Abstract
AIMS
To characterize patients with urate measurements by urate-lowering therapy (ULT) use and to study the impact of allopurinol treatment on cardiovascular and mortality outcomes.
METHODS
A cohort study using a record-linkage database. The study included 7135 patients aged ≥60 years with urate measurements between 2000 and 2002 followed up until 2007. A Cox regression model was used. The association between urate levels, dispensed allopurinol and cardiovascular hospitalization and mortality was determined.
RESULTS
Six thousand and forty-two patients were not taking ULT and 45.9% of those (2774 of 6042) had urate concentrations ≤6 mg dl−1. Among 1035 allopurinol users, 44.7% (45.6% for men and 43.3% for women) reached target urate concentration. There was no significant increased risk of cardiovascular events for allopurinol users when compared with non-ULT users [adjusted hazard ratio (HR) 1.10, 95% confidence interval (CI) 0.95–1.26] and the non-ULT group with urate >6 mg dl−1 (adjusted HR 1.07, 95% CI 0.89–1.28). Within the allopurinol use cohort, cardiovascular event rates were 74.0 (95% CI 61.9–86.1) per 1000 person years for the 100 mg group, 69.7 (49.6–89.8) for the 200 mg group and 47.6 (38.4–56.9) for the ≥300 mg group. Compared with low-dose (100 mg) users, high-dose (≥300 mg) users had significant reductions in the risk of cardiovascular events (adjusted HR 0.69, 95% CI 0.50–0.94) and mortality (adjusted HR 0.75, 95% CI 0.59–0.94).
CONCLUSIONS
Less than 50% of patients taking allopurinol reached target urate concentration. Higher doses of allopurinol were associated with better control of urate and lower risks of both cardiovascular events and mortality.
Keywords: allopurinol, cardiovascular event, mortality, urate
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Guidelines recommend that the therapeutic goal of urate-lowering therapy (ULT) is to achieve a urate concentration of ≤6 mg dl−1.
High-dose allopurinol is associated with reduced cardiovascular events and mortality in heart failure patients.
WHAT THIS STUDY ADDS
Less than 50% of patients taking allopurinol reached target urate concentration.
Higher doses of allopurinol were associated with better control of urate and lower risks of both cardiovascular events and mortality in all patients on allopurinol treatment.
Introduction
Allopurinol is a widely used urate-lowering therapy (ULT) in patients with gout and hyperuricaemia. The therapeutic aim is to lower serum urate levels and prevent the precipitation of urate crystals. Clinical studies have shown that maintaining serum uric acid levels ≤6.0 mg dl−1 (or ≤0.36 µmol l−1) results in a reduction in the incidence of gout flares, decreased numbers of urate crystals in aspirated fluid from joints and reductions in size and number of tophi [1–3].
However, a significant limitation of this therapy is that the target urate level of ≤6.0 mg dl−1 is achieved in less than 50% of patients with gout receiving a standard dose of 300 mg of allopurinol [4]. Recently it has been shown that dose escalation may help to increase the success rate of lowering urate to target levels [5].
Uric acid is an independent predictor of mortality and cardiovascular events in both high-risk patients, such as those with heart failure [6] and diabetes [7], and the general population [8–10]. Allopurinol improves endothelial function, defects in which are known to be associated with progression of cardiovascular disease [11]. We have previously found that high-dose allopurinol is associated with reduced cardiovascular events and mortality in heart failure patients [12]. However, it is not clear whether this finding extends to all patients on allopurinol treatment. The present study is a cohort study in all patients having urate concentration measurements to determine the effect of allopurinol on cardiovascular hospitalization and mortality outcome.
Patients and methods
This study was carried out in the Tayside population of Scotland, using the MEMO (MEdicines MOnitoring Unit) record-linkage database [13]. The MEMO database covers a population that is geographically compact and serves about 400 000 National Health Service in Scotland (NHSiS) patients, 97% of whom are of white ethnic origin. The NHSiS is tax funded, free at the point of consumption and covers the entire population. In Tayside, there is almost no healthcare delivered outwith the NHS and there is a low rate of patient migration (less than 3% of patients aged 60 years or over left the Tayside region over a 5 year period of 2004–2008). In brief, the database contains several data sets including all dispensed community prescriptions, hospital discharge data, mortality data, biochemistry data and other data that are linked by a unique patient identifier, the community health index number. The data have been validated and made anonymous for the purposes of research as approved by the government-appointed guardians of patient confidentiality (the Tayside Caldecott Guardians). The project was also approved by the Tayside Research Ethics Committee.
Study design
This was a cohort study using a UK record-linkage database.
Study subjects
The study subjects included all patients from the hospitals and communities who had urate concentration measurements between January 2000 and December 2002 and were aged 60 years old or over (i.e. the entire cohort of those who had urate measured within the region). They were either resident in Tayside throughout the study period of January 2000 to December 2007 (i.e. a fixed cohort with complete follow-up for each patient) or died during the study period.
