Abstract
AIMS
To determine the effects of the CYP2C9*1/*13 genotype on the pharmacokinetics and pharmacodynamics of meloxicam in Korean subjects.
METHODS
Meloxicam (15 mg) was orally administered to 21 healthy Korean volunteers with either the CYP2C9*1/*1 or the CYP2C9*1/*13 genotype. Plasma meloxicam concentrations were analysed by HPLC-UV for 72 h after drug administration. The pharmacodynamic effects of meloxicam were determined by measuring TXB2 generated in blood.
RESULTS
The AUC(0,∞) and Cmax of meloxicam were 2.43- and 1.46-fold higher in the CYP2C9*1/*13 group than in the CYP2C9*1/*1 group, respectively. The oral clearance of meloxicam was significantly lower in the CYP2C9*1/*13 group (37.9% of wild type) than in the CYP2C9*1/*1 group. The t1/2 of meloxicam was 1.84-fold longer in the CYP2C9*1/*13 group than in the CYP2C9*1/*1 group. The rate of TXB2 production was significantly lower in the CYP2C9*1/*13 group than in the CYP2C9*1/*1 group.
CONCLUSIONS
The CYP2C9*1/*13 genotype is associated with decreased metabolism and increased pharmacodynamic effects of meloxicam.
Keywords: CYP2C9, genetic polymorphism, meloxicam, pharmacodynamics, pharmacokinetics
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Meloxicam is a substrate for the CYP2C9 and CYP3A4 enzymes.
We have previously reported that the frequency of the CYP2C9*1/*13 genotype in the Korean population is 1.1%.
WHAT THIS STUDY ADDS
The CYP2C9*1/*13 genotype is associated with decreased metabolism and increased pharmacodynamic effects of meloxicam.
This is the first report that evaluates the in vivo effects of the CYP2C9*13 allele on the pharmacokinetics and pharmacodynamics of CYP2C9 substrates with a sufficient sample size.
Introduction
Meloxicam is a non-steroidal anti-inflammatory drug (NSAID) that inhibits the synthesis of prostaglandins through preferential cyclo-oxygenase-2 (COX-2) inhibition, imparting analgesic, antipyretic and anti-inflammatory properties [1] for the treatment of osteoarthritis and rheumatoid arthritis. Meloxicam is primarily metabolized to a 5′-hydroxymethyl metabolite by CYP2C9 (major) and CYP3A4 (minor) [2, 3]. In humans, the CYP2C subfamily is a drug metabolizing enzyme that accounts for approximately 18% of the CYP protein content in human liver microsomes and catalyzes approximately 20% of the CYP-mediated metabolic reactions of drugs currently on the market [4]. CYP2C9 is polymorphic and is involved in the oxidation of a wide range of drugs, including S-warfarin, phenytoin, losartan, tolbutamide and torsemide. Further, CYP2C9 also metabolizes NSAIDs including diclofenac, naproxen, piroxicam, tenoxicam and ibuprofen, as well as the selective COX-2 inhibitor celecoxib [5]. Thirty-four allelic variants of the CYP2C9 gene [6] show differences in enzyme expression and activity, leading to differences in dosing and the potential for unpredictable adverse effects [7]. Among these mutations, the CYP2C9*2 (p.Arg144Cys) and CYP2C9*3 (p.Ile359Leu) alleles show impaired activity towards a number of substrates both in vivo and in vitro[4, 5, 8]. CYP2C9*13 was first described in a male Chinese individual, and a large decrease in enzyme activity is observed in individuals with the CYP2C9*3/*13 genotype [9]. The CYP2C9*13 allele involves a c.269T>C transition in exon 2, leading to a p.Leu90Pro substitution in the encoded protein [9]. The CYP2C9*13 allele has been detected in Chinese, Japanese and Korean samples at low frequencies of 0.2% to 1.0% [9–11]. However, it is not detected in other populations including European, African-American, Hispanic and Ashkenazi Jewish [12]. Although the CYP2C9*13 allele was reported to show decreased enzymatic activity towards some CYP2C9 substrates in vivo and/or in vitro[11, 13–17], the effects of the CYP2C9*13 allele on the pharmacokinetics of CYP2C9 substrate drugs have not been sufficiently studied because of the low frequency with which the allele is found in the general population.
