Abstract
Objective: To examine the influence of cannabis use on long-term outcome in patients with a first psychotic episode, comparing patients who have never used cannabis with (a) those who used cannabis before the first episode but stopped using it during follow-up and (b) those who used cannabis both before the first episode and during follow-up. Methods: Patients were studied following their first admission for psychosis. They were interviewed at years 1, 3, and 5. At follow-up after 8 years, functional outcome and alcohol and drug abuse were recorded. Patients were classified according to cannabis use: 25 had cannabis use before their first psychotic episode and continuous use during follow-up (CU), 27 had cannabis use before their first episode but stopped its use during follow-up (CUS), and 40 never used cannabis (NU). Results: The 3 groups did not differ significantly in symptoms or functional outcome at baseline or during short-term follow-up. The CUS group exhibited better long-term functional outcome compared with the other 2 groups and had fewer negative symptoms than the CU group, after adjusting for potential confounders. For the CUS group, the effect size was 1.26 (95% confidence interval [CI] = 0.65 to 1.86) for functional outcome and −0.72 (95% CI = −1.27 to −0.14) for negative symptoms. All patients experienced improvements in positive symptoms during long-term follow-up. Conclusion: Cannabis has a deleterious effect, but stopping use after the first psychotic episode contributes to a clear improvement in outcome. The positive effects of stopping cannabis use can be seen more clearly in the long term.
Keywords: cannabis, first-episode psychosis, outcome, follow-up, discontinued use
Introduction
The consistent association between cannabis use and psychosis establishes cannabis as a harmful drug, especially in young people. Nevertheless, the nature of the association is not fully understood. Some genetic factors probably predispose individuals to cannabis use, especially in midadulthood.1 More importantly, however, cannabis use may act as an environmental factor that influences age at onset of psychosis,2,3 increasing the risk of developing psychosis both in the general population and, particularly, in vulnerable individuals,4 as well as potentially worsening the outcome of patients with psychosis.5–7 Moreover, a greater brain volume reduction has been demonstrated over a 5-year follow-up in patients with schizophrenia who are cannabis users compared with nonusers.8
First-episode patients with schizophrenia who use cannabis have been reported to have a poorer outcome than patients who do not use cannabis. In a systematic review, the majority of 7 follow-up studies of first-episode psychosis patients found that cannabis use was associated with poorer outcomes in the short term and medium term, although differences in outcome between cannabis users and nonusers were more modest when the studies controlled for the use of other drugs and baseline illness severity.9 In the 6-month follow-up study by Hides,10 there was a poorer outcome in patients with a first psychotic episode and cannabis use, after adjusting for use of other drugs and baseline symptoms. Similar results were found in another elegant study with a 15-month follow-up.11 Moreover, in a study comparing the effects of cannabis use in first-episode manic patients who began to use cannabis before vs after their first episode, the percentage of time in remission was inversely associated with the percentage of weeks with cannabis abuse.12
Findings so far may seem pessimistic for patients with comorbid cannabis use, and it can be inferred that one or more of the components of cannabis, such as delta-9-tetrahydrocannabinol,13,14 may produce permanent changes in the central nervous system. However, little is known about outcomes in patients who quit using cannabis and in those who continue to use cannabis after a first episode of psychosis. The issue is important as, according to recent studies, about half of the patients who use cannabis are able to cease cannabis use with usual psychopharmacological treatments6,15 and with specific programs designed to prevent abuse.16,17 Given the high prevalence of substance misuse in first-episode psychotic patients,18 the outcome in patients who continue to use cannabis and in those who stop using cannabis should be investigated and differentiated from that of patients who have never used cannabis. In a short-term follow-up study with 110 early-onset first-episode psychosis patients, a greater improvement in psychopathology was seen in those who ceased using cannabis.19
In a cohort of first-episode psychosis patients followed for 8 years, we hypothesized that patients who continued to use cannabis would have a poorer long-term outcome and that those who stopped using cannabis would have a similar outcome to never users. The aim of this prospective observational study was to examine the influence of cannabis use on long-term outcome in patients with a recent-onset first psychotic episode, comparing those patients who used and then stopped cannabis with those who never used cannabis and with those who continued to use cannabis during follow-up.
Methods
Subjects
Data were gathered on recent-onset first-episode psychosis patients admitted consecutively to a general hospital psychiatric ward between February 1997 and January 1999. The hospital provides psychiatric care to all inhabitants (300 000) of the catchment area around Vitoria in the Spanish Basque Country, regardless of their socioeconomic status. There is only one emergency room for psychiatric patients, and, if necessary, patients are hospitalized in the psychiatric department of the general hospital as there are no other inpatient units (public or private) in the area. Therefore, the study sample represents the entire population of patients with a first psychotic episode who need inpatient psychiatric treatment. Patients were enrolled in the study after providing informed consent.
A first psychotic episode was defined as the first time a patient displayed positive psychotic symptoms of delusions and/or hallucinations. All subjects were aged 15–65 years and met the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV)20 criteria for schizophreniform disorder, schizoaffective disorder, schizophrenia, delusional disorder, brief psychotic disorder, psychotic disorder not otherwise specified, bipolar disorder with psychotic features, or major depressive disorder with psychotic features. Subjects with mental retardation or organic brain disorders were excluded from the study, as were subjects with substance-induced psychotic disorders. No subjects had shared psychotic disorder. The DSM-IV axis I diagnosis was made using the Structured Clinical Interview for DSM-IV (SCID-I).21 Patients with positive symptoms lasting longer than 6 months were excluded from the study.
