Abstract
AIM: To examine trends in and correlates of liver disease and viral hepatitis in an human immunodeficiency virus (HIV)-infected cohort.
METHODS: The multi-site adult/adolescent spectrum of HIV-related diseases (ASD) followed 29 490 HIV-infected individuals receiving medical care in 11 U.S. metropolitan areas for an average of 2.4 years, and a total of 69 487 person-years, between 1998 and 2004. ASD collected data on the presentation, treatment, and outcomes of HIV, including liver disease, hepatitis screening, and hepatitis diagnoses.
RESULTS: Incident liver disease, chronic hepatitis B virus (HBV), and hepatitis C virus (HCV) were diagnosed in 0.9, 1.8, and 4.7 per 100 person-years. HBV and HCV screening increased from fewer than 20% to over 60% during this period of observation (P < 0.001). Deaths occurred in 57% of those diagnosed with liver disease relative to 15% overall (P < 0.001). Overall 10% of deaths occurred among individuals with a diagnosis of liver disease. Despite care guidelines promoting screening and vaccination for HBV and screening for HCV, screening and vaccination were not universally conducted or, if conducted, not documented.
CONCLUSION: Due to high rates of incident liver disease, viral hepatitis screening, vaccination, and treatment among HIV-infected individuals should be a priority.
Keywords: Human immunodeficiency virus, Hepatitis B, Hepatitis C, Liver disease
INTRODUCTION
As highly active antiretroviral therapy (HAART) has improved the health of people living with human immunodeficiency virus (HIV), acquired immure deficiency syndrome (AIDS)-related morbidity and mortality have decreased and mortality from other illnesses, including hepatitis and liver disease, has grown[1-4]. Among HIV-infected individuals, chronic co-infection with hepatitis B virus (HBV) and/or hepatitis C virus (HCV) are associated with excess morbidity and mortality[1,4,5]. Liver disease, from HBV/HCV or other etiologies, is now one of the most frequent causes of death among HIV-infected people[6,7].
Current care treatment guidelines for people living with HIV include HBV screening, plus vaccination as indicated, and HCV screening[8]. However, it is not known how widely these guidelines are followed, nor are there clear recommendations regarding the frequency of repeat screening following a negative test for HBV and HCV when risk of infection remains present. Screening for HBV is particularly important before initiating antiviral therapy for HIV, because several therapeutic agents are active against both viruses and use of these agents may cause HBV resistance and subsequent hepatitis flares, if the provider is unaware of HBV infection. Similarly, initiation of HBV treatment without the protection of HAART can lead to HIV antiretroviral resistance.
It is also important to follow trends in HBV, HCV, and hepatic disease diagnoses among people living with HIV for care planning purposes, prevention, education, and treatment of preventable and modifiable factors contributing to liver disease. Although most serious liver morbidity among people living with HIV is attributed to HBV and HCV[9,10], other factors contributing to liver morbidity may include the HIV virus itself, HIV-induced immunosuppression and/or inflammation, cirrhosis, hepatic carcinoma, alcohol use, aging, injection drug use, and hepatotoxic therapies-including some antiretroviral therapies.
To examine trends in, and factors associated with, both incident and prevalent liver disease and hepatitis diagnoses and screening in a modern HAART-era cohort, we analyzed data from a longitudinal medical record review cohort study, the national adult/adolescent spectrum of HIV-related diseases project 1998-2004.
MATERIALS AND METHODS
The adult/adolescent spectrum of HIV related diseases (ASD) project was a multicenter medical record review surveillance project funded by the centers for disease control and prevention (CDC) and conducted in Atlanta, Detroit, Seattle, Denver, Houston, Dallas, San Antonio, Puerto Rico, New Orleans, Los Angeles, and New York City[11]. HIV-infected persons of at least 13 years of age who attended participating clinics were eligible for inclusion. ASD began data collection in 1990 and continued until 2004. Data for this manuscript were left truncated to start in 1998 due to protocol revisions at that time-including the addition of hepatitis screening-which remained in place through the project end in 2004. Medical records of ASD enrollees were retrospectively reviewed for the 12-mo period prior to their enrollment date, and at subsequent six month intervals until death, relocation, or loss to follow-up (no contact for 18 mon). Information collected included basic demographic data, mode of exposure to HIV, prescription of antiretrovirals and other medications, CD4+ T-lymphocyte (CD4+) counts, HIV RNA viral load, complete history of AIDS-defining opportunistic illnesses (OIs)[12], other infections, selected medical and psychosocial conditions, and behaviors of medical importance. Over 100 medical facilities participated in ASD, including HIV specialty clinics, private practitioners’ offices, and community clinics, both hospital-based and freestanding. Men of color and women were oversampled at selected sites. All data were collected by trained abstractors; sites conducted a variety of quality assurance activities, including duplicate record abstraction audits and double data entry.