Here, we evaluated the effects of the CYP2C9*13 allele on the pharmacokinetics and pharmacodynamics of meloxicam in a healthy Korean cohort.
Methods
Subjects
Among 1213 male Korean subjects tested for the CYP2C9 genotype, 21 healthy subjects carrying either the CYP2C9*1/*1 or CYP2C9*1/*13 genotype were selected. All subjects were healthy according to medical history, physical examination and routine laboratory tests (blood chemistry, haematology and urine analysis). The subjects were asked to refrain from taking any medication, caffeine, grapefruit products, smoking and alcoholic beverages for at least 1 week before and throughout the study period. All subjects provided verbal and written informed consent. The study was performed according to the guidelines of the Declaration of Helsinki and was approved by the institutional ethics committee of the School of Pharmacy, Sungkyunkwan University, Suwon, Korea.
Study protocol
On the day of the study, each subject received a 15 mg oral dose of meloxicam (Mobic®, Boehringer Ingelheim Korea, Seoul, Korea) with 240 ml of water after an overnight fast. The subjects were maintained in the fasting state for 4 h after administering the drug. Venous blood samples (10 ml) were obtained before and at 1, 2, 3, 4, 5, 6, 8, 10, 12, 24, 48 and 72 h after meloxicam administration. The blood samples were centrifuged immediately and plasma samples were stored at –70°C until needed.
CYP2C9 genotyping
Genomic DNA was isolated from peripheral blood leukocytes using the Wizard® Genomic DNA Purification Kit (Promega, Madison, WI, USA). CYP2C9 genotyping for CYP2C9*2, *3, *4, *5, *11 and *13 was performed using polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP) and direct sequence analysis described in our previous study [10]. The CYP2C9*1 allele was assigned in the absence of other detectable variant alleles. CYP2C9 sequencing was performed for all subjects participating in this study in order to identify additional CYP2C9 coding region changes. Direct sequencing of the CYP2C9 gene for the amplification of all nine exons and exon/intron junctions were performed according to a previously reported method with slight modifications [10, 18]. PCR products were purified using a PCR purification kit (AxyPrep® PCR Clean-up Kit, Axygen Bioscience Inc., Union City, CA, USA) and were sequenced on an ABI3730 automatic sequencer (Applied Biosystems Inc., Foster City, CA, USA) using a BigDye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems Inc.). There were no subjects carrying the CYP2C9*2, *4, *5, or *11 alleles in our study.
Assay of meloxicam plasma concentrations
The plasma concentrations of meloxicam were determined using a validated high-performance liquid chromatography (HPLC) assay with ultraviolet detection [19]. Briefly, 0.5 ml of plasma was spiked with 400 ng of an internal standard (piroxicam), acidified with 0.1 ml 5 m HCl, then extracted with 6 ml of diethyl ether. The organic phase was evaporated at 40°C under a constant flow of nitrogen gas. The residue was reconstituted with 500 µl of the mobile phase. A 65 µl aliquot was then injected into the analytical column (Sunfire, C18, 5 µm, 4.5 mm × 150 mm, Waters, MA, USA). The mobile phase consisted of a mixture of 20 mm phosphate buffer : acetonitrile (60 : 40, v : v, pH 3.5). The flow rate was 1.2 ml min–1 and the column oven temperature was 30°C. The effluents were detected with ultraviolet detection at 355 nm. The detection limit was 10 ng ml–1 for meloxicam. The linear ranges of the standard curves for meloxicam ranged from 10 to 2400 ng ml–1 in plasma (r2 > 0.9998). The mean accuracy of meloxicam was 98–114% in plasma. The coefficients of the variation (within-day and between-day precision) of meloxicam were <8% in plasma.
Thromboxane B2 (TXB2) analysis
To assess the effects of meloxicam on platelet COX (cyclo-oxygenase), the level of TXA2 generated during the clotting of whole blood was determined by measuring the concentration of its stable breakdown product, TXB2, as described previously [20]. Briefly, blood samples without anticoagulant were obtained before and at 1, 2, 3, 4, 5, 6, 8, 10, 12, 24, 48 and 72 h after meloxicam administration. Within 5 min of blood collection, each sample was incubated for 1 h at 37°C to allow the blood to clot. The serum was then collected, immediately centrifuged, and stored at –70°C until assayed for TXB2. Serum TXB2 concentrations were determined using a commercially available TXB2 enzyme immunoassay kit (Assay Designs, Ann Arbor, MI, USA).
