Abstract
AIM
The primary objective was to evaluate the pharmacokinetics of a single dose of neratinib, a potent, low-molecular-weight, orally administered, irreversible pan-ErbB (ErbB-1, -2, -4) receptor tyrosine kinase inhibitor, during co-administration with ketoconazole, a potent CYP3A4 inhibitor.
METHODS
This was an open-label, randomized, two-period, crossover study. Fasting healthy adults received a single oral dose of neratinib 240 mg alone and with multiple oral doses of ketoconazole 400 mg. Blood samples were collected up to 72 h after each neratinib dose. Plasma concentration data were analyzed using a noncompartmental method. The least square geometric mean ratios [90% confidence interval (CI)] of Cmax(neratinib+ketoconazole) : Cmax(neratinib alone), and AUC(neratinib+ketoconazole) : AUC(neratinib alone) were assessed.
RESULTS
Twenty-four subjects were enrolled. Compared with neratinib administered alone, co-administration of ketoconazole increased neratinib Cmax by 3.2-fold (90% CI: 2.4, 4.3) and AUC by 4.8-fold (3.6, 6.5). Median tmax was 6.0 h with both regimens. Ketoconazole decreased mean apparent oral clearance of neratinib from 346 l h−1 to 87.1 l h−1 and increased mean elimination half-life from 11.7 h to 18.0 h. The incidence of adverse events was comparable between the two regimens (50% neratinib alone, 65% co-administration with ketoconazole).
CONCLUSION
Co-administration of neratinib with ketoconazole, a potent CYP3A inhibitor, increased neratinib Cmax by 3.2-fold and AUC by 4.8-fold compared with administration of neratinib alone. These results indicate that neratinib is a substrate of CYP3A and is susceptible to interaction with potent CYP3A inhibitors and, thus, dose adjustments may be needed if neratinib is administered with such compounds.
Keywords: CYP3A4 protein, drug interactions, healthy, HKI-272, human, ketoconazole, neratinib, pharmacokinetics
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Neratinib is a potent, low-molecular-weight, orally administered, irreversible pan-ErbB (ErbB-1, -2, -4) receptor tyrosine kinase inhibitor that has shown anti-tumour activity in patients with advanced ErbB-2-positive breast cancer both with and without prior trastuzumab exposure.
Neratinib is in phase III development for the treatment of patients with ErbB-2-positive breast cancer.
Preclinical data suggest that neratinib is predominantly metabolized by CYP3A4.
WHAT THIS STUDY ADDS
The pharmacokinetic profile of neratinib was evaluated in healthy subjects after a single oral administration of neratinib with and without the potent CYP3A4 inhibitor, ketoconazole.
Exposure to neratinib increased more than 3-fold during co-administration of ketoconazole, indicating that neratinib is a substrate of CYP3A and is susceptible to interactions with potent CYP3A inhibitors and, thus, dose adjustments may be needed if neratinib is administered with such compounds.
Introduction
Receptor tyrosine kinases are important in controlling critical cellular processes such as the cell cycle, cell migration, cell metabolism and survival, and cell proliferation and differentiation [1]. The epidermal growth factor receptor (EGFR) family of receptor tyrosine kinases is hyperactivated in a variety of cancers. The EGFR family consists of human epidermal growth factor receptor (HER)-1 (also known as EGFR and ErbB-1), HER-2 (also known as ErbB-2 or neu), HER-3 (ErbB-3), and HER-4 (ErbB-4) [2]. ErbB-2 is amplified in approximately 30% of cases of breast cancer and over-expression is correlated with poor prognosis [3]. The standard treatment for ErbB-2-positive breast cancer is trastuzumab, which provides response rates of only 15% as monotherapy, 41% in combination with paclitaxel, and 50% in combination with chemotherapy [4–6]. Furthermore, the development of resistance to trastuzumab is a common problem.
Neratinib (HKI-272) is a potent [7], low-molecular-weight, orally administered, irreversible pan-ErbB (ErbB-1, -2, -4) receptor tyrosine kinase inhibitor [2, 7, 8]. Neratinib, when used either alone or in combination with trastuzumab or paclitaxel, showed antitumor activity in patients with ErbB-2-positive breast cancer [9–12].