If patients had more than one urate measurement, the last measurement was used for the study. They were divided into three groups according to whether they were on ULT during the same period: (i) allopurinol use group; (ii) other ULT use group; and (iii) non-ULT use group. Within the allopurinol use group, subjects were further divided into three groups according to allopurinol daily dose (i.e. 100, 200 and ≥300 mg). There were only two patients who had a daily dose of 150 mg and they were categorized into the 200 mg group.
Patients with a diagnosis of cancer or hospitalization malignancy (International Classification of Disease (ICD) codes of C00-C97 and D00-D09) before the time of entry into the study were excluded from the analysis.
Definition of APTC end-point
The Antiplatelet Trialists' Collaboration (APTC) combined end-point of nonfatal myocardial infarction, nonfatal stroke and cardiovascular mortality was used in this study [14]. Outcomes were ascertained from the hospital discharge diagnosis data, Scottish Mortality Record-1 (SMR1) coded by primary International Classification of Diseases (ICD)-9 codes and ICD-10 code and from death certification from the General Register Office in Scotland.
Outcome variables
The outcomes of the study were the APTC combined end-point and all-cause mortality during the follow-up period until December 2007. The minimal follow-up for each subject was 5 years.
Covariates
Covariates included age, sex and Carstair's deprivation code (derived from patients' postcode and 2001 census data comprising social class, overcrowding, male unemployment and car ownership), prior hospitalization for cardiovascular disease, gout or hyperuricaemia, hypertension, renal disease and diabetes mellitus, cardiovascular drugs including aspirin, anticoagulants, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers, β-blockers, cardiac glycosides, thiazide diuretics, other diuretics, nitrates and calcium-channel blockers, diabetes drugs, nonsteroidal anti-inflammatory drugs (NSAIDs), steroids or colchicine at baseline or any time during the follow-up.
Statistical analysis
Data were summarized as means (standard deviations) for continuous variables and numbers of subjects (percentages) for categorical variables. Data distributions and Cox model assumption were checked before any statistical tests were carried out. Chi-square and t-tests were performed to determine significant differences. The Cox proportional hazard model was used to compare risks of cardiovascular event or mortality between the allopurinol daily dosage groups. To minimize confounding by indication, where patients with more severe illness are more likely to be treated with allopurinol, we calculated a propensity index for each subject, i.e. the likelihood that a patient would be treated by allopurinol based on known baseline characteristics. A propensity score matched cohort analysis was carried out for the high-dose allopurinol group vs. the low-dose allopurinol group. All statistical analyses were carried out using SAS (Statistical Analysis System) software version 9.1 (SAS Institute, Cary, NC, USA).
Prespecified sensitivity analysis
A number of sensitivity analyses were performed subsequent to the primary analysis of the data. Firstly, we matched for baseline urate level for the incident high-dose allopurinol users and non-ULT users to see whether high-dose allopurinol reduced events/mortality over non-ULT takers (sensitivity 1). Secondly, we used the first urate measurement instead of the last measurement for the analysis (sensitivity 2). Thirdly, we re-ran the analysis by excluding patients with renal disease (sensitivity 3). Fourthly, we repeated the analysis by removing patients with previous cardiovascular disease (sensitivity 4).
Results
There were 7135 study subjects. Of these, 1093 (15.3%) were taking ULT treatment. Among the ULT users, 94.7% (n = 1035) were taking allopurinol treatment and only 5.3% (n = 58) were on other ULT treatments (colchicine, sulfinpyrazone and probenecid). Table 1 shows the characteristics of patients who were on allopurinol and those who were not on any ULT treatment. There were no significant differences in age, urate concentration, social deprivation and previous hospitalizations for diabetes mellitus or hypertension between the allopurinol and non-ULT groups. However, patients in the allopurinol group were more likely to be male, had more co-morbidities and more concurrent drug use than patients in the non-ULT group (P < 0.05; Table 1). Table 2 shows the patient characteristics of the different allopurinol dose groups. Patients in the high daily dose group (432 patients were prescribed 300 mg or more but of these, only 12 patients were prescribed more than 300 mg allopurinol per day) were significantly younger, more likely to be male, had less renal disease and diabetes, less use of anticoagulants and diuretics and more use of NSAIDs than the lower dose groups.
Table 1.