Assessments
After admission, patients with first-episode psychotic symptoms were assessed using a protocol that included the SCID-I, urine drug screens (including cannabis derivatives), and the following clinical scales: the Positive and Negative Symptoms Scale (PANSS),22 the Hamilton Depression Rating Scale (HDRS)-21,23 the Young Mania Rating Scale (YMRS),24 the Phillips Premorbid Adjustment Scale,25 and the Global Assessment of Functioning (GAF),26 which was used to assess general functioning. The original GAF instructions call for rating of symptoms or functioning, but as symptoms were already evaluated with other scales, the raters were trained to evaluate psychosocial functioning, as in some previous studies.27 The Addiction Severity Index (ASI) and SCID-I were administered at every visit (baseline and 1, 2, 3, 4, 5, and 8 y). This ASI is based on a 9-point scale (0–1, no real problem; 2–3, slight problem, substance abuse treatment probably not necessary; 4–5, moderate problem, some treatment indicated; 6–7, considerable problem, treatment necessary; and 8–9, extreme problem, treatment absolutely necessary). Using the information obtained from the patient, the key informant, the medical record, and drug screens, we determined whether the patient had used cannabis, how often the patient had used cannabis, and when that use had occurred. This information on cannabis use was grouped into 4 categories: no use, use, abuse, and dependence (table 1). The same method was used to establish use, abuse, or dependence of other drugs and of alcohol. Other relevant clinical and demographic variables were also collected, ie, gender, age, civil status, residential status, and comorbidity with alcohol and/or drug abuse. The evaluations were performed during a clinical interview that lasted about 90 minutes and pertained to the previous week. The interview was conducted by 2 psychiatrists (A.G.-P.; F.M.) who had good interrater reliability for SCID-I diagnoses (κ = 0.88) and for the scales used (PANSS, κ = 0.80; GAF, κ = 0.95; Phillips Premorbid Scale, κ = 0.77; YMRS, κ = 0.83; HDRS-21, κ = 0.79).
Table 1.
Information Used to Define the 4 Categories of Cannabis Use
| Cannabis Use | DSM-IV Abuse or Dependence Cannabis | ASI Cannabis Scores |
| Dependence | Meet minimal or more DSM-IV criteria for cannabis dependence | 8–9 |
| Abuse | Meet ≥1 criteria for cannabis abuse | 4–7 |
| Use | Abuse criteria but do not meet temporal criteria (at least 12 mo) or use 12 mo but not fulfilling any criteria of DSM-IV abuse | 2–3 |
| Not use | No significant symptoms | 0–1 |
Note: DSM-IV, Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition); ASI, Addiction Severity Index.
The research team (S.B.; P.V.) that determined drug abuse was blind to the clinical ratings; they discussed any inconsistencies and selected the most reliable source. Cannabis use was defined according to DSM-IV using SCID-I,21 ASI, and the information obtained from urine analyses.
At years 1, 3, and 5, patients were evaluated by direct interview using the same methods as those used at baseline, obtaining information using the GAF, PANSS positive, PANSS negative, YMRS, and HDRS-21. At year 8, only the GAF was completed, which evaluated psychosocial functioning from the previous visit to the present visit. Hospitalizations were recorded at all visits. Patients were also interviewed using the SCID-I and the ASI every second year (ie, at years 2 and 4), and urine analyses were performed. A patient was defined as a cannabis user if, during follow-up, they took cannabis at least 4 times in the previous year and once in the month before each interview. The first 5 years of follow-up ended in January 2004. Patients who could not be contacted during the study period were considered lost to follow-up.
Other drugs of use or abuse were most commonly cocaine and amphetamines. With regard to treatment for psychosis, all patients received pharmacological treatment, mainly low doses of atypical antipsychotics, according to clinical guidelines and irrespective of cannabis use status. The majority of patients were treated with monotherapy (61/92, 66.3%), and one-third were treated with 2 or more drugs at some point during the study (31/92, 33.7%). Use of polytherapy did not differ between the cannabis use groups (P = .483). Similarly, use of benzodiazepines did not differ among groups (P = .742).
Patients received standard care at their community mental health center after hospital discharge, usually one visit per month. They received family interventions, if required, and psychological support. More care was prescribed if needed, and hospitalizations were available for all patients who required them, irrespective of socioeconomic status. If immediate attention was needed, an emergency room was available on a 24-hour basis.
The study was approved by the Ethics Committee (Institutional Research Board) of Santiago Hospital.
Patient Classification
Patients were classified according to their cannabis use into 1 of 3 groups: (1) patients who had never used cannabis (NU); (2) patients who used cannabis before the baseline evaluation and continued to use it throughout the follow-up period (CU), including those who began using cannabis during follow-up (1 patient) and those who stopped cannabis use and then started it again (4 patients); and (3) patients who used cannabis before the baseline evaluation but stopped using it definitively during the follow-up period (CUS), including 1 patient who began to use cannabis in year 1 but stopped definitively in year 2. This classification was determined by integrating baseline and follow-up information on the same variables. Of the patients in the CUS group, 85.2% (23/27) stopped using cannabis in the first 3 years of follow-up and 14.8% (4/27) stopped in the fourth year.
