many male cultures expressed sex and roughly twice as many male cultures produced at least five inflorescences relative to females. In addition, final inflorescence density was an order of magnitude greater in males (0·30) than females (0·03).
Fig. 5.
Sex expression by genotype in Bryum argenteum cultured for 92 d from individual shootlets (bulbils). Values are means ± s.e. (the s.e. for the NM male is zero). NM = New Mexico; AZ = Arizona; KY = Kentucky.
Mean biomass of a single perigonium (n = 104) was 26·05 µg, whereas the mean biomass of a single perichaetium (n = 53) was 11·36 µg. (NB: variance could not be calculated because single inflorescences could not be weighed.) Prezygotic reproductive effort, taken as the biomass of inflorescences produced per unit area of culture media, was about 24 times greater in males than females (7·76 ± 0·69 vs. 0·32 ± 0·05 µg mm−2; Table 2).
DISCUSSION
Males and females of Bryum argenteum, when regenerated from shootlets (bulbils) from three localities, were remarkably similar in vegetative growth rates over a 92-d period under moderate light, a 12-h photoperiod and moderately humid conditions. No sex differences in protonemal emergence and growth rate, shoot proliferation and density, and above- and below-ground biomass were found. These findings correspond with a similar inquiry into sex-specific vegetative growth rates for the dioecious Pleurozium schreberi by Longton and Greene (1979), who found no sex differences in shoot elongation rates, branch numbers produced and protonemal regeneration rates from leaf fragments. Similarly, in both the weedy moss Ceratodon purpureus and the boreal wetland moss Pseudocalliergon trifarium, sexes exhibited similar shoot formation rates (Shaw and Beer, 1999; Bisang et al., 2006). However, the present findings contrast with those of Polytrichum commune (faster rates of growth for males; Wyatt and Derda, 1997), Pogonatum dentatum (females grow faster; Hassel et al., 2005), Syntrichia caninervis (females regenerate twice as fast from detached leaves but males regenerate faster from detached shoots following a thermal stress; Stark et al., 2005, 2006, 2009b), Marchantia inflexa (females grow faster; McLetchie and Puterbaugh, 2000) and Lophozia silvicola (females produced 3 times more branches than males; Laaka-Lindberg, 2001).
Production of specialized asexual propagules (shootlets) by B. argenteum was also equivalent between the sexes in the day of first appearance, the rate of production as judged by the time to produce ten shootlets, and in final shootlet density per culture. This contrasts with two dioecious liverwort species, where males tended to produce more gemma cups than females (Marchantia inflexa; McLetchie and Puterbaugh, 2000), and males produced twice the number of gemmae as females (Lophozia silvicola; Laaka-Lindberg, 2001). Findings from the latter two studies indicate that females may trade-off sexual RI with asexual RI (discussed below). In B. argenteum, the absence of sexual dimorphic life-history traits that can lead to differential population growth rates between males and females is surprising, considering that primary sex ratios are approx. 1 : 1 and field sex ratios are strongly female biased (Stark et al., 2010). Higher male mortality under natural conditions where stress events are likely to occur might reconcile these laboratory results with the field observations.
The only trade-off detected was that between a growth trait (shoot density) and an asexual reproductive trait (shootlet density), but this trade-off did not differ by sex (Fig. 3). Rarely (Kimmerer, 1991; McLetchie and Puterbaugh, 2000) is the vegetative function (as shoot proliferation here) compared with a specialized asexual function (as shootlet production here), in part because both correspond to the more general function of clonal expansion. Whereas shoot and protonemal proliferation allow the clone to occupy and compete for adjacent space, shootlet production and subsequent dispersal by water allow the clone to colonize more distant space and possibly contact the opposite sex, and may be more common than previously assumed (During, 2007). The strong positive relationship between protonemal growth rate and shootlet production is difficult to explain because shootlets are produced by shoots and, to a lesser extent, by protonemata. One hypothesis is that protonemal expansion and shootlet production are selected for the occupation of horizontal space, whereas shoot density responds to selection for the occupation of vertical space.
