Abstract
AIMS
The aim of the study was to determine the relative lung and systemic bioavailability of terbutaline.
METHODS
On separate days healthy volunteers received 500 µg terbutaline study doses either inhaled from a metered dose inhaler or swallowed as a solution with and without oral charcoal. Urine samples were provided at timed intervals post dosing.
RESULTS
Mean (SD) urinary terbutaline 0.5 h post inhalation, in 12 volunteers, with (IC) and without (I) oral charcoal and oral (O) dosing was 7.4 (2.2), 6.5 (2.1) and 0.2 (0.2) µg. I and IC were similar and both significantly greater than O (P < 0.001). Urinary 24 h terbutaline post I was similar to IC + O. The method was linear and reproducible, similar to that of the urinary salbutamol method.
CONCLUSIONS
The urinary salbutamol pharmacokinetic method post inhalation applies to terbutaline. Terbutaline study doses can replace routine salbutamol during these studies when patients are studied.
Keywords: inhalation, lung, terbutaline, urinary excretion
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
The relative bioavailability of salbutamol to the lung and body following inhalation can be identified by a urinary pharmacokinetic method.
WHAT THIS STUDY ADDS
The amount of terbutaline excreted in the urine during the first 30 min and over the 24 h period post inhalation represents the relative bioavailability of terbutaline to the lung and the body following an inhalation.
Terbutaline study doses can replace a routine salbutamol dose during studies in patients when comparing different inhalation methods.
Introduction
Urinary terbutaline has been shown to be an index of relative lung bioavailability following inhalation with the co-administration of charcoal [1]. However this method cannot be used with patients. Hindle & Chrystyn have shown that the amount of salbutamol excreted in the urine 0.5 h post inhalation represents the relative bioavailability to the lungs (an index of lung deposition) and the cumulative amount excreted upto 24 h post-inhalation identifies the relative bioavailability to the body (an index of systemic delivery) [2]. We have therefore extended this urinary salbutamol pharmacokinetic method to terbutaline.
Method
Ethics approval was granted from the University of Bradford Research Ethics Committee. All subjects were healthy and gave signed informed consent.
Twelve volunteers inhaled two 250 µg doses of terbutaline sulphate from a metered dose inhaler (AstraZeneca, UK) with (IC) and without (I) the co-administration of 20 g oral activated charcoal (10 g in 50 ml water before and after dosing). They also swallowed 500 µg of terbutaline sulphate dissolved in 20 ml water with (OC) and without oral charcoal (O). The order of the study days was randomized and urine samples were collected at 0.5, 1, 2, 4, 6, 12, and 24 h post dosing (Study A).
In study B each subject repeated I on five separate occasions and provided urine samples at 30 min post-inhalation. In study C, each subject randomly inhaled either one, two, three or four 250 µg doses from a terbutaline sulphate MDI. Urine samples were provided at 30 min post-inhalation and pooled for the next 24 h. There was a minimum of 7 days between each study dosing. Urinary terbutaline excretion was determined using a published assay [3].
Two way analysis of variance (anova) using SPSS V15.0 (SPSS Inc., Chicago, USA) was used to compare data from study A and the mean differences with 95% confidence intervals (CI) were calculated. The coefficient of variation (CV%) was calculated for intra and inter-subject variability from study B. Linear regression was used to identify the correlation coefficient (r) for the dose–response relationship of study C. To identify equivalence between the inhalation doses of study C, the % nominal dose for all 0.5 and 24 h excretions were log transformed. From the mean square error of the anova, using patients and inhalation method as the main factors, the mean ratio (90% CI) was calculated with respect to the two doses.