Pharmacokinetic and pharmacodynamic analysis
The pharmacokinetic parameters of meloxicam were estimated using non-compartmental methods with the BA Calc 2002 analysis program (KFDA, Seoul, Korea); actual blood sampling times were used for analysis. The maximum plasma concentration (Cmax) and time to reach Cmax (tmax) were used as the observed values. The area under the plasma concentration–time curve (AUC) was calculated using the linear trapezoidal rule. The elimination rate constant (λz) was estimated from the least-squares regression slope of the terminal plasma concentration. The AUC from 0 to infinity [AUC(0,∞)] was calculated as AUC(0,∞) = AUC +Ct/λz (Ct is the last plasma concentration measured). The half-life (t1/2) was calculated as ln 2/λz, and the apparent oral clearance (CL/F) of meloxicam was calculated as CL/F = Dose/AUC(0,∞).
The inhibition of TXB2 generation was calculated as the percent inhibition of TXB2 generation in the serum relative to the baseline (pre-dose) values [TXB2 inhibition % = (TXB2 serum concentration at 0 h – TXB2 serum concentration at t h)/(TXB2 serum concentration at 0 h)]. The area under the effect (TXB2 inhibition %)–time curve (AUEC) up to 72 h was calculated using the linear trapezoidal rule.
Statistical analysis
The number of subjects in each genotype group was estimated to be sufficient to detect a 50% difference in AUC(0,∞) of meloxicam between the two genotype groups with a statistical power of at least 80% (α level of 0.05). The power and sample size were calculated with the Power and Sample Size Program, PS (version 3.0.17) [21]. All pharmacokinetic data are expressed as the mean ± SD, except for tmax, which is presented as the median value and range. Differences in pharmacokinetic parameters between the genotype groups were assessed using Student's t-test or the Mann-Whitney rank sum test after normality and equal variance tests. Data were analysed using the statistical program Sigmastat for Windows (version 2.3; SPSS Inc., Chicago, IL, USA). P values of <0.05 were considered statistically significant.
Results
All subjects completed the study according to the protocol, and there were no clinically undesirable signs or symptoms attributed to the administration of meloxicam. In addition, there were no significant differences in the demographic parameters between the two genotyped groups (Table 1).
Table 1.
Individual demographic characteristics (mean ± SD) according to CYP2C9 genotype in 21 healthy Korean subjects
| Genotype | Number of subjects | Age (years) | Height (cm) | Weight (kg) | Body mass index (kg m–2) |
|---|---|---|---|---|---|
| CYP2C9*1/*1 | 12 | 23.2 ± 2.6 | 176.0 ± 4.4 | 71.8 ± 8.1 | 23.1 ± 2.1 |
| CYP2C9*1/*13 | 9 | 24.4 ± 2.5 | 174.1 ± 3.6 | 70.7 ± 7.3 | 23.3 ± 2.4 |
After a single 15 mg oral dose of meloxicam, heterozygous CYP2C9*13 subjects showed a higher mean plasma meloxicam concentration curve than the wild-type CYP2C9 subjects (Figure 1). Meloxicam pharmacokinetics were significantly different in subjects homozygous for the CYP2C9*1 allele (Table 2). The mean AUC(0,∞) of meloxicam in the CYP2C9*1/*13 subjects was 2.43-fold higher than in the CYP2C9*1/*1 subjects (P < 0.001). Similarly, the t1/2 of meloxicam was 1.84-fold longer in the CYP2C9*1/*13 subjects than in the CYP2C9*1/*1 subjects (P < 0.001). The mean CL/F value of meloxicam in the CYP2C9*1/*13 subjects was decreased to 37.9% compared with that in the CYP2C9*1/*1 subjects (P < 0.001). The Cmax of meloxicam in the CYP2C9*1/*13 subjects was 1.46 fold higher in the CYP2C9*1/*1 subjects (P < 0.01).
Figure 1.