The same procedure was used to classify patients into 3 groups for other drug use (other drugs NU, other drugs CU, other drugs CUS) and for alcohol abuse (alcohol abuse NU, alcohol abuse CU, alcohol abuse CUS), so the effect of such substance abuse could be taken into account in the analyses.
Statistical Analyses
The primary outcome variable was functional outcome measured using the GAF, and the secondary outcome variables were positive and negative symptoms measured using the PANSS. Baseline sociodemographic and clinical characteristics for the total sample and by cannabis use group were described using frequencies and percentages for the categorical variables and means and SDs or medians and interquartile ranges for the continuous variables, depending on the distributional characteristics of each variable. Comparisons among the 3 cannabis use groups were made using χ2 tests or the Fisher exact test for categorical data and the analysis of variance (ANOVA) test or Kruskal-Wallis test for continuous data, depending on whether normality and size assumptions held. Furthermore, any differences between patients followed up for less than 5 years vs those followed up for more than 5 years were analyzed using χ2 tests, Fisher exact tests, Student t tests and Mann-Whitney tests, as appropriate.
Analyses of Clinical and Functional Outcomes.
Differences in clinical and functional outcome throughout the follow-up period were assessed using mixed-effects models for the analyses of repeated-measures data. The response variables were the scores obtained for each scale (GAF, PANSS positive, and PANSS negative) at each evaluation. The approach comprised a 3-step procedure. First, to analyze the apparent individual effect that stopping cannabis use may have on outcome, univariate mixed effect models were fitted using time, cannabis group, and the interaction term. In the second step, the same modeling approach was used to assess the individual effect of other variables on the response variables; the variables evaluated were gender, age, civil status, as well as the effect of stopping other drug use and stopping alcohol abuse. When the ANOVA test for the corresponding terms had a P value <.1, they were included in the final model together with cannabis use. Finally, multivariate mixed-effect models were fitted, which included the cannabis group, the confounding variables selected in the second step, the continuous variable time, and the appropriate interaction terms. The CUS group was used as the reference category to facilitate interpretation and comparisons. All models included a random effect to account for the repeated-measure structure of the data and were fitted using maximum likelihood techniques assuming normality in the error term. Diagnosis of the models was carried out to assess whether or not the underlying normality assumptions held. Additionally, the same methodology was used to assess short-term outcome but considered only baseline values and data at 1-year follow-up.
All statistical analyses were carried out using the statistical package R 2.5.1.28
Results
Study Sample and Baseline Characteristics
Patients were included in the analysis if they were followed up for at least 5 years. Of the 127 patients with a first psychotic episode, 15 did not meet the inclusion criteria: They were excluded due to organic disease (n = 5), a diagnosis of drug-induced psychosis (n = 4), and not giving informed consent (n = 6). Of the 112 patients enrolled at baseline, 92 (82%) were followed up for at least 5 years and comprise the total study sample; 82 patients were interviewed at 8-year follow-up.
The mean (±SD) age of the patients at baseline was 29.78 ± 10.77 years (range, 16–61 y), and 45 (48.9%) were women. The sociodemographic characteristics and baseline clinical data of the study sample are given in table 2. Of the total sample (n = 92), 56% were cannabis users at baseline.
Table 2.
Sociodemographic and Baseline Clinical Data of the Total Sample and by Cannabis Use Group
| Total (n=92) | Never Used (n = 40) | Continued to Use (n = 25) | Used and Stopped (n = 27) | P Value | |
| Gender | |||||
| Female | 45 (48.9%) | 21 (52.5%) | 12 (48%) | 12 (44.4%) | χ2 = 0.43 (P = .807) |
| Male | 47 (51.1%) | 19 (47.5%) | 13 (52%) | 15 (55.6%) | |
| Age, y | 29.78 ± 10.77 | 35.43 ± 12.59 | 26.00 ± 5.99 | 24.93 ± 6.91 | F = 12.17 (P < .001) |
| Civil status | |||||
| Married | 16 (17.4%) | 12 (30%) | 1 (4%) | 3 (11.1%) | χ2 = 8.28 (P = .016) |
| Other | 76 (82.6%) | 28 (70%) | 24 (96%) | 24 (88.9%) | |
| Residency | |||||
| With relatives | 69 (75%) | 31 (77.5%) | 19 (76%) | 19 (70.4%) | Fisher (P = .29) |
| Alone | 8 (8.7%) | 4 (10%) | 0 (0%) | 4 (14.8%) | |
| Other | 15 (16.3%) | 5 (12.5%) | 6 (24%) | 4 (14.8%) | |
| Philips | 5.63 ± 3.01 | 5.60 ± 3.70 | 6.04 ± 2.30 | 5.30 ± 2.45 | F = 0.39 (P = .67) |
| PANSS positive | 24.76 ± 6.86 | 23.63 ± 6.88 | 25.44 ± 6.96 | 25.81 ± 6.74 | F = 0.99 (P = .37) |
| PANSS negative | 18.88 ± 9.39 | 19.13 ± 10.00 | 16.96 ± 9.06 | 20.30 ± 8.79 | F = 0.84 (P = .43) |
| GAF | 55.18 ± 13.17 | 56.13 ± 13.50 | 55.08 ± 14.04 | 53.89 ± 12.18 | F = 0.23 (P = .79) |
| Alcohol abuse | 49 (53.3%) | 11 (27.5%) | 19 (76%) | 19 (70.4%) | Fisher (P < .001) |
| Drugs | 30 (32.6%) | 3 (7.5%) | 16 (64%) | 11 (40.7%) | Fisher (P < .001) |
Note: PANSS, Positive and Negative Symptoms Scale; GAF, Global Assessment of Functioning.