To describe the mode of HIV infection, we used four categories: men who have sex with men (MSM), injection drug users (IDU), individuals with both exposures (MSM-IDU), and other, including heterosexual exposure to an individual with HIV infection or with a known HIV risk and blood/blood product exposure (transfusion, blood products to treat hemophilia, organ transplant recipient, health care workers with needle stick exposure). For these analyses, substance use included those with a history of IDU as an HIV risk factor, and those with ongoing IDU, non-injection use of illicit drugs (including cocaine and marijuana), and problem alcohol use (defined as a diagnosis of alcoholism or treatment for past or current alcoholism). Cigarette smoking and other tobacco use was not collected in the national ASD project.
HBV and HCV screening for each year was defined as screening conducted up to and including each year individuals contributed follow-time to the project. A prior diagnosis of HCV or HBV was also considered indicative of screening for each infection. To examine trends in prevalence over time and overall death rates, individuals with a prior diagnosis of chronic HBV or any diagnosis of HCV or liver disease were considered to have these conditions in their diagnosis year and all subsequent years of follow time. Acute and chronic HBV and HCV infection were defined by provider diagnosis or laboratory evidence. IgM anti-HBc was considered diagnostic of acute HBV and IgG anti-HBc, or other characteristic antigen and antibody combinations, distinguished chronic HBV. Age at entry was coded as 14-29, 30-39, 40-49, 50-59, and 60 years and greater. Nadir CD4+ was aggregated to < 200, 200-499, and 500+ CD4+ cells/μL. Indicator (dummy) variables were used for mode, race/ethnicity, and nadir CD4+ cell count with MSM, Blacks, and CD4+ ≥ 500 cells/μL as reference groups. Intensity of health services utilization was measured with a grouped continuous variable of the total number of outpatient visits (in 10 visit increments).
An aggregate variable, “liver disease”, was created and included cirrhosis (both alcoholic and non-alcoholic), necrosis, abscess, primary liver cancers and liver failure. Liver disease included International Classification of Diseases 9th Revision (ICD-9) codes 570 (acute and subacute necrosis of liver), 571 (chronic liver disease and cirrhosis), 572 (liver abscess and sequelae of chronic liver disease), 573 (other disorders of liver), 572.3 (portal hypertension), 277.1 (α 1 antitrypsin deficiency), 456.0-456.2 (esophageal varices), 789.5 (ascites), and 275.1 (Wilson’s disease). Esophageal varices and ascites may not be specific for liver disease; therefore, we compared characteristics of individuals with liver disease including and excluding these additional diagnoses, and found the populations were nearly identical before making the decision to include these with liver disease. HBV and/or HCV alone, when we were able to distinguish these, were not sufficient to define liver disease. A dichotomous variable used only through 1999 indicating liver failure was also included with liver disease. Liver failure was defined by medical practitioner diagnoses recorded in the medical record and supported by abnormal liver function tests, jaundice, and/or special hepatic dietary management. Liver neoplasms and morphology codes of malignant and not-otherwise-specified (NOS) neoplasms were collected.
Death rates
Follow time from 1/1/98 or start of observation until death or censure was calculated overall and for individuals diagnosed with chronic HBV, HCV, or liver disease-starting from the follow-up interval of diagnosis if after 12/31/1997. Death rates were calculated due to any cause and separately for deaths that may have been due to liver disease. Deaths that may have been due to liver disease were defined as those listing liver disease as a contributing factor on a death certificate or individuals with a diagnosis of liver disease during follow-up interval during in which death occurred (thus including individuals with a new or ongoing diagnosis of liver disease at the time of death or within six months of death).