Although the findings that the NM population had higher RI rates and lower asexual reproductive rates than the KY population suggesting a trade-off between RI and asexual reproduction, no compelling evidence of this trade-off was found. In fact, a positive relationship between protonemal growth rate and inflorescence density was found when the other variables were statistically controlled. Trade-offs between sexual and clonal reproductive modes in bryophytes are well documented (Kimmerer, 1991; Laaka-Lindberg, 2001; Bisang and Ehrlén, 2002; Fuselier and McLetchie, 2002; Rydgren and Økland, 2003; McLetchie and Stark, 2006; Hedderson and Longton, 2008; Stark et al., 2009a). Increasing the number of genotypes in a study at the expense of using more locations might improve the ability to detect relationships among traits.
Most studies assessing potential sex dimorphism in bryophyte size find females to be either taller or larger than males (Shaw and Gaughan, 1993; McLetchie and Puterbaugh, 2000; Laaka-Lindberg, 2001; McLetchie, 2001; Pohjamo and Laaka-Lindberg, 2004; McDaniel, 2005), although in some cases differences are minimal (e.g. Shaw and Beer, 1999; Stark et al., 2001; Bisang et al., 2006) or male plants are larger (Laaka-Lindberg, 2001; Rydgren and Økland, 2002). Several hypotheses attempt to account for sexually dimorphic plant size in angiosperms, including the costs of reproduction (Delph, 1999), sexual selection (Delph, 1999), physiological differentiation (Obeso and Retuerto, 2002), size-advantage (Ghiselin, 1969) and time commitment hypotheses (Day and Aarssen, 1997). Sexually dimorphic traits may reflect an adaptive response to selection for different phenotypic optima for males versus females or reflect nonadaptive trade-offs deriving from differential costs of reproduction (Wheelwright and Logan, 2004). In B. argenteum, males were smaller at initial sex expression than females of the same age, consistent with male precociousness and smaller size at reproduction often found in angiosperms (Delph, 1999).
While males and females of B. argenteum were essentially similar in the timeline of expression (confirming results in Chopra and Bhatla, 1981) and rate of expression (inflorescence appearance and the time to produce five inflorescences), significant sex differences were detected in the proportion of male cultures expressing and the magnitude of the expression (prezygotic RI). Twice as many male cultures expressed sex and, similarly, twice as many male cultures produced at least five inflorescences, than females. The disparity in expression by sex was greater than that observed after 40 d on supplemented agar (53 vs. 43 % for male and female clones, respectively; Bhatla and Chopra, 1981). More telling, given that the biomass of an individual inflorescence was larger (2·3 times) in males, and males produced a higher density (10 times) of inflorescences than females, the average effort devoted to prezygotic sexual investment was 24 times greater for males (7·76 vs. 0·32 µg mm−2, male and female, respectively). The majority of the tissue mass of a male shoot is perigonial in origin (66 %), whereas only 19 % of a female shoot is dedicated to perichaetial tissue. Mechanisms for this divergence in expression by sex probably involve relative amounts of auxin and gibberellins produced in combination with the availability of Fe and Cu: male expression is stimulated, and female expression inhibited, by auxin (IAA) and gibberellins (Bhatla and Chopra, 1981). In addition, male expression is promoted to a greater extent by chelating agents including Fe and Cu compared with female expression (Bhatla and Chopra, 1983), which may indicate that male plants of B. argenteum accumulate more Fe and Cu than female plants prior to expression.
In Bryum, females are more constrained in expression than males. Females are less likely to express and, when they express, they produce far fewer inflorescences. In Ceratodon purpureus, cultures of males and females also revealed a higher proportion of males that expressed and males produced more inflorescences (Shaw and Beer, 1999). In the desert moss Syntrichia caninervis, prezygotic RI was 6·3 times greater for males (Stark et al., 2000). However, in Lophozia silvicola, prezygotic RI was 17·4 times greater for females (Laaka-Lindberg, 2001, noting that young embryos were included in female RI), while in Pseudocalliergon trifarium, prezygotic RI, controlling for field shoot size, was 1·3 times higher in females (Bisang et al., 2006). Notable in the latter study, the authors did not detect a prezygotic ‘cost of sex’ as measured by the size of the current growth interval, the size of the following growth interval, nor in the probability of expressing sex, all of which were unaffected by the current year expression. The next step in this course of research should be to determine (a) post-zygotic RI for females and, critically, to estimate how often a female realizes her full sexual RI (i.e. becomes fertilized and matures a sporophyte) in mixed-sex patches; infrequent sporophyte production by females can explain female-biased sex ratios (Rydgren et al., 2010).
