Abstract
Background: Schizophrenia is characterized by a lack of integration between thought, emotion, and behavior. A disruption in the connectivity between brain processes may underlie this schism. Functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI) were used to evaluate functional and anatomical brain connectivity in schizophrenia. Methods: In all, 29 chronic schizophrenia patients (11 females, age: mean = 41.3, SD = 9.28) and 29 controls (11 females, age: mean = 41.1, SD = 10.6) were recruited. Schizophrenia patients were assessed for severity of negative and positive symptoms and general cognitive abilities of attention/concentration and memory. Participants underwent a resting-fMRI scan and a DTI scan. For fMRI data, a hybrid independent components analysis was used to extract the group default mode network (DMN) and accompanying time-courses. Voxel-wise whole-brain multiple regressions with corresponding DMN time-courses was conducted for each subject. A t-test was conducted on resulting DMN correlation maps to look between-group differences. For DTI data, voxel-wise statistical analysis of the fractional anisotropy data was carried out to look for between-group differences. Voxel-wise correlations were conducted to investigate the relationship between brain connectivity and behavioral measures. Results: Results revealed altered functional and anatomical connectivity in medial frontal and anterior cingulate gyri of schizophrenia patients. In addition, frontal connectivity in schizophrenia patients was positively associated with symptoms as well as with general cognitive ability measures. Discussion: The present study shows convergent fMRI and DTI findings that are consistent with the disconnection hypothesis in schizophrenia, particularly in medial frontal regions, while adding some insight of the relationship between brain disconnectivity and behavior.
Keywords: fMRI, DTI, default mode network, medial frontal, behavioral correlates
Introduction
Schizophrenia is a mental illness characterized by a lack of integration between thought, emotion, and behavior.1 It is hypothesized that this schism may be the result of a disruption in the connectivity between brain regions that mediate appropriate information processing. Neuroimaging studies have confirmed that schizophrenia patients show abnormal interactions or connectivity problems between brain regions.2 The present study uses functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI) to evaluate both functional and anatomical connectivity in schizophrenia and their relationship to clinical symptoms and cognition.
Functional Connectivity in Schizophrenia
“Functional connectivity” is a term used to refer to correlations in brain activity among spatially distinct brain regions, either in a resting state or when processing external stimuli.3 Neuroimaging studies examining functional connectivity have traditionally examined changes in the blood-oxygen-level dependent (BOLD) response across brain regions during task performance. Such task-related brain activity typically accounts for only 0.5%–1% of brain activity. By contrast, it is estimated that 60%–80% of brain's energy demand is used to support neural communication during rest.4 Individual differences in this broader ongoing neural activity may reveal impairments present in schizophrenia patients before engaging in a specific task.
Based on positron emission tomography data, Raichle et al5 identified a default mode network (DMN) consisting of posterior cingulate, precuneus, inferior parietal lobule, and medial frontal cortex. It is hypothesized that activity within this network is heightened during rest and decreases or is suppressed during task performance. Recent fMRI studies that analyze resting-state correlations using independent component analysis (ICA) or related techniques have observed several reproducible resting-state networks. One prominent network is formally similar to the DMN originally identified by Raichle et al.5
fMRI data previously collected to investigate functional connectivity of the DMN in schizophrenia patients have been examined using 2 approaches. One is a hypothesis-driven data analysis approach that uses the mean time series from preselected of regions of interest (ROIs) as regressors in a correlation analysis in order to examine temporal correlations between chosen ROIs and/or between one selected ROI and the rest of the voxels in the brain.6,7 The second data analysis approach is a data-driven method that uses ICA to extract spatially and temporally organized networks.8,9 Each methodology, however, has its own caveats. While the ROI analysis may be biased by the selection of the predetermined seeds, ICA may fail to disclose a direct relationship between extracted components and a previously defined hypothesis.
In order to investigate functional connectivity of the DMN in schizophrenia patients more thoroughly, the present study adopted a comprehensive approach that incorporates both methodologies. This novel approach is a variant of a hybrid ICA proposed by McKeown.10 McKeown points out that the 2 methods typically used to analyze fMRI data (general linear model [GLM] or ICA) represent 2 extremes of data analysis with their own limitations. The GLM technique strongly relies on the assumption that the extracted time series is precise and may lack the characterization of other sources of variability in the fMRI signal that may be omitted from the model and possibly bias the results. The ICA technique may fail to disclose a direct means to test the relationship of a hypothesized time series model of interest. McKeown proposes a balanced “hybrid” approach that combines these 2 extremes by taking advantage of ICA's power to accurately characterize the intrinsic structure of the data and GLM's power to guide further analysis to test questions of interest. This more balanced approach eliminates the need of predetermined seeds (as required by GLM on ROI) while it directly tests for the relationship of a time series model of interest.
We used McKeown's hybrid ICA approach to (1) use ICA to identify and remove data-derived sources of noise and variability (movement, scanner-related noise or physiological artifacts) on an individual basis, (2) use ICA to identify a data-derived spatial map with its accompanying model or time series representing DMN fluctuations for each individual, and (3) enter time series of each individual's spatial map representing DMN fluctuations into a conventional GLM for statistical inference. The resulting individual spatial maps were used as the basis of further analysis investigating between-group differences in DMN fluctuations as well as correlations between DMN fluctuations and symptoms and signs in schizophrenia patients.
Anatomical Connectivity in Schizophrenia
In order to fully examine interactions or connectivity between brain regions, it is important that in addition to examining the functional connectivity of brain regions in gray matter, we examine the underlying anatomical system in white matter. White matter constitutes the underlying “wiring” between functional brain regions. White matter organization and integrity can be evaluated with DTI. DTI provides a quantitative method to assess the integrity of anatomical connectivity in white matter based on patterns of water diffusion in neural tissue.11 White matter integrity can be studied by examining the degree of fractional anisotropy (FA).