In fasting healthy subjects, after oral administration of neratinib 120 mg to 800 mg, the median time to peak plasma neratinib concentration (tmax) is 4–7 h, the half-life is approximately 9–17 h and the exposure appears to plateau at the 400-mg dose [13]. Preclinical data suggest that neratinib is predominantly metabolized by cytochrome P450 (CYP) 3A4 and to a lesser extent by flavin-containing mono-oxygenases. CYP3A4 is a common pathway for the metabolism of many drugs [14], and because patients with cancer are frequently prescribed multiple medications, it is important to determine whether co-administration of a strong CYP3A4 inhibitor with neratinib would result in a drug–drug interaction that would decrease the clearance of neratinib and, thus, increase exposure to neratinib. The broad-spectrum antifungal agent, ketoconazole, is a potent inhibitor of CYP3A4 [15] and is recommended for studies conducted to evaluate the potential for CYP3A4-mediated drug–drug interactions [16, 17].
The current study used ketoconazole to evaluate the effect of CYP3A4 inhibition on neratinib pharmacokinetics (PK). The recommended daily oral dose of ketoconazole as an antifungal agent for humans is 200 to 400 mg. In the current study, the regimen selected for ketoconazole was 400 mg once daily, which is a regimen that is recommended to achieve maximum inhibition of CYP3A4 activity [18, 19]. Ketoconazole 400 mg was administered once daily for 5 days in this study. Neratinib has been shown to be generally safe and well tolerated after single oral doses up to and including 400 mg in healthy subjects who were fed and 640 mg in healthy subjects who were fasting [13]. In the current study, neratinib was administered at a dose of 240 mg, which is the dose that has been effective in patients with advanced ErbB-2-positive breast cancer [9]. The primary objective of the study was to evaluate the effects of co-administration of ketoconazole and a single dose of neratinib on the PK profile of neratinib in healthy subjects. The secondary objective of the study was to assess the safety and tolerability of single doses of neratinib when neratinib is co-administered with ketoconazole in healthy subjects.
Methods
The study protocol was approved by Aspire Institutional Review Board (La Mesa, California). Written informed consent was obtained from all subjects prior to their enrolment in the study. The study number in the http://www.clinicaltrials.gov registry is NCT00380328.
Study participants
Healthy men and women of non-childbearing potential aged 18 to 50 years were eligible to enrol if they had a body mass index (BMI) in the range of 18.0 to 30.0 kg m−2 and bodyweight ≥50 kg. Sexually active men agreed to the use of a medically acceptable form of contraception during the study and for 12 weeks after the last dose of any study drug. Subjects must have been nonsmokers or smoked <10 cigarettes a day and been able to abstain from smoking during the inpatient stay.
Exclusion criteria included history of significant medical or surgical conditions, acute disease within 7 days before study day 1; clinically important deviations from normal limits on physical examination, vital signs measurements, 12-lead electrocardiogram (ECG), or clinical laboratory test results; QTc duration ≥450 ms in men or ≥470 ms in women at screening or on day −1; history of known hypersensitivity to ketoconazole or any clinically important drug allergy; use of any investigational or prescription drug within 30 days before administration of study drug, consumption of grapefruit within 48 h before day 1; and use of any over-the-counter drugs, including herbal supplements (except for the occasional use of acetaminophen and vitamins ≤100% recommended daily allowance) within 14 days before day 1.