The cannabis use groups consisted of a large group of never users (NU, 40/92, 43.5%), followed by 2 similarly sized groups of continuous users (CU, 25/92, 27.2%) and use and stop patients (CUS, 27/92, 29.3%). The latter 2 groups of cannabis users had similar severity of cannabis use at baseline: About 88.9% (24/27) of patients in the CUS group and 92% (23/25) of the CU group met the criteria for abuse or dependence at baseline, whereas only 7.4% (2/27) of the CUS group and 4% (1/25) of the CU group used cannabis without meeting the criteria for abuse or dependence. At year 8, the frequency of cannabis use, abuse, and dependence in the CU group was 16% (4/25), 56% (14/25), and 28% (7/25), respectively.
There were no significant differences between the cannabis use groups with respect to diagnoses (Fisher, P = .54), but some sociodemographic variables differed between groups. Patients in the NU group were more frequently married and were older compared with the CU and CUS groups (table 2). The differences between the NU and CU groups and between the NU and CUS groups were significant (P < .001 in both cases).
At baseline, there were no significant differences between the 3 groups in premorbid adjustment, positive or negative symptoms, or functioning. Rates of both alcohol abuse and other drug abuse were significantly higher in the CU and CUS groups, compared with the NU group (table 2).
Follow-up Data and Comparisons Between Patients With and Without Long-term Follow-up.
The mean duration of follow-up was 7.67 ± 0.94 years. The mean number of hospitalizations for the total sample was 3.39 ± 4.50. The total number of hospitalizations did not differ significantly among the 3 cannabis use groups (Kruskal-Wallis = 3.273, P = .195; means: 2.48 ± 3.04 for NU, 5.50 ± 7.51 for CU, 3.22 ± 2.80 for CUS).
The 3 cannabis use groups reduced their alcohol and drug intake during follow-up. At the end of the study, 19.5% (18/92) of the total sample were abusing alcohol, and 9.2% (10/92) were using other drugs. Medication adherence did not differ between the cannabis use groups (χ2 = 3.84, P = .06, for CUS vs NU; χ2 = 1.23, P = .268, for CUS vs CU).
There were no differences between patients followed up for at least 5 years and those with a shorter follow-up with respect to baseline symptoms and cannabis use group: age (t = −0.622, P = .535), gender (χ2 = 0.986, P = .321), civil status (Fisher, P = .750), residential status (Fisher, P = .409), and cannabis use (Fisher, P = .252).
Clinical and Functional Outcomes During Follow-up by Cannabis Use Group
Univariate Models.
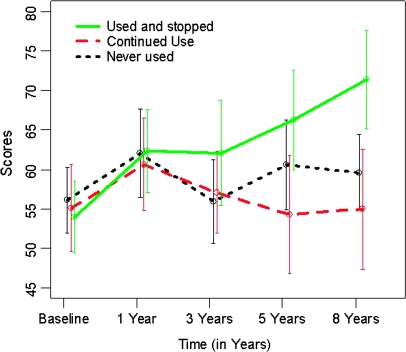
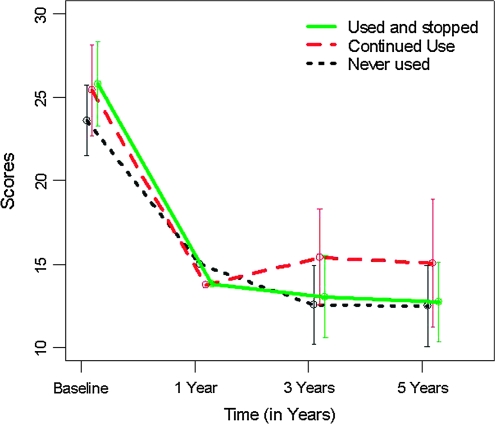
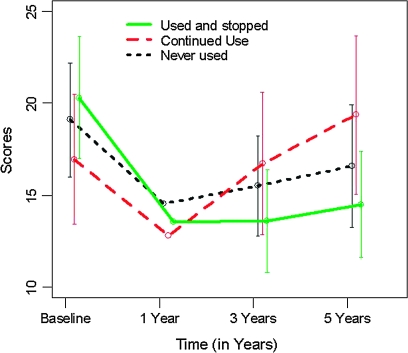
The evolution of the GAF, PANSS positive, and PANSS negative scores over time by cannabis use group are shown in figures 1–3. The univariate models fitted in the first step showed that the cannabis group variable was significant on both the GAF scores (F2,332 = 9.938, P < .001) and the PANSS negative scores (F2,241 = 4.828, P = .009) but not on the PANSS positive scores (F2,241 = 0.436, P = .647). These univariate analyses showed a significant improvement (reduction) from baseline in PANSS positive scores in all 3 cannabis use groups (all P‘s <.001) (figure 2), whereas only the CUS group showed a significant improvement (increase) in the GAF score (figure 1, CUS, b = 1.902, P < .001; CU, b = −0.271, P = .465; NU, b = 0.262, P = .370). There was a significant improvement (reduction) in the PANSS negative scores in the CUS group only, while the CU group showed a nonsignificant trend to increase (figure 3, CUS, b = −0.965, P = .012; NU, b = −0.392, P = .226; CU, b = 0.744, P = .069). Regarding the affective symptoms, the univariate models showed that the cannabis group variable was not significant on the HDRS-21 scores (F2,236 = 0.535, P = .587) or on the YMRS scores (F2,240 = 0.855, P = .427).