Previous studies investigating the congruence between functional and anatomical connectivity measures have reported strong agreement between resting fMRI and DTI connectivity. A study by Skudlarski et al12 collected resting fMRI and DTI data and generated functional and anatomical connectivity matrices in order to examine the agreement between resting fMRI and DTI connectivity. Skudlarski reported that there was a significant agreement between resting fMRI and DTI connectivity and that the agreement was parametric, such that the regions that had stronger functional connectivity during rest (ie, DMN regions) also showed increased anatomical connectivity. Another study13 that examined resting fMRI and DTI connectivity generated DMN regions from functional coherence maps and examined the white matter tracts that connected these regions. Greicius reported that particular regions that have strong functional connectivity within the DMN have direct anatomical projections (ie, posterior cingulate projects to medial frontal gyrus [MFG] and medial temporal lobe), suggesting an agreement of resting fMRI and DTI connectivity. Given the reports of these previous studies, the present study examines both functional as well as anatomical connectivity.
Disruptions in white matter integrity have been associated with schizophrenia.14 Evidence from DTI studies evaluating FA abnormalities in schizophrenia, however, is not consistent. Even though most studies have found lower FA values in the schizophrenia patients when compared with healthy controls, the spatial characteristics of this difference have varied. Studies have found lower FA in widespread white matter regions15,16 and more specifically in splenium of corpus callosum,17 anterior cingulum,18–21 posterior cinguluml,21 uncinate, and arcuate fasciculi.22
Relationship Between Brain Connectivity and Behavioral Measures
Because regions comprising the DMN (ie, MFG, anterior cingulate gyrus [ACG]) are also involved in behavior such as monitoring internal thoughts, emotion, and cognition, the organization of neural activation and white matter integrity in these regions may affect these aspects of behavior when the person is not at rest. Hence, if DMN regions have impaired connectivity at baseline, performance on behavioral tasks that also engage these DMN regions may be compromised. FMRI studies on healthy controls have found correlations between performance of a task requiring executive functioning and the strength of functional connectivity of regions within the DMN.23,24
Abnormalities in the intrinsic fluctuations of the DMN may be relevant to understanding clinical symptoms and poor cognitive performance of schizophrenia patients. Reported findings, however, have varied in the nature of this relationship, possibly due to differences in methodologies used as described above. Using ICA to extract a component that represented DMN fluctuations during auditory oddball task performance in schizophrenia patients, Garrity et al9 found that positive symptoms were positively correlated with task-related deactivation in the MFG, the precuneus, and the left middle temporal gyrus in schizophrenia patients. Using a seed in posterior cingulate to analyze resting fMRI from blocks of only rest, Bluhm et al6 found that both positive and negative symptoms had positive correlations with connectivity of posterior cingulate seed region and other regions of posterior cingulate, bilateral premotor areas, and bilateral regions of the temporal gyrus. Bluhm et al also found that symptoms were negatively correlated with connectivity of posterior cingulate seed region and right temporal lobe, right premotor areas, right middle and left superior temporal gyri, left inferior frontal gyrus, right dorsal ACG, and the brain stem. Using seeds in DMN regions to analyze resting fMRI data extracted from blocks of task performance, Whitfield-Gabrieli et al7 found that altered intrinsic fluctuations of the DMN during task performance were associated with severity of positive symptoms, as well as working memory performance.
Anatomical connectivity studies have also found a relationship between white matter integrity and behavioral measures such as symptoms and task performance. A preliminary DTI study found an inverse relationship between severity of negative symptoms and FA values in inferior frontal white matter.25 A recent study by Skelly and colleagues26 found an inverse relationship between severity of positive symptoms and FA values in uncinate fasciculus, sagittal stratum, and superior longitudinal fasciculus. White matter integrity has also been associated with performance in tasks that assess cognitive functioning such as attention and memory in schizophrenia patients.27–30
This literature suggests that brain connectivity abnormalities examined with fMRI and DTI are good indicators of patient impairments. Data from relatives of schizophrenia patients7 showed that altered resting functional connectivity, particularly in medial prefrontal cortex, may be related to risk for the disease, suggesting that this abnormality may be an endophenotype candidate associated with schizophrenia.
The purpose of this study was to integrate neuroimaging methods to further analyze functional and anatomical connectivity in schizophrenia patients. First, fMRI data were analyzed with a novel hybrid ICA approach to investigate functional connectivity differences in the DMN between schizophrenia patients and a control group. Second, DTI data were analyzed to look for anatomical connectivity differences between schizophrenia patients and a control group. Third, an exploratory analysis was conducted to look for associations between the connectivity measures and behavioral measures in schizophrenia. It is hypothesized that schizophrenia patients will show (a) reduced functional connectivity within the DMN when compared with the control group, (b) reduced anatomical connectivity when compared with the control group, and (c) significant correlations between brain connectivity and behavior.
Methods
Participants
Twenty-nine chronic schizophrenia patients (11 females, age: mean [M] = 41.3, SD = 9.3) and 29 healthy participants (11 females, age: M = 41.1, SD = 10.6) were recruited (see table 1 for detailed characteristics of participants). All participants provided written informed consent and received payment for the time they spent participating. Schizophrenia patients were diagnosed with the Structured Clinical Interview for DSM-IV31 and assessed for negative and positive symptoms using the Scale for the Assessment of Negative Symptoms (SANS) and Scale for the Assessment of Positive Symptoms (SAPS).32 Out of the 29 chronic schizophrenia patients: 16 were taking 1 atypical antipsychotic, 8 were taking 2 atypical antipsychotics, 1 was taking 1 typical antipsychotic, 1 was taking 1 atypical and 1 typical antipsychotic, 1 was taking 2 atypical and 1 atypical antipsychotics, and 2 were not taking any antipsychotic. Participants were excluded if they fulfilled the criteria for Alcohol or Substance Abuse or Dependence described in the Diagnostic and Statistical Manual of Mental Disorder, Fourth Edition within 3 months prior to scanning, significant medical illness, or head injury resulting in loss of consciousness exceeding 30 min. Education and occupational status were determined with the Socioeconomic Status questionnaire.33 Groups were matched by levels of education (t(55) = 0.06, P = .952) and occupational status (t(55) = 1.191, P = .239) of primary caregivers. Healthy participants, however, had significantly higher levels of education (t(55) = 5.01, P < .001) and occupational status (t(55) = 5.78, P < .001).