Characteristics of patients by allopurinol use status
| Allopurinol group n (%) | Non-ULT group n (%) | P-value | |
|---|---|---|---|
| Number | 1035 | 6042 | – |
| Gender | |||
| Men | 647 (62.5) | 2843 (47.1) | <0.01 |
| Women | 388 (37.5) | 3199 (52.9) | – |
| Age (mean, SD) | 72.6 (7.8) | 72.4 (7.1) | 0.43 |
| Social deprivation | |||
| 1 | 64 (6.2) | 393 (6.6) | 0.60 |
| 2 | 192 (18.6) | 1175 (19.7) | – |
| 3 | 268 (26.0) | 1658 (27.8) | – |
| 4 | 102 (9.9) | 563 (9.4) | – |
| 5 | 116 (11.2) | 580 (9.7) | – |
| 6 and 7 | 290 (28.1) | 1101 (26.8) | – |
| Urate concentration (mg dl−1) | |||
| ≤6 | 463 (44.7) | 2774 (45.9) | 0.65 |
| 6.01–7 | 200 (19.3) | 1162 (19.2) | – |
| 7.01–8 | 140 (13.5) | 871 (14.4) | – |
| 8.01–9 | 104 (10.1) | 544 (9.0) | – |
| >9 | 128 (12.4) | 691 (11.4) | – |
| Previous hospitalization | |||
| Diabetes mellitus | 6 (0.6) | 17 (0.3) | 0.12 |
| Gout or hyperuricaemia | 19 (1.8) | 26 (0.4) | <0.01 |
| Renal disease | 77 (7.4) | 209 (3.5) | <0.01 |
| Cardiovascular disease | 100 (9.7) | 437 (7.2) | <0.01 |
| Hypertension | 8 (0.8) | 38 (0.6) | 0.59 |
| Drugs in concurrent use | |||
| Aspirin | 506 (48.9) | 2312 (38.3) | <0.01 |
| Anticoagulants | 127 (12.3) | 360 (6.0) | <0.01 |
| Angiotensin-converting enzyme inhibitors | 373 (36.0) | 1556 (25.8) | <0.01 |
| β-Blockers | 274 (26.5) | 1188 (19.7) | <0.01 |
| Cardiac glycosides | 131 (12.7) | 329 (5.5) | <0.01 |
| Calcium-channel blockers | 314 (30.3) | 1553 (25.7) | <0.01 |
| Thiazides | 106 (10.2) | 1068 (17.7) | <0.01 |
| Other diuretics | 513 (49.6) | 1503 (24.9) | <0.01 |
| Nitrates | 273 (26.4) | 936 (15.5) | <0.01 |
| Statins | 371 (35.9) | 1602 (26.5) | <0.01 |
| Nonsteroidal anti-inflammatory drugs | 328 (31.7) | 1490 (24.7) | <0.01 |
| Colchicine | 66 (6.0) | 1 (0.2) | <0.01 |
Table 2.
Characteristics of patients by daily dose in the allopurinol users
| 100 mg n (%) | 200 mg n (%) | ≥300 mg n (%) | P-value | |
|---|---|---|---|---|
| Number | 449 | 154 | 432 | – |
| Gender | ||||
| Men | 248 (55.2) | 96 (62.3) | 303 (70.1) | <0.01 |
| Women | 201 (44.8) | 58 (37.7) | 129 (29.9) | – |
| Age (mean, SD) | 73.2 (7.8) | 72.9 (7.8) | 71.8 (7.8) | 0.02 |
| Social deprivation | ||||
| 1 | 31 (6.9) | 8 (5.2) | 25 (5.8) | 0.56 |
| 2 | 88 (19.6) | 35 (22.7) | 69 (16.1) | – |
| 3 | 105 (23.4) | 39 (25.3) | 124 (28.4) | – |
| 4 | 49 (10.9) | 15 (9.7) | 38 (8.8) | – |
| 5 | 54 (12.1) | 18 (11.7) | 44 (10.2) | – |
| 6 and 7 | 121 (27.0) | 39 (25.3) | 130 (30.2) | – |
| Urate concentration (mg dl−1) | ||||
| ≤6 | 109 (24.3) | 72 (46.7) | 282 (65.3) | <0.01 |
| 6.01–7 | 104 (23.2) | 42 (27.3) | 54 (12.5) | – |
| 7.01–8 | 91 (20.3) | 13 (8.4) | 36 (8.3) | – |
| 8.01–9 | 66 (14.7) | 18 (11.7) | 20 (4.6) | – |
| >9 | 79 (17.6) | 9 (5.8) | 40 (9.3) | – |
| Previous hospitalization | ||||
| Diabetes mellitus | 2 (0.5) | 3 (2.0) | 1 (0.2) | 0.05 |
| Gout or hyperuricaemia | 10 (2.2) | 2 (1.3) | 7 (1.6) | 0.69 |
| Renal disease | 54 (12.0) | 15 (9.7) | 8 (1.9) | <0.01 |
| Cardiovascular disease | 46 (10.2) | 13 (8.4) | 41 (9.5) | 0.80 |
| Hypertension | 5 (1.1) | 1 (0.7) | 2 (0.5) | 0.53 |
| Drugs in concurrent use | ||||
| Aspirin | 208 (46.3) | 83 (53.9) | 215 (49.8) | 0.24 |
| Anticoagulants | 69 (15.4) | 18 (11.7) | 40 (9.3) | 0.02 |
| Angiotensin-converting enzyme inhibitors | 160 (35.6) | 55 (35.7) | 158 (36.6) | 0.95 |
| β-Blockers | 124 (27.6) | 42 (27.3) | 108 (25.0) | 0.66 |
| Cardiac glycosides | 63 (14.0) | 15 (9.7) | 53 (12.3) | 0.37 |
| Calcium-channel blockers | 147 (32.7) | 50 (32.5) | 117 (27.1) | 0.16 |
| Thiazides | 48 (10.7) | 15 (9.7) | 43 (10.0) | 0.91 |
| Other diuretics | 257 (57.2) | 76 (49.4) | 180 (41.7) | <0.01 |
| Nitrates | 125 (27.8) | 37 (24.0) | 111 (25.7) | 0.60 |
| Statins | 153 (34.1) | 65 (42.2) | 153 (35.4) | 0.19 |