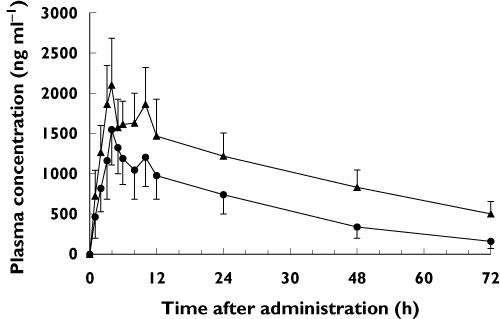
Plasma concentration–time profiles for meloxicam in CYP2C9 genotype groups after a single 15 mg oral dose of meloxicam. Each value represents the mean ± SD. •, CYP2C9*1/*1 (n = 12); ▴, CYP2C9*1/*13 (n = 9)
Table 2.
Pharmacokinetic parameters of meloxicam determined after oral administration of 15 mg meloxicam according to CYP2C9 genotype
| Variable | CYP2C9*1/*1 (n = 12) | CYP2C9*1/*13 (n = 9) |
|---|---|---|
| Cmax (ng ml–1) | 1558 ± 394 | 2270 ± 472** |
| (1308, 1808) | (1907, 2633) | |
| tmax (h) | 4.2 (4.0–5.0) | 4.0 (3.0–5.0) |
| t1/2 (h) | 21.5 ± 4.6 | 39.5 ± 5.5*** |
| (18.8, 24.4) | (35.3, 43.8) | |
| CL/F (ml h–1) | 367 ± 117 | 139 ± 21*** |
| (292, 441) | (122, 155) | |
| AUC(0,72 h) (µg ml–1 h) | 40.3 ± 12.3 | 78.6 ± 10.1*** |
| (32.5, 48.1) | (70.9, 86.3) | |
| AUC(0,∞) (µg ml–1 h) | 45.2 ± 15.2 | 109.7 ± 15.8*** |
| (35.5, 54.8) | (97.6, 121.8) |
Each value represents the mean ± SD apart from tmax which is given as a median (range). Values in parentheses represent 95% confidence intervals. Cmax maximum plasma concentration; tmax time to reach the maximum plasma concentration; AUC(0,72 h) area under the plasma concentration–time curve from time 0 to 72 h; AUC(0,∞) area under the plasma concentration–time curve from time 0 to infinity; t1/2 elimination half-life; CL/F apparent oral clearance.
P < 0.01
P < 0.001 compared with the CYP2C9*1/*1 group.
The meloxicam-induced inhibition of TXB2 generation was assessed from baseline after the oral administration of meloxicam. Subjects with CYP2C9*1/*13 tended to show greater inhibition of TXB2 generation relative to baseline than subjects with CYP2C9*1/*1 (Figure 2). Likewise, CYP2C9*1/*13 subjects tended to have significantly higher values for area under the effect curve from 0 to 72 h than the subjects with CYP2C9*1/*1 (Table 3).
Figure 2.
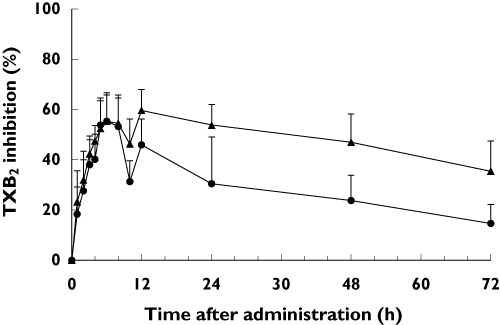
Percent inhibition of thromboxane B2 (TXB2) from baseline in the CYP2C9 genotype groups after a single 15 mg oral dose of meloxicam. Each value represents the mean ± SD. •, CYP2C9*1/*1 (n = 12); ▴, CYP2C9*1/*13 (n = 9)
Table 3.
Pharmacodynamic parameters (mean ± SD) of meloxicam according to CYP2C9 genotype
| Variable | CYP2C9*1/*1 (n = 12) | CYP2C9*1/*13 (n = 9) |
|---|---|---|
| Maximum observed TXB2 inhibition (%) | 60.3 ± 8.3 | 63.5 ± 8.5 |
| (55.0, 65.6) | (56.9, 70.0) | |
| AUEC (%·h) | 2033 ± 631 | 3337 ± 552*** |
| (1633, 2435) | (3013, 3862) |
Values in parentheses represent 95% confidence intervals. AUEC, area under the effect (percent of inhibition of TXB2 generation)–time curve.