Table 1.
Mean (and SD) Demographic and Diagnostic Characteristics of Participants
| Age | Education | SES | SANS | SAPS | Chronicity of Illness | |
| Schizophrenia patients (N = 29) | 41.3(9.28) | 3.25(0.80) | 3.62(0.98) | 10.31(3.66) | 6.86(3.15) | 20.21(8.96) |
| Healthy controls (N = 29) | 41.1(10.6) | 2.28(0.75) | 5.46(1.40) | N/A | N/A | N/A |
Note: SES, Socioeconomic Status; SANS, Scales for Assessment of Negative Symptoms; SAPS, Scales for Assessment of Positive Symptoms.
Imaging Data Acquisition
Participants underwent a 6-min resting-state fMRI scan and were instructed to be as still as possible, keep their eyes closed, and stay awake. Images were collected using a Siemens Trio 3T scanner (Erlangen, Germany). Sequence parameters: gradient-echo echo-planar imaging (EPI) 180 volumes, repetition time (TR) = 2 s, echo time (TE) = 30 ms, flip angle = 90°, 34 contiguous AC-PC aligned axial slices, voxel size = 3.4 × 3.4 × 4.0 mm, matrix = 64 × 64 × 34. Participants were debriefed at the end of the scan to find out if they fell asleep. FMRI scan was repeated on the 1 participant who reported to have fallen asleep. A high-resolution T1-weighted anatomical image was acquired using a magnetization prepared rapid gradient-echo sequence. A field map acquisition was collected and used to correct the fMRI data for geometric distortion caused by magnetic field inhomogeneities (TR = 300 ms, TE = 1.91 ms/4.37 ms, flip angle = 55°, voxel size = 3.4 × 3.4 × 4.0 mm).
DTI data were acquired axially using a dual spin echo, single-shot, pulsed-gradient EPI sequence (TR = 8.3 s, TE = 86 ms, 64 slices, voxel size = 2 × 2 × 2 mm, 0 mm skip, field of view = 256 mm, b value = 1000 s/mm2, number of averages = 2). Diffusion was measured along 30 noncollinear directions.
In a separate session prior to the scan, schizophrenia subjects completed a cognitive battery that included tests to measure 2 domains of general cognitive abilities proposed by Carroll34: (1) attention and concentration and (2) memory. Each cognitive ability domain proposed by Carroll should reflect a more general measure of thought processes rather than specific performance in a given task. These domains were chosen for this study because of extensive schizophrenia literature showing impairments in these domains.35–39 In order to measure attention and concentration abilities, specific subtests of the Weschler Adult Intelligence Scale—III (digit symbol, digit span, symbol search, letter-number sequence) and the Delis-Kaplan Executive Function System (trails numbers-letters test, tower test) were administered. In order to measure memory abilities, the California Verbal Learning Test II and the Weschler Memory Scales were administered. The scores for each test were scaled and averaged within each domain, resulting in one composite score representing a measure of attention and concentration and one score representing a measure of memory ability for each subject.
FMRI Imaging Analysis
First-Level Analysis.
Preprocessing was conducted with FEAT (FMRIB's Software Library [FSL]). The following prestatistics processing was applied for each subject: first 3 volumes deleted to account for magnetization stabilization, motion correction, B0 field map unwarping, slice-timing correction, non-brain removal, spatial smoothing (with a 6-mm full-width half-maximum kernel), grand mean and intensity normalization, high-pass temporal filtering, registration of all images to standard space. Probabilistic independent component analysis (PICA) analysis was conducted for each individual to denoise individual data by removing components that represented noise such as head motion (which appear as “rim-like” artifacts around the brain), scanner artifacts (such as slice dropouts, high-frequency noise, and field inhomogeneities), and physiological noise (components with time courses corresponding to respiration and cardiac frequencies). Noise components were selected by spatial and temporal characteristics detailed in MELODIC (FSL) manual (http://www.fmrib.ox.ac.uk/fslcourse/lectures/melodic.pdf).
Default Mode Component Identification.
A hybrid ICA10,40 was performed on the denoised individual data. This approach uses ICA to derive a data-driven model that can be used to create a reference function for use in a GLM analysis.10,40 Multisession temporal concatenation41 was run on all 58 participants as a group, where a standard (space × time) ICA decomposition was conducted. PICA yielded 29 spatially independent components for all participants as a group. A DMN mask was created by generating ROIs (spheres of 10-mm radius) with center of mass coordinates from the literature including MFG,5,42,43 posterior parietal cortex,5,42,43 posterior cingulate cortex,5,42,43 and inferior temporal cortex.42,43 This DMN mask was then spatially correlated with all 29 components, and the component that had the highest spatial correlation was selected (see figure 1a).
Fig. 1.
(a) Axial images showing the default mode network component extracted from group independent component analysis for both patients with schizophrenia and healthy controls. See table 1 for coordinates. (b) Axial images showing the group DMN correlation map in the control group. (c) Axial images showing the group DMN correlation map in the schizophrenia group. All values at each voxel are z values. All images are radiologically oriented (left is right).