Lloyd and Webb (1977) predicted that the higher female RI among flowering plants should lead to both delayed female flowering relative to males and also to less frequent female flowering relative to males. Indeed, among angiosperms, males flower more frequently in 63 % of species examined with no known cases where the reverse is true, and females are larger and older upon first flowering (Delph, 1999). The ‘constrained female’ in B. argenteum can be understood as aligning with an optimal reproductive allocation sensu Williams (1966). Should total female RI exceed total male RI, and a cost of reproduction is triggered that reduces female fitness when female RI is high, then selection should act to keep female prezygotic RI low and male prezygotic (and total) RI relatively high (Jönsson and Tuomi, 1994). A female genotype investing highly in both pre- and post-zygotic RI is likely to suffer a cost in terms of fewer spores (this should be subject to test) in the current or subsequent years (balancing pre- and post-breeding costs; Jönsson et al., 1995). One way to cap total female RI is to limit the number of inflorescences produced, i.e. keep prezygotic female RI low [a variation of the bet-hedging hypothesis (Stearns, 1976) that in this case applies to one sex].
Sex-specific life-history patterns and sexual dimorphisms may be subject to rapid evolution in bryophytes based on the Ceratodon model, where male and female traits were frequently uncorrelated (McDaniel, 2005). For Bryum, this may mean that males compete for access to females, and thus early and frequent expression is selected; whereas in females, a larger size may translate into better nutritional support for the dependent offspring (Roff, 1992). Because males probably have a higher Fe or N requirement for expression than females (Chopra and Bhatla, 1983), nutrient-rich substrates may favour males. An alternative strategy is for females to maintain a higher prezygotic RI (similar to males), and then selectively abort embryos that cannot be matured with resources at hand, a system operating efficiently in many seed plants (Kozlowski and Stearns, 1989) and possibly in some monoecious species of bryophytes (e.g. Stark and Stephenson, 1983). Our unpublished data on post-zygotic RI indicate that for every fertilization event resulting in a mature capsule, a female expends well over an order of magnitude effort (as biomass) compared with a single perigonium. Given this high total female RI, females should be expected to express sex at a lower rate and produce fewer inflorescences that may be fertilized (both sustained by the data given here).
Site of origin had a marked effect on the tendency of a clone to reproduce asexually versus sexually, and on the tendency to spread vegetatively. Genotypes from the southern New Mexico desert showed higher levels of sexual reproduction and lower levels of asexual reproduction when compared with genotypes from Kentucky, and genotypes from montane Arizona were intermediate (but closer to Kentucky genotypes) in their tendencies. Plants from the New Mexico population also grew faster during the protonemal extension phase than the other two populations (Table 3). Such data are consistent with (a) a genetic basis of specializing in either sexual or asexual RI and (b) the presence of ecotypes in sexual, asexual and vegetative life history traits. For B. argenteum, other workers have also found evidence of ecotypes in vegetative growth capacity, although populations can be remarkably similar in temperature relationships and metal tolerance (Longton and MacIver, 1977; Longton, 1981; Shaw et al., 1989; Shaw and Albright, 1990). Interestingly, the foregoing workers found the most vigorous populations for vegetative proliferation originated from harsh environments (Antarctica and on metal mine tailings), a finding consistent with the present results of the desert population growing most vigorously vegetatively and sexually (but not asexually) in culture. Two populations of Ceratodon purpureus diverged in sex expression tendencies (under controlled conditions) by a factor of ×1·6 (Shaw and Beer, 1999), indicating that expression can vary ecotypically not unlike the present findings for Bryum. The foregoing authors also presented clear evidence of the heritability and ecotypic divergence of life-history traits in bryophytes, evidence that is yet scant and understudied in this group of plants. In the present study, the clones derived from single spore isolates, even though they had been raised from sporeling to shootlet, subcultured and raised from shootlet to shootlet, and then the shootlets used in the current experiment, still showed a marked tendency toward sexual reproduction relative to the other clones. However, the clones derived from field-collected shoots showed a marked tendency toward asexual reproduction. This raises the issue of whether colonies derived from spores (a product of sexual reproduction) tend to reproduce sexually, whereas colonies derived from vegetative means (shoots, shootlets, etc.) tend to reproduce asexually. Given that the sexually derived clones in the present experiment were taken through two asexual generations (similar to the asexually derived clones), this is improbable and is more likely to represent variation from population to population.
