Abstract
AIMS
Pharmacokinetic studies suggest that trimethoprim (TMP) can inhibit the hepatic metabolism of phenytoin, but the clinical relevance of this is uncertain. We studied the risk of phenytoin toxicity following the prescription of trimethoprim/sulfamethoxazole (TMP/SMX), a commonly used antibiotic, among elderly patients receiving phenytoin.
METHODS
We conducted a population-based, nested case–control study of a cohort of Ontario residents aged 66 years of age or older treated with phenytoin over a 17-year period (April 1 1992 to March 31 2009). Within this group, case patients were those hospitalized with phenytoin toxicity. For each case, we identified up to four control patients from the same cohort, matched for age and sex, and determined the odds ratio (OR) for the association between phenytoin toxicity and receipt of TMP/SMX in the preceding 30 days.
RESULTS
Among 58 429 elderly patients receiving phenytoin during the study period, we identified 796 case patients hospitalized for phenytoin toxicity and 3148 matched controls. Following multivariable adjustment for potential confounders, we observed a more than doubling of the risk of phenytoin toxicity following the receipt of TMP/SMX [adjusted OR 2.11, 95% confidence interval (CI) 1.24, 3.60]. In contrast, we observed no such risk with amoxicillin, an antibiotic with similar indications but not expected to interact with phenytoin (adjusted OR 1.12, 95% CI 0.64, 1.98).
CONCLUSION
Among older patients receiving phenytoin, treatment with TMP/SMX is associated with a more than twofold increase in the risk of phenytoin toxicity. When clinically appropriate, alternate antibiotics should be considered for these patients.
Keywords: drug interaction, nested case–control, patient safety, pharmacoepidemiology, phenytoin, population-based, trimethoprim/sulfamethoxazole
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Drug interactions are an important and avoidable, yet underappreciated cause of phenytoin toxicity.
Trimethoprim (TMP), a potent inhibitor of the CYP2C8 isoenzyme that is commonly administered with sulfamethoxazole (SMX) for the treatment of urinary tract infections, is known to reduce phenytoin clearance by 30%. Given the saturable nature of phenytoin metabolism, decreases in phenytoin clearance of this magnitude may be clinically significant.
WHAT THIS STUDY ADDS
Prescription of TMP/SMX was associated with a more than doubling of the risk of hospitalization for phenytoin toxicity [adjusted odds ratio 2.11, 95% confidence interval (CI) 1.24, 3.60].
Co-prescription of TMP/SMX and phenytoin is common. In our study, approximately 20% of phenytoin users received at least one prescription for TMP/SMX, thereby being placed at excess risk of phenytoin toxicity.
Introduction
Phenytoin is a commonly prescribed anticonvulsant medication for patients with seizure disorders [1, 2]. However, phenytoin use in the elderly is complicated by both age-related changes in pharmacokinetics and concentration-dependent, saturable metabolism [3–8]. As a result, serum concentrations of phenytoin can increase unpredictably even after small increases in dose.
Drug interactions are an important yet avoidable cause of phenytoin toxicity. Phenytoin biotransformation is catalyzed by the cytochrome P450 enzymes 2C9, 2C8 and 2C19, and the concomitant administration of drugs that can inhibit these enzymes may predispose patients to phenytoin toxicity [9–11]. Trimethoprim (TMP) is a potent inhibitor of the CYP2C8 isoenzyme at clinically relevant concentrations, and is commonly administered with sulfamethoxazole (SMX) for the treatment of urinary tract infections [9]. In a small pharmacokinetic study, phenytoin clearance was reduced by 30% when combined with TMP [12]. Given the saturable nature of phenytoin metabolism, decreases in phenytoin clearance of this magnitude may lead to clinically significant increases in phenytoin concentrations. However, the clinical evidence supporting a meaningful interaction between TMP/SMX and phenytoin is presently limited to case reports [13, 14].
Because phenytoin and TMP/SMX are commonly used medications, the likelihood of co-prescription is high. However, the risk of phenytoin toxicity associated with the combined use of phenytoin and TMP/SMX has not been studied. We sought to characterize the significance of this drug interaction in clinical practice.
