Abstract
Background and Aims
Enhancing the zinc (Zn) concentration in wheat (Triticum aestivum) grain is a breeding objective in order to improve human Zn nutrition. At enhanced plant Zn uptake, grain Zn levels do not increase proportionally and within the grain the endosperm Zn levels remain below grain Zn levels. This study analysed the temporal dynamics of Zn concentrations in grain tissues during grain filling to find major bottlenecks.
Methods
Plants of two cultivars were grown at 1 and 5 mg Zn kg−1 soil. Individual panicles were harvested 7, 14, 24 or 34 d after their flowering or at maturity and seeds were dissected into constituting tissues, which were analysed for Zn and other minerals.
Key Results
The Zn concentration of the crease was found to increase five- to nine-fold between 7 and 34 d after anthesis, while that of the endosperm decreased by 7 and 45 % when grown at 1 or 5 mg Zn kg−1, respectively. The Zn turnover rate (d−1) in the crease tissues was either independent of the Zn application level or higher at the lower Zn application level, and the Zn concentration increased in the crease tissues with time during grain filling while the turnover rate gradually decreased.
Conclusions
There is significant within-seed control over Zn entering the seed endosperm. While the seed crease Zn concentration can be raised to very high levels by increasing external Zn supply, the endosperm Zn concentrations will not increase correspondingly. The limited transfer of Zn beyond the crease requires more research to provide further insight into the rate-determining processes and their location along the pathway from crease to the deeper endosperm
Keywords: Triticum aestivum, grain zinc allocation, crease, vascular bundle, nucellar projection, grain zinc distribution, sink limitation
INTRODUCTION
Zinc (Zn) is an essential trace element for plant growth (Marschner, 1993) as well as human and animal nutrition (Hambidge, 2000). Recent efforts in biofortification have aimed to enhance the Zn nutrition of wheat (comprising Triticum aestivum and T. durum), to improve the Zn intake of poorer consumers whose mineral intake depends on this staple food (Ortiz-Monasterio et al., 2007). The micronutrient density of cereal grains can be increased by using natural genetic variation for both improved root uptake and improved long-distance transport to the grain (Graham and Welch, 1996; Palmgren et al., 2008). Two recent reviews have summarized the vast body of work on transport (Palmgren et al., 2008) and nutrient loading (Zhang et al., 2007). While the former reviews our current understanding at the molecular level, the latter emphasizes that our understanding of transport limitations for minerals such as Zn to and in the developing grain is in its infancy.
The localization of Zn in grain tissues in mature wheat grains has been reported (Ozturk et al., 2006; Choi et al., 2007). Recent studies have further revealed that Zn concentrations in cereal grains are linked to levels of proteins (Kutman et al., 2010). Detailed work by Persson et al. (2009) has shown that Zn in barley grains is mainly co-located with sulphur-containing peptides. It therefore seems relevant to analyse potential differences between treatments in Zn allocation also in terms of sulphur allocation. Studies on the mechanisms of Zn allocation to the grain (Pearson and Rengel, 1994; Pearson et al., 1998; Garnett and Graham, 2005) have revealed the role of the phloem, but little is known about the dynamics of internal Zn transport in wheat grains during grain filling. It is also unclear whether the Zn distribution within and between major grain tissues differs between genotypes or can be influenced by the application of Zn fertilizer. Zn fertilizers have been clearly shown to improve plant Zn uptake, growth and yield (Cakmak, 2008).
Within-grain allocation of sugars has been extensively studied (e.g. Ugalde and Jenner, 1990; Wolswinkel, 1992; Wang et al., 1995; Patrick and Offler, 2001). Thorne (1985) has given a comprehensive summary of the tissues involved in, among others, the wheat grain. Much less work seems to have been done on minerals, but transport pathways can be expected to be comparable. Although Zn is transported in the wheat plant via both the xylem and the phloem, the xylem discontinuity (Zee and O'Brien, 1970) forces all transport into the grains to pass through the phloem. At the base of the grain the phloem splits into proto-phloem strands that provide the outer and inner pericarp and possibly the seed coat on the dorsal side of the wheat grain with nutrients (Fisher, 1990; Pearson et al., 1998), while a phloem-only strand provides nutrients for the embryo (Zee and O'Brien, 1970; O'Brien et al., 1985; Pearson et al., 1995). The major transport route follows the vascular bundle in the crease, which provides nutrients to the endosperm and possibly the ventral part of the pericarp and seed coat (Fisher, 1990). When the dorsal pericarp tissues re-translocate solutes, including minerals (towards the end of the grain-filling stage), these seem to flow through the proto-phloem back to the phloem at the base of the seeds (Fisher, 1990). In more detail, we can surmise the major transport routes indicated in Fig. 1. From the phloem in the vascular bundle, the minerals move through transfer cells in the chalaza to the nucellar projection and from there into the endosperm cavity (for diagrams of the tissues see Thorne, 1985). The minerals are taken up from the endosperm cavity by the aleurone cells bordering the endosperm cavity at the endosperm side and from there the minerals enter the endosperm. It is unclear if some minerals also enter the seed coat from the endosperm, yet the cuticullar layer on the seed coat appears to limit exchange of minerals between endosperm and epidermis (Patrick and Offler, 1995).
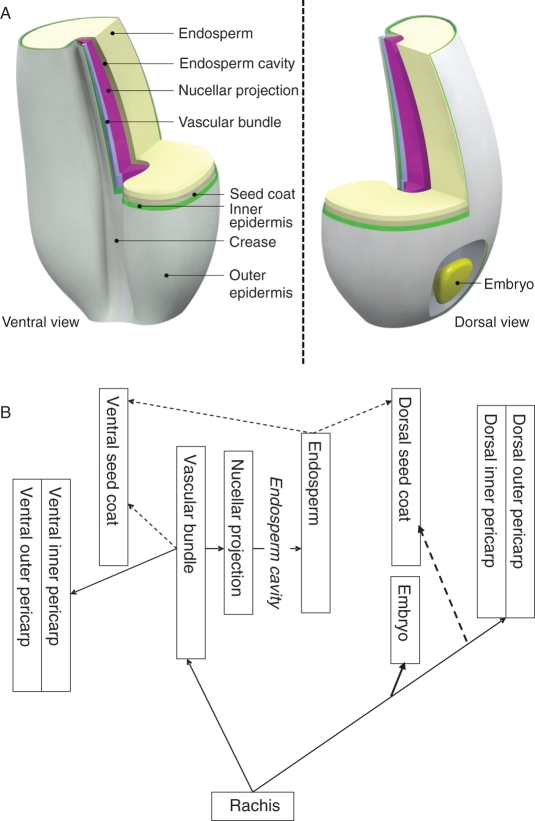
Fig. 1.