ACKNOWLEDGEMENTS
We thank Richard Castetter for field assistance in locating critical populations, Allen (‘Koop’) Gibbs for evolutionary discussions and the use of his microbalance, Crystal Erickson for providing sterile water, media, and Hoagland's solution, and John Brinda and Sarah Eppley for insightful discussion and literature acquisitions. This research was supported by the National Science Foundation (IOB 0416407 to D.N.M., IOB 0416281 to L.R.S., and an undergraduate research award from the Experimental Program to Stimulate Competitive Research to K.H.).
LITERATURE CITED
- Ashman T-L. A dynamic perspective on the physiological cost of reproduction in plants. American Naturalist. 1994;144:300–316. [Google Scholar]
- Bhatla SC, Chopra RN. Hormonal regulation of gametangial formation in the moss Bryum argenteum Hedw. Journal of Experimental Botany. 1981;32:1243–1256. [Google Scholar]
- Bhatla SC, Chopra RN. Effect of chelating agents and metal ions on gametangial formation in the moss Bryum argenteum Hedw. Annals of Botany. 1983;52:755–761. [Google Scholar]
- Bisang I, Ehrlén J. Reproductive effort and cost of sexual reproduction in female Dicranum polysetum Sw. Bryologist. 2002;105:384–397. [Google Scholar]
- Bisang I, Ehrlén J, Hedenäs L. Reproductive effort and costs of reproduction do not explain female-biased sex ratios in the moss Pseudocalliergon trifarium (Amblystegiaceae) American Journal of Botany. 2006;93:1313–1319. doi: 10.3732/ajb.93.9.1313. [DOI] [PubMed] [Google Scholar]
- Burnell KD, Yelverton FH, Neal JC, Gannon TW, McElroy JS. Control of silvery-thread moss (Bryum argenteum Hedw.) in creeping bentgrass (Agrostis plaustris Huds.) putting greens. Weed Technology. 2004;18:560–565. [Google Scholar]
- Chopra RN, Bhatla SC. Effect of physical factors on gametangial induction, fertilization and sporophyte development in the moss Bryum argenteum grown in vitro. New Phytologist. 1981;89:439–447. [Google Scholar]
- Chopra RN, Bhatla SC. Regulation of gametangial formation in bryophytes. Botanical Review. 1983;49:29–63. [Google Scholar]
- Cronberg N, Andersson K, Wyatt R, Odrzykoski IJ. Clonal distribution, fertility and sex ratios of the moss Plagiomnium affine (Bland.) T. Kop. in forests of contrasting age. Journal of Bryology. 2003;25:155–162. [Google Scholar]
- Cronberg N, Rydgren K, Økland RH. Clonal structure and genet-level sex ratios suggest different roles of vegetative and sexual reproduction in the clonal moss Hylocomium splendens. Ecography. 2006;29:95–103. [Google Scholar]
- Crum HA, Anderson LE. Mosses of eastern North America. 2 vols. New York, NY: Columbia University Press; 1981. [Google Scholar]
- Day T, Aarssen LW. A time commitment hypothesis for size-dependent gender allocation. Evolution. 1997;51:988–993. doi: 10.1111/j.1558-5646.1997.tb03680.x. [DOI] [PubMed] [Google Scholar]
- Delph L. Sex differential resource allocation patterns in the subdioecious shrub Hebe subalpina. Ecology. 1990;71:1342–1351. [Google Scholar]
- Delph LF. Sexual dimorphism in life history. In: Geber MA, Dawson TE, Delph LF, editors. Gender and sexual dimorphism in flowering plants. Berlin: Springer-Verlag; 1999. pp. 149–173. [Google Scholar]
- During HJ. Relations between clonal growth, reproduction and breeding system in the bryophytes of Belgium and The Netherlands. Nova Hedwigia. 2007;131:133–145. [Google Scholar]
- Ehrlén J, van Groenendael J. Storage and the delayed costs of reproduction in the understorey perennial Lathyrus vernus. Journal of Ecology. 2001;89:237–246. [Google Scholar]
- Ehrlén J, Bisang I, Hedenäs L. Costs of sporophyte production in the moss, Dicranum polysetum. Plant Ecology. 2000;149:207–217. [Google Scholar]