Results
Twelve healthy volunteers (six females), whose mean (SD) age, weight and height were 29.1 (4.6) years, 65.6 (12.7) kg and 166.8 (5.1) cm, respectively, with a FEV1 of 96.1 (3.7) % of predicted completed study A. No terbutaline was detected in all urine samples following oral dosing with the co-administration of oral charcoal (OC). Individual amounts of terbutaline excreted in the urine post I, IC and O dosing are shown in Figure 1. The mean (SD) amounts excreted (expressed as amounts and % nominal dose) are shown in Table 1. I and IC urinary excretion 0.5 h post-dosing were similar and both greater (P < 0.001) than O. The mean difference (95% CI) between I and IC was 7.2 (5.7, 8.7) µg. The mean (SD) combined excretion following IC and O over the 24 h post dosing was 233.5 (100.7) µg which is similar to I with a mean difference (95% CI) of 5.29 (85.26, −74.69) µg. The mean (SD) 0–24 h urinary terbutaline excretion following IC was 16.0 (7.9) % of the nominal dose (Table 1), and this represents the total lung bioavailability for the MDI.
Figure 1.
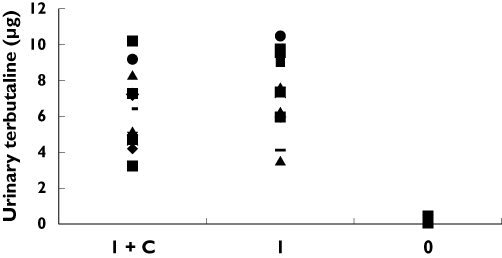
Individual amounts of terbutaline (in µg) excreted in the first 30 min post-inhaled (I), inhaled with charcoal (IC) and after oral dosing of 500 µg terbutaline.
Table 1.
Mean (SD) urinary terbutaline post dosing
| Amount in µg | % nominal dose | |||
|---|---|---|---|---|
| 0.5 h | 24 h | 0.5 h | 24 h | |
| Study A | ||||
| I | 7.4 (2.2) | 230 (86.2) | 1.8 (0.5) | 56.2 (21.0) |
| I + C | 6.5 (2.1) | 65.8 (32.6) | 1.6 (0.5) | 16.0 (7.9) |
| O | 0.2 (0.2) | 167.7 (68.1) | 0.04 (0.04) | 40.8 (16.6) |
| Study C | ||||
| One dose | 4.2 (2.1) | 103.9 (52.2) | 2.1 (1.0) | 50.6 (25.4) |
| Two doses | 8.9 (2.7) | 219.6 (67.4) | 2.2 (0.7) | 53.5 (16.4) |
| Three doses | 13.1 (3.1) | 320.6 (76.1) | 2.1 (0.5) | 52.1 (12.4) |
| Four doses | 18.3 (4.7) | 448.6 (115) | 2.2 (0.6) | 54.6 (14.0) |
In study B 12 (six females) healthy non-smoking subjects with a mean (SD) age, weight and height of 29.2 (4.7) years, 66.8 (13.3) kg and 168.2 (6.2) cm, respectively, and FEV1 96.8 (3.2) % of predicted completed the reproducibility study. The mean (SD) urinary terbutaline in the 0.5 h post inhalation was 10.4 (2.9) µg following the two doses and the mean (SD) intra-subject coefficient of variation was 9.2 (2.1) %. The mean (SD) inter-subject coefficient of variation was 28.8 (1.0) %.
Twelve (six females) healthy subjects with a mean (SD) age, weight and height of 28.5 (4.0) years, 67.3 (13.9) kg and 168.2 (6.2) cm, respectively, and FEV1 96.8 (3.2) % of predicted completed study C. Although there was inter-subject variability the amount of terbutaline excreted in the urine during the first 0.5 h and 24 h post dosing was linear (P < 0.002) with the dose. Table 1 summarizes the urinary terbutaline excretion. Using the nominal doses for the two doses compared with one, three and four doses the 0.5 h urinary terbutaline excretion was similar with mean ratios (90% CI) of 109.1 (90.3, 129.5), 99.7 (89.2, 107.1) and 93.4 (80.5, 106.6) %, respectively. Similar results were demonstrated for the cumulative 24 h excretions with mean ratios (90% CI) of 111.2 (91.5, 131.0), 100.2 (91.2, 109.3) and 95.8 (82.9, 108.7) %, respectively.