The following additional ICA analyses were conducted separately on the denoised individual data in order to verify that one group did not have a stronger DMN representation in the main ICA analysis described above. First, an ICA analysis was carried out in which group membership was specified for each participant. Based on this, MELODIC conducted a between-groups test to look for differences in DMN representation by group. Groups did not differ in their mean effect (or representation) to the DMN identified component (z(56) = 0.43, P < .34). Second, an ICA analysis was conducted for each group, from which a DMN component was identified. Spatial characteristics of the DMN component for each group were not significantly different.
The chosen DMN component provided a time course that represented DMN fluctuations for each individual. This individual time course served as a regressor and was used as a reference function for GLM analysis. For each subject, a voxelwise multiple regression analysis was conducted by correlating the BOLD signal change across time with the individual DMN time course. Resulting correlation maps were transformed into z-maps for score standardization. The end result was the generation of a statistical z spatial map, from now on referred to as the DMN correlation map, which quantifies the degree of the correlation of each voxel to the selected custom DMN model for each participant. A 1-sample t test was performed for each group using individual z-maps in order to illustrate a DMN for each group (figures 1b and 1c).
FMRI Group Differences.
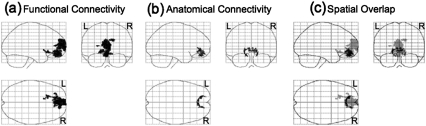
To compare functional connectivity between groups, we used a whole-brain approach to create statistical parametric maps of the t statistics (using FSL).44 These unpaired t statistics tested, at each voxel in the brain, for differences in coupling with the default mode, as indexed by DMN correlation coefficients. To protect for false positives, a threshold/cluster method derived from Monte Carlo simulations was applied to z maps.45 The resulting clustered and thresholded z maps were used to identify group differences in DMN correlation maps. Statistical parametric mapping was used for the purposes of illustrating summarized results in 1 figure from 3 outline views (glass brain; see figures 2 and 3). The center of mass for the regions showing between-group differences on these DMN correlation maps were then used to generate ROIs to test for clinical and behavioral correlates of DMN in schizophrenia patients.
Fig. 2.
Glass brain showing regions in which schizophrenia subjects had (a) lower functional connectivity in medial frontal gyrus and anterior cingulate gyrus manifested as lower correlation with DMN time course in these regions, (b) lower anatomical connectivity in anterior cingulate bundle manifested as lower FA values in this region than healthy comparison subjects, and (c) spatial overlap of altered functional and anatomical connectivity in schizophrenia patients; gray is functional connectivity and black is anatomical connectivity. All voxels displayed for P < .05 (2 tailed) corrected for multiple comparisons.
Fig. 3.
Glass brain showing regions in which schizophrenia subjects showed positive correlations of resting functional magnetic resonance imaging (fMRI) connectivity in region of interest with (a) positive symptoms, (b) negative symptoms, (c) attention and concentration general ability, and (d) memory general ability. Center of mass in (a) medial frontal cortex (7, 66, 22 and 8, 53, 5), left superior frontal cortex (−19, 48, 4), and right superior frontal cortex (17, 68, −15 and 23, 52, −18); (b) right anterior cingulate cortex (11, 38 −5); (c) right medial frontal cortex (15, 49, 4), left superior frontal cortex (15, 49, 4), and left anterior cingulate cortex (−17, 39, 12); (d) right medial frontal gyrus (31, 43, −11). Scatter plots show correlation between resting fMRI connectivity and behavioral measures.
DTI Imaging Analysis
Data were preprocessed with FMRIB's Diffusion Toolbox.46 Data were corrected for effects of head movement and eddy currents by using affine registration to a reference volume.47 Voxelwise statistical analysis of the FA data was carried out (tract-based spatial statistics in FSL48). First, raw DTI data were brain extracted,49 and then FA images were created by fitting a tensor model to the raw diffusion data. All subjects’ FA data were then aligned into a common space (Montreal Neurological Institute-152 brain).50 Next, the mean FA image was created and thinned to create a mean FA skeleton, which represents the centres of all tracts common to the group. Each subject's aligned FA data were then projected onto this skeleton and the resulting data fed into voxelwise cross-subject statistics.
Voxelwise statistics were conducted with Randomise from FSL.44 Randomise is a permutation program that enables modeling and inference using cluster-based thresholding and was used to test for between-group differences in FA. The Randomise option for cluster-based thresholding corrected for multiple comparisons that uses the null distribution of the max (across the image) “cluster mass” was used with t ≥ 2.00 (http://www.fmrib.ox.ac.uk/fsl/randomise/index.html).
Clinical and Behavioral Correlates.
An exploratory analysis examining the nature of the relationship between altered brain connectivity and behavioral measures in schizophrenia patients was conducted. Randomise (FSL)51 was used to examine the correlation between (1) behavioral measures and DMN correlation values and (2) behavioral measures and FA values in schizophrenia patients. Randomise was used to correct for multiple comparisons by using the null distribution of the max (across the image) cluster size (critical t value of 1.7).51 Covariates of interest included in the permutation test were total scores for clinical measure (SANS and SAPS) and cognitive ability (attention and concentration and memory). The resulting statistical maps show regions in which individual scores varied together with (1) DMN correlation maps and (2) FA maps.
Results
Functional Connectivity—fMRI Results
ICA on all participants as a group revealed 29 independent components, from which one showed the highest correlation with the DMN mask. This component included bilateral: posterior cingulate cortex, precuneus, anterior cingulate, parietal lobule, inferior and medial prefrontal cortex, middle temporal gyrus, and thalamus (see figure 1; table 2).
Table 2.