| Nonsteroidal anti-inflammatory drugs | 144 (32.1) | 26 (16.9) | 158 (36.6) | <0.01 |
| Colchicine | 17 (3.8) | 6 (3.9) | 8 (1.9) | 0.19 |
Urate concentration target and allopurinol treatment
The proportion of patients reaching target urate concentrations (defined as ≤6 mg dl−1) in the allopurinol use group was 44.7% overall [45.6% of men (295 of 647) and 43.3% of women (168 of 388); P < 0.01]. Looking at the effects of daily dose of allopurinol, the proportions of patients with urate concentrations attaining target levels for men and women were 24.2 and 24.4% for 100 mg, 40.6 and 56.9% for 200 mg and 64.7 and 66.7% for 300 mg or over, respectively (Table 3). Women tended to be on lower doses of allopurinol compared with men [51.8 (201 on 100 mg) vs. 38.3% (248 on 100 mg), P < 0.01; Table 3]. The median age was 73 years old (interquartile range 67–79) for the 100 mg group, 73 years old (interquartile range 66–79) for the 200 mg group and 71 years old (interquartile range 65–77) for the 300 mg or over group.
Table 3.
Distribution of urate concentration (mg dl−1) in patients aged 60 years and over by allopurinol daily dose
| Daily dose (mg) | Urate concentration (mg dl−1) n (%) | Percentage of patients reaching target (≤6 mg dl−1) | ||||
|---|---|---|---|---|---|---|
| ≤6 | 6.01–7 | 7.01–8 | 8.01–9 | >9 | ||
| Men (n = 647) | ||||||
| 100 | 60 (20.34) | 60 (43.48) | 50 (60.24) | 41 (63.08) | 37 (56.06) | 24.19 |
| 200 | 39 (13.22) | 33 (23.91) | 9 (10.84) | 13 (20.00) | 2 (3.03) | 40.63 |
| ≥300 | 196 (66.44) | 45 (32.60) | 24 (28.91) | 11 (16.92) | 27 (40.91) | 64.69 |
| Total | 295 (100.00) | 138 (100.00) | 83 (100.00) | 65 (100.00) | 66 (100.00) | 45.60 |
| Average dose | 253 mg | 191 mg | 172 mg | 153 mg | 184 mg | |
| Women (n = 388) | ||||||
| 100 | 49 (29.17) | 44 (70.97) | 41 (71.93) | 25 (64.10) | 42 (67.74) | 24.38 |
| 200 | 33 (19.64) | 9 (14.52) | 4 (7.02) | 5 (12.82) | 7 (11.29) | 56.90 |
| ≥300 | 860 (51.19) | 9 (14.52) | 12 (21.05) | 9 (23.08) | 13 (20.97) | 66.67 |
| Total | 168 (100.00) | 62 (100.00) | 57 (100.00) | 39 (100.00) | 62 (100.00) | 43.30 |
| Average dose | 226 mg | 144 mg | 149 mg | 158 mg | 152 mg | |
Cardiovascular event rates and all-cause mortality
Comparison between the allopurinol cohort and non-ULT cohort
There were 1145 cardiovascular events and 1943 deaths in the non-ULT use group (with 732 cardiovascular events and 1218 deaths in those with a urate level >6 mg dl−1) and 273 cardiovascular events and 475 deaths in the allopurinol group during a median follow-up time of 5.6 years. The cardiovascular event rates were 38.5 per 1000 person years [95% confidence interval (CI) 36.3–40.7] for the non-ULT use group, 48.5 per 1000 person years (95% CI 45.1–51.9) for the non-ULT group with urate >6 mg dl−1 and 61.4 per 1000 person years (95% CI 54.3–68.4) for the allopurinol use group. There was no significant increased risk of cardiovascular events for allopurinol users when compared with the non-ULT users [adjusted hazard ratio (HR) 1.10, 95% CI 0.95–1.26] and the non-ULT group with urate >6 mg dl−1 (adjusted HR 1.07, 95% CI 0.89–1.28). A further propensity score matched analysis of allopurinol users vs. non-ULT users showed that the adjusted HR was 0.88, 95% CI 0.73–1.05. Within the non-ULT use group, there was a strong relationship between urate concentration and cardiovascular outcome (Figure 1). However, in the allopurinol use group, the risk of adverse outcome decreased with increased ULT dose (Figure 2), and the effect of allopurinol did not depend on the urate concentration. There was no clear relationship between urate concentration and cardiovascular outcome in Figure 2 except with the high-dose group, and this is probably due to small sample size and different cut-off limits used in the stratification of urate concentration.