Methods
We conducted a population-based, nested case–control study of Ontario residents 66 years of age or older treated with phenytoin between January 1 1992 and March 1 2009. The study was approved by the research ethics board of Sunnybrook Health Sciences Centre. Prescription medications were identified using the records of the Ontario Drug Benefit Program (ODBP), which records prescriptions dispensed to all Ontario residents aged 65 years or older. Hospitalization data and demographic information were obtained from the Canadian Institute for Health Information Discharge Abstract Database (DAD) and Registered Persons Database (RPDB), respectively. The DAD contains demographic and clinical information regarding hospital admissions, discharges and same-day surgeries from participating hospitals in Canada. The RPDB contains demographic information, including age, gender and date of death, on all Ontarians ever issued a health card. Finally, we used the Ontario Health Insurance Plan database to identify claims for inpatient and outpatient physician services. These databases were linked in an anonymous fashion using encrypted health card numbers, and have been used previously to study population based health outcomes, including the consequences of drug–drug interactions [15–18].
For each patient, we identified a period of continuous phenytoin use beginning with the first prescription for phenytoin following the patient's 66th birthday. We did not study medication use during the first year of eligibility for coverage (age 65 years) to avoid incomplete medication records. The observation period ended with the first hospitalization for phenytoin toxicity, death, end of study period (March 31 2009), or cessation of phenytoin treatment, defined as a lapse of more than 100 days between prescriptions; in these instances, we extended the observation period 180 days beyond the date of the last prescription, in order to identify outcomes which may have precipitated the cessation of treatment.
Within the cohort of continuous users of phenytoin, we defined cases as those hospitalized with a diagnosis of phenytoin toxicity (International Classifications of Diseases, 9th and 10th editions, codes 966.1 and T42.0, respectively). We included only patients who had phenytoin toxicity coded as an admission diagnosis, and excluded those in whom toxicity emerged during the course of hospitalization. The date of hospital admission served as the index date for all analyses, and only the first instance of hospitalization with phenytoin toxicity was considered for patients with more than one such admission during the study period.
From within the cohort of patients receiving phenytoin, we selected up to four controls for each case patient using incidence density sampling [19]. Controls and cases were matched for age at the index date (±3 years) and gender, and controls were assigned the index date of their matched case. When fewer than four potential controls were available for each case, we analyzed only those controls and maintained the matching process.
We used the ODB database to identify prescriptions for TMP/SMX in the 30 days prior to the index date for each case and control patient. Because TMP is invariably used in combination with SMX in Canada, we did not include prescriptions for TMP monotherapy. We selected a 30 day exposure window to provide sufficient time for complete enzyme inhibition, achievement of maximal phenytoin concentrations, and presentation to hospital following treatment with TMP/SMX. To test the specificity of our findings, we repeated our analysis using exposure to amoxicillin, which has similar clinical indications as TMP/SMX but is not expected to inhibit phenytoin metabolism or provoke phenytoin toxicity. Case and control patients who filled prescriptions for multiple exposure antibiotics in the 45 days preceding the index date were excluded from the analysis to avoid the potential contaminating effects of illness and multiple antibiotic exposures.
Statistical analysis
We compared the baseline demographic and clinical characteristics between cases and controls by using conditional logistic regression models. We also computed the standardized difference between the two groups for each variable, with differences of less than 0.1 taken to indicate good balance between the cases and controls for a given covariate [20].
We used conditional logistic regression to estimate the odds ratio (OR) and 95% confidence intervals (CIs) for the association between hospitalization for phenytoin toxicity and receipt of TMP/SMX or amoxicillin in the preceding 30 days. Patients treated with neither of the exposure antibiotics served as the reference group. We used multivariable conditional logistic regression analysis to adjust for concomitant medical conditions and prescription drugs that might influence the risk of phenytoin toxicity (Appendix 1). We also adjusted for age category, income quintile, residence in a long-term care facility, number of prescription drugs dispensed in the preceding year [21], history of hospitalization for phenytoin toxicity in the 1 year prior to cohort entry, and number of years receiving phenytoin treatment. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, North Carolina).