- Flowers S. Mosses: Utah and the West. Provo, UT: Brigham Young University Press; 1973. [Google Scholar]
- Fuselier L, McLetchie DN. Maintenance of sexually dimorphic pre-adult traits in Marchantia inflexa (Marchantiaceae) American Journal of Botany. 2002;89:592–601. doi: 10.3732/ajb.89.4.592. [DOI] [PubMed] [Google Scholar]
- García-Ramos G, Stieha C, McLetchie DN, Crowley PH. Persistence of the sexes in metapopulations under intense asymmetric competition. Journal of Ecology. 2007;95:937–950. [Google Scholar]
- Geber MA, de Kroon H, Watson MA. Organ preformation in mayapple as a mechanism for historical effects on demography. Journal of Ecology. 1997;85:211–223. [Google Scholar]
- Ghiselin MT. The evolution of hermaphroditism among animals. Quarterly Review of Biology. 1969;44:189–208. doi: 10.1086/406066. [DOI] [PubMed] [Google Scholar]
- Hassel K, Pedersen B, Söderstrom L. Changes in life-history traits in an expanding moss species: phenotypic plasticity or genetic differentiation? A reciprocal transplantation experiment with Pogonatum dentatum. Ecography. 2005;28:71–80. [Google Scholar]
- Hedderson TA, Longton RE. Local adaptation in moss life histories: population-level variation and a reciprocal transplant experiment. Journal of Bryology. 2008;30:1–11. [Google Scholar]
- Hoagland D, Arnon DI. The water culture method for growing plants without soil. California Agricultural Experiment Station Circular. 1938:1–39. No. 347. [Google Scholar]
- Jönsson KI, Tuomi J. Costs of reproduction in a historical perspective. Trends in Ecology and Evolution. 1994;9:304–307. doi: 10.1016/0169-5347(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Jönsson KI, Tuomi J, Järemo J. Reproductive effort tactics: balancing pre- and postbreeding costs of reproduction. Oikos. 1995;74:35–44. [Google Scholar]
- Kimmerer RW. Reproductive ecology of Tetraphis pellucida. I. Population density and reproductive mode. Bryologist. 1991;94:255–260. [Google Scholar]
- Korpelainen H. Patterns of resource allocation in male and female plants of Rumex acetosa and R. acetosella. Oecologia. 1992;89:133–139. doi: 10.1007/BF00319025. [DOI] [PubMed] [Google Scholar]
- Kozlowski J, Stearns SC. Hypotheses for the production of excess zygotes: models of bet-hedging and selective abortion. Evolution. 1989;43:1369–1377. doi: 10.1111/j.1558-5646.1989.tb02588.x. [DOI] [PubMed] [Google Scholar]
- Laaka-Lindberg S. Biomass allocation to sexual and asexual reproduction in a leafy hepatic Lophozia silvicola Buch. Journal of Bryology. 2001;23:3–8. [Google Scholar]
- Ligrone R, Gambardella R. The sporophyte–gametophyte junction in bryophytes. Advances in Bryology. 1988;3:225–274. [Google Scholar]
- Lloyd DG, Webb CJ. Secondary sex characters in plants. Botanical Review. 1977;43:177–216. [Google Scholar]
- Longton RE. Inter-population variation in morphology and physiology in the cosmopolitan moss Bryum argenteum Hedw. Journal of Bryology. 1981;11:501–520. [Google Scholar]
- Longton RE, Greene SW. Experimental studies of growth and reproduction in the moss Pleurozium schreberi (Brid.) Mitt. Journal of Bryology. 1979;10:321–338. [Google Scholar]
- Longton RE, MacIver MA. Climatic adaptation in Antarctic and northern hemisphere populations of a cosmopolitan moss, Bryum argenteum Hedw. In: Llano GA, editor. Adaptations within Antarctic ecosystems. Washington, WA: Smithsonian Institution; 1977. pp. 899–919. [Google Scholar]
- Lovett Doust J, LaPorte G. Population sex ratios, population mixtures and fecundity in a clonal dioecious macrophyte, Vallisneria americana. Journal of Ecology. 1991;79:477–489. [Google Scholar]