(A) A stylized representation of the wheat grain with tissues distinguished in this study through manual dissection under a light microscope (16×). The bulk of the grain consists of the endosperm, which from inside to outside is enveloped by a seed coat, an inner pericarp and an outer pericarp. On the dorsal side the embryo is found embedded in the seed coat, while on the ventral side the crease is found with the main vascular tissues. The vascular tissue comprises the vascular bundle and the nucellar projection. The seed coat surrounding the endosperm discontinues where the vascular bundle and nucellar projection join (the chalaza, not shown separately). Between the nucellar projection and the endosperm is the so-called endosperm cavity, a space filled with water, minerals and nutrients that feed the endosperm. (B) Schematic representation of the pathway that minerals and nutrients follow during internal grain transport as reported in the literature (see Introduction). The major uncertainty lies in the pathway to the seed coat, and hence the broken arrows. The endosperm cavity is not a tissue, and although the endosperm cavity can be tapped for analyses (cf. Ugalde and Jenner, 1990), this was not done in this study.
To investigate the barriers to Zn transport within the grain and more specifically into the endosperm, this study will look at the turnover rate for Zn between individual grain tissues. The following hypotheses are proposed. (1) Wheat seeds that differ in grain Zn concentrations in the endosperm differ in Zn turnover rates in the vascular grain tissues rather than in Zn levels in these tissues. (2) Within the seed, the Zn concentration gradually decreases between the vascular bundle, the nucellar projection and the endosperm, but also between the vascular bundle on the one hand and the inner and outer epidermis and the seed coat on the other hand. (3) At higher levels of Zn nutrition, the concentration gradients as well as the Zn turnover rates are maintained, while all grain tissues increase in Zn level.
MATERIALS AND METHODS
Two replicate experiments were conducted to observe the distribution pattern of Zn in grain tissues. A single wheat genotype (Triticum aestivum ‘SAMNYT-16’), which was found to be of good grain Zn loading capacity in field trials, was grown at sufficient soil Zn status to observe the distribution patterns around mid grain filling stage (expt 1). In a second experiment a second wheat genotype (‘Carnamah’) with a more average grain Zn loading ability was grown in addition to ‘SAMNYT-16’. Plants were grown at two soil Zn levels (sufficient: 1 mg Zn kg−1 soil; high: 5 mg Zn kg−1 soil) to study the grain Zn distribution patterns in more detail throughout grain filling and dependent upon plant nutrition level (expt 2).
In both experiments, the wheat seeds were first surface sterilized by washing in 70 % ethanol for 1 min and soaking in 1 % sodium hypochlorite for 5 min, then rinsed and finally pre-germinated in Petri dishes for 48 h. Both experiments were set up in a completely randomized design, with seven and three replications for expts 1 and 2, respectively. Results were analysed by the regression and analysis of variance routines of the GENSTAT computer program.
Experiment 1: within-grain Zn distribution at 21 DAA
Wheat genotype ‘SAMNYT-16’ was grown in 254-mm-diameter PVC pots containing the equivalent of 13 kg of air-dried UC potting mix (Baker, 1957) with the following nutritional characteristics (mg kg−1 soil): NO3-N (228), NH4-N (195), P (78), K (140), S (57), Fe (278), DTPA-extractable Cu (0·75), DTPA-extractable Zn (0·38), DTPA-extractable Mn (0·31) and DTPA-extractable Fe (12·9). Four plants per pot were grown, while each replication consisted of one pot. Three weeks after sowing, 90 g of the slow-release fertilizer Osmocote – Long Life Plus Trace Elements was added to each pot, supplying the following nutrients (mg kg−1 soil): Zn (1), total N (1038), P (305), K (692), S (173), Mg (83), B (1), Cu (3), Fe (28), Mn (4), Mo (1). Plants were grown in a temperature-controlled glasshouse at 25/20 °C day/night. Plants were watered daily with double-deionized water to keep the soil moist, allowing water to be drained through the base of the pots. Anthesis date (the appearance of anthers) was recorded and whole spikes were harvested at 21 d after their individual anthesis (DAA) for the separation of various tissues (rachis, embryo, outer pericarp, inner pericarp and seed coat combined, endosperm and crease). One replication consisted of in total eight grains sampled from florets of the four central spikelets (two per spikelet) of a single spike and dissected within 1–2 min of harvesting. Crease tissues (including vascular bundle and nucellar projection) were sampled according to Ugalde and Jenner (1990). A schematic representation of the wheat seed that details the various seed tissues sampled in expts 1 and 2 is given in Fig. 1.
Experiment 2: Zn distribution dynamics during grain filling
Two wheat genotypes, ‘SAMNYT-16’ and ‘Carnamah’, were grown in PVC pots (20 cm diameter × 15 cm depth), sealed at the base and filled with the same UC potting mix as used in expt 1. The use of sealed pots in this experiment in contrast to the previous experiment was based on the need to avoid any leaching of Zn from the soil during the experiment. Preparation of the soil included the addition of Zn (ZnSO4.7H2O) at rates of 1 (sufficient Zn) and 5 (high Zn) mg Zn kg−1 soil with a total of 5 kg of air-dried soil in each pot. Soil without added Zn was packed into the top 20-cm section of each pot and the entire soil was watered to 7·5 % (w/w) moisture content by adding deionized water three times weekly to each of the pots. In each of the three replications of all treatments two pots were available with five plants each. Anthesis date was recorded for individual spikes as the appearance of anthers in ‘SAMNYT-16’ and when pollinating anthers were observed upon opening of the top spikelets on ‘Carnamah’ as this cultivar retained the anthers mostly in the spikes. As sampling started at an earlier stage during grain growth when tissue weights were smaller compared with expt 1, two spikes were sampled per replication from two pots so a single sample was composed of tissues from 16 seeds. Selection of spikes for harvesting was based purely on available time per day for dissection and flowering time of the different spikes. Whole spikes were harvested at 7, 14, 24 and 34 DAA for the separation of various tissues as described for expt 1, except for the following: the crease was separated into nucellar projection and vascular bundle by microscope dissection at 14, 24 and 34 DAA. At harvest maturity (55 DAA) dissection of individual grain tissues was no longer possible and only full grains were analysed.