Figure 1.
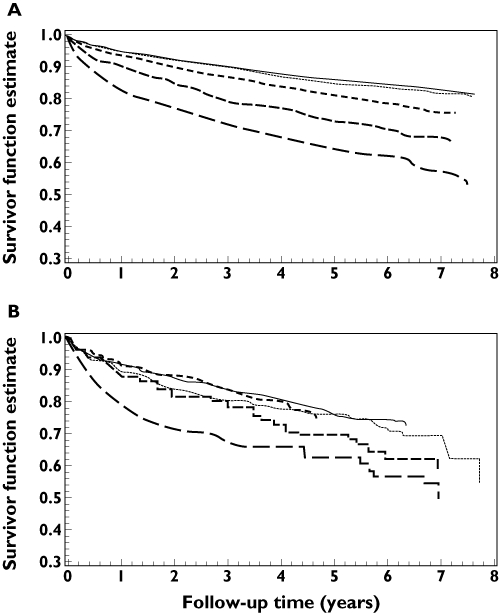
Kaplan–Meier plot of the Antiplatelet Trialists' Collaboration (APTC) event rate by urate concentration in non-users of urate-lowering therapy (non-ULT users; A) and in allopurinol users (B). ≤6 mg dl−1 ( ); 6.01–7 mg dl−1 (
); 6.01–7 mg dl−1 ( ); 7.01–8 mg dl−1 (
); 7.01–8 mg dl−1 ( ); 8.01–9 mg dl−1 (
); 8.01–9 mg dl−1 ( ); >9 mg dl−1 (
); >9 mg dl−1 ( )
)
Figure 2.
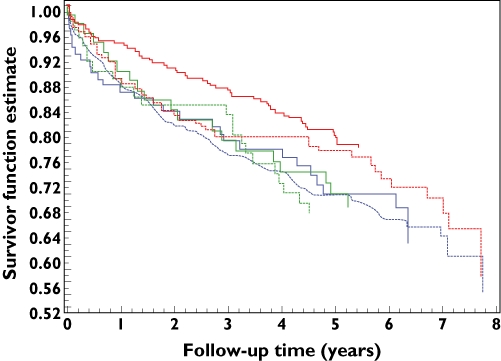
Kaplan–Meier plot of the APTC event rate by urate concentration and allopurinol daily dose. Dose = 100 mg: ≤6 mg dl−1 ( ); >6 mg dl−1 (
); >6 mg dl−1 ( ); Dose = 200 mg: ≤6 mg dl−1 (
); Dose = 200 mg: ≤6 mg dl−1 ( ); >6 mg dl−1 (
); >6 mg dl−1 ( ); Dose >= 300 mg: ≤6 mg dl−1 (
); Dose >= 300 mg: ≤6 mg dl−1 ( ); >6 mg dl−1 (
); >6 mg dl−1 ( )
)
Comparison within the allopurinol cohort
In the allopurinol use group, cardiovascular event rates were 61.3 per 1000 person years (95% CI 54.3–68.4), 74.0 per 1000 person years (95% CI 61.9–86.1) for the 100 mg daily group, 69.7 per 1000 person years (95% CI 49.6–89.8) for the 200 mg group and 47.6 per 1000 person years (95% CI 38.4–56.9) for the 300 mg and over group. Only one cardiovascular event occurred in the 12 patients who took more than 300 mg allopurinol per day. Table 4 shows the hazard ratios of cardiovascular disease and all-cause mortality in the different dose groups. The 300 mg and over group had significantly lower risk for both cardiovascular events and all-cause mortality than the 100 mg group (adjusted HR 0.69, 95% CI 0.50–0.94 and adjusted HR 0.75, 95% CI 0.59–0.94, respectively). The adjusted HR of cardiovascular events was 0.63, 95% CI 0.44–0.91, for a propensity score matched analysis of high-dose vs. low-dose allopurinol therapy. The death rates per 1000 person years in the allopurinol use group were 100.7 (95% CI 92.2–109.3) overall, 120.3 (95% CI 105.8–134.9) for the 100 mg group, 108.6 (95% CI 84.9–132.3) for the 200 mg group and 73.2 (95% CI 62.2–84.2) for the 300 mg and over group.
Table 4.
Univariate and multivariate hazard ratios (HR) for cardiovascular disease and all-cause mortality in patients who had urate concentration measurement
| Allopurinol daily dose | Univariate | Multivariate† | ||
|---|---|---|---|---|
| HR | 95% Confidence interval | HR | 95% Confidence interval | |
| Cardiovascular outcome | ||||
| 100 mg | 1.00 | – | 1.00 | – |
| 200 mg | 0.94 | 0.67–1.33 | 1.01 | 0.70–1.45 |
| ≥300 mg | 0.66 | 0.51–0.86** | 0.69 | 0.50–0.94** |
| All-cause mortality | ||||
| 100 mg | 1.00 | – | 1.00 | – |
| 200 mg | 0.92 | 0.71–1.20 | 0.92 | 0.70–1.21 |
| ≥300 mg | 0.63 | 0.52–0.77** | 0.75 | 0.59–0.94* |
P < 0.05
P < 0.01.