Fig. 1.
Global Assessment of Functioning (GAF) Outcome by Cannabis Use Group.
Fig. 2.
Positive and Negative Symptoms Scale (PANSS) Positive Symptoms Outcome by Cannabis Use Group.
Fig. 3.
Positive and Negative Symptoms Scale (PANSS) Negative Symptoms Outcome by Cannabis Use Group.
In the second step of the analysis, the univariate models showed that gender, age, and civil status did not have a significant effect on any of the outcome measures, whereas other drug use and alcohol abuse had a significant effect on the GAF score only (other drug use, ANOVA F2,332 = 5.772, P = .003; alcohol abuse, F2,332 = 6.597, P = .002). This indicated that stopping alcohol abuse and stopping other drug use significantly increased the GAF score (ie, improved functioning). On the other hand, quitting alcohol abuse or ceasing use of other drugs did not have a significant effect on the PANSS positive and PANSS negative scores (all ANOVA P values were greater than .1).
Multivariate Models.
The results of the multivariate mixed-effect models for PANSS positive, PANSS negative, and GAF evolution assessments are given in table 3.
Table 3.
Results Derived From the Mixed Effect Models of the Evolution for the Scales PANSS Positive, PANSS Negative, and GAF by Group
| Variable Parameter (*) | GAF |
PANSS Negative |
PANSS Positive |
|||
| β | P Value | β | P Value | β | P Value | |
| Baseline | ||||||
| Intercept (reference = CUS group for all variables) | 57.70 | <.001 | 17.61 | <.001 | 21.23 | <.001 |
| Cannabis | ||||||
| CU group (vs reference) | 1.60 | .70 | −2.64 | .21 | 0.01 | .99 |
| NU group (vs reference) | 3.78 | .36 | −0.42 | .82 | −0.78 | .61 |
| Other drugs | ||||||
| CU group (vs reference) | 0.03 | .99 | Not included | Not included | ||
| NU group (vs reference) | −0.56 | .88 | Not included | Not included | ||
| Alcohol abuse | ||||||
| CU group (vs reference) | −2.06 | .60 | Not included | Not included | ||
| NU group (vs reference) | −3.16 | .41 | Not included | Not included | ||
| Follow-up | ||||||
| Time (reference = CUS evolution for all variables) | 2.10 | <.001 | −0.96 | .01 | −2.15 | <.001 |
| Cannabis | ||||||
| Time* CU (vs reference) | −1.43 | .01 | 1.70 | .00 | 0.54 | .36 |
| Time* NU (vs reference) | −1.42 | .01 | 0.57 | .25 | 0.16 | .76 |
| Other drugs | ||||||
| Time* CU (vs ref) | −1.59 | .02 | Not included | Not included | ||
| Time* NU (vs reference) | 0.14 | .78 | Not included | Not included | ||
| Alcohol abuse | ||||||
| Time* CU (vs reference) | −0.58 | .31 | Not included | Not included | ||
| Time* NU (vs reference) | −0.73 | .17 | Not included | Not included | ||
Note: GAF, Global Assessment of Functioning; PANSS, Positive and Negative Symptoms Scale. Asterisk—the reference categories are continuous use and stop (CUS) groups for cannabis, for other drug use, and for alcohol abuse, whereas groups CU and NU refer to continuous use and never use for the same 3 substances.
For GAF, all substance use variables (cannabis, alcohol, and other drugs) were significant in the second step of the analyses and so were included in the final model. At baseline, the GAF scores did not differ significantly between the reference group (CUS) and the other 2 cannabis use groups (CU and NU). Similar nonsignificant results were obtained for the comparison between categories of other drug use and alcohol abuse at baseline (table 3). In contrast, the changes in GAF scores during follow-up differed significantly between the groups. For the cannabis use groups, the slope of the curve for the CUS group was positive and significant (b = 2.102, P < .001), indicating an increase in GAF over time. For the CU and NU groups, the GAF outcome was significantly different from the CUS group (b = −1.438, P = .014, for CU and b = −1.421, P = .016, for NU). In addition, continuous use of other drugs had a worse effect on the GAF outcome compared with stopping other drugs (b = −1.598, P = .025). No other significant between-group changes were observed (table 3). The effect size for the CUS group was 1.26 (95% confidence interval [CI] = 0.65 to 1.86).
For the PANSS negative subscale, only cannabis use was statistically significant in the univariate models, so the univariate and multivariate models did not differ. At baseline, there were no significant differences in PANSS negative scores between the cannabis use groups. During follow-up, however, there were between-group differences on this scale. In the CUS group, the PANSS negative score decreased significantly (b = −0.965, P = .012). The changes in the PANSS negative score of the CUS group differed significantly from those of the CU group (b = 1.709, P = .003) but not from those of the NU group (b = 0.573, P = .253). The effect size for the CUS group was −0.72 (95% CI = −1.27 to −0.14).