Chemical analyses
Collected samples in both experiments were dried at 80 °C and dry weights were measured. Tissue weights ranged between 0·3 and 850 mg with the lower weights for the embryo, at 7 and 14 DAA. Weights of nucellar projection and vascular bundle tissues ranged between 1·1 and 5·9 mg. The smaller samples (<150 mg) were digested in three steps: overnight in 1·5 mL nitric acid and 0·5 mL hydrogen peroxide, the following morning in a DigiPrep HotBlock for 30 min at 80 °C followed by 2 h at 125 °C in the same blocks. After cooling, the volume was brought up to 20 mL. Larger samples (≥150 mg) were digested in the same way but with 2·0 mL nitric acid and 0·5 mL hydrogen peroxide and volumes were brought up to 25 mL after digestion. Mineral levels in the digests were determined by inductively coupled plasma optical emission spectroscopy (ICP-OES) according to Zarcinas et al. (1987), with the limit of reporting greater than ten times the standard deviation of the blank. Quality assurance was also maintained through the use of the wheat reference standards from NIST (US National Institute of Standards and Technology). For the lower sample weights the Zn concentrations of the reference samples were within the indicated range for the reference samples. All concentrations reported and discussed below are mass concentrations based on dry weight.
Calculations
The Zn turnover rates of the vascular bundle and nucellar projection have been calculated as:
where T is turnover rate (d−1), ΔZnb is the change in Zn content of the tissues provided over a period (ng per seed d−1) and Zna is the average Zn content of the tissue provided over a period (ng per seed).
RESULTS
Dry weights of grain tissue
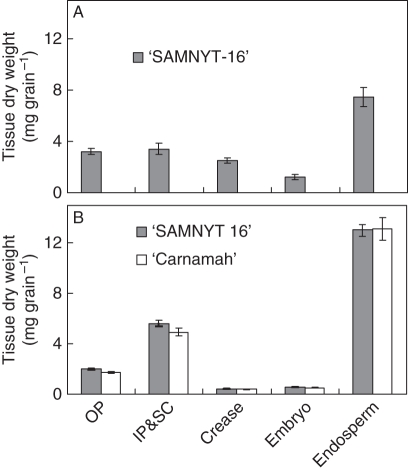
Analysis of various grain tissues sampled at 21 DAA in expt 1 and 24 DAA in expt 2 (Fig. 2) indicated that the endosperm had the highest dry weights at these sampling times and that filling of the endosperm had started. In expt 2, an increase in dry weight of individual grains followed the expected pattern during grain filling (Fig. 3). There was no effect of Zn treatment on individual seed and tissue dry weights (data not shown). The gradual increase in grain weight was mainly due to an increase in endosperm, inner pericarp and seed coat weight, but the weight of the embryo also increased with time. The dry weight of the outer pericarp hardly changed beyond 7 DAA while that of the crease was too small to play any role in the total grain weight. The crease weight increased from 0·279 ± 0·012 to 0·397 ±0·021 mg per seed between 7 and 14 DAA and remained roughly the same between 14 and 34 DAA (0·403 ± 0·017 mg per seed). There were no Zn treatment effects (P > 0·05) on the final weight of individual grains selected for tissue analysis; ‘SAMNYT-16’ had a significantly (P = 0·012) higher individual grain weight than ‘Carnamah’ at 34 DAA.
Fig. 2.
Dry weights of the various sections of wheat grain [outer pericarp (OP), inner pericarp plus seed coat (IP&SC), crease (combination of vascular bundle and nucellar projection), embryo and endosperm], in (A) expt 1 (21 DAA) and (B) expt 2 (24 DAA). Data in expt 2 represent averages of the two Zn levels as no significant differences were observed. Error bars represent standard errors of, respectively, seven and three replications.
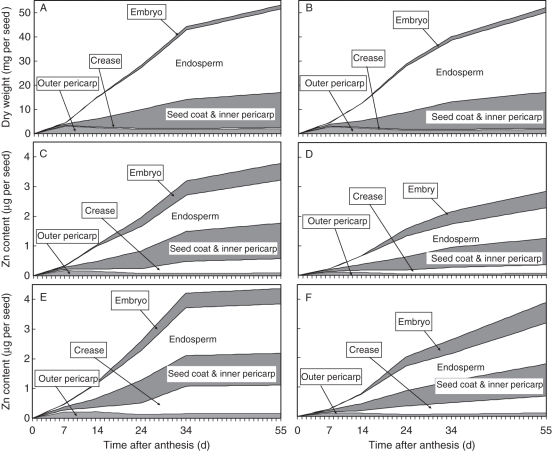
Fig. 3.
Accumulated grain dry weight (A, B) and grain Zn content at sufficient (C, D) and high (E, F) Zn supply level over time after anthesis (days) for ‘SAMNYT-16’ (A, C, E) and ‘Carnamah’ (B, D, F). The division of the accumulated grain dry weight and Zn content over the composing tissues until 34 DAA is based on observed data. The totals at maturity (55 DAA) are observed data; the division over the tissues between 34 and 55 DAA is based on their relative weights at 34 DAA.
The grain weight and vegetative dry matter per plant differed significantly between the two cultivars (Table 1). Plant grain weight differences were caused by a significantly higher grain number for ‘SAMNYT-16’. The interaction between cultivar and Zn treatment for grain number did not overrule this main effect, and was caused by a significantly larger number of grains under the high Zn treatment in ‘SAMNYT-16’ than under the sufficient Zn treatment while no differences in grain number between Zn treatments were observed for ‘Carnamah’.
Table 1.
Grain yield, vegetative dry matter yield and grain number per plant for expt 2 at grain maturity
| Cultivar | Grain yield (g per plant) | Vegetative dry matter (g per plant) | Zinc treatment | Grain number (per plant) |
|---|---|---|---|---|
| ‘SAMNYT-16’ | 1·42 | 1·37 | Sufficient | 114 |
| High | 139 | |||
| ‘Carnamah’ | 1·01 | 1·15 | Sufficient | 91 |
| High | 73 | |||
| Cultivar | <0·001 | 0·01 | <0·001 | |
| Zinc | n.s. | n.s. | n.s. | |
| Interaction | n.s. | n.s. | 0·009 | |
| Cultivar SED | 0·062 | 0·067 | Interaction SED | 9·0 |
Standard errors of differences (SED) between means are only indicated for treatments or interactions when these are significantly different. n.s. = not significant.
Grain zinc content
The time course of Zn content in individual seeds was similar to that of individual grain weight, although the relative contribution of the tissues showed marked differences; in particular, the contribution of the crease to total grain Zn content was much larger than its weight contribution (Fig. 3). This effect was observed in both experiments and for both cultivars in expt 2 (Table 2).