- McDaniel SF. Genetic correlations do not constrain the evolution of sexual dimorphism in the moss Ceratodon purpureus. Evolution. 2005;59:2353–2361. [PubMed] [Google Scholar]
- McLetchie DN. Sex ratio from germination through maturity and its reproductive consequences in the liverwort Sphaerocarpos texanus. Oecologia. 1992;92:273–278. doi: 10.1007/BF00317375. [DOI] [PubMed] [Google Scholar]
- McLetchie DN. Sex-specific germination response in the liverwort Sphaerocarpos texanus (Sphaerocarpaceae) Bryologist. 2001;104:69–71. [Google Scholar]
- McLetchie DN, Puterbaugh MN. Population sex ratios, sex-specific clonal traits and tradeoffs among these traits in the liverwort. Marchantia inflexa. Oikos. 2000;90:227–237. [Google Scholar]
- McLetchie DN, Stark LR. Sporophyte and gametophyte generations differ in responses to thermotolerance in the moss Microbryum. Annals of Botany. 2006;97:505–511. doi: 10.1093/aob/mcl011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso JR. The costs of reproduction in plants. New Phytologist. 2002;155:321–348. doi: 10.1046/j.1469-8137.2002.00477.x. [DOI] [PubMed] [Google Scholar]
- Obeso JR, Retuerto R. Dimorfismo sexual en el acebo, Ilex aquifolium: ¿coste de la reproducción, selección sexual o diferenciación fisiológica? Revista Chilena de Historia Natural. 2002;75:67–77. [Google Scholar]
- Ochi H. Sharp A, Crum H, Eckel P, editors. Bryum. 1994;69:454–489. The moss flora of Mexico, Pt 1. Memoirs of the New York Botanical Garden. [Google Scholar]
- Piquot Y, Petit D, Valero M, Cuguen J, de Laguerie P, Vernet P. Variation in sexual and asexual reproduction among young and old populations of the perennial macrophyte Sparganium erectum. Oikos. 1998;82:139–148. [Google Scholar]
- Pohjamo M, Laaka-Lindberg S. Demographic population structure of the leafy hepatic Anastrophyllum hellerianum. Plant Ecology. 2004;173:73–81. [Google Scholar]
- Proctor MCF. Evidence on the carbon nutrition of moss sporophytes from 14CO2 uptake and the subsequent movement of labelled assimilate. Journal of Bryology. 1977;9:375–386. [Google Scholar]
- Queenborough SA, Burslem DFRP, Garwood NC, Valencia R. Determinants of biased sex ratios and inter-sex costs of reproduction in dioecious tropical forest trees. American Journal of Botany. 2007;94:67–78. doi: 10.3732/ajb.94.1.67. [DOI] [PubMed] [Google Scholar]
- Roff DA. The evolution of life histories: theory and analysis. New York, NY: Chapman and Hall; 1992. [Google Scholar]
- Rydgren K, Økland RH. Sex distribution and sporophyte frequency in a population of the clonal moss Hylocomium splendens. Journal of Bryology. 2002;24:207–214. [Google Scholar]
- Rydgren K, Økland RH. Short-term costs of sexual reproduction in the clonal moss Hylocomium splendens. Bryologist. 2003;106:212–220. [Google Scholar]
- Rydgren K, Halvorsen R, Cronberg N. Infrequent sporophyte production maintains a female-biased sex ratio in the unisexual clonal moss Hylocomium splendens. Journal of Ecology. 2010;98:1224–1231. [Google Scholar]
- Scheiner SM. MANOVA: multiple response variables and multispecies interactions. In: Scheiner SM, Gurevitch J, editors. Design and analysis of ecological experiments. New York, NY: Chapman and Hall; 1993. pp. 94–112. [Google Scholar]
- Selkirk PM, Skotnicki ML, Ninham JA, Connett MB, Armstrong J. Genetic variation and dispersal of Bryum argenteum and Hennediella heimii populations in the Garwood Valley, Southern Victoria Land, Antarctica. Antarctic Science. 1998;10:423–430. [Google Scholar]
- Shaw AJ, Albright DL. Potential for the evolution of heavy metal tolerance in Bryum argenteum, a moss. II. Generalized tolerances among diverse populations. Bryologist. 1990;93:187–192. [Google Scholar]