P < 0.001 compared with the CYP2C9*1/*1 group.
Discussion
Genetic polymorphisms in drug-metabolizing enzymes cause significant inter-individual variability in dose–concentration relationships and drug response. In the present study, we examined the effect of the CYP2C9*13 allele on the pharmacokinetics and pharmacodynamics of meloxicam. Here, the mean CL/F value of meloxicam was significantly lower in CYP2C9*1/*13 subjects than in CYP2C9*1/*1 subjects.
The CYP2C9*13 allele was observed in 13 subjects from a population sample of 1213, and all 13 subjects with the CYP2C9*13 allele were the heterozygous variant with CYP2C9*1/*13 (data not shown). Among the 13 subjects with CYP2C9*1/*13, two had a liver health problem (abnormal aspartate transaminase and alanine transaminase concentrations) and two subjects did not consent to participate in the pharmacokinetic study of meloxicam. Thus, a total of nine subjects with CYP2C9*1/*13 were enrolled in the clinical study of meloxicam. The frequency of the CYP2C9*13 allele in Koreans (0.54%) is lower than in the Chinese population (1.02%) [9]. Interestingly, a Chinese subject who was a CYP2C9*3/*13 heterozygote demonstrated a significantly longer elimination half-life for lornoxicam and tolbutamide compared with CYP2C9*1/*1[9]. In the three-dimensional structure models study, the trans configuration of the bond between p.Pro90 and p.Asp89 in CYP2C9*13 was identified. In addition, the backbone of residues p.Ala106–p.Arg108 in CYP2C9*13 turns over and their side chains block the entrance for accessing substrates so that the entrance of CYP2C9*13 shrinks greatly compared with that in the wild-type, which is believed to be the dominant mechanism of the catalytic activity reduction [16]. Only two studies have previously evaluated whether the CYP2C9*13 allele influences the pharmacokinetics of lornoxicam [13] or losartan [15]. However, small sample sizes were a limitation for both of these studies. Thus, our study is the first to evaluate the in vivo effects of the CYP2C9*13 allele on the pharmacokinetics and pharmacodynamics of a CYP2C9 substrate with a sample size sufficient for a statistical power above 0.80. Here, the AUC of meloxicam was increased 2.43-fold and its oral clearance was decreased by 62.1% in two CYP2C9*1/*13 subjects compared with CYP2C9*1/*1, indicating a significant decrease in meloxicam metabolism and suggesting potential toxicity with other CYP2C9 substrates that have a narrow therapeutic index.
CYP2C9 sequencing was performed in order to identify additional changes of the CYP2C9 coding region in all subjects participating in this clinical study of meloxicam. The genotype results obtained by PCR-RFLP fully corresponded to the sequencing results. Further, no additional changes in the CYP2C9 coding region were identified in any of the subjects.
Most plasma concentration–time curves exhibited two peaks after oral administration of meloxicam, with a small second peak occurring 10 to 12 h after administration. This result has also been reported previously for the disposition of piroxicam [22, 23] and meloxicam [24–27]. The AUC and the elimination half-life of intravenous meloxicam are reduced by cholestyramine, indicating involvement of the entero-hepatic or entero-enteric circulation [28], which may explain the observed double peaks and prolonged absorption of meloxicam.
The enolic acid of meloxicam preferentially inhibits COX-2 over COX-1 [29–31], and the inhibition of COX-1 by meloxicam was determined by measuring TXB2 concentrations in clotted whole blood ex vivo. The pharmacodynamic data from our study suggested that a single 15 mg dose of meloxicam was sufficient to significantly inhibit TXB2 generation, which is consistent with a previous report [32]. The CYP2C9*1/*13 genotype significantly increased the inhibition of TXB2 generation (AUEC) in this study. Taken together with the pharmacokinetic and pharmacodynamic results of this study, the CYP2C9*13 allele may increase the potential for unpredictable adverse effects of meloxicam.