Results
Over the course of the 18 year study period, we identified 58 429 individuals aged 66 years or older treated with phenytoin. Of these, 11 545 (19.8%) received at least one prescription for TMP/SMX while receiving treatment with phenytoin, indicating that concomitant use of these drugs is common. A total of 796 patients from the cohort were hospitalized with phenytoin toxicity during follow-up. All cases were matched to four controls and were included in the analysis. Overall, cases and controls were similar with respect to baseline characteristics (Table 1). However, as expected, case patients exhibited a greater degree of co-morbidity, received more prescription drugs in the preceding year, and were more likely to have been previously hospitalized for phenytoin toxicity. The proportion of cases admitted each year with phenytoin toxicity is summarized in Figure 1.
Table 1.
Characteristics of cases and controls
| Variable | Cases (n = 796) | Controls (n = 3148) | Standardized difference† | P value |
|---|---|---|---|---|
| Age (median, IQR) (years) | 75 (70–80) | 74 (70–80) | 0.00 | 0.8475 |
| 66–75 | 438 (55.0%) | 1,757 (55.2%) | 0.00 | 0.8672 |
| 76–85 | 294 (36.9%) | 1,196 (37.6%) | 0.01 | 0.5628 |
| ≥86 | 65 (7.8%) | 230 (7.3%) | 0.02 | 0.1505 |
| Male | 367 (46.1%) | 1,468 (46.1%) | 0.00 | 1.0000 |
| Number of years of phenytoin treatment (median, IQR) | 1 (0–3) | 2 (1–5) | 0.37 | <0.0001 |
| Residence in a long-term care facility, n (%) | 116 (14.6%) | 832 (26.1%) | 0.27 | <0.0001 |
| Number of prescription drugs in previous year, (median, IQR) | 11 (7–15) | 9 (6–13) | 0.27 | <0.0001 |
| Previous hospitalization for phenytoin toxicity (1 year) | 8 (1.0%) | ≤5 (0.1%) | 0.17 | 0.0005 |
| Chronic liver disease in preceding year | 8 (1.0%) | 12 (0.4%) | 0.09 | 0.0316 |
| Chronic renal disease in preceding year | 36 (4.5%) | 58 (1.8%) | 0.18 | <0.0001 |
| Chronic alcoholism in preceding year | 53 (6.7%) | 92 (2.9%) | 0.20 | <0.0001 |
| Malignancy in preceding year | 52 (6.5%) | 112 (3.5%) | 0.15 | 0.0001 |
| Medication use in preceding 90 days | ||||
| CYP2C8/2C9 inhibitors | 89 (11.2%) | 272 (8.5%) | 0.09 | 0.0205 |
| CYP2C8/2C9 inducers | 106 (13.3%) | 390 (12.2%) | 0.03 | 0.4100 |
| CYP2C19 inhibitors | 152 (19.1%) | 484 (15.2%) | 0.11 | 0.0073 |
| CYP2C19 inducers | 32 (4.0%) | 82 (2.6%) | 0.09 | 0.0286 |
| Income quintile, n (%) | ||||
| 1 (lowest) | 211 (26.5%) | 652 (20.5%) | 0.15 | 0.0003 |
| 2 | 155 (19.5%) | 681 (21.4%) | 0.05 | 0.2371 |
| 3 | 156 (19.6%) | 690 (21.7%) | 0.05 | 0.1965 |
| 4 | 140 (17.6%) | 556 (17.5%) | 0.00 | 0.9336 |
| 5 | 117 (14.7%) | 544 (17.1%) | 0.06 | 0.1013 |
| Missing | 17 (2.1%) | 61 (1.9%) | 0.02 | 0.6780 |
Difference between cases and controls divided by standard deviation.
Figure 1.