Regions in Default Mode Network That Showed Synchronized Fluctuation From Independent Component Analysis
| Anatomy | MNI Coordinates: x, y, z | |
| Posterior cingulate extending to precuneus | Right | 1, −57, 31 |
| Posterior cingulate extending to cingulate | Right | 1, −18, 36 |
| Anterior cingulate gyrus | Right | 1, 38, 12 |
| Parietal lobule | Right | 42, −54, 36 |
| Left | −34, -62, 36 | |
| Cingulate gyrus | Right | 2, 14, 36 |
| Inferior frontal gyrus | Left | −42, 14, 28 |
| Middle frontal gyrus | Right | 22, 58, -4 |
| Left | −26, 54, 0 | |
| Middle temporal gyrus | Right | 50, −50, 16 |
| Left | −50, −50, 16 | |
| Thalamus | Right | 10, −18, 8 |
| Left | −6, −14, 4 |
Note: MNI, Montréal Neurological Institute.
Unpaired t test results corrected for multiple comparisons on DMN correlation maps revealed significant between-group differences in a cluster comprised of MFG and ACG (x = 2, y = 54, z = 1). Schizophrenia patients’ DMN correlation maps showed reduced correlation with their DMN time courses in MFG and ACG when compared with healthy controls (see figure 2a). Mean MFG and ACG signal extracted from individuals’ data showed that there were no group differences in variance in these regions (Levene test for equality of variance t = 3.21, P = .79). A medial frontal ROI was derived from this group difference map to test for correlations with clinical and cognitive measures in schizophrenia patients.
Anatomical Connectivity—DTI Results
t Test results corrected for multiple comparisons revealed significant between-group differences in the anterior cingulate bundle (P < .05). Schizophrenia patients showed reduced FA in this region when compared with healthy controls (see figure 2b). Mean FA values for the schizophrenia patients (M = 6267.62, SE = 79.36) were significantly lower than mean FA values for healthy controls (M = 6630.70, SE = 64.59); (t(2,56) = 3.55, P = .001) within voxels shown in figure 2b. There were no regions in which schizophrenia patients had increased FA when compared with healthy controls.
Clinical and Cognitive Measures Results
Schizophrenia patients’ total scores for negative and positive symptom assessment were M = 10.31 and SD = 3.66 for SANS and M = 6.86 and SD = 3.15 for SAPS. Schizophrenia patients showed significantly lower scores in tasks measuring attention and concentration abilities (M = 9.11, SD = 1.78) when compared with the HC group (M = 12.43, SD = 1.69; t(56) = 7.28, P < .001). Schizophrenia patients showed significantly lower scores in tasks measuring memory ability (M = 13.80, SD = 3.37) when compared with the HC group (M = 19.01, SD = 2.80; t(56) = 6.39, P < .001).
Relationship Between Behavioral Measures and Brain Connectivity
Functional Connectivity.
There were significant associations between DMN correlation map and clinical symptoms within medial frontal ROI in schizophrenia patients. Results revealed positive correlations between local measures of connectivity in medial frontal ROI and behavior (negative and positive symptoms). Schizophrenia patients with higher SAPS scores showed increased values in DMN correlation maps in MFG (x = 7, y = 66, z = 22 and x = 8, y = 53, z = 5) and superior frontal gyrus (SFG; x = −19, y = 48, z = 4; x = 17, y = 68, z = −15; x = 23, y = 52, z = −18; see figure 3a). Schizophrenia participants with higher SANS scores showed increased values in DMN correlation maps in right ventral ACG (x = 11, y = 38, z = −5; see figure 3b).
Schizophrenia patients showed positive correlations between cognitive measures and DMN correlation maps. Attention and concentration scores of SZ participants varied together with DMN correlation maps in MFG (x = 15, y = 49, z = 4), SFG (x = −15, y = 52, z = −20) and in right dorsal ACG (x = −17, y = 39, z = 12; see figure 3c). Memory scores of SZ participants varied together with DMN correlation maps in right MFG (x = 31, y = 43, z = −11; see figure 3d).
Anatomical Connectivity.
Schizophrenia patients showed significant positive correlations (P < .05) between FA values in genu of corpus callosum and SANS scores (x = −11, y = −29, z = 6 and x = 11, y = −33, z = 4). Patients had no significant correlation between FA values and SAPS scores or cognitive measures.
Discussion
The main goal of the present study was to examine brain connectivity abnormalities in schizophrenia and its relationship to behavior. In order to investigate this, we integrated 2 neuroimaging techniques: fMRI to examine resting functional connectivity and DTI to examine anatomical connectivity in schizophrenia patients. In addition, we utilized an innovative hybrid ICA approach that incorporated both hypothesis-driven and data-driven components in order to address previous conflicting results of reduced,6 increased,7 or normal connectivity8 within the DMN of schizophrenia patients.
Both functional and anatomical connectivity results showed altered gray and white matter organization, respectively, manifested as reduced connectivity in schizophrenia patients. In addition, altered connectivity in medial frontal regions of schizophrenia patients was found to be associated with symptomatology and cognition.
FMRI data confirmed the existence of a fronto-parietal-occipital network in schizophrenia, and healthy controls comprised of brain regions that have previously shown synchronized intrinsic fluctuations during rest known as the DMN.5,43,52 Consistent with a previous study that used ICA to examine resting state networks in schizophrenia patients,8 groups did not differ in their representation within the chosen DMN network extracted from ICA. When comparing DMN correlation maps between groups, however, schizophrenia patients had reduced functional connectivity in medial frontal regions that included MFG and ACG when compared with healthy controls. Present results are consistent with other studies that have reported reduced connectivity of frontal regions and previously defined seeds within the DMN during rest.6,53 From a broader perspective, present results provide further evidence to the hypofrontality hypothesis in schizophrenia patients.54 Results from the present study, however, are inconsistent with previous studies that have found hyperconnectivity in frontal regions in schizophrenia patients.7 Inconsistency of findings may be related to the type of resting data collected (ie, pure resting scan versus resting periods extracted during task performance). The level of self-awareness and internal thought can be potentially different if an individual is either asked to only rest during the whole duration of the scan (ie, 6 min in the present study) or asked to rest during smaller blocks of time in between task performance (eg, Whitfield-Gabrieli et al7 collected resting data between performance of an N-back working memory task). Further research needs to be conducted examining functional connectivity in frontal regions in schizophrenia patients in which different types of resting scans are collected and compared.