Finally, the analyses carried out to assess if the aforementioned differences among groups were also observed in the short term showed that stopping cannabis use had no influence on short-term outcome. The results were similar for all 3 outcome measures: The outcome was positive and highly significant for the whole sample regardless of cannabis use group (for GAF, F1,84 = 13.464, P < .001; for PANSS negative, F1,78 = 18.351, P < .001; for PANSS positive, F1,78 = 88.127, P < .001), whereas the cannabis group effect was not significant on any of the changes in scores (for GAF, F2,84 = 0.465, P = .630; for PANSS negative, F2,78 = 0.344, P = .710; for PANSS positive, F2,78 = 1.041, P = .358).
Discussion
The most important finding of this study is that the functional outcome of CUS patients improved more than that of NU patients. Moreover, the functional outcome of CUS patients improved progressively, while their negative symptoms diminished significantly. These differences were apparent in the long term but not after 1 year of follow-up. Consistent with our hypothesis, continued use of cannabis (CU) had a deleterious effect on outcome. CU patients only improved in their positive symptoms and showed a nonsignificant tendency to increase their negative symptoms. Taking all the findings together, and considering that the influence of other potential factors (such as other drug use and alcohol abuse) was also evaluated, we conclude that cannabis has a complex effect on patients with psychosis. In addition, both the negative effect of continuous cannabis use and the positive effects of cannabis cessation can be seen more clearly in the long term, with the differences between groups being more marked after 3 years of follow-up.
To our knowledge, this is the first study that has measured long-term functional outcome while controlling for use of alcohol and other drugs and in which those patients who are able to stop using cannabis are separated from those who cannot do so. In the only previous study that separated patients with recent-onset psychosis into groups based on duration of cannabis use, the patients who continued to use cannabis over the 4-year follow-up had a more chronic course of disease.29 In addition, those patients who used cannabis but stopped using it during follow-up had fewer negative symptoms, although the difference was not significant.29 Furthermore, in a group of children and adolescents with a first psychotic episode followed up for 6 months, quitting cannabis was associated with a better outcome.19 Other studies evaluating functional outcome in patients with a first psychotic episode and continued use of cannabis generally found a worse outcome in such patients.15,30
As in a previous study,31 cannabis users (both CU and CUS) were not distinguishable from nonusers with respect to premorbid adjustment or baseline symptoms. Although some previous studies found slight short-term increases in positive symptoms with cannabis use, these were generally modest when adjusted for other variables.9 As previously reported,15 we found a greater reduction in positive and negative symptoms and an improvement in functional outcome in the first year in all groups. However, only patients who used and then stopped using cannabis (CUS) had significant reductions in negative symptoms in the long term. Patients who continued to use cannabis (CU) did not experience reductions in their negative symptoms, so it is questionable that they used cannabis to diminish negative symptoms, as proposed by the self-medication hypothesis.32 Likewise, functional outcome was clearly improved after 8 years of follow-up in patients who stopped using cannabis. Indeed, there was a cannabis time improvement interaction with respect to functional outcome in the CUS group, with a progressive improvement during follow-up. Therefore, there seems to be an association between cannabis use and worse outcome, although the direction of the relationship (ie, causality) is not known due to the naturalistic design of the study.
Just over half of the total sample (56%) used cannabis at baseline, which is similar to the rate reported by Veen et al2 (52%) in their incident sample. Also, a comparative general population study found that cannabis use was similar in Arizona and Spain.33 Consistent with a previous study,6 we found that all but 2 of the cannabis users in our sample used cannabis before their first psychotic episode was diagnosed. However, the temporal pattern of cannabis use may vary from country to country; eg, in a UK study, a subgroup of patients stopped cannabis use before the baseline assessment.29 Also, in a well-designed study comparing different types of cannabis use, some patients began to use cannabis after their first episode of mania.12 Some of the differences noted between studies could be related to the age of the patients and to the sample size.
The majority of our sample was only able to quit using cannabis after 3 years of follow-up. Thus, it is probably only at this stage that a decision can be made about the need for specific treatment for patients who continue to use cannabis. Our results are in line with previous findings that almost half of the patients receiving psychopharmacological treatment are able to cease cannabis abuse after 2 years of treatment.15 Similarly, in the European Mania in Bipolar Longitudinal Evaluation of Medication study, cannabis use was overrepresented in first-episode manic patients, compared with patients who had experienced multiple episodes.34
The deleterious effect of continued cannabis use on symptoms and functional outcome could, in part, be explained as an additive effect of psychosis and cannabis use. Use of other drugs, mainly stimulants, also contributes to worsening functional outcome. It is difficult to understand why those patients who were able to quit cannabis use during follow-up had a better long-term functional outcome than those who never used cannabis. It could be that at initial hospitalization with a first psychotic episode, cannabis users have a phenotypic presentation that is as severe as nonusers (although they could have a lower genetic loading) but that quitting cannabis reveals a more benign disease after some years of follow-up, at least for functional outcome and negative symptoms. If so, continued use of cannabis causes even more damage than is currently thought. The progressive improvement of both symptoms and functioning during follow-up in the CUS group is not consistent with the theory that cannabis causes permanent and residual effects in the long term. As cannabis use among patients with a first psychotic episode is associated with a greater brain volume loss than in nonusers, it would be interesting to monitor the long-term brain volume of patients who cease using cannabis after a first psychotic episode. Curiously, in the study by Rais et al,8 half of the nonuser patients had used cannabis prior to their baseline evaluation. It would also be interesting to analyze separately those patients who were able to quit cannabis use and those who never used cannabis.