The oral clearance of meloxicam in subjects with CYP2C9*1/*13 was significantly decreased by 62% compared with subjects with CYP2C9*1/*1. Although different from the results of in vitro studies on CYP2C9 substrates, the CYP2C9*13 allele appeared to have lower activity than the CYP2C9*1 allele, which has been extensively studied in humans. In addition to meloxicam, CYP2C9 is involved in the metabolism of hypoglycaemic, anticonvulsant, anticoagulants and antihypertensive drugs. Interestingly, the CYP2C9*1/*13 genotype was recently found to be associated with a lower warfarin dose following mechanical heart valve replacement in a Korean patient [17]. In addition, phenytoin clearance is impaired by CYP2C9*2, *3, *5, *6 and *11 variants [33–35]. Based on these results, the CYP2C9*13 allele is expected to have lower activity than the CYP2C9*1 allele, and may be at risk for narrow therapeutic indices, e.g. warfarin and phenytoin. Although the frequency of the CYP2C9*13 allele is low, it should nevertheless be considered for warfarin and phenytoin dosing predictions in East Asian populations.
In conclusion, the CYP2C9*1/*13 genotype is associated with decreased metabolism and increased pharmacodynamic effects of meloxicam.
Acknowledgments
This study was supported by the research fund from Sungkyunkwan University, Republic of Korea. The authors thank Ho-Kyun Han, Joon-Ho Sa, Yee Song, Sung-Min Moon and Do-Hoon Kim for their assistance in these experiments.
Competing Interests
All of the authors declare that there are no financial or personal relationships connected with the research described herein that would lead to a conflict of interest.
REFERENCES
- 1.Engelhardt G, Homma D, Schlegel K, Utzmann R, Schnitzler C. Anti-inflammatory, analgesic, antipyretic and related properties of meloxicam, a new non-steroidal anti-inflammatory agent with favourable gastrointestinal tolerance. Inflamm Res. 1995;44:423–33. doi: 10.1007/BF01757699. [DOI] [PubMed] [Google Scholar]
- 2.Schmid J, Busch U, Heinzel G, Bozler G, Kaschke S, Kummer M. Pharmacokinetics and metabolic pattern after intravenous infusion and oral administration to healthy subjects. Drug Metab Dispos. 1995;23:1206–13. [PubMed] [Google Scholar]
- 3.Chesne C, Guyomard C, Guillouzo A, Schmid J, Ludwig E, Sauter T. Metabolism of Meloxicam in human liver involves cytochromes P4502C9 and 3A4. Xenobiotica. 1998;28:1–13. doi: 10.1080/004982598239704. [DOI] [PubMed] [Google Scholar]
- 4.Lee CR, Goldstein JA, Pieper JA. Cytochrome P450 2C9 polymorphisms: a comprehensive review of the in-vitro and human data. Pharmacogenetics. 2002;12:251–63. doi: 10.1097/00008571-200204000-00010. Erratum in: Pharmacogenetics 2002; 12: 343. [DOI] [PubMed] [Google Scholar]
- 5.Miners JO, Birkett DJ. Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. Br J Clin Pharmacol. 1998;45:525–38. doi: 10.1046/j.1365-2125.1998.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ingelman-Sundberg M, Daly AK, Nebe DW, editors. Human Cytochrome P450(CYP) Allele Nomenclature Committee Website. Available at http://www.cypalleles.ki.se/cyp2c9.htm (last accessed 1 March 2010)
- 7.Xie HG, Prasad HC, Kim RB, Stein CM. CYP2C9 allelic variants: ethnic distribution and functional significance. Adv Drug Deliv Rev. 2002;54:1257–70. doi: 10.1016/s0169-409x(02)00076-5. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein JA. Clinical relevance of genetic polymorphisms in the human CYP2C subfamily. Br J Clin Pharmacol. 2001;52:349–55. doi: 10.1046/j.0306-5251.2001.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Si D, Guo Y, Zhang Y, Yang L, Zhou H, Zhong D. Identification of a novel variant CYP2C9 allele in Chinese. Pharmacogenetics. 2004;14:465–9. doi: 10.1097/01.fpc.0000114749.08559.e4. [DOI] [PubMed] [Google Scholar]