Study design
This was an open-label, randomized, two-period, crossover study conducted at a single study centre. Healthy subjects received a single oral dose of neratinib 240 mg alone (treatment A) and in combination with multiple oral doses of ketoconazole 400 mg (treatment B). Approximately 24 subjects were planned for enrolment to allow for the completion of the study with 20 subjects if a 15% attrition rate occurred. Subject enrolment occurred via a web-based computerized randomization and enrolment system. Subjects were randomly assigned to one of two treatment sequences for periods 1 and 2: treatment A followed by treatment B or treatment B followed by treatment A. In both periods, neratinib was administered at approximately 08.00 h after an overnight fast of at least 10 h. The day neratinib was administered was considered day 1 of the treatment period. For treatment B, the first dose of ketoconazole was administered in the evening approximately 12 h before the anticipated administration of neratinib and the second dose was administered on day 1 concomitantly with neratinib at approximately 08.00 h; the third, fourth, and fifth doses of ketoconazole 400 mg were administered at approximately 08.00 h on days 2, 3, and 4. The two study periods were separated by a washout interval of 13 days between neratinib doses. Subjects were discharged from the study centre after the last PK blood sample collection of each 5-day/4-night inpatient period. Neratinib was supplied as three 80-mg capsules and ketoconazole was supplied as two 200-mg tablets.
Safety and tolerability were evaluated based on reported signs and symptoms, physical examinations, vital signs measurements, ECGs, and clinical laboratory test results. On-treatment adverse events (AEs) were AEs that occurred any time after the first dose of any study drug until 15 days after the last dose of any study drug, regardless of relationship to study drug.
Sample collection and analytical methods
In both study periods, 5-ml venous blood samples were collected from an indwelling catheter or by direct venepuncture into potassium EDTA-treated tubes at 0, 0.5, 1, 2, 3, 4, 6, 8, 12, 18, 24, 36, 48, 60 and 72 h after neratinib administration for quantitation of plasma neratinib concentrations. The contents of the tubes were gently mixed and the tubes were immediately placed on ice. Samples were centrifuged within 15 min after collection at approximately 1000 g for about 10 min at 4°C. The separated plasma was transferred to watertight polypropylene tubes and stored frozen at approximately −70°C until tubes were shipped on dry ice to Covance Bioanalytical Services, LLC (Indianapolis, IN) for assay.
The plasma samples were analyzed for neratinib by a validated liquid chromatography/tandem mass spectrometry (LC/MS/MS) assay using 0.25 ml of plasma. The method was linear over the range 3.00 to 250 ng ml−1 and the lower limit of quantitation was 3 ng ml−1. Proteins were removed from plasma by precipitation, and neratinib and the internal standard, WAY-178357, were isolated on a Genesis C18 column (50 × 2.1 mm, 4 µm particle size) (Grace Vydac, Columbia, Maryland) using mobile phases of 50 mm ammonium acetate (pH 4.5) : acetonitrile : methanol (8 : 1 : 1 and 1 : 5 : 4, v : v : v). Detection was accomplished using a Sciex API 4000® mass spectrometer (Sciex, Foster City, CA) equipped with a turbo ion spray interface, which was operated in positive ion mode with multiple reaction monitoring using the mass transitions 557.2→112.2 for neratinib, and 574.2→339.0 for the internal standard. No interfering peaks occurred in the areas of interest that significantly impacted the data. For the low, medium, and high quality control samples that were analyzed with the study samples, the inter-assay accuracy ranged from 96.1% to 104.0%, and the inter-assay precision (expressed as percent relative standard deviation) ranged from 5.9% to 9.0%.
Pharmacokinetics
The plasma concentration data for neratinib were analyzed for each subject using a noncompartmental method with WinNonlin Enterprise application version 4.1 (Pharsight Corporation, Mountain View, CA) [20]. The peak plasma concentration (Cmax) and tmax were determined directly from observed concentration data. The terminal-phase disposition rate constant (λz) was estimated by a log-linear regression of the terminal monoexponential portion of the observed plasma concentration–time curve. The half-life was calculated as 0.693/λz.
The area under the concentration–time curve truncated at the last reported plasma concentration (Ct) at time t (AUC0,t), was calculated using the trapezoidal rule during the ascending portion of the curve and the log-trapezoidal rule during the descending portion of the curve. Total AUC was estimated as AUC = AUCt+Ct/λz. The clearance (CL/F) was calculated as a ratio of dose : AUC. The apparent volume of distribution for the terminal disposition phase (Vz/F) was calculated as the ratio of CL/F to λz.