Furthermore, as already proposed,35 there might be some interaction between cannabis and antipsychotic treatment that results in reduced antipsychotic efficacy. Although such an interaction may play a role, we did not find any differences between cannabis use groups in the area where antipsychotics are most effective, ie, positive symptoms.
Several limitations of this study should be taken in consideration. First, the number of patients included was limited, which can produce type II errors (wrongly concluding that there is no difference between groups when there is a difference). Nevertheless, the rates of follow-up were very high despite the long follow-up period (8 y). Second, the results cannot be generalized to first-episode patients who do not need hospital admission or to patients with cannabis- or drug-induced psychosis. However, the use of a population-based inpatient sample gives strength to the results. Third, as the cannabis composition varies slightly between batches, it is impossible to determine the actual amount of cannabis consumed by each patient. We estimated the amount of cannabis taken from the information provided by the patients and their relatives in the semistructured interviews, and cannabis use was confirmed by urine analyses carried out at least once a year in the entire sample. This method, and the high rates of cannabis and alcohol use within the sample, suggests that bias in the estimates of cannabis use is minimal.
Despite these potential limitations, this is the first study of patients with recent-onset psychosis to consider patients who use and then stop using cannabis as a separate group, as well as controlling for comorbid use of alcohol and other drugs.
In conclusion, half of the patients with a first psychotic episode who used cannabis were able to stop using it during follow-up. Those patients who stopped using cannabis experienced a slow but steady improvement over time both in functional outcome and negative symptoms. On the other hand, patients who continued using cannabis had a trend to increasing negative symptoms over time and no improvement in functional outcome. All patients had improvements in positive symptoms over the long term.
Funding
Spanish Government (Health Research Funds FIS: PI052761, PI061416, RD06/0011/0014, FI05/00763; CIBER Network, which is an initiative of ISCIII CB07/09/0024, EC07/90435, EC07/90666, PI080873, PI081213, PI08/90224, PI08/90439); European Regional Development Funds; local grants (006111025, 2007/04).
Acknowledgments
We thank Fernando Mosquera, MD, for interviewing the patients. We also want to thank everyone who helped to make the realization of this article possible. A.G-.P. is responsible for a specific collaborative agreement between the Spanish Government (Instituto Carlos III) and the Basque Government to stabilize and intensify research in the National Health System (Boletin Oficial del Estado no. 21, January 24, 2007). The psychiatric research department in Santiago Apóstol Hospital is supported by the Ministry of Science and the Basque Government. These institutions had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The authors report no conflicts of interest in connection with the manuscript.
References
- 1.Kendler KSM, Schmitt EB, Aggen S, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Arch Gen Psychiatry. 2008;65:674–682. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veen ND, Selten JP, van der Tweel I, Feller WG, Hoek HW, Kahn RS. Cannabis use and age at onset of schizophrenia. Am J Psychiatry. 2004;161:501–516. doi: 10.1176/appi.ajp.161.3.501. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Pinto A, Vega P, Ibañez B, et al. Impact of cannabis and other drugs on age at onset of psychosis. J Clin Psychiatry. 2008;69:1210–1216. doi: 10.4088/jcp.v69n0802. [DOI] [PubMed] [Google Scholar]
- 4.Henquet C, Murray R, Linszen D, Van OJ. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005;31:608–612. doi: 10.1093/schbul/sbi027. [DOI] [PubMed] [Google Scholar]
- 5.Linszen DH, Dingemans PM, Lenior ME. Cannabis abuse and the course of recent-onset schizophrenic disorders. Arch Gen Psychiatry. 1994;51:273–279. doi: 10.1001/archpsyc.1994.03950040017002. [DOI] [PubMed] [Google Scholar]
- 6.Dekker N, de Hann L, Berq S, Gier M, Becker H, Linzen DH. Cessation of cannabis use by patients with recent-onset schizophrenia and related disorders. Psychopharmacol Bull. 2008;41(1):142–153. [PubMed] [Google Scholar]
- 7.Selten JP, Veen ND, Hoek HW, et al. Early course of schizophrenia in a representative Dutch incidence cohort. Schizophr Res. 2007;97(1–3):79–87. doi: 10.1016/j.schres.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Rais M, Cahn W, Van Haren N, et al. Excessive brain volume loss over time in cannabis-using first-episode schizophrenia patients. Am J Psychiatry. 2008;165:490–496. doi: 10.1176/appi.ajp.2007.07071110. [DOI] [PubMed] [Google Scholar]
- 9.Zammit S, Moore TH, Lingford-Hughes A, et al. Effects of cannabis use on outcomes of psychotic disorders: systematic review. Br J Psychiatry. 2008;193:357–363. doi: 10.1192/bjp.bp.107.046375. [DOI] [PubMed] [Google Scholar]