- 10.Bae JW, Kim HK, Kim JH, Yang SI, Kim MJ, Jang CG, Lee SY. Allele and genotype frequencies of CYP2C9 in a Korean population. Br J Clin Pharmacol. 2005;60:418–22. doi: 10.1111/j.1365-2125.2005.02448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maekawa K, Harakawa N, Sugiyama E, Tohkin M, Kim SR, Kaniwa N, Katori N, Hasegawa R, Yasuda K, Kamide K, Miyata T, Saito Y, Sawada J. Substrate-dependent functional alterations of seven CYP2C9 variants found in Japanese subjects. Drug Metab Dispos. 2009;37:1895–903. doi: 10.1124/dmd.109.027003. [DOI] [PubMed] [Google Scholar]
- 12.Scott SA, Khasawneh R, Peter I, Kornreich R, Desnick RJ. Combined CYP2C9, VKORC1 and CYP4F2 frequencies among racial and ethnic groups. Pharmacogenomics. 2010;11:781–91. doi: 10.2217/pgs.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Y, Zhang Y, Wang Y, Chen X, Si D, Zhong D, Fawcett JP, Zhou H. Role of CYP2C9 and its variants (CYP2C9*3 and CYP2C9*13) in the metabolism of lornoxicam in humans. Drug Metab Dispos. 2005;33:749–53. doi: 10.1124/dmd.105.003616. [DOI] [PubMed] [Google Scholar]
- 14.Guo Y, Wang Y, Si D, Fawcett PJ, Zhong D, Zhou H. Catalytic activities of human cytochrome P450 2C9*1, 2C9*3 and 2C9*13. Xenobiotica. 2005;35:853–61. doi: 10.1080/00498250500256367. [DOI] [PubMed] [Google Scholar]
- 15.Li Z, Wang G, Wang LS, Zhang W, Tan ZR, Fan L, Chen BL, Li Q, Liu J, Tu JH, Hu DL, Liu ZQ, Zhou HH. Effects of the CYP2C9*13 allele on the pharmacokinetics of losartan in healthy male subjects. Xenobiotica. 2009;39:788–93. doi: 10.1080/00498250903134435. [DOI] [PubMed] [Google Scholar]
- 16.Zhou YH, Zheng QC, Li ZS, Zhang Y, Sun M, Sun CC, Si D, Cai L, Guo Y, Zhou H. On the human CYP2C9*13 variant activity reduction: a molecular dynamics simulation and docking study. Biochimie. 2006;88:1457–65. doi: 10.1016/j.biochi.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Kim HS, Lee SS, Oh M, Jang YJ, Kim EY, Han IY, Cho KH, Shin JG. Effect of CYP2C9 and VKORC1 genotypes on early-phase and steady-state warfarin dosing in Korean patients with mechanical heart valve replacement. Pharmacogenet Genomics. 2009;19:103–12. doi: 10.1097/FPC.0b013e32831a9ae3. [DOI] [PubMed] [Google Scholar]
- 18.Maekawa K, Fukushima-Uesaka H, Tohkin M, Hasegawa R, Kajio H, Kuzuya N, Yasuda K, Kawamoto M, Kamatani N, Suzuki K, Yanagawa T, Saito Y, Sawada J. Four novel defective alleles and comprehensive haplotype analysis of CYP2C9 in Japanese. Pharmacogenet Genomics. 2006;16:497–514. doi: 10.1097/01.fpc.0000215069.14095.c6. [DOI] [PubMed] [Google Scholar]
- 19.Bae JW, Kim MJ, Jang CG, Lee SY. Determination of meloxicam in human plasma using a HPLC method with UV detection and its application to a pharmacokinetic study. J Chromatogr B. 2007;859:69–73. doi: 10.1016/j.jchromb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Patrono C, Ciabattoni G, Pinca E, Pugliese F, Castrucci G, De Salvo A, Satta MA, Peskar BA. Low dose aspirin and inhibition of thromboxane B2 production in healthy subjects. Thromb Res. 1980;17:317–27. doi: 10.1016/0049-3848(80)90066-3. [DOI] [PubMed] [Google Scholar]
- 21.Dupont WD, Plummer WD. Power and sample size calculations for studies involving linear regression. Control Clin Trials. 1998;19:589–601. doi: 10.1016/s0197-2456(98)00037-3. [DOI] [PubMed] [Google Scholar]