Table 2.
The Zn content in the grain tissue parts of wheat plants harvested at 21 DAA (expt 1) and 24 DAA at sufficient Zn (expt 2) expressed as total content per grain and as a percentage of this total content
| Expt 2 |
|||
|---|---|---|---|
| Expt 1: ‘SAMNYT-16’ | ‘SAMNYT-16’ | ‘Carnamah’ | |
| Zn content (μg per grain) | 1·00 ± 0·07 | 1·58 ± 0·16 | 1·5 3 ± 0·11 |
| Percentage | |||
| Crease | 32 ± 2·5 | 7·8 ± 2·8 | 8·6 ± 0·8 |
| OP 1 | 16 ± 1·2 | 4·0 ± 0·4 | 2·9 ± 0·7 |
| IP&SC1 | 22 ± 2·1 | 31·0 ± 3·7 | 28·0 ± 1·7 |
| Embryo | 11 ± 1·6 | 16·0 ± 0·2 | 16·0 ± 0·7 |
| Endosperm | 21 ± 1·7) | 42·0 ± 1·0 | 45·0 ± 2·7 |
Data represent means (± s.e.) of seven (expt 1) or three (expt 2) replications. OP, outer pericarp; IP&SC, combination of inner pericarp and seed coat.
Grain Zn concentration
The Zn concentrations in rachis and grain tissues in expt 1 (Fig. 4) were much higher in the crease than in any other tissue, with next highest levels in the embryo and the rachis; this pattern was also observed in expt 2. The Zn concentration of the individual grain tissues and the rachis followed different trends over time (Fig. 5). The concentration did not change much for the combination of inner pericarp and seed coat (Fig. 5E). The concentration in the endosperm (Fig. 5D) appeared to decrease slightly between 7 and 34 DAA, while that in the embryo remained roughly the same between 14 and 34 DAA. At 7 DAA it was difficult to distinguish the embryo from the endosperm and its weight was often too low for proper chemical analyses, and hence no data on embryo tissue Zn concentration at 7 DAA are presented.
Fig. 4.
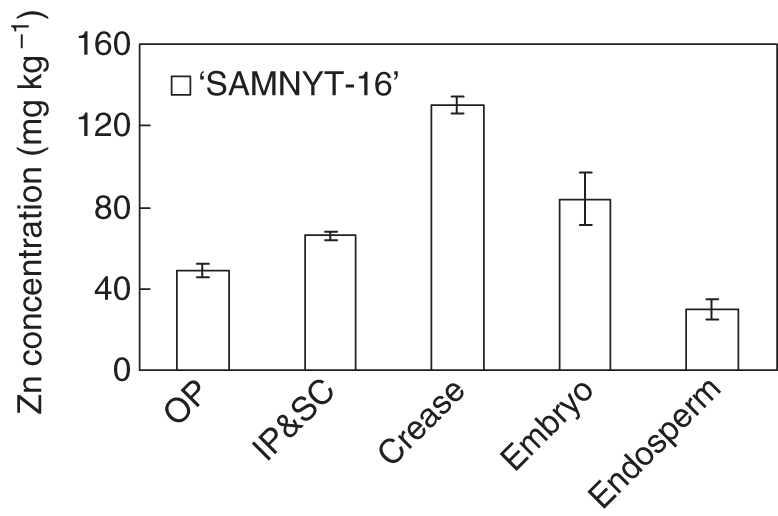
The Zn concentration in the distinguished grain tissues [outer pericarp (OP), inner pericarp plus seed coat (IP&SC), crease, embryo and endosperm] harvested at 21 DAA in expt 1. The mature grains were harvested at 60 DAA. Error bars represent the s.e. of seven replications.
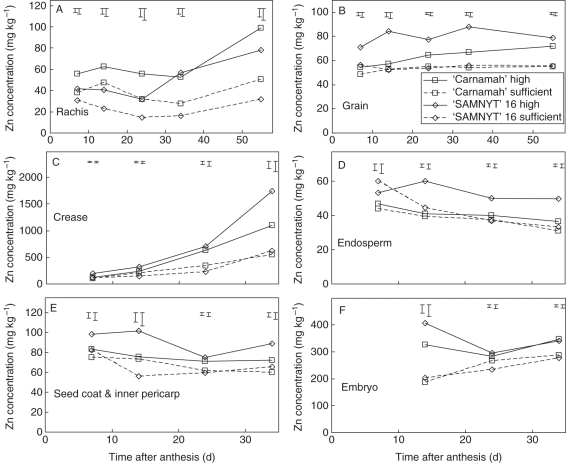
Fig. 5.
Zn concentration over time after anthesis for the rachis (A), the total grain (B) and different grain tissues (C–F) separately for the four treatments. The y-axis scale differs with the grain tissue. The error bars indicate s.e. of differences between any means (larger bars) or between means within treatments (smaller bars). The corresponding analysis of variance probabilities are given in Table 3.
The Zn concentration in the crease tissue showed the largest variation over time with an increase from 100–200 mg kg−1 at 7 DAA to 550–1750 mg kg−1 at 34 DAA (Fig. 5C). The trends for the vascular bundle and nucellar projection were comparable between 14 and 34 DAA. The application of Zn fertilizer had a significant (P < 0·05) positive effect on the accumulation of Zn in the major tissue components of the grain (endosperm, inner pericarp plus seed coat, and crease) for ‘SAMNYT-16’ from 14 DAA onwards (Table 3, Fig. 5). At this time there was no effect on ‘Carnamah’, as revealed by a significant interaction effect between Zn treatment and cultivar treatment for all four tissues. From 24 DAA onwards, the effect of Zn application was also significant (P < 0·05) for ‘Carnamah’ except for the endosperm at 24 DAA, yet in quantitative terms the effect was much lower for ‘SAMNYT-16’ (Fig. 5), as indicated by the significant interaction effect (Table 3). At 14 DAA, in all tissues there was no difference between the two genotypes when no Zn was applied, while the Zn concentration in the major tissues was higher for ‘SAMNYT-16’ than for ‘Carnamah’ at the high Zn level. The same was observed in the endosperm at 24 and 34 DAA. Only at 34 DAA was the Zn concentration also higher in ‘SAMNYT-16’ than in ‘Carnamah’ at the low Zn level, for the crease and inner pericarp plus seed coat. In the rachis, the opposite was observed: the Zn concentration was higher in ‘Carnamah’ than in ‘SAMNYT-16’ at both Zn levels, at 7, 14 and 24 DAA (Fig. 5A, Table 3).