Discussion
The 0.5 h and the cumulative 24 h urinary terbutaline excretion post-inhaled, inhaled with charcoal and oral dosing are similar to the results previously published for the urinary salbutamol pharmacokinetic method [2]. Thus urinary terbutaline pharmacokinetics can be used to determine the relative bioavailability to the lung and the body following terbutaline inhalation. The comparable mean ratios in the dose–response arm of the study (part C) highlights that larger doses can be used for this index. This dose–response relationship was similar to that demonstrated for the urinary salbutamol pharmacokinetic method [4].
The amount excreted in the urine following inhaled with charcoal did not differ significantly from that following inhaled dosing during the first 0.5 h urine collection period. Hence there is no need to use oral charcoal for this urinary terbutaline pharmacokinetic method.
The 24 h terbutaline excreted post-oral dosing was relatively lower than that post inhalation even though the doses were the same. This might be due to the incomplete absorption of the oral dose compared with the inhaled dose. The mean of 16.5% cumulative excretion over the 24 h, following inhaled with charcoal dosing was comparable with the 14.8% previously reported for salbutamol [3] and the 16.0% for terbutaline reported by Borgstrom & Nilsson [1].
The inter-subject variability was high [28.8 (1.0) %] due to between subject variability of lung deposition together with their renal excretion. This variability between subjects is consistent with previous reports [2, 4, 5]. Also, the intra-subject variability was similar to that reported by Hindle & Chrystyn [2] when volunteers inhaled four doses.
Although terbutaline metered dose inhalers have been discontinued we have shown how the urinary salbutamol pharmacokinetic method can be used for nebulized therapy [6]. Hence we can apply this method by using terbutaline respiratory solution to compare different nebulizers in patients without having to withhold their salbutamol therapy. This method could be the solution to the ERS Consensus Guidelines comment for the need to compare different nebulized methods using patient studies rather than in vitro comparisons of the characteristics of the droplets in the nebulized doses [7].
In conclusion, the urinary salbutamol pharmacokinetic method following inhalation can be extended to terbutaline. This method could be used in patient studies without having to alter or withhold their salbutamol prescription.
Acknowledgments
Dr Abdelrahim was sponsored by the Egyptian government for his PhD studies. This study was part of his thesis.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Borgstrom L, Nilsson M. A method for determination of the absolute pulmonary bioavailability of inhaled drugs: terbutaline. Pharm Res. 1990;7:1068–70. doi: 10.1023/a:1015951402799. [DOI] [PubMed] [Google Scholar]
- 2.Hindle M, Chrystyn H. Determination of the relative bioavailability of salbutamol to the lung following inhalation. Br J Clin Pharmacol. 1992;34:311–5. doi: 10.1111/j.1365-2125.1992.tb05921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazhar S, Chrystyn H. New HPLC assay for urinary salbutamol concentrations in samples collected post inhalation. J Pharm Biomed Anal. 2009;50:175–82. doi: 10.1016/j.jpba.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Tomlinson HS, Corlett SA, Chrystyn H. Dose–response relationship and reproducibility of urinary salbutamol excretion during the first 30 min after an inhalation. Br J Clin Pharmacol. 2003;56:225–7. doi: 10.1046/j.1365-2125.2003.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hindle M, Chrystyn H, Newton DA. Relative bioavailability of salbutamol to the lung following inhalation using metered dose inhalation methods and spacer devices. Thorax. 1994;49:549–53. doi: 10.1136/thx.49.6.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silkstone VL, Corlett SA, Chrystyn H, Tomlinson HS. Determination of the relative bioavailability of salbutamol to the lungs and systemic circulation following nebulization. Br J Clin Pharmacol. 2002;54:115–9. doi: 10.1046/j.1365-2125.2002.01632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boe J, Dennis J, O'Driscoll B, Members of Task Force. Bauer T, Carone M, Dautzenberg B, Diot P, Heslop K, Lannefors L. European Respiratory Society Guidelines on the use of nebulizers: guidelines prepared by a European Respiratory Society Task Force on the use of nebulizers. Eur Respir J. 2001;18:228–42. doi: 10.1183/09031936.01.00220001. [DOI] [PubMed] [Google Scholar]