When examining anatomical connectivity, schizophrenia patients showed reduced white matter organization in medial frontal regions when compared with healthy controls as has been reported in previous DTI studies.15,19–21 It is worthy of note that present results were internally consistent, in that connectivity abnormalities found in medial frontal regions were manifested both in gray and white matter. Previous studies that have examined brain connectivity using both DTI and fMRI have also found an agreement between anatomical and functional connectivity properties.12,13,55,56 Internal consistency between anatomical and functional connectivity abnormalities can imply 2 possible mechanisms: (1) that poor white matter integrity contributes to disturbances in functional connectivity in adjacent gray matter or (2) that consistently reduced neuronal activity in gray matter contributes to loss of myelination in white matter. A study examining the thickness of myelin sheath in mice has provided evidence for the latter mechanism by reporting a relationship between neuronal signaling and axons’ myelination.57 Michailov et al57 found that the thickness of myelin sheaths in axons depended on the degree of nerve signaling. Based on this evidence, it is possible that reduced white matter integrity found in medial frontal lobe in schizophrenia patients is the result of reduced neuronal firing in adjacent medial frontal cortex. Further research, however, needs to be conducted to fully understand specifics of this relationship.
Because results found in the present study were consistent across neuroimaging methods, reduced frontal connectivity may be a good candidate for a biological marker for developing schizophrenia. In order to further investigate this, however, individuals at high genetic risk for developing schizophrenia need to be examined. Results from previous studies examining brain connectivity on individuals at high genetic risk for schizophrenia, however, are inconsistent. While Whitfield-Gabrieli et al7 found abnormally increased levels of functional connectivity in frontal regions, Hoptman et al58 found reduced anatomical connectivity in frontal regions of individuals at high genetic risk for schizophrenia. Again, differences in methodologies may underlie discrepancies in results.
To investigate the effects of altered medial frontal brain connectivity on schizophrenia behavior, the present study examined the correlations between brain connectivity in this region and behavioral measures in schizophrenia participants. Significant correlations between schizophrenia-related psychopathology and DMN fluctuations found in the present study are consistent with previous studies in which schizophrenia participants with more severe symptomatology had increased MFG and ventral ACG connectivity.7,9 In other words, schizophrenia patients that deviate the most from healthy controls in terms of symptomatology were more similar to healthy controls in terms of increased medial frontal connectivity. The nature of this association may be related to the involvement of medial frontal regions in regulating internal thoughts. It has been reported that in healthy controls, decreased regulation of internal thoughts (manifested as reports of increased mind wandering) is associated with increased activation in the DMN.59 This concept can be applicable to present findings in that schizophrenia patients that had exaggerated disorganized internal thoughts (manifested as more severe schizophrenia-like symptoms such as hallucinations and delusions) show increased engagement of the DMN. This association seems to exist regardless of diagnosis because Whitfield-Gabrieli et al7 also reported that relatives of schizophrenia patients as well as healthy participants who had higher levels of schizophrenia-like symptoms also had increased MFG connectivity.
Positive correlations found between cognitive abilities assessed outside the scanner and functional connectivity in medial frontal regions suggest that functional connectivity of brain regions during rest mediate general cognitive performance in schizophrenia patients. One of the medial frontal regions particularly associated with attention and concentration abilities in the present study included a dorsal region of ACG. Dorsal ACG has been termed as the cognitive division of the ACG60 because it is often activated by cognitively demanding tasks that involve stimulus-response selection in the face of competing streams of information (ie, Stroop task). Dorsal ACG has been previously implicated in cognitive abilities that require attention and concentration such as conflict monitoring,61–63 error detection,64 and response selection.65,66
It is interesting to note that, in the present study, ventral and dorsal ACG regions were found to be associated with different aspects of behavior in schizophrenia patients. While ventral ACG was found to be positively correlated to severity of negative symptoms, dorsal ACG was found to be positively correlated with attention and concentration composite scores. A study that specifically examined the functional connectivity of ACG during rest67 proposed that ACG's connections to other brain regions depend on which ACG subdivision is involved. While ventral/rostral portions of the ACG were found to be functionally correlated to regions mediating affective processes in amygdala, hippocampus, ventromedial prefrontal cortex, and posterior cingulate cortex, more dorsal regions of ACG were found to be functionally correlated to regions mediating attentional processes in dorsolateral and inferior prefrontal cortices and sensorimotor processes in frontoparietal cortices.67 Findings from the current study follow this distinction in that ventral ACG was associated with emotional aspects of behavior (negative symptomatology) and dorsal ACG was associated with cognitive aspects of behavior (attention and concentration).
The MFG was also positively associated with memory abilities in schizophrenia patients. Our results are consistent with previous studies that have found positive associations between working memory performance and strength of MFG functional connectivity during rest.23 Present results, however, are not consistent with a previous fMRI study by Whitfield-Gabrieli et al7 examining brain connectivity during rest and during a working memory task (N-back).7 Whitfield-Gabrieli et al7 found that increased resting activity in MFG is associated with better working memory performance in schizophrenia patients and their relatives. As mentioned before, inconsistency in results may be related to differences in methodology. While both Whitfield-Gabrieli and Hampson used seeded ROIs in medial frontal cortex and posterior cingulate cortex, they differed in that the resting data were either extracted from scans that included an N-back task alternated with blocks of rest7 or collected in scans separate from N-back task collection.23 The context in which resting data is collected may affect ongoing intrinsic brain activity because in the former, participants may be consolidating performance during previous trials or preparing for future performance, while in the latter, the participant is not involved in a task at all during the whole resting scan. These methodological differences need to be addressed in future studies.