Table 3.
Calculated probabilities of treatment effects or their interaction from analysis of variance on the zinc concentration of the rachis and grain and of the various grain tissues
| 7 DAA |
14 DAA |
24 DAA |
34 DAA |
55 DAA |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| INT | Zn | cv | INT | Zn | cv | INT | Zn | cv | INT | Zn | cv | INT | Zn | cv | |
| Rachis | n.s. | 0·003 | 0·01 | n.s. | 0·01 | 0·02 | n.s. | 0·03 | 0·03 | n.s. | <0·001 | n.s. | n.s. | <0·001 | 0·03 |
| Grain | n.s. | 0·03 | 0·01 | 0·01* | 0·04 | 0·03 | 0·01* | <0·001 | 0·01 | 0·01† | <0·001 | 0·003 | n.s. | <0·001 | n.s. |
| OP | n.s. | 0·02 | 0·01 | 0·02* | 0·003 | 0·002 | n.s. | 0·01 | 0·03 | 0·04† | <0·001 | 0·04 | – | – | – |
| Embryo | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 0·005 | n.s. | n.s. | <0·001 | n.s. | – | – | – |
| IP&SC | n.s. | n.s. | n.s. | 0·04* | 0·03 | n.s. | n.s. | 0·001 | n.s. | n.s. | 0·004 | 0·04 | – | – | – |
| Crease | n.s. | n.s. | n.s. | 0·03* | 0·01 | n.s. | n.s. | <0·001 | n.s. | n.s. | <0·001 | 0·03 | – | – | – |
| VB | – | – | – | n.s. | n.s. | n.s. | n.s. | <0·001 | n.s. | 0·02 | <0·001 | 0·003 | – | – | – |
| NP | – | – | – | n.s. | n.s. | n.s. | n.s. | 0·005 | n.s. | n.s. | 0·002 | n.s. | – | – | – |
| Endosperm | n.s. | n.s. | 00·02 | 0·01* | 0·003 | <0·001 | 0·01* | 0·001 | 0·02 | 0·01† | <0·001 | 0·001 | – | – | – |
OP, outer pericarp; IP&SC, embryo, inner pericarp plus seed coat; VB, vascular bundle; NP, nucellar projection. Treatments are zinc application (Zn) or cultivar (cv) and their interaction (INT).
* The significant interaction effect was caused by the fact that only ‘SAMNYT-16’ showed a higher Zn concentration when Zn was applied. For ‘Carnamah’ the Zn application had no significant effect on the Zn concentration and the Zn concentration between ‘Carnamah’ and ‘SAMNYT-16’ without Zn application was not significantly different.
† The significant interaction effect was caused by a lack of difference between the two cultivars when no zinc was applied. ‘SAMNYT-16’ showed a higher concentration than ‘Carnamah’ when Zn was applied and both cultivars showed a higher concentration when Zn was applied than when it was not.
Grain Zn concentration gradient and turnover rates
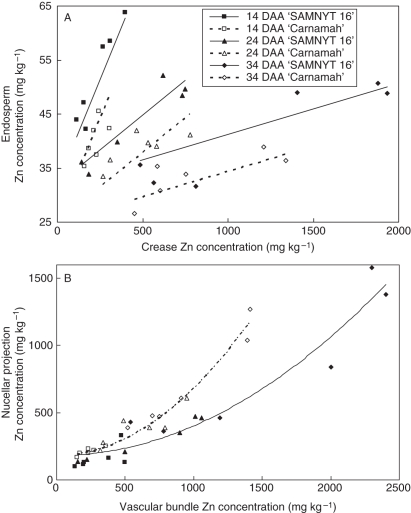
The concentration gradient of Zn in grain tissues of ‘SAMNYT-16’ and ‘Carnamah’ was consistent between the two experiments. Regardless of soil Zn status and time during grain filling, the Zn concentration ranked in the following order: vascular bundle > nucellar projection > embryo > inner pericarp plus seed coat > endosperm. As indicated in Fig. 1, the endosperm receives its Zn from the crease region and from the nucellar projection. In other words all the Zn that accumulated in the endosperm had to move along the concentration gradient that existed between the nucellar projection and endosperm. When the endosperm Zn concentration was plotted against the Zn concentration of the crease (Fig. 6A), the slopes of the regression lines differed significantly (P < 0·01) between the three harvests with the steepest slope at 14 DAA and shallowest at 34 DAA. Furthermore, the intercept for ‘SAMNYT-16’ was significantly (P < 0·01) higher than for ‘Carnamah’. Including separate slopes for the two cultivars did not improve the regression model.
Fig. 6.
Optimum regression model explaining 83 % of the variance in endosperm concentration was obtained with a linear regression of endosperm Zn concentration on crease Zn concentrations (A), showing significantly (P < 0·01) different slopes between harvests (0·0776, 0·0258 and 0·0094 for harvests 14, 24 and 34 DAA, respectively, with a standard error of 0·0063) and significantly (P < 0·01) different intercepts between the two cultivars (31·8 and 25·0 for ‘SAMNYT-16’ and ‘Carnamah’, respectively, independent of the harvest time, with a standard error of 1·34). An optimum regression model explaining 94·0 % of the variance in nucellar projection Zn concentration was obtained with a regression on vascular bundle Zn concentration (B) allowing for a quadratic term (P < 0·001) with a different coefficient for the two cultivars (P < 0·001).
The accumulation of Zn in the crease compared with the endosperm was consistent for both the vascular bundle and the nucellar projection. Regression of the Zn concentrations of the nucellar projection on those of the vascular bundle for the data for 14, 24 and 34 DAA (Fig. 6B) revealed nucellar projection Zn concentrations were best explained when allowing for a quadratic term for the vascular bundle. In doing so a single line was found for all three harvesting dates, while the regression coefficient of the quadratic term (P < 0·001) differed between the cultivars, with the higher coefficient for ‘Carnamah’.