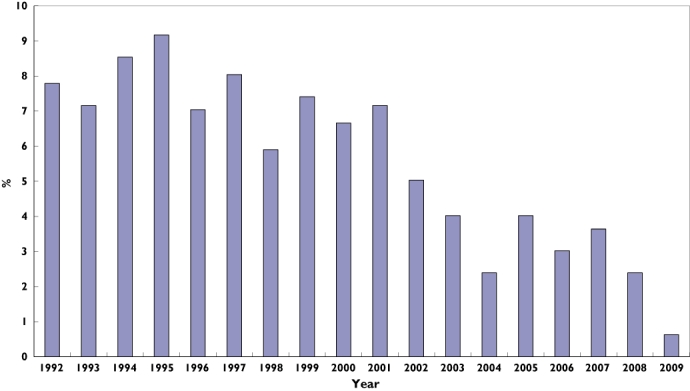
Distribution of cases by year of cohort entry. Each bar represents the proportion of elderly patients hospitalized with phenytoin toxicity in Ontario for a specific year over a 17-year period (1992 to 2009). Proportion of Cases (%) ( )
)
In the primary analysis, a recent prescription for TMP/SMX was significantly associated with hospitalization for phenytoin toxicity (Table 2). Following multivariable adjustment, patients with a recent prescription for TMP/SMX were more than twice as likely to be hospitalized for phenytoin toxicity [adjusted odds ratio (aOR) 2.11, 95% CI 1.24, 3.60] as compared with no antibiotic exposure. As expected, we found no such association with amoxicillin (aOR 1.12, 95% CI 0.64, 1.98).
Table 2.
Association between antibiotic use and hospitalization for phenytoin toxicity
| Antibiotic | Patients Cases n (%) (n = 796) | Controls n (%) (n = 3148) | Crude odds ratio (95% confidence interval) | Adjusted odds ratio† (95% confidence interval) |
|---|---|---|---|---|
| TMP/SMX | 25 (3.1) | 47 (1.5) | 2.18 (1.33, 3.57) | 2.11 (1.24, 3.60) |
| Amoxicillin | 25 (3.1) | 61 (1.9) | 1.14 (0.66, 1.96) | 1.12 (0.64, 1.98) |
TMP/SMX, trimethoprim-sulfamethoxazole.
Adjusted for age category, hospitalization for phenytoin toxicity in previous year, chronic disease (malignancy, hepatic, renal, alcoholism), income quintile, living in long-term care facility, number of prescription drugs in previous year, number of years of phenytoin treatment, and interacting medications (Appendix 1).
Discussion
In this population-based study spanning 18 years, we found a significant association between use of TMP/SMX and hospitalization for phenytoin toxicity. In contrast, no such risk was seen for amoxicillin, a commonly used antibiotic with similar indications to TMP/SMX but not expected to provoke phenytoin toxicity. Overall, our findings support the notion of a clinically meaningful drug interaction between TMP/SMX and phenytoin at the population level, and augment the findings of previous in vitro and pharmacokinetic investigations in this area by providing an estimate of the risk of phenytoin toxicity associated with this drug combination in clinical practice.
Our findings have important clinical implications. Among our cohort of continuous phenytoin users, roughly 1 in 5 received at least one prescription for TMP/SMX during the study period, thereby being placed at excess risk of phenytoin toxicity. Although phenytoin-associated toxicity is rarely fatal, it is a uniformly avoidable form of drug-related harm attended by considerable morbidity and cost to the health care system. Consequently, strategies aimed at minimizing the risk of phenytoin toxicity are necessary co-requisites of treatment, particularly in vulnerable groups such as the elderly. Therefore, minimizing the use of drugs that inhibit phenytoin metabolism is desirable. When patients receiving phenytoin also require treatment with antibiotics, avoidance of TMP/SMX may be prudent when other options exist.