Adjusted for age, gender, social deprivation, urate concentration, gout/hyperuricaemia, renal disease, cardiovascular disease, diabetes mellitus, concurrent use of colchicine, nonsteroidal anti-inflammatory drugs, diabetic medication and cardiovascular drugs during the follow-up and number of cardiovascular prescriptions.
Results of sensitivity analyses
The risks of cardiovascular and mortality outcomes in the allopurinol group changed little when compared with the non-ULT group. The results of sensitivity analysis 1 (i.e. incident high-dose group vs. non-ULT group) showed that the adjusted HRs for cardiovascular events and all-cause mortality were 0.54 (95% CI 0.32–0.92) and 0.96 (95% CI 0.55–1.68), respectively. Compared with the 100 mg group, the HRs were 0.73 (95% CI 0.54–0.99) and 0.76 (95% CI 0.61–0.96) for sensitivity analysis 2, 0.68 (95% CI 0.49–0.95) and 0.76 (95% CI 0.59–0.96) for sensitivity analysis 3 and 0.62 (95% CI 0.44–0.86) and 0.75 (95% CI 0.58–0.97) for sensitivity analysis 4, respectively.
Discussion
In this study, we found that less than half of patients reached target urate concentration, and high-dose allopurinol was associated with reduced cardiovascular events and all-cause mortality. The results suggest that the benefit of high-dose allopurinol observed in the high-risk patients, such as those with diabetes or heart failure [6, 12, 15], could be extended to all patients who taking allopurinol. We have done a number of post hoc sensitivity analyses for the study to make sure our results were consistent. All the results were similar to the primary results except that the risk reduction of mortality for the incident high-dose group became statistically nonsignificant when compared with the non-ULT group in sensitivity 1.
Previous evidence has suggested that high-dose allopurinol may be associated with reduced risk of mortality and cardiovascular events through its patho-physiological pathway [16] by the following two mechanisms: higher doses of allopurinol may have an increased ability to improve endothelial function; and higher doses may also improve cardiac structure [17]. These two mechanisms are noteworthy because both endothelial function and cardiac function are independent predictors of mortality [7, 18, 19]. Other mechanisms could contribute, such as allopurinol reducing myocardial oxygen consumption for a particular stroke volume, and this has been confirmed in a randomized clinical trial in patients with chronic stable angina [20]. Notably, a recent crossover trial in 30 adolescents with newly diagnosed, never-treated stage 1 essential hypertension and serum uric acid levels ≥6 mg dl−1 showed that allopurinol reduced mean 24 h ambulatory systolic and diastolic blood pressures [21]. This implies that treatment with allopurinol may reduce blood pressure, which could potentially reduce the risk of cardiovascular disease. More recently, Luk et al. reported a significant survival benefit of allopurinol treatment in hyperuricaemic patients (adjusted HR 0.78, 95% CI 0.67–0.91) [22]. We also observed this benefit in our high-dose group when compared with non-ULT patients (sensitivity 1).
The EULAR (European League Against Rheumatism) guideline has recommended that the therapeutic goal of ULT is to achieve a urate concentration ≤6 mg dl−1[23]. The allopurinol dose should be increased progressively until a target urate concentration or maximal tolerated doses are achieved [24]. Our study showed that less than half of the patients achieved this goal. However, there was a strong dose–response relationship between allopurinol dose and urate concentration, with about two-thirds of patients who were on 300 mg daily dose achieving this goal. This highlights that dose adjustment should be considered in order to achieve the maximal benefit of the therapy in routine clinical practice. Our study result was consistent with the finding that the proportion of gout patients achieving their target urate concentration level (≤6 mg dl−1) was higher in those taking recommended doses (based on creatinine clearance) compared with those taking lower than recommended doses (38 vs. 19%; P < 0.01) [25]. A recent clinical trial in stroke patients also showed that there was a dose–response relationship for reduction of uric acid level by allopurinol at 6 weeks (reductions in uric acid were 0.14 mmol l−1 for the 300 mg group and 0.02 mmol l−1 for the 100 mg group) [26].
The strengths of the present study are as follows. (i) The study was a population-based cohort design with complete follow-up over the study period. Unlike clinical trials, which focus on highly selected patients, our study method allows for a ‘real-world’ population to be studied, representing all socio-economic groups and within a universal healthcare coverage scheme [27]. (ii) A complete biochemistry database was available for this population. (iii) MEMO only collects dispensed prescribing data and so primary noncompliance was eliminated as a source of error [28]. (iv) Our study also had details of concomitant drug treatment.