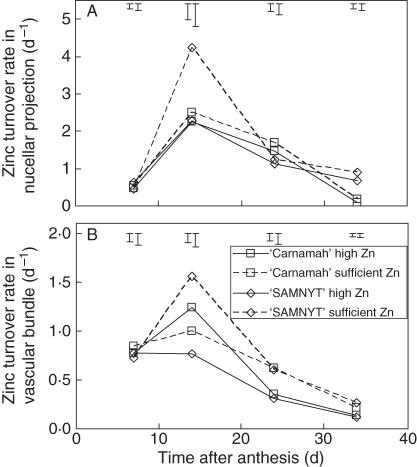
The dynamics in accumulation of Zn in the endosperm over time (Fig. 3B, D) can also be analysed in terms of rate of allocation of Zn to the endosperm (Fig. 7A) indicating that the maximum rate of allocation is observed at 14 DAA and more so in ‘SAMNYT-16’, while it was reached somewhat later in ‘Carnamah’. After 34 DAA the rate could only be estimated for the vascular bundle, as at harvest the Zn concentration was only analysed for the whole grain. All additional accumulated Zn must have passed through the vascular bundle but not necessarily through the nucellar projection. Whereas the allocation rate to the endosperm was maintained after 14 DAA in at least some treatments, the turnover rates of both the nucellar projection and the vascular bundle decreased from 14 DAA onwards (Fig. 7B, C). The turnover rate was often not different between cultivars and was either the same for the application rates or slightly higher for the sufficient Zn conditions compared with the high Zn conditions.
Fig. 7.
The rate of allocation of Zn to the endosperm (A) and the Zn turnover rates in the nucellar projection (B) and the vascular bundle (C) during grain filling for the four treatments. The error bars indicate standard errors of differences between any means (larger bars) or between means within treatments (smaller bars).
The steep gradient in Zn concentration between the crease tissue and the endosperm can be compared with the concentrations of other minerals (Table 4). An even higher gradient was observed for Mn than for Zn, while that for Fe was comparable with Zn, and for Ca, Mg and S the gradients were much less steep than for Zn. The Zn concentration in the vascular bundle was higher than in the nucellar projection whereas the opposite was found for Fe and Mn.
Table 4.
Average mineral concentrations at 34 DAA from expt 2
| Zn (μg g−1) |
Fe (μg g−1) | Mn (μg g−1) | Ca (mg g−1) | Mg (mg g−1) | P (mg g−1) | S (mg g−1) | ||
|---|---|---|---|---|---|---|---|---|
| Organ | Sufficient | High | ||||||
| Rachis | 22·0 ± 7·9 | 54·7 ± 10·1 | 20·4 ± 4·7 | 74·9 ± 21·8 | 2·62 ± 0·77 | 0·55 ± 0·09 | 3·09 ± 0·98 | 3·77 ± 1·31 |
| Grain | 55·0 ± 2·6 | 77·5 ± 12·7 | 53·3 ± 2·6 | 67·9 ± 3·4 | 0·97 ± 0·05 | 1·72 ± 0·03 | 5·47 ± 0·28 | 2·03 ± 0·11 |
| Embryo | 282 ± 14·7 | 343 ± 15·1 | 289 ± 10 | 384 ± 26 | 1·71 ± 0·11 | 6·00 ± 0·17 | 23·9 ± 1·00 | 3·33 ± 0·05 |
| Outer pericarp | 36·0 ± 5·8 | 69·7 ± 16·2 | 26·8 ± 2·9 | 234 ± 22 | 4·46 ± 0·91 | 0·63 ± 0·12 | 1·58 ± 0·36 | 1·25 ± 0·11 |
| Inner pericarp plus seed coat | 62·7 ± 4·5 | 80·7 ± 13·2 | 111 ± 4·9 | 83·1 ± 6·7 | 1·51 ± 0·06 | 4·64 ± 0·37 | 12·2 ± 1·5 | 2·39 ± 0·14 |
| Vascular bundle | 747 ± 243 | 1735 ± 589 | 143 ± 40 | 1260 ± 116 | 4·54 ± 0·67 | 2·69 ± 0·29 | 9·09 ± 1·52 | 3·55 ± 0·23 |
| Nucellar projection | 432 ± 48 | 1120 ± 360 | 942 ± 100 | 3120 ± 200 | 4·19 ± 0·39 | 2·07 ± 0·31 | 6·74 ± 1·64 | 4·72 ± 0·52 |
| Endosperm | 32·0 ± 3·3 | 42·9 ± 7·4 | 15·1 ± 2·2 | 12·2 ± 1·5 | 0·46 ± 0·03 | 0·38 ± 0·04 | 2·01 ± 0·20 | 1·84 ± 0·12 |
For Zn, data are shown separately for the two zinc levels and averaged over the two cultivars; for all other elements data are shown as means of all treatments, in rachis, and total grain and several grain tissues. Values are shown as mean ± s.e.
DISCUSSION
The aim of this study was to determine whether temporal variation and effects of Zn application exist in the distribution of Zn within the wheat grain and to investigate the barriers to providing more Zn to the endosperm.
Contrary to our first hypothesis, the turnover rate (Fig. 7B, C) in the vascular bundle or the nucellar projection of the two cultivars was lower when the endosperm concentration was higher. The Zn turnover rates were higher at sufficient Zn supply level when endosperm Zn concentrations were lower compared with the higher Zn supply and thus also hypothesis 3 is rejected: the Zn turnover rate is not maintained at higher Zn concentrations in the vascular bundle. The Zn allocation to the endosperm (Fig. 7A) tends to be higher for plants at high Zn and for ‘SAMNYT-16’ and the decrease in Zn concentration along the gradient of vascular bundle to nucellar projection to endosperm is much larger at the higher Zn concentrations in the tissues. These observations suggest that as the Zn status of the wheat plant is improved through increased Zn supply, Zn transport into the endosperm becomes relatively more difficult as compared with Zn unloading from the rachis to the seed vascular tissue or the internal transport in the vascular tissue. There appears to be a major internal transport limitation between the vascular tissue and the endosperm.
In testing hypothesis 2, the Zn concentration gradient in the seed tissues did decline from the vascular bundle to the nucellar projection and to the endosperm, and also from the vascular bundle to the inner and outer epidermis and the seed coat. Transport within the seed does not always follow a Zn concentration gradient, as the decrease in concentration between crease tissues and endosperm is very large, especially towards the end of grain filling. Even when we correct the endosperm concentrations with a factor 4–6 to account for 75–85 % starch (Ugalde and Jenner, 1990; Jenner et al., 1991) – assuming that starch is not metabolically active, as argued by Stomph et al. (2009) – a huge gradient remains between the nucellar projection and the endosperm. This is equally true for Fe and Mn, but would bring the level of S in the endosperm to a comparable or even higher level than in the nucellar projection (Table 4).