Statistical analyses
Demographic characteristics of individuals with chronic HBV, any diagnosis of HCV, and liver disease were compared to individuals without these diagnoses by χ2 testing (SAS version 9.1, Cary NC). Multivariate Cox proportional hazards analyses were performed to calculate estimates of the relative risks (proportional hazards ratio) of HCV, chronic HBV, and liver disease (excluding those with prevalent diagnoses at baseline) and simultaneously adjusting for HIV risk mode (specifically, IDU, MSM, and hemophiliac), race/ethnicity, nadir CD4+ group (nadirs of < 200 cells/μL and 200-499 cells/μL), age, gender, alcohol use, IDU, antiretroviral use, intensity of health services utilization, and year entered cohort. Ninety-five percent confidence intervals are given for the proportional hazard ratios; intervals that do not include the value “one” are considered statistically significant. Individuals included in the multivariate analyses were required to have two or more outpatient visits documented in follow-up intervals ending in 1998 or later to minimize the contributions of persons unlikely to be assessed for any of the potential risk factors for liver disease (hepatitis, alcohol use, etc.). In the multivariate analyses examining liver disease as an outcome, all exposures were limited to those occurring prior to the diagnosis of liver disease. For chronic HBV and HCV, co-variates could be simultaneous with infection.
RESULTS
From January 1, 1998 to June 30, 2004, 29 490 individuals were followed for an average of 2.4 years per person, and for a total of 69 487 person-years. The proportion of the 29 490 observed each year from 1998 to 2004 was 56% in 1998, gradually declining to 42% in 2003; in 2004 (due to project end) 5% were followed. Liver disease was diagnosed in 3% of the cohort, HCV in 19%, and chronic HBV in 8% (Table 1). Individuals diagnosed with HBV, HCV, and liver disease were more likely to be male (P < 0.001), have a history of IDU (P < 0.001), use other illicit drugs (P < 0.001), be diagnosed with problem alcohol use (P < 0.001), and have more advanced HIV disease as measured by diagnoses of AIDS, including low nadir CD4 counts (P < 0.001). Sixty-five percent of individuals diagnosed with HCV had a history of IDU, relative to 25% overall. Twenty-five percent of HCV infected individuals were current IDU (medical records documented ongoing IDU over the course of observation) relative to 8% overall. Over half (57%) of individuals diagnosed with liver disease died over the period of observation, compared to a 15% overall mortality rate (P < 0.001).
Table 1.
Characteristics of human immunodeficiency virus-infected individuals with chronic hepatitis B, hepatitis C, liver disease, and overall; Adult/adolescent spectrum of human immunodeficiency virus-related diseases project, 11 U.S. metropolitan areas; 1998-2004 n (%)
| Total | Chronic hepatitis B | Hepatitis C | Liver disease | |
| Characteristic | = 29 490 | = 23321 | = 54632 | = 8321 |
| (100%) | (8%) | (19%) | (3%) | |
| Gender | ||||
| Male | 72 | 84 | 74 | 78 |
| Female | 28 | 16 | 26 | 22 |
| Race/Ethnicity | ||||
| White non-Hispanic | 25 | 31 | 24 | 34 |
| Black non-Hispanic | 43 | 49 | 42 | 34 |
| Hispanic | 19 | 11 | 22 | 29 |