Statistical methods
A sample size of 24 was selected to quantify the drug-drug interaction of ketoconazole on neratinib. Descriptive statistics were computed for plasma concentrations at each scheduled time point and derived PK parameters for each treatment separately. The PK parameters of neratinib were compared between neratinib administered alone and administered with ketoconazole using an anova for a two-period crossover design. Additionally, the geometric mean (log-transformed) ratios, AUC(neratinib+ketoconazole) : AUC(neratinib alone) and Cmax(neratinib+ketoconazole) : Cmax(neratinib alone) and 90% confidence intervals (CIs) were calculated to estimate the magnitude of the effect of ketoconazole on the PK profile of neratinib.
Results
The study was conducted from October 27 2006 to November 12 2006. Twenty-four subjects (12 in each treatment sequence) were enrolled. Subjects were aged 19 to 48 years (mean [SD], 31.2 [9.3] years), 23 (96%) were men, 16 (67%) were White, 7 (29%) were Black or African American, and 1 (4%) was Asian. The BMI ranged from 18.9 to 30.5 kg m−2 (mean [SD] 25.08 [3.66]). Twenty-one subjects completed the study. One subject discontinued because of an AE and two discontinued for other reasons (positive urine drug screen result, n = 1; lost to follow-up, n = 1).
Pharmacokinetics
Blood samples for PK analysis were available for 22 subjects. Mean plasma neratinib concentrations were higher after administration of neratinib with ketoconazole (treatment B) than after neratinib alone (treatment A), as shown in Figure 1. PK parameters for both regimens are summarized in Table 1. Co-administration of ketoconazole increased exposure to neratinib. For Cmax, the mean (coefficient of variation % [CV%]) value increased from 55 (36) ng ml−1 to 201 (58) ng ml−1. For AUC, the mean (CV%) increased from 903 (45) ng ml−1 h to 4660 (53) ng· ml−1 h. Log-transformed anova showed that the treatment effect was significant (P < 0.001) for Cmax and AUC. The least squares geometric mean ratio (90% CI) of neratinib co-administered with ketoconazole to neratinib administered alone (treatment B : treatment A) for Cmax was 3.2 (2.4, 4.3) and for AUC it was 4.8 (3.6, 6.5). The median tmax was 6.0 h with both regimens (Table 1, Figure 1). Co-administration of ketoconazole decreased mean apparent oral clearance of neratinib from 346 l h−1 to 87.1 l h−1 and increased mean elimination half-life from 11.7 h to 18.0 h (Table 1).
Figure 1.
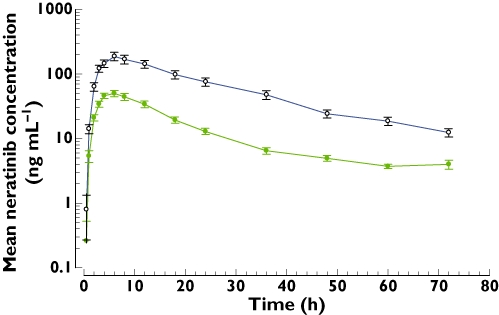
Mean (SE) plasma neratinib concentrations after administration of a single oral dose of neratinib 240 mg alone and with multiple oral doses of ketoconazole 400 mg in healthy adults. 240 mg neratinib ( ); 240 mg neratinib + ketoconazole (
); 240 mg neratinib + ketoconazole ( )
)
Table 1.
Neratinib pharmacokinetics after administration of a single oral dose of neratinib 240 mg alone and with multiple oral doses of ketoconazole 400 mg in healthy adults
| Pharmacokinetic variable | Neratinib alone (A) | Neratinib with ketoconazole (B) | Ratio (90% CI) of LS Geometric means (B/A) |
|---|---|---|---|
| Mean (CV%) | Mean (CV%) | ||
| Cmax (ng ml−1) | 55.32 (36) | 201 (58) | 3.21 (2.41, 4.28)*** |
| AUC (ng ml−1 h) | 903 (45) | 4660 (53) | 4.81 (3.59, 6.45)*** |
| tmax (h)† | 6.00 (4.00, 8.00) | 6.00 (3.00, 8.00) | NA |
| Half-life (h) | 11.65 (26) | 18.02 (22) | NA |
| CL/F (l h−1) | 346 (61) | 87.10 (101) | NA |
| Vz/F (l) | 5476 (59) | 2365 (113) | NA |
AUC, total area under the concentration–time curve; CL/F, apparent oral dose clearance; CI, confidence interval; Cmax, peak plasma neratinib concentration; LS, least squares; tmax, time to Cmax; Vz/F, apparent volume of distribution.