The regression of endosperm Zn concentration on crease tissue Zn concentration (Fig. 6A) clearly shows that Zn accumulates in the crease. Regression of nucellar projection Zn concentrations on vascular bundle Zn concentrations (Fig. 6B) suggests that the accumulation in the crease is not specific for either of the two tissues, or is stronger in the nucellar projection. At adequate to high Zn supply, the limitation in the transport of Zn to the endosperm therefore does not appear to be due to the phloem unloading step or the mainly symplastic (Thorne, 1985; Wang and Fisher, 1994) transport within the crease tissue but seems to occur at the transfer step between the nucellar projection and the endosperm. The manual separation of vascular bundle and nucellar projection was the most difficult of all dissections made. As the concentrations in both tissues follow the same trends over time and are an order of magnitude different from that of the endosperm, the above conclusion on transfer to the endosperm remains valid. As we failed to sample the endosperm cavity sap in sufficient quantity to analyse the mineral nutrition it remains to be tested whether the mainly symplastic transfer to the cavity sap or the apoplastic transport across the aleurone layer cells of the endosperm is the rate-limiting step at higher crease Zn levels. In wheat a difference in Zn concentration has been observed between the aleurone cell layer and the endosperm cells (Ozturk et al., 2006). In their study the aleurone cell layer was not sampled separately, with the cells directly adjacent to the endosperm cavity being sampled together with the endosperm and the remainder probably being sampled partly with the endosperm and partly with the seed coat. Given this, it is possible that we have underestimated the Zn concentration in the endosperm plus aleurone slightly. This could not, however, explain the large concentration difference between the crease tissue and the endosperm, and so does not affect our main conclusions. The seeds studied by Ozturk et al. (2006) were mature, whereas in the present study analysis of the location of Zn within the seeds was not done at maturity. In theory, the Zn present in the vascular bundle may have been transported to the endosperm after the last sampling. Interestingly, however, the analysis of micronutrients in mature wheat seeds published by Mazzolini et al. (1985) also shows a relatively high concentration of Zn and Mn in the vascular bundle compared with the endosperm. Further detailed studies to understand the processes determining the transport limitation should therefore target this region of the seed.
The weight data for ‘SAMNYT-16’ differed slightly between the two experiments. This is probably due to the slight differences in temperature between the two grain filling periods and the slightly later sampling in expt 2. The Zn concentrations in the various tissues were comparable between the two experiments (compare Figs 4 and 5). In expt 2 ‘Carnamah’ had a slightly higher rachis Zn concentration than ‘SAMNYT-16’, while the concentrations in the grain tissues between 7 and 34 DAA were in general comparable for the cultivars when sufficient Zn was applied and were higher for ‘SAMNYT-16’ at the higher soil Zn level. By contrast, ‘SAMNYT-16’ plants produced more grains and therefore a higher per-plant grain weight. So, while concentrations in the central grains on the spikes were comparable, the total grain weight and thus accumulated Zn in the grains of ‘SAMNYT-16’ differed from that in ‘Carnamah’. The interactions between cultivar and Zn supply were significant for most important grain tissue data but not for the rachis (Table 3). The differences between cultivars in expt 2 appeared to be related to within-seed dynamics rather than to differences in the supply of Zn to the grains: a higher Zn concentration was observed in the rachis of ‘Carnamah’ at both Zn levels (Fig. 5), while this cultivar otherwise had often lower Zn concentrations in grain tissues. This corresponds well with the findings of Herren and Feller (1994) and Pearson et al. (1995) that translocation of Zn from the xylem to the phloem in the peduncle and rachis does not seem a major transport limitation. In our experiment, a complication may have been the lower number of seeds in ‘Carnamah’, indicating that the lower Zn level in the rachis of ‘SAMNYT-16’ may have been caused by a higher demand for Zn from the larger number of grains.
Although we only manipulated the Zn concentration by supplying different Zn levels, the study also indicates that an accumulation of other minerals occurs in the vascular tissue during grain filling. The extent of the transfer limitation beyond the crease seems to be comparable for Fe, Mn and Zn, but does not seem to be of the same order of magnitude for Ca and Mg and even appears absent for S. There was no correlation between Zn and S concentrations in the endosperm (data not shown). Previously, it has been observed that the speciation of Zn in seeds correlates with the presence of protein thiol groups (Persson et al., 2009). In the current study additional accumulation of Zn in the endosperm upon Zn fertilization changes the S/Zn ratio, indicating that there is need to further understand the ways in which the additional Zn is stored in the endosperm.
For progress in breeding it also seems relevant to elucidate whether the limited transfer rate between crease and endosperm is due to a source limitation or whether the sink capacity for Zn in the endosperm is too limited to increase uptake rates. A source limitation could either be due to a limited transport capacity or a very high Zn sequestration capacity in the vascular tissues through, for instance, storage in vacuoles of the crease cells. Storage in the vacuoles would be regulated by transporters in the tonoplast, such as the VIT1 transporter present within the cells of the crease region in barley (Tauris et al., 2009) and, given their morphological similarity, is probably also found in the crease tissues of wheat. More importantly, Tauris et al. (2009) found that VIT1 was not present in the endosperm and we might speculate that the lack of a tonoplast transporter for Zn may contribute toward a lack of Zn transport deeper into the endosperm. Sink capacity could also potentially be related to proteins that use Zn as co-factor and that have been found to co-locate with Zn (Persson et al., 2009) or other binding sites for Zn. Zn uptake rates of the endosperm reach their maximum level and Zn turnover rates in the crease tissue are clearly highest during the first 14 DAA when cell activity includes cell division and cell expansion as the endosperm cells are formed and expand. From 14 DAA a decrease in Zn turnover rate was seen that corresponded to a significant change in endosperm properties, as starch deposition started to be visible from a change in colour and texture. Between 14 and 34 DAA Zn concentration in the crease increases but remains more or less stable (Fig. 5D) in the endosperm, and therefore it appears that during the most active starch deposition the demand for Zn from the endosperm cells remains stable or is slightly reduced, while Zn availability builds up in the crease. It cannot be ruled out that there is simply a reduced demand or a reduced sink for Zn and that therefore enhanced levels can only be reached when an additional sink is created in the endosperm.
Although this study has improved our knowledge of limitations to Zn translocation into wheat seeds, further research is needed to fully understand the barriers to transport deep into the seed endosperm, with a focus on: (1) whether the limitation to enhanced endosperm levels is transport- or sink-determined, and (2) which genes are involved in the regulation and possible alleviation of the sink or transport limitation between vascular tissue and endosperm.
ACKNOWLEDGEMENTS
We thank HarvestPlus for partial funding, Dr Colin Jenner for providing advice on grain dissection, Dr Ivan Ortiz-Monasterio from CIMMYT for wheat seeds and the Waite Analytical Service for chemical analyses.