There are limitations to this study which should be considered and addressed in future work. First, while between-group DMN connectivity differences were found, these differences could be further addressed by measuring brain connectivity during task performance in the same scanning session. In this study, however, we focused on examining connectivity during rest because we were particularly interested in understanding connectivity differences when an individual is not engaged in task performance. Second, the present study did not specifically address whether altered functional and anatomical brain connectivity in schizophrenia patients precedes or is subsequent to manifestation of disease-related processes such as active symptom subtypes, illness duration, and/or medication effects. The inclusion of individuals at risk for the disease such as relatives of patients would help to address these possible effects. Finally, groups differed in their level of education and occupation. Because many individuals with schizophrenia have their education interrupted due to the manifestation of the illness, it is difficult to find a large sample of patients and controls that are matched on education and occupation levels. In the present study we conducted a separate analysis to look for between group differences in functional and anatomical connectivity with these variables as regressors of no interest. Data still showed differences between groups in medial frontal regions, but in smaller clusters that did not survive correction for multiple comparisons. Future studies should try to recruit groups that are matched in education and occupation.
The present study shows convergent fMRI and DTI findings that are consistent with the disconnection hypothesis in schizophrenia, particularly in medial frontal regions, while adding some insight to the relationship between altered brain connectivity and behavior. First, there is reduced functional connectivity in medial frontal regions during rest in schizophrenia patients. Second, reduced anatomical connectivity is also found in white matter adjacent to these medial frontal regions in schizophrenia patients. Third, altered connectivity in medial frontal regions may underlie schizophrenia psychopathology as well as poor cognitive performance characteristic to schizophrenia.
Funding
National Institute of Mental Health (R21MH79262, MH-060662 to K.O.L., A.W.M. III); the General Clinical Research Center (M01 RR00400); the Center for Magnetic Resonance Research (BTRR—P41 RR008079 and P30 NS057091); the Mind Research Network, Albuquerque, NM (to K.O.L, A.W.M. III).
Acknowledgments
The authors would like to thank the subjects who participated in this study. The authors report no potential conflicts of interest.
References
- 1.Bleuler E. Dementia Praecox or the Group of Schizophrenias. New York: International Universities Press (Original work published 1911); 1950. [Google Scholar]
- 2.Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- 3.Zhou D, Thompson WK, Siegle G. Matlab toolbox for functional connectivity. Neuroimage. 2009;47:1590–1607. doi: 10.1016/j.neuroimage.2009.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–1099. [DOI] [PubMed] [Google Scholar]
- 5.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bluhm RL, Miller J, Lanius RA, et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33:1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 10.McKeown MJ. Detection of consistently task-related activations in fMRI data with hybrid independent component analysis. Neuroimage. 2000;11:24–35. doi: 10.1006/nimg.1999.0518. [DOI] [PubMed] [Google Scholar]
- 11.Lim KO, Helpern JA. Neuropsychiatric applications of DTI—a review. NMR Biomed. 2002;15:587–593. doi: 10.1002/nbm.789. [DOI] [PubMed] [Google Scholar]
- 12.Skudlarski P, Jagannathan K, Calhoun VD, Hampson M, Skudlarska BA, Pearlson G. Measuring brain connectivity: diffusion tensor imaging validates resting state temporal correlations. Neuroimage. 2008;43:554–561. doi: 10.1016/j.neuroimage.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis KL, Stewart DG, Friedman JI, et al. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60:443–56. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- 15.Ardekani BA, Nierenberg J, Hoptman MJ, Javitt DC, Lim KO. MRI study of white matter diffusion anisotropy in schizophrenia. Neuroreport. 2003;14:2025–2029. doi: 10.1097/00001756-200311140-00004. [DOI] [PubMed] [Google Scholar]
- 16.Minami T, Nobuhara K, Okugawa G, et al. Diffusion tensor magnetic resonance imaging of disruption of regional white matter in schizophrenia. Neuropsychobiology. 2003;47:141–145. doi: 10.1159/000070583. [DOI] [PubMed] [Google Scholar]
- 17.Foong J, Maier M, Clark CA, Barker GJ, Miller DH, Ron MA. Neuropathological abnormalities of the corpus callosum in schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2000;68:242–244. doi: 10.1136/jnnp.68.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Z, Wang F, Cui L, et al. Abnormal anterior cingulum in patients with schizophrenia: a diffusion tensor imaging study. Neuroreport. 2003;14:1833–1836. doi: 10.1097/00001756-200310060-00015. [DOI] [PubMed] [Google Scholar]
- 19.Kubicki M, Westin CF, Nestor PG, et al. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol Psychiatry. 2003;54:1171–1180. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang F, Sun Z, Cui L, et al. Anterior cingulum abnormalities in male patients with schizophrenia determined through diffusion tensor imaging. Am J Psychiatry. 2004;161:573–575. doi: 10.1176/appi.ajp.161.3.573. [DOI] [PubMed] [Google Scholar]
- 21.Fujiwara H, Namiki C, Hirao K, et al. Anterior and posterior cingulum abnormalities and their association with psychopathology in schizophrenia: a diffusion tensor imaging study. Schizophr Res. 2007;95:215–222. doi: 10.1016/j.schres.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 22.Burns J, Job D, Bastin ME, et al. Structural disconnectivity in schizophrenia: a diffusion tensor magnetic resonance imaging study. Br J Psychiatry. 2003;182:439–443. [PubMed] [Google Scholar]