| Asian/Pacific Islander non-Hispanic | 1 | 1 | < 1 | 1 |
| Native American non-Hispanic | < 1 | < 1 | 10 | 1 |
| Other/unknown | 11 | 8 | < 1 | 2 |
| Age (yr) | ||||
| 13-29 | 24 | 22 | 11 | 13 |
| 30-39 | 43 | 46 | 41 | 40 |
| 40-49 | 25 | 24 | 37 | 34 |
| 50+ | 8 | 7 | 11 | 13 |
| HIV transmission mode: | ||||
| MSM | 39 | 48 | 17 | 30 |
| IDU | 18 | 18 | 49 | 39 |
| IDU and MSM | 7 | 12 | 15 | 12 |
| Other (i.e. heterosexual or blood product exposure) | 16 | 9 | 9 | 9 |
| Unknown | 20 | 13 | 10 | 10 |
| Ever prescribed HAART | ||||
| Yes | 66 | 75 | 67 | 72 |
| No | 34 | 25 | 33 | 28 |
| Nadir CD4 count in cells/µL | ||||
| ≥ 500 | 11 | 6 | 10 | 5 |
| 200-499 | 34 | 30 | 32 | 22 |
| < 200 | 55 | 64 | 58 | 74 |
| Highest viral load in copies per mL | ||||
| Undetectable to 9999 | 28 | 23 | 25 | 19 |
| 10 000-99 999 | 33 | 33 | 32 | 32 |
| 100 000-999 999 | 33 | 38 | 35 | 42 |
| 1 000 000 and higher | 7 | 7 | 8 | 7 |
| Vital status | ||||
| Alive | 85 | 80 | 80 | 43 |
| Died | 15 | 20 | 20 | 57 |
| AIDS diagnosis | ||||
| Never diagnosed with AIDS | 37 | 26 | 32 | 19 |
| AIDS | 63 | 74 | 68 | 81 |
| Substance use | ||||
| Alcohol use/problem drinking | 23 | 31 | 40 | 49 |
| Non-IDU | 29 | 39 | 44 | 37 |
| Ongoing IDU | 8 | 10 | 25 | 17 |
All comparisons of clinical and demographic characteristics presented in this table between individuals with and without chronic Hepatitis B virus and with and without liver disease are highly statistically significant (P values ≤ 0.001);
All except two comparisons between individuals with and without chronic hepatitis C virus (HCV) are highly statistically significant (P values ≤ 0.001). These exceptions are gender and HCV (P = 0.004) and highly active antiretroviral (HAART) and HCV (P = 0.05). MSM: Male-male sex; IDU: Injection drug use.
Trends in hepatitis screening, HBV vaccination, and diagnoses of HBV and HCV
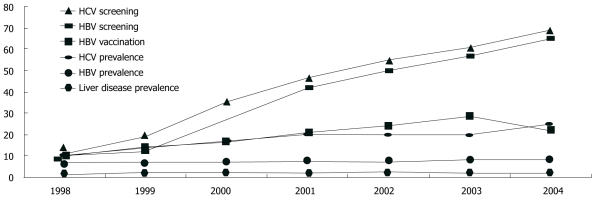
Cumulatively, 38% of the cohort was documented to have been screened for HBV and 37% had been screened for HCV. Screening increased significantly over the seven years (P < 0.0001 χ2 for trend test for HBV and HCV, Figure 1). Repeat screenings were uncommon, even in the presence of ongoing risk. For example, for individuals without any documented HBV vaccination and with ongoing injection drug use noted in their medical record, only 12% of those followed for two or more years had two or more HBV screenings documented.
Figure 1.
Screening and prevalence rates for hepatitis B and hepatitis C, liver disease prevalence, and hepatitis B vaccinations among human immunodeficiency virus-infected individuals; Adult/adolescent Spectrum of human immunodeficiency virus disease project, 11 U.S. metropolitan areas; 1998-2004.HCV: Hepatitis C virus; HBV: Hepatitis B virus.
The proportion of the cohort diagnosed with chronic HBV (at any time up to and including the observation year) increased from 7% in 1998 to 8.5% in 2004 (P < 0.0001). Similarly 9% were diagnosed with HCV in 1998, increasing to 24% in 2004 (P < 0.0001). Over the course of follow-up, chronic HBV was diagnosed at a rate of 1.8 per 100 person-years, and HCV was diagnosed in 4.7 per 100 person-years (Table 2). Between 1998 and 2003, vaccination for HBV among those without a prior diagnosis of HBV increased from 10% to 28%.
Table 2.