The limitations of this study are as follows. (i) MEMO does not have information on certain risk factors, such as lifestyle, i.e. body mass index, smoking, alcohol and exercise. These limitations are not unique to MEMO's record-linkage database; other databases also do not have routinely collected information on lifestyle or drug indication. However, we used a socio-economic deprivation score as a surrogate marker for this, as data from Scotland have shown that there is a significant correlation between body mass index, smoking and social deprivation [29]. (ii) We assumed that if a prescription was filled then patients would adhere to treatment, but we had no way of knowing whether or not subjects took the medication. (iii) Our study also did not account for whether subsequent to the urate measurement used in the analysis, patients might have had a change in their allopurinol dosage. (iv) Although we have used multivariate analyses, observational studies cannot exclude residual confounding factors. (v) We did not obtain information on adverse secondary effects of high-dose allopurinol use in this study. Further investigation is needed to clarify this issue.
In conclusion, more than half of the patients taking allopurinol did not reach the recommended target urate levels. High-dose allopurinol use was associated with a lower risk of cardiovascular events and all-cause mortality than low-dose allopurinol use, suggesting that higher doses of allopurinol may be of benefit by reducing cardiovascular disease or death.
Acknowledgments
Financial support: None.
Competing Interests
The University of Dundee and Professor AD Struthers have filed a patent for the use of xanthine oxidase inhibitors to relieve chest pain in angina. University of Dundee and Professor TM MacDonald have contracted to carry out a large study of allopurinol vs. comparators. LW, ISM and YC declare no competing interest. TMM received consultancy fees, honoraria and travel expenses in the past 3 years from Pfizer, Servier, Novartis, Wyeth, Kaiser Permanante, Takeda, Recordati, NiCox, Quintiles and Speedel. ADS received consultancy fees, honoraria and travel expenses from Pfizer in the past 3 years.
REFERENCES
- 1.Shoji A, Yamanaka H, Kamatani N. A retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: evidence for reduction of recurrent gouty arthritis with antihyperuricemic therapy. Arthritis Rheum. 2004;51:321–5. doi: 10.1002/art.20405. [DOI] [PubMed] [Google Scholar]
- 2.Li-Yu J, Clayburne G, Sieck M, Beutler A, Rull M, Eisner E, Schumacher HR., Jr Treatment of chronic gout. Can we determine when urate stores are depleted enough to prevent attacks of gout? J Rheumatol. 2001;28:577–80. [PubMed] [Google Scholar]
- 3.Perez-Ruiz F, Calabozo M, Pijoan JI, Herrero-Beites AM, Ruibal A. Effect of urate-lowering therapy on the velocity of size reduction of tophi in chronic gout. Arthritis Rheum. 2002;47:356–60. doi: 10.1002/art.10511. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Ruiz F, Alonso-Ruiz A, Calabozo M, Herrero-Beites A, García-Erauskin G, Ruiz-Lucea E. Efficacy of allopurinol and benzbromarone for the control of hyperuricaemia. A pathogenic approach to the treatment of primary chronic gout. Ann Rheum Dis. 1998;57:545–9. doi: 10.1136/ard.57.9.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinders MK, Haagsma C, Jansen TL, van Roon EN, Delsing J, van de Laar MA, Brouwers JRA. randomised controlled trial on the efficacy and tolerability with dose escalation of allopurinol 300-600 mg/day versus benzbromarone 100-200 mg/day in patients with gout. Ann Rheum Dis. 2009;68:892–7. doi: 10.1136/ard.2008.091462. [DOI] [PubMed] [Google Scholar]
- 6.Zoppini G, Targher G, Negri C, Stoico V, Perrone F, Muggeo M, Bonora E. Elevated serum uric acid concentrations independently predict cardiovascular mortality in type 2 diabetic patients. Diabetes Care. 2009;32:1716–20. doi: 10.2337/dc09-0625. Epub 2009 Jun 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anker SD, Doehner W, Rauchhaus M, Sharma R, Francis D, Knosalla C, Davos CH, Cicoira M, Shamim W, Kemp M, Segal R, Osterziel KJ, Leyva F, Hetzer R, Ponikowski P, Coats AJ. Uric acid and survival in chronic heart failure: validation and application in metabolic, functional, and hemodynamic staging. Circulation. 2003;107:1991–7. doi: 10.1161/01.CIR.0000065637.10517.A0. [DOI] [PubMed] [Google Scholar]
- 8.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971-1992. National Health and Nutrition Examination Survey. JAMA. 2000;283:2404–10. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 9.Chen JH, Chuang SY, Chen HJ, Yeh WT, Pan WH. Serum uric acid level as an independent risk factor for all-cause, cardiovascular, and ischemic stroke mortality: a Chinese cohort study. Arthritis Rheum. 2009;61:225–32. doi: 10.1002/art.24164. [DOI] [PubMed] [Google Scholar]