- 23.Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambataro F, Murty VP, Callicott JH, et al. Age-related alterations in default mode network: impact on working memory performance. doi: 10.1016/j.neurobiolaging.2008.05.022. [published online ahead of print July 30, 2008]. Neurobiol Aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolkin A, Choi SJ, Szilagyi S, Sanfilipo M, Rotrosen JP, Lim KO. Inferior frontal white matter anisotropy and negative symptoms of schizophrenia: a diffusion tensor imaging study. Am J Psychiatry. 2003;160:572–574. doi: 10.1176/appi.ajp.160.3.572. [DOI] [PubMed] [Google Scholar]
- 26.Skelly LR, Calhoun V, Meda SA, Kim J, Mathalon DH, Pearlson GD. Diffusion tensor imaging in schizophrenia: relationship to symptoms. Schizophr Res. 2008;98:157–162. doi: 10.1016/j.schres.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manoach DS, Ketwaroo GA, Polli FE, et al. Reduced microstructural integrity of the white matter underlying anterior cingulate cortex is associated with increased saccadic latency in schizophrenia. Neuroimage. 2007;37:599–610. doi: 10.1016/j.neuroimage.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 28.Nestor PG, Kubicki M, Spencer KM, Niznikiewicz M, McCarley RW, Shenton ME. Attentional networks and cingulum bundle in chronic schizophrenia. Schizophr Res. 2007;90:308–315. doi: 10.1016/j.schres.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takei K, Yamasue H, Abe O, et al. Disrupted integrity of the fornix is associated with impaired memory organization in schizophrenia. Schizophr Res. 2008;103:52–61. doi: 10.1016/j.schres.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Lim KO, Ardekani BA, Nierenberg J, Butler PD, Javitt DC, Hoptman MJ. Voxelwise correlational analyses of white matter integrity in multiple cognitive domains in schizophrenia. Am J Psychiatry. 2006;163:2008–2010. doi: 10.1176/appi.ajp.163.11.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition (SCID-I/P), Version 2.0. New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- 32.Andreasen NC, Olsen S. Negative V positive schizophrenia. Definition and validation. Arch Gen Psychiatry. 1982;39:789–94. doi: 10.1001/archpsyc.1982.04290070025006. [DOI] [PubMed] [Google Scholar]
- 33.Hollingshead A, Redlich F. Social Class and Mental Illness. New York: Wiley; 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carroll JB. Human Cognitive Abilities, A Survey of Factor-Analytic Studies. New York: Cambridge University Press; 1993. [Google Scholar]
- 35.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 36.Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- 37.Fuller RL, Luck SJ, Braun EL, Robinson BM, McMahon RP, Gold JM. Impaired control of visual attention in schizophrenia. J Abnorm Psychol. 2006;115:266–275. doi: 10.1037/0021-843X.115.2.266. [DOI] [PubMed] [Google Scholar]
- 38.Carter CS, Mintun M, Nichols T, Cohen JD. Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task performance. Am J Psychiatry. 1997;154:1670–1675. doi: 10.1176/ajp.154.12.1670. [DOI] [PubMed] [Google Scholar]
- 39.Potts GF, O'Donnell BF, Hirayasu Y, McCarley RW. Disruption of neural systems of visual attention in schizophrenia. Arch Gen Psychiatry. 2002;59:418–424. doi: 10.1001/archpsyc.59.5.418. [DOI] [PubMed] [Google Scholar]
- 40.Dyckman KA, Camchong J, Clementz BA, McDowell JE. An effect of context on saccade-related behavior and brain activity. Neuroimage. 2007;36:774–784. doi: 10.1016/j.neuroimage.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 41.Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 42.Long XY, Zuo XN, Kiviniemi V, et al. Default mode network as revealed with multiple methods for resting-state functional MRI analysis. J Neurosci Methods. 2008;171:349–395. doi: 10.1016/j.jneumeth.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 43.Shulman GL, Fiez JA, Corbetta M, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;5:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 44.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 45.Ward . Alphasim Program Documentation for Afni. Simultaneous inference for fMRI data. Medical College of Wisconsin; 2000. http://afni.nimh.nih.gov/pub/dist/doc/program_help/AlphaSim.html. Accessed January 2, 2008. [Google Scholar]
- 46.Behrens TE, Woolrich MW, Jenkinson M, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 47.Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- 48.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 49.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–21. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- 51.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 53.Zhou Y, Liang M, Tian L, et al. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97:194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 54.Hill K, Mann L, Laws KR, Stephenson CM, Nimmo-Smith I, McKenna PJ. Hypofrontality in schizophrenia: A meta-analysis of functional imaging studies. Acta Psychiatr Scand. 2004;110:243–156. doi: 10.1111/j.1600-0447.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- 55.Jeong B, Wible CG, Hashimoto RI, Kubicki M. Functional and anatomical connectivity abnormalities in left inferior frontal gyrus in schizophrenia. doi: 10.1002/hbm.20835. [published online ahead of print June 30, 2009]. Hum Brain Mapp. doi: 10.1002/hbm.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang F, Kalmar JH, He Y, et al. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry. 2009;66:516–521. doi: 10.1016/j.biopsych.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Michailov GV, Sereda MW, Brinkmann BG, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 58.Hoptman MJ, Nierenberg J, Bertisch HC, et al. A DTI study of white matter microstructure in individuals at high genetic risk for schizophrenia. Schizophr Res. 2008;106:115–124. doi: 10.1016/j.schres.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 59.Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 61.Carter CS, Macdonald AM, Botvinick M, et al. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci USA. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 63.MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 64.Bush G, Vogt BA, Holmes J, et al. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci USA. 2002;99:523–8. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Erickson KI, Milham MP, Colcombe SJ, et al. Behavioral conflict, anterior cingulate cortex, and experiment duration: implications of diverging data. Hum Brain Mapp. 2004;21:98–107. doi: 10.1002/hbm.10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- 67.Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]