P < 0.001.
Values for tmax are median (range).
Safety and tolerability
On-treatment AEs occurred in 20 (83.3%) of the 24 subjects enrolled (11 subjects after administration of neratinib alone, 15 subjects after co-administration of neratinib with ketoconazole) as shown in Table 2. There were no clinically relevant differences in the frequency or severity of on-treatment AEs between the two treatment regimens. Gastrointestinal AEs were the most common AEs, were mild or moderate, and except for two instances of nausea, were considered by the investigator to be at least possibly drug related. Four subjects reported headaches, and these were considered by the investigator to be mild and at least possibly related to study drug. One subject was discontinued from the study because of an AE of chills (this subject had grade 2 nausea, back pain, and chills approximately 5.5 h after receipt of ketoconazole but before administration of neratinib in period 1). There were no serious AEs.
Table 2.
On-treatment adverse events (AEs) occurring in >5% of subjects
| AE | Neratinib alone (n = 22) | Neratinib with ketoconazole (n = 23) | Total (n = 24) |
|---|---|---|---|
| Any AE, n (%) | 11 (50.0) | 15 (65.2) | 20 (83.3) |
| Specific AEs, n (%) | |||
| Diarrhoea | 6 (27.3) | 4 (17.4) | 8 (33.3) |
| Headache | 4 (18.2) | 1 (4.3) | 4 (16.7) |
| Nausea | 1 (4.5) | 3 (13.0) | 4 (16.7) |
| Eosinophilia | 1 (4.5) | 2 (8.7) | 3 (12.5) |
| Abdominal pain | 0 | 2 (8.7) | 2 (8.3) |
| Flatulence | 1 (4.5) | 1 (4.3) | 2 (8.3) |
| Neutrophil count, decreased | 0 | 2 (8.7) | 2 (8.3) |
Some subjects experienced the same adverse event in both treatment periods, therefore, numbers in the total column are not the sums of numbers in the individual columns.
Discussion
Neratinib is in development for the treatment of ErbB-2-postive malignancies. In phase II studies, the therapeutic regimen for the treatment of patients with advanced ErbB-2-positive breast cancer is once-daily oral doses of neratinib 240 mg with food [9]. In the current study, healthy subjects received two 240-mg doses in a two-period crossover fashion to evaluate the interaction of neratinib with ketoconazole, a potent CYP3A4 inhibitor.
Maximum CYP3A4 inhibition is achieved with a ketoconazole dosing regimen of 400 mg once daily [18, 19]. Chien et al. predicted that using this regimen, the CYP3A inhibitory response increases and reaches a maximum plateau [18]. In our study, ketoconazole 400 mg was administered once daily for 5 days (i.e. on day −1 at 12 h before neratinib administration, on day 1 concomitantly with neratinib, and on days 2–4) to maximally inhibit CYP3A4 activity during and after neratinib co-administration. The reported tmax for oral ketoconazole (3 h [21]) is shorter than the tmax for neratinib (4–7 h). The last dose of ketoconazole was administered on day 4, providing a CYP3A4-inhibitory state through the last PK blood sampling time point of 72 h. Thus, the plasma exposure to neratinib was evaluated over an entire single dose interval under conditions of maximum CYP3A inhibition.
Co-administration of ketoconazole increased exposure to neratinib by 3.2-fold for Cmax and 4.8-fold for AUC. Interindividual variability was modest, with %CVs falling within the range of 36% to 58% for Cmax and AUC. Consistent with the observations of increased exposure, the mean apparent oral clearance of neratinib decreased from 346 l h−1 to 87 l h−1 and the mean elimination half-life was prolonged by approximately 6 h. These findings confirm the preclinical observations that CYP3A4 plays a major role in the metabolism of neratinib.