LITERATURE CITED
- Baker KF. The U.C. system for producing healthy container-grown plants. Berkeley, CA: University of California Press; 1957. [Google Scholar]
- Cakmak I. Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant and Soil. 2008;302:1–17. [Google Scholar]
- Choi EY, Graham RD, Stangoulis JCR. Semi-quantitative analysis for selecting Fe- and Zn-dense genotypes of staple food crops. Journal of Food Composition and Analysis. 2007;20:496–505. [Google Scholar]
- Fisher DB. Persistence of non-crease protophloem strands in the developing wheat grain. Australian Journal of Plant Physiology. 1990;17:223–227. [Google Scholar]
- Garnett TP, Graham RD. Distribution and remobilization of iron and copper in wheat. Annals of Botany. 2005;95:817–826. doi: 10.1093/aob/mci085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham RD, Welch RM. Breeding for staple food crops with high micronutrient density. Washington, DC: International Food Policy Research Institute; 1996. Working Papers on Agricultural Strategies for Micronutrients No.3. [Google Scholar]
- Hambidge M. Human zinc deficiency. Journal of Nutrition. 2000;130 doi: 10.1093/jn/130.5.1344S. [DOI] [PubMed] [Google Scholar]
- Herren T, Feller U. Transfer of zinc from xylem to phloem in the peduncle of wheat. Journal of Plant Nutrition. 1994;17:1587–1598. [Google Scholar]
- Jenner CF, Ugalde TD, Aspinall D. The physiology of starch and protein deposition in the endosperm of wheat. Australian Journal of Plant Physiology. 1991;18:211–226. [Google Scholar]
- Kutman UB, Yildiz B, Ozturk L, Cakmak I. Biofortification of durum wheat with zinc through soil and foliar applications of nitrogen. Cereal Chemistry. 2010;87:1–9. [Google Scholar]
- Marschner H. Zinc uptake from soils. In: Robinson AD, editor. Zinc in soils and plants. Dordrecht: Kluwer Academic Publishers; 1993. pp. 59–77. [Google Scholar]
- Mazzolini AP, Pallaghy CK, Legge GJF. Quantitative microanalysis of Mn, Zn and other elements in mature wheat seed. New Phytologist. 1985;100:483–509. [Google Scholar]
- O'Brien TP, Sammut ME, Lee JW, Smart MG. The vascular system of the wheat spikelet. Australian Journal of Plant Physiology. 1985;12:487–511. [Google Scholar]
- Ortiz-Monasterio JI, Palacios-Rojas N, Meng E, Pixley K, Trethowan R, Peña RJ. Enhancing the mineral and vitamin content of wheat and maize through plant breeding. Journal of Cereal Science. 2007;46:293–307. [Google Scholar]
- Ozturk L, Yazici MA, Yucel C, et al. Concentration and localization of zinc during seed development and germination in wheat. Physiologia Plantarum. 2006;128:144–152. [Google Scholar]
- Palmgren MG, Clemens S, Williams LE, et al. Zinc biofortification of cereals problems and solutions. Trends in Plant Science. 2008;13:464–473. doi: 10.1016/j.tplants.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Patrick JW, Offler CE. Post-sieve element transport of sucrose in developing seed. Australian Journal of Plant Physiology. 1995;22:681–702. [Google Scholar]
- Patrick JW, Offler CE. Compartmentation of transport and transfer events in developing seeds. Journal of Experimental Botany. 2001;52:551–564. [PubMed] [Google Scholar]
- Pearson JN, Rengel Z. Distribution and remobilization of Zn and Mn during grain development in wheat. Journal of Experimental Botany. 1994;45:1829–1835. [Google Scholar]
- Pearson JN, Rengel Z, Jenner CF, Graham RD. Transport of zinc and manganese to developing wheat grains. Physiologia Plantarum. 1995;95:449–455. [Google Scholar]
- Pearson JN, Rengel Z, Jenner CF, Graham RD. Dynamics of zinc and manganese movement in developing wheat grains. Australian Journal of Plant Physiology. 1998;25:139–144. [Google Scholar]
- Persson DP, Hansen TH, Laursen KH, Schjoerring JK, Husted S. Simultaneous iron, zinc sulphur and phosphorus speciation analysis of barley grain tissues using SEC-ICP- MS and IP-ICP-MS. Metallomics. 2009;1:418–426. doi: 10.1039/b905688b. [DOI] [PubMed] [Google Scholar]
- Stomph TJ, Jiang W, Struik PC. Zinc biofortification of cereals, rice differs from wheat. Trends in Plant Science. 2009;14:123–124. doi: 10.1016/j.tplants.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Tauris B, Borg S, Gregersen PL, Holm PB. A roadmap for zinc trafficking in the developing barley grain based on laser capture microdissection and gene expression profiling. Journal of Experimental Botany. 2009;60:1333–1347. doi: 10.1093/jxb/erp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne JH. Phloem unloading of C and N assimilates in developing seeds. Annual Review of Plant Physiology. 1985;36:317–343. [Google Scholar]
- Ugalde TD, Jenner CF. Substrate gradients and regional patterns of dry mater deposition within developing wheat endosperm. I. Carbohydrates. Australian Journal of Plant Physiology. 1990;17:377–394. [Google Scholar]
- Wang HL, Patrick JW, Offler CE, Wang XD. The cellular pathway of photosynthate transfer in the developing grain III A structural analysis and physiological studies of the pathway from the endosperm cavity to the starchy endosperm. Plant, Cell and Environment. 1995;18:389–407. [Google Scholar]
- Wang N, Fisher DB. Monitoring phloem unloading and post-phloem transport by microperfusion of attached wheat grains. Plant Physiology. 1994;104:7–16. doi: 10.1104/pp.104.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolswinkel P. Transport of nutrients into developing seeds: a review of physiological mechanisms. Seed Science Research. 1992;2:59–73. [Google Scholar]
- Zarcinas BA, Cartwright B, Spouncer LR. Nitric acid digestion and multi-element analysis of plant material by inductively coupled plasma spectrometry. Communications in Soil Science and Plant Analysis. 1987;18:131–146. [Google Scholar]
- Zhang W-H, Zhou Y, Dibley KE, Tyerman SD, Furbank RT, Patrick JW. Nutrient loading of developing seeds. Functional Plant Biology. 2007;34:314–331. doi: 10.1071/FP06271. [DOI] [PubMed] [Google Scholar]
- Zee SY, O'Brien TP. A special type of tracheary element associated with ‘xylem discontinuity’ in the floral axis of wheat. Australian Journal of Biological Science. 1970;23:783–791. [Google Scholar]