- 10.Niskanen LK, Laaksonen DE, Nyyssönen K, Alfthan G, Lakka HM, Lakka TA, Salonen JT. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: a prospective cohort study. Arch Intern Med. 2004;164:1546–51. doi: 10.1001/archinte.164.14.1546. [DOI] [PubMed] [Google Scholar]
- 11.Butler R, Morris AD, Belch JJ, Hill A, Struthers AD. Allopurinol Normalizes Endothelial Dysfunction in Type 2 Diabetics with Mild Hypertension. Hypertension. 2000;35:746–51. doi: 10.1161/01.hyp.35.3.746. [DOI] [PubMed] [Google Scholar]
- 12.Struthers AD, Donnan PT, Lindsay P, McNaughton D, Broomhall J, MacDonald TM. Effect of allopurinol on mortality and hospitalisations in chronic heart failure: a retrospective cohort study. Heart. 2002;87:229–34. doi: 10.1136/heart.87.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei L, Parkinson J, MacDonald TM. The Tayside Medicines Monitoring Unit (MEMO) In: Strom BL, editor. Pharmacoepidemiology. 4th edn. Chichester: John Wiley and Sons; 2005. pp. 323–36. [Google Scholar]
- 14.Antiplatelet Trialists' Collaboration. Collaborative overview of randomised trials of antiplatelet therapy – I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 15.Wei L, Fahey T, Struthers AD, MacDonald TM. Association between Allopurinol and mortality in heart failure patients: a long-term follow-up study. Int J Clin Pract. 2009;63:1327–33. doi: 10.1111/j.1742-1241.2009.02118.x. [DOI] [PubMed] [Google Scholar]
- 16.Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58:87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George J, Carr E, Davies J, Belch JJ, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114:2508–16. doi: 10.1161/CIRCULATIONAHA.106.651117. Epub 2006 Nov 27. [DOI] [PubMed] [Google Scholar]
- 18.Short RA, Johnson RJ, Tuttle KR. Uric acid, microalbuminuria and cardiovascular events in high-risk patients. Am J Nephrol. 2005;25:36–44. doi: 10.1159/000084073. Epub 2005 Feb 21. [DOI] [PubMed] [Google Scholar]
- 19.Aengevaeren WR. Beyond lipids – the role of the endothelium in coronary artery disease. Atherosclerosis. 1999;147(Suppl 1):S11–6. doi: 10.1016/s0021-9150(99)00250-6. Review. [DOI] [PubMed] [Google Scholar]
- 20.Noman A, Ang DS, Ogston S, Belch JJ, Struthers A. Effect of high-dose allopurinol on exercise in patients with chronic stable angina: a randomised, placebo controlled crossover trial. Lancet. 2010;375:2161–7. doi: 10.1016/S0140-6736(10)60391-1. Epub 2010 Jun 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300:924–32. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luk AJ, Levin GP, Moore EE, Zhou XH, Kestenbaum BR, Choi HK. Allopurinol and mortality in hyperuricaemic patients. Rheumatology. 2009;48:804–6. doi: 10.1093/rheumatology/kep069. Epub 2009 May 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang W, Doherty M, Pascual E, Zhang W, Doherty M, Pascual E. EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part I: diagnosis. Report of a task force of the Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT) Ann Rheum Dis. 2006;65:1301–11. doi: 10.1136/ard.2006.055251. Epub 2006 May 17. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richette P, Bardin T. Gout. Lancet. 2010;375:318–28. doi: 10.1016/S0140-6736(09)60883-7. Epub 2009 August 17. Review. [DOI] [PubMed] [Google Scholar]
- 25.Dalbeth N, Kumar S, Stamp L, Gow P. Dose adjustment of allopurinol according to creatinine clearance does not provide adequate control of hyperuricemia in patients with gout. J Rheumatol. 2006;33:1646–50. [PubMed] [Google Scholar]
- 26.Muir SW, Harrow C, Dawson J, Lees KR, Weir CJ, Sattar N, Walters MR. Allopurinol use yields potentially beneficial effects on inflammatory indices in those with recent ischemic stroke: a randomized, double-blind, placebo-controlled trial. Stroke. 2008;39:3303–7. doi: 10.1161/STROKEAHA.108.519793. Epub 2008 Oct 9. [DOI] [PubMed] [Google Scholar]
- 27.MacDonald TM, Morant SV, Robinson GC, Shield MJ, McGilchrist MM, Murray FE, McDevitt DG. Association of upper gastrointestinal toxicity of non-steroidal anti-inflammatory drugs with continued exposure: cohort study. BMJ. 1997;315:1333–7. doi: 10.1136/bmj.315.7119.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beardon PH, McGilchrist MM, McKendrick AD, McDevitt DG, MacDonald TM. Primary non-compliance with prescribed medication in primary care. BMJ. 1993;307:846–8. doi: 10.1136/bmj.307.6908.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scottish health Survey. 2003. Available at http://www.scotland.gov.uk/Resource/Doc/76169/0019729.pdf. (last accessed on 5 January 2011)