Some limitations of our work merit emphasis. We used administrative data and had no information regarding concentrations of serum phenytoin or albumin, renal or hepatic function or medication adherence. In addition, the accuracy of hospital discharge coding for phenytoin toxicity is unknown. Importantly, however, these limitations apply equally to both TMP/SMX and amoxicillin. Our analysis focused on outcomes involving hospital admission, and we therefore did not identify cases of phenytoin toxicity managed in emergency departments or in the ambulatory setting. Our study may therefore underestimate the clinical consequences of this drug interaction. Furthermore, our findings may not apply to younger patients with fewer risk factors for phenytoin toxicity. Finally, as expected in a case-control study, our cases and controls differed at baseline with respect to variables that may influence the relationship between antibiotic use and hospitalization for phenytoin toxicity. However, this applies to both TMP/SMX and amoxicillin, rendering it an unlikely explanation for our findings.
In conclusion, we found that prescription of TMP/SMX to elderly patients receiving phenytoin was associated with a more than doubling of the risk of hospitalization for phenytoin toxicity. A similar risk was not observed with amoxicillin. Increased awareness of this drug interaction among pharmacists and physicians is necessary to ensure that the potential for this avoidable adverse drug reaction is minimized, either by selection of alternative antibiotics when clinically appropriate, or by close monitoring of patients for phenytoin toxicity when TMP/SMX is required.
Acknowledgments
Tony Antoniou is supported by a scholarship from the Canadian Observational Cohort (CANOC) Collaboration. This project was supported by research funds from the Ontario Drug Policy Research Network and by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred.
Appendix 1 Medications included in the multivariable model
Medication use in the 90 days preceding index date
-
CYP2C8/2C9 inhibitors
Amiodarone, chloramphenicol, cimetidine, delavirdine, disulfiram, efavirenz, fluconazole, fluvastatin, gemfibrozil, isoniazid, metronidazole, montelukast, phenylbutazone, sulfinpyrazone, sulfadiazine, valproic acid, voriconazole, zafilukast
-
CYP2C8/2C9 inducers
Amobarbital, carbamazepine, dexamethasone, nevirapine, phenobarbital, primidone, rifampin, secobarbital
-
CYP2C19 inhibitors
Felbamate, indomethacin, ketoconazole, moclobemide, modafinil, oxcarbazepine, probenecid, ticlopidine, topiramate
-
CYP2C19 inducers
Prednisone, lopinavir/ritonavir, ritonavir
Competing Interests
Tony Antoniou has received unrestricted research grants from Glaxo-Smith-Kline Inc, Merck and Pfizer for different studies.
REFERENCES
- 1.Pugh MJ, Van Cott AC, Cramer JA, Knoefel JE, Amuan ME, Tabares J, Ramsay RE, Berlowitz DR, Treatment In Geriatric Epilepsy Research (TIGER) Team Trends in antiepileptic drug prescribing for older patients with new-onset epilepsy: 2000–2004. Neurology. 2008;70:2171–8. doi: 10.1212/01.wnl.0000313157.15089.e6. [DOI] [PubMed] [Google Scholar]
- 2.Garrard J, Harms S, Hardie N, Eberly LE, Nitz N, Bland P, Gross CR, Leppik IE. Antiepileptic drug use in nursing home admissions. Ann Neurol. 2003;54:75–85. doi: 10.1002/ana.10593. [DOI] [PubMed] [Google Scholar]
- 3.Bach B, Hansen JM, Kampmann JP, Rasmussen SN, Skovsted L. Disposition of antipyrine and phenytoin correlated with age and liver volume in man. Clin Pharmacokinet. 1981;6:389–96. doi: 10.2165/00003088-198106050-00005. [DOI] [PubMed] [Google Scholar]