- Shaw AJ, Beer SC. Life history variation in gametophyte populations of the moss Ceratodon purpureus (Ditrichaceae) American Journal of Botany. 1999;86:512–521. [PubMed] [Google Scholar]
- Shaw AJ, Gaughan JF. Control of sex ratios in haploid populations of the moss, Ceratodon purpureus. American Journal of Botany. 1993;80:584–591. doi: 10.1002/j.1537-2197.1993.tb13844.x. [DOI] [PubMed] [Google Scholar]
- Shaw AJ, Beer SC, Lutz J. Potential for the evolution of heavy metal tolerance in Bryum argenteum, a moss. I. Variation within and among populations. Bryologist. 1989;92:73–80. [Google Scholar]
- Sletvold N. Effects of plant size on reproductive output and offspring performance in the facultative biennial Digitalis purpurea. Journal of Ecology. 2002;90:958–966. [Google Scholar]
- Stark LR, McLetchie DN. Gender-specific heat-shock tolerance of hydrated leaves in the desert moss Syntrichia caninervis. Physiologia Plantarum. 2006;126:187–195. [Google Scholar]
- Stark LR, Stephenson AG. Reproductive biology in Entodon cladorrhizans (Bryopsida, Entodontaceae). II. Resource-limited reproduction and sporophyte abortion. Systematic Botany. 1983;8:389–394. [Google Scholar]
- Stark LR, Mishler BD, McLetchie DN. The cost of realized sexual reproduction: assessing patterns of reproductive allocation and sporophyte abortion in a desert moss. American Journal of Botany. 2000;87:1599–1608. [PubMed] [Google Scholar]
- Stark LR, McLetchie DN, Mishler BD. Sex expression and sex dimorphism in sporophytic populations of the desert moss Syntrichia caninervis. Plant Ecology. 2001;157:183–196. [Google Scholar]
- Stark LR, McLetchie DN, Mishler BD. Sex expression, plant size, and spatial segregation of the sexes across a stress gradient in the desert moss Syntrichia caninervis. Bryologist. 2005;108:183–193. [Google Scholar]
- Stark LR, Brinda J, McLetchie DN. An experimental demonstration of the cost of sex and a potential resource limitation on reproduction in the moss Pterygoneurum. American Journal of Botany. 2009a;96:1712–1721. doi: 10.3732/ajb.0900084. [DOI] [PubMed] [Google Scholar]
- Stark LR, McLetchie DN, Roberts SP. Gender differences and a new adult eukaryotic record for upper thermotolerance in the desert moss Syntrichia caninervis. Journal of Thermal Biology. 2009b;34:131–137. [Google Scholar]
- Stark LR, McLetchie DN, Eppley SM. Sex ratios and the shy male hypothesis in the moss Bryum argenteum (Bryaceae) Bryologist. 2010;113:788–797. [Google Scholar]
- Stearns SC. Life-history tactics: a review of the ideas. Quarterly Review of Biology. 1976;51:3–47. doi: 10.1086/409052. [DOI] [PubMed] [Google Scholar]
- Sutherland S, Vickery RK. Trade-offs between sexual and asexual reproduction in the genus Mimulus. Oecologia. 1988;76:330–335. doi: 10.1007/BF00377025. [DOI] [PubMed] [Google Scholar]
- Wheelwright NT, Logan BA. Previous-year reproduction reduces photosynthetic capacity and slows lifetime growth in females of a neotropical tree. Proceedings of the National Academy of Sciences of the USA. 2004;101:8051–8055. doi: 10.1073/pnas.0402735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GC. Natural selection, the cost of reproduction and a refinement of Lack's principle. American Naturalist. 1966;100:687–690. [Google Scholar]
- Wyatt R, Derda GS. Population biology of the Polytrichaceae. Advances in Bryology. 1997;6:265–296. [Google Scholar]
- van Zanten BO. Experimental studies on trans-oceanic long-range dispersal of moss spores in the southern hemisphere. Journal of the Hattori Botanical Laboratory. 1978;44:455–482. [Google Scholar]