- 10.Hides LD. Psychotic symptom and cannabis relapse in recent-onset psychosis: prospective study. Br J Psychiatry. 2006;189:137–143. doi: 10.1192/bjp.bp.105.014308. [DOI] [PubMed] [Google Scholar]
- 11.Wade D, Harrigan S, Edwards J, Burgess PM, Whelan G, McGorry PD. Course of substance misuse and daily tobacco use in first-episode psychosis. Schizophr Res. 2006;81(2–3):145–150. doi: 10.1016/j.schres.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Strakowski SM, DelBello MP, Fleck DE, et al. Effects of co-occurring cannabis use disorders on the course of bipolar disorder after a first hospitalization for mania. Arch Gen Psychiatry. 2007;64:57–64. doi: 10.1001/archpsyc.64.1.57. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharyya S, Fusar-Poli P, Borgwardt S, et al. Modulation of mediotemporal and ventrostriatal function in humans by Delta9-tetrahydrocannabinol: a neural basis for the effects of Cannabis sativa on learning and psychosis. Arch Gen Psychiatry. 2009;66:442–451. doi: 10.1001/archgenpsychiatry.2009.17. [DOI] [PubMed] [Google Scholar]
- 14.Fusar-Poli P, Crippa JA, Bhattacharyya S, et al. Distinct effects of {delta}9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry. 2009;66:95–105. doi: 10.1001/archgenpsychiatry.2008.519. [DOI] [PubMed] [Google Scholar]
- 15.Addington J, Addington D. Patterns, predictors and impact of substance use in early psychosis: a longitudinal study. Acta Psychiatr Scand. 2007;115:304–309. doi: 10.1111/j.1600-0447.2006.00900.x. [DOI] [PubMed] [Google Scholar]
- 16.Edwards J, Elkins K, Hinton M, et al. Randomized controlled trial of a cannabis-focused intervention for young people with first-episode psychosis. Acta Psychiatr Scand. 2006;114:109–117. doi: 10.1111/j.1600-0447.2006.00783.x. [DOI] [PubMed] [Google Scholar]
- 17.Archie S, Rush BR, Akhtar-Danesh N, et al. Substance use and abuse in first-episode psychosis: prevalence before and after early intervention. Schizophr Bull. 2007;33:1354–1363. doi: 10.1093/schbul/sbm011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salvatore P, Baldessarini JR, Tohen M, et al. McLean-Harvard First-Episode project: two-year stability of DSM-IV diagnosis in 500 first-episode psychotic disorder patients. J Clin Psychiatry. 2009;70:458–466. doi: 10.4088/jcp.08m04227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baeza I, Graell M, Moreno D, et al. Cannabis use in children and adolescents with first episode psychosis: influence on psychopathology and short-term outcome (CAFEPS study) Schizophr Res. 2009;113:129–137. doi: 10.1016/j.schres.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 21.First MB, Spitzer RL, Williams JBW, Gibbon M. SCID-I: Version 2.0 for DSM-IV. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 22.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 25.Harris JG., Jr An abbreviated form of the Philips rating scale of premorbid adjustment in schizophrenia. J Abnorm Psychol. 1975;84:129–137. doi: 10.1037/h0076983. [DOI] [PubMed] [Google Scholar]
- 26.Endincott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 27.Martínez-Arán A, Vieta E, Colom F, et al. Cognitive impairment in euthymic bipolar patients: implications for clinical and functional outcome. Bipolar Disord. 2004;6:224–232. doi: 10.1111/j.1399-5618.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- 28. R Development Core Team (2007). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. ISBN 3-900051-07-0. http://www.R-project.org Accessed December 21, 2008. [Google Scholar]
- 29.Grech A, Van OJ, Jones PB, Lewis SW, Murray RM. Cannabis use and outcome of recent onset psychosis. Eur Psychiatry. 2005;20:349–353. doi: 10.1016/j.eurpsy.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Casper ES, Regan JR. Reasons for admission among six profile subgroups of recidivists of inpatient services. Can J Psychiatry. 1993;38:657–661. doi: 10.1177/070674379303801006. [DOI] [PubMed] [Google Scholar]
- 31.Stirling J, Lewis S, Hopkins R, White C. Cannabis use prior to first onset psychosis predicts spared neurocognition at 10-year follow-up. Schizophr Res. 2005;75(1):135–137. doi: 10.1016/j.schres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Peralta V, Cuesta MJ. Influence of cannabis abuse on schizophrenic psychopathology. Acta Psychiatr Scand. 1992;85:127–130. doi: 10.1111/j.1600-0447.1992.tb01456.x. [DOI] [PubMed] [Google Scholar]
- 33.Luengo MA, Kulis S, Marsiglia FF, et al. A cross-national study of preadolescent substance use: exploring differences between youth in Spain and Arizona. Subst Use Misuse. 2008;43:1571–1593. doi: 10.1080/10826080802241078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tohen M, Vieta E, Gonzalez-Pinto A, Reed C, Lin D for the European mania in Biopolar Longitudinal Evaluation of medication (EMBLEM) Advisory Board. Baseline characteristics and outcomes in patients with first episode or multiple episodes of acute mania [Epub ahead of print August 25, 2009] J Clin Psychiatry. doi: 10.4088/JCP.08m04580. PMID: 19709503. [DOI] [PubMed] [Google Scholar]
- 35.Lieberman JA, Kane JM, Safferman AZ, et al. Predictors of response to clozapine. J Clin Psychiatry. 1994;55(suppl B):126–128. [PubMed] [Google Scholar]