- 4.Bauer LA, Blouin RA. Age and phenytoin kinetics in adult epileptics. Clin Pharmacol Ther. 1982;31:301–4. doi: 10.1038/clpt.1982.37. [DOI] [PubMed] [Google Scholar]
- 5.Battino D, Croci D, Mamoli D, Messina S, Perucca E. Influence of aging on serum phenytoin concentrations: a pharmacokinetic analysis based on therapeutic drug monitoring data. Epilepsy Res. 2004;59:155–65. doi: 10.1016/j.eplepsyres.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Morrell MJ. Antiepileptic medications for the treatment of epilepsy. Semin Neurol. 2002;22:247–58. doi: 10.1055/s-2002-36645. [DOI] [PubMed] [Google Scholar]
- 7.Hung CC, Lin CJ, Chen CC, Chang CJ, Liou HH. Dosage recommendation of phenytoin for patients with epilepsy with different CYP2C9/CYP2C19 polymorphisms. Ther Drug Monit. 2004;25:534–40. doi: 10.1097/00007691-200410000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Osorio I, Burnstine TH, Remler B, Manon-Espaillat R, Reed RC. Phenytoin-induced seizures: a paradoxical effect at toxic concentrations in epileptic patients. Epilepsia. 1989;30:230–4. doi: 10.1111/j.1528-1157.1989.tb05459.x. [DOI] [PubMed] [Google Scholar]
- 9.Wen X, Wang JS, Backman JT, Laitila J, Neuvonen PJ. Trimethoprim and sulfamethoxazole are selective inhibitors of CYP2C8 and CYP2C9, respectively. Drug Metab Dispos. 2002;30:631–5. doi: 10.1124/dmd.30.6.631. [DOI] [PubMed] [Google Scholar]
- 10.Doecke CJ, Veronese ME, Pond SM, Miners JO, Birkett DJ, Sansom LN, McManus ME. Relationship between phenytoin and tolbutamide hydroxylations in human liver microsomes. Br J Clin Pharmacol. 1991;31:125–30. doi: 10.1111/j.1365-2125.1991.tb05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bajpai M, Roskos LK, Shen DD, Levy RH. Roles of cytochrome P4502C9 and cytochrome P4502C19 in the stereoselective metabolism of phenytoin to its major metabolite. Drug Metab Dispos. 1996;24:1401–3. [PubMed] [Google Scholar]
- 12.Hansen JM, Kampmann JP, Siersbaek-Nielsen K, Lumholtz IB, Arrøe M, Abildgaard U, Skovsted L. The effect of different sulfonamides on phenytoin metabolism in man. Acta Med Scand Suppl. 1979;624:106–10. doi: 10.1111/j.0954-6820.1979.tb00729.x. [DOI] [PubMed] [Google Scholar]
- 13.Gillman MA, Sandyk R. Phenytoin toxicity and co-trimoxazole. Ann Intern Med. 1985;102:559. doi: 10.7326/0003-4819-102-4-559_2. [DOI] [PubMed] [Google Scholar]
- 14.Wilcox JB. Phenytoin intoxication and cotrimoxazole. N Z Med J. 1981;94:235–6. [PubMed] [Google Scholar]
- 15.Juurlink DN, Mamdani M, Kopp A, Laupacis A, Redelmeier DA. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289:1652–8. doi: 10.1001/jama.289.13.1652. [DOI] [PubMed] [Google Scholar]
- 16.Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351:543–51. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- 17.Park-Wyllie LY, Juurlink DN, Kopp A, Shah BR, Stukel TA, Stumpo C, Dresser L, Low DE, Mamdani MM. Outpatient gatifloxacin therapy and dysglycemia in older adults. N Engl J Med. 2006;354:1352–61. doi: 10.1056/NEJMoa055191. [DOI] [PubMed] [Google Scholar]
- 18.Lipscombe LL, Gomes T, Lévesque LE, Hux JE, Juurlink DN, Alter DA. Thiazolidinediones and cardiovascular outcomes in older patients with diabetes. JAMA. 2007;298:2634–43. doi: 10.1001/jama.298.22.2634. [DOI] [PubMed] [Google Scholar]
- 19.Lubin JH, Gail MH. Biased selection of controls for case-control analyses of cohort studies. Biometrics. 1984;40:63–75. [PubMed] [Google Scholar]
- 20.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med. 2007;26:734–53. doi: 10.1002/sim.2580. [DOI] [PubMed] [Google Scholar]
- 21.Schneeweiss S, Seeger JD, Maclure M, Wang PS, Avorn J, Glynn RJ. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol. 2001;154:854–64. doi: 10.1093/aje/154.9.854. [DOI] [PubMed] [Google Scholar]