The incidence of on-treatment AEs was similar between the two treatment regimens (50% of subjects after administration of neratinib alone and 65% after co-administration of neratinib with ketoconazole). Seven (32%) subjects and seven (30%) subjects had one or more gastrointestinal on-treatment AEs after receiving neratinib alone and with ketoconazole, respectively. Despite a 4.8-fold difference in exposure (AUC) between the two regimens, there were no clinically relevant differences in the frequency or severity of on-treatment AEs between the two regimens.
In conclusion, co-administration of neratinib, an investigational anti-cancer agent, with ketoconazole, a potent CYP3A inhibitor, increased neratinib Cmax by 3.2-fold and AUC by 4.8-fold compared with administration of neratinib alone. Nevertheless, the safety and tolerability of neratinib 240 mg was unchanged by co-administration of multiple doses of ketoconazole. These results indicate that neratinib is a substrate of CYP3A and is susceptible to interaction with potent CYP3A inhibitors and, thus, dose adjustments may be needed if neratinib is administered with such compounds.
Acknowledgments
The authors thank Tuli Ahmed, Medical Writer, for professional medical writing assistance that was funded by Wyeth and Pfizer Inc; the investigator Paula Shaw, Northwest Kinetics, Tacoma, Washington; Ann Gooding, Principal Research Scientist at Wyeth Research at the time of the study; and Covance Bioanalytical Services, LLC, Indianapolis, Indiana.
Competing Interests
This study was sponsored by Wyeth Research, which was acquired by Pfizer Inc in October 2009. All authors are current or former employees of Wyeth and Pfizer.
REFERENCES
- 1.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–25. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 2.Rabindran SK. Antitumor activity of HER-2 inhibitors. Cancer Lett. 2005;227:9–23. doi: 10.1016/j.canlet.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 4.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–48. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 5.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 6.HERCEPTIN® (trastuzumab) (United States package insert) South San Francisco, CA: Genentech; 2008. [Google Scholar]
- 7.Rabindran SK, Discafani CM, Rosfjord EC, Baxter M, Floyd MB, Golas J, Hallett WA, Johnson BD, Nilakantan R, Overbeek E, Reich MF, Shen R, Shi X, Tsou HR, Wang YF, Wissner A. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64:3958–65. doi: 10.1158/0008-5472.CAN-03-2868. [DOI] [PubMed] [Google Scholar]
- 8.Tsou HR, Overbeek-Klumpers EG, Hallett WA, Reich MF, Floyd MB, Johnson BD, Michalak RS, Nilakantan R, Discafani C, Golas J, Rabindran SK, Shen R, Shi X, Wang YF, Upeslacis J, Wissner A. Optimization of 6,7-disubstituted-4-(arylamino)quinoline-3-carbonitriles as orally active, irreversible inhibitors of human epidermal growth factor receptor-2 kinase activity. J Med Chem. 2005;48:1107–31. doi: 10.1021/jm040159c. [DOI] [PubMed] [Google Scholar]
- 9.Paridaens R, Badwe R, Sun Y, Dirix L, Cardoso F, Powell C, Zacharchuk C, Burstein HJ. Neratinib (HKI-272), an irreversible pan erbB2+ receptor tyrosine kinase inhibitor: phase 2 results in patients with erbB2+ advanced breast cancer [abstract 186P] Ann Oncol. 2009;20(Suppl 2):ii61. Available at http://annonc.oxfordjournals.org/cgi/reprint/20/suppl_2/ii61 (last accessed 23 December 2009) [Google Scholar]