- 22.Brogden RN, Heel RC, Speight TM, Avery GS. Piroxicam. A reappraisal of its pharmacology and therapeutic efficacy. Drugs. 1984;28:292–323. doi: 10.2165/00003495-198428040-00002. [DOI] [PubMed] [Google Scholar]
- 23.Polli JE, Bigora S, Piscitelli DA, Straughn AB, Young D. ‘Pavlovian’ food effect on the enterohepatic recirculation of piroxicam. Biopharm Drug Dispos. 1996;17:635–41. doi: 10.1002/(SICI)1099-081X(199610)17:7<635::AID-BDD981>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Turck D, Roth W, Busch U. A review of the clinical pharmacokinetics of meloxicam. Br J Rheumatol. 1996;35(Suppl. 1):13–6. doi: 10.1093/rheumatology/35.suppl_1.13. [DOI] [PubMed] [Google Scholar]
- 25.Turck D, Busch U, Heinzel G, Narjes H. Clinical pharmacokinetics of meloxicam. Arzneimittelforschung. 1997;47:253–8. [PubMed] [Google Scholar]
- 26.Rani S, Guttikar S, Rathod R, Cherian B, Nivsarkar M, Padh H. Determination of oral meloxicam pharmacokinetics parameters in Asian Indians: comparison with a German population. Saudi Pharm J. 2004;12:144–9. [Google Scholar]
- 27.Medvedovici A, Albu F, Georgita C, Mircioiu C, David V. A non-extracting procedure for the determination of meloxicam in plasma samples by HPLC-diode array detection. Arzneimittelforschung. 2005;55:326–31. doi: 10.1055/s-0031-1296867. [DOI] [PubMed] [Google Scholar]
- 28.Busch U, Heinzel G, Narjes H. The effect of cholestyramine on the pharmacokinetics of meloxicam, a new non-steroidal anti-inflammatory drug (NSAID), in man. Eur J Clin Pharmacol. 1995;48:269–72. doi: 10.1007/BF00198310. [DOI] [PubMed] [Google Scholar]
- 29.Engelhardt G, Bogel R, Schnitzer C, Utzmann R. Meloxicam: influence on arachidonic acid metabolism. Part 1. In vitro findings. Biochem Pharmacol. 1996;51:21–8. doi: 10.1016/0006-2952(95)02111-6. [DOI] [PubMed] [Google Scholar]
- 30.Engelhardt G, Bogel R, Schnitzer C, Utzmann R. Meloxicam: influence on arachidonic acid metabolism. Part II. In vivo findings. Biochem Pharmacol. 1996;51:29–38. doi: 10.1016/0006-2952(95)02110-8. [DOI] [PubMed] [Google Scholar]
- 31.Ogino K, Hatanaka K, Kawamura M, Katori M, Harada Y. Evaluation of pharmacological profile of meloxicam as an anti-inflammatory agent, with particular reference to its relative selectivity for cyclooxygenase-2 over cyclooxygenase-1. Pharmacology. 1997;55:44–53. doi: 10.1159/000139511. [DOI] [PubMed] [Google Scholar]
- 32.Tegeder I, Lotsch J, Krebs S, Muth-Selbach U, Brune K, Geisslinger G. Comparison of inhibitory effects of meloxicam and diclofenac on human thromboxane biosynthesis after single doses and at steady state. Clin Pharmacol Ther. 1999;65:533–44. doi: 10.1016/S0009-9236(99)70073-1. [DOI] [PubMed] [Google Scholar]
- 33.Odani A, Hashimoto Y, Otsuki Y, Uwai Y, Hattori H, Furusho K, Inui K. Genetic polymorphism of the CYP2C subfamily and its effect on the pharmacokinetics of phenytoin in Japanese patients with epilepsy. Clin Pharmacol Ther. 1997;62:287–92. doi: 10.1016/S0009-9236(97)90031-X. [DOI] [PubMed] [Google Scholar]
- 34.Kidd RS, Curry TB, Gallagher S, Edeki T, Blaisdell J, Goldstein JA. Identification of a null allele of CYP2C9 in an African-American exhibiting toxicity to phenytoin. Pharmacogenetics. 2001;11:803–8. doi: 10.1097/00008571-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Allabi AC, Gala JL, Horsmans Y. CYP2C9, CYP2C19, ABCB1 (MDR1) genetic polymorphisms and phenytoin metabolism in a Black Beninese population. Pharmacogenet Genomics. 2005;15:779–86. doi: 10.1097/01.fpc.0000174787.92861.91. [DOI] [PubMed] [Google Scholar]