- 10.Wong KK, Fracasso PM, Bukowski RM, Lynch TJ, Munster PN, Shapiro GI, Jänne PA, Eder JP, Naughton MJ, Ellis MJ, Jones SF, Mekhail T, Zacharchuk C, Vermette J, Abbas R, Quinn S, Powell C, Burris HA. A phase I study with neratinib (HKI-272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumors. Clin Cancer Res. 2009;15:2552–8. doi: 10.1158/1078-0432.CCR-08-1978. [DOI] [PubMed] [Google Scholar]
- 11.Chow L, Jiang Z, Epstein R, Bondarenko I, Awada A, Coughlin C, Gauthier E, Zhao Y, Abbas R, Hershman D. Safety and efficacy of neratinib (HKI-272) in combination with paclitaxel in patients with solid tumors [abstract 3557] J Clin Oncol. 2009;27(Suppl):S15. Available at http://meeting.ascopubs.org/content/vol27/15_suppl/ (last accessed 28 October 2009) [Google Scholar]
- 12.Swaby R, Blackwell K, Jiang Z, Sun Y, Dieras V, Zaman K, Zacharchuk C, Powell C, Abbas R, Thakuria M. Neratinib in combination with trastuzumab for the treatment of advanced breast cancer: a phase I/II study [ASCO 2009 abstract 1004] J Clin Oncol. 2009;27(Suppl):S15. Available at http://meeting.ascopubs.org/content/vol27/15_suppl/ (last accessed 28 October 2009) [Google Scholar]
- 13.Abbas R, Hug BA, Leister C, Burns J, Sonnichsen D. Single-ascending-dose (SAD) study of safety, tolerability, and pharmacokinetics (PK) of HKI-272 in healthy subjects [AAPS abstract 906] AAPS J. 2009;11(Suppl 2) Available at http://www.aapsj.org/abstracts/abstr2009/AM/am_year_abstr.asp (last accessed 22 December 2009) [Google Scholar]
- 14.Dresser GK, Spence JD, Bailey DG. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet. 2000;38:41–57. doi: 10.2165/00003088-200038010-00003. [DOI] [PubMed] [Google Scholar]
- 15.von Moltke LL, Greenblatt DJ, Schmider J, Duan SX, Wright CE, Harmatz JS, Shader RI. Midazolam hydroxylation by human liver microsomes in vitro: inhibition by fluoxetine, norfluoxetine, and by azole antifungal agents. J Clin Pharmacol. 1996;36:783–91. doi: 10.1002/j.1552-4604.1996.tb04251.x. [DOI] [PubMed] [Google Scholar]
- 16.United States Food and Drug Administration. Drug Interaction Studies – Study Design, Data Analysis, and Implications for Dosing and Labeling. Rockville, MD: FDA Center for Drug Evaluation and Research; 2006. Available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072101.pdf (last accessed 30 July 2009) [Google Scholar]
- 17.Bjornsson TD, Callaghan JT, Einolf HJ, Fischer V, Gan L, Grimm S, Kao J, King SP, Miwa G, Ni L, Kumar G, McLeod J, Obach SR, Roberts S, Roe A, Shah A, Snikeris F, Sullivan JT, Tweedie D, Vega JM, Walsh J, Wrighton SA, Pharmaceutical Research and Manufacturers of America Drug Metabolism/Clinical Pharmacology Technical Working Groups The conduct of in vitro and in vivo drug-drug interaction studies: a PhRMA perspective. J Clin Pharmacol. 2003;43:443–69. [PubMed] [Google Scholar]
- 18.Chien JY, Lucksiri A, Ernest CS, Gorski JC, Wrighton SA, Hall SD. Stochastic prediction of CYP3A-mediated inhibition of midazolam clearance by ketoconazole. Drug Metab Dispos. 2006;34:1208–19. doi: 10.1124/dmd.105.008730. [DOI] [PubMed] [Google Scholar]
- 19.Oo C, Chen YC. The need for multiple doses of 400 mg ketoconazole as a precipitant inhibitor of a CYP3A substrate in an in vivo drug-drug interaction study. J Clin Pharmacol. 2009;49:368–9. doi: 10.1177/0091270008325931. [DOI] [PubMed] [Google Scholar]
- 20.Evans WE, Schentag JJ, Jusko WJ. Principles of Therapeutic Drug Monitoring. 3rd edn. Vancouver, WA: Applied Therapeutics, Inc; 1992. [Google Scholar]
- 21.Daneshmend TK, Warnock DW, Turner A, Roberts CJ. Pharmacokinetics of ketoconazole in normal subjects. J Antimicrob Chemother. 1981;8:299–304. doi: 10.1093/jac/8.4.299. [DOI] [PubMed] [Google Scholar]