Chronic and acute hepatitis B, hepatitis C, and liver disease prevalence versus incidence (and rates of disease occurrence) among human immunodeficiency virus-infected individuals: Adult/adolescent spectrum of human immunodeficiency virus-related diseases project, 11 U.S. metropolitan areas; 1998-2004 (n)
| Diagnoses | Total | Incident | Present at baseline |
| (% of 29 490) | [rate per 100 person-years (py)] | (% of 29 490) | |
| All liver disease | 832 (3) | 621 (0.9/100 py) | 212 (1) |
| Chronic hepatitis B | 2332 (8) | 1212 (1.8/100 py) | 1120 (4) |
| Hepatitis C | 5463 (19) | 3129 (4.7/100 py) | 2334 (8) |
| Acute hepatitis B | 862 (3) | 575 (0.8/100 py) | 287 (1) |
Liver disease
Among the 832 individuals diagnosed with liver disease, 262 (31%) were diagnosed with non-alcoholic (or alcohol not specified) cirrhosis, 165 (20%) with alcoholic cirrhosis, 3% with a primary liver cancer, 3% with liver failure not otherwise specified, (these numbers and percents include 7% with two or more of these conditions diagnosed), and 244 (29%) with other liver diseases (Table 3). Of the 832 with liver disease, 212 individuals (25% of those with liver disease, 1% of the cohort) had a liver disease diagnosis present at baseline (prevalent disease) and 621 individuals (75% with liver disease, 2% of the cohort) had new or incident diagnoses over follow-up (Table 2). Between 1998 and 2004, the proportion of individuals followed each year with a diagnosis of liver disease increased from 1.8% to 2.2% (χ2 for trend P value = 0.03, Figure 1). Overall, 496 of the 5005 people who died (9.9%) had a diagnosis of liver disease. This ranged from 8.6% to 11.0% over the seven years of the study, with no significant trend (data not shown).
Table 3.
Types of liver disease diagnosed among human immunodeficiency virus-infected individuals. Adult/adolescent spectrum of human immunodeficiency virus-related diseases project, 11 U.S. metropolitan areas; 1998-2004 n (%)
| Liver diseases diagnosed | Not exclusive categories |
| All liver disease | 832 (100) |
| All cirrhosis | 389 (47) |
| Non-alcoholic cirrhosis | 262 (31) |
| Alcoholic cirrhosis | 165 (20) |
| Both | 38 (5) |
| Other alcoholic liver disease excluding cirrhosis | 39 (5) |
| Liver cancer | 25 (3) |
| Liver failure (not otherwise specified) | 22 (3) |
| Other liver diseases | 244 (29) |
Death rates
Table 4 presents the death rates per 1000 person-years observed among the entire ASD cohort from 1998-2004, and separately for people with diagnoses of liver disease, chronic HBV, HCV, and none of these diagnoses. Death rates ranged from 61/1000 for individuals without liver disease, HCV, and chronic HBV diagnoses to 74/1000 for people diagnosed with HCV. About 1% of overall deaths may have been due to liver disease among the entire cohort. Among those with HCV or chronic HBV, about 2% of deaths may have been due to liver disease (defined as liver disease as a contributing illness on a death certificate or as a new or ongoing diagnosis within six months of death). Death rates potentially attributable to liver disease ranged from 3/1000 for the entire cohort to 31/1000 among those with a prior diagnosis of liver disease.
Table 4.
Overall death rates and deaths potentially due to liver disease among individuals with chronic hepatitis B, hepatitis C, and liver disease diagnoses among human immunodeficiency virus-infected individuals followed by the Adult/adolescent spectrum of human immunodeficiency virus-related diseases project, 11 U.S. metropolitan areas; 1998-2004
| Diagnoses | Number of deaths | Overall death rate [Per 1000 person-years (py)] | Potential liver disease death rate [(Per 1000 person-years py)]1 |
| Entire cohort | 4461 | 64.2 | 2.9 |
| Liver disease | 475 | 73.0 | 31.0 |
| HCV | 1073 | 74.4 | 7.0 |
| Chronic hepatitis B | 455 | 68.9 | 6.4 |
| No HCV, no chronic HBV | 3099 | 61.4 | 1.5 |
A potential liver disease death was a death where liver disease was included as a contributing factor on a death certificate or a death occurring within approximately six months of a new or ongoing diagnosis of liver disease. HCV: Hepatitis C virus; HBV: Hepatitis B virus.
Overlapping diagnoses and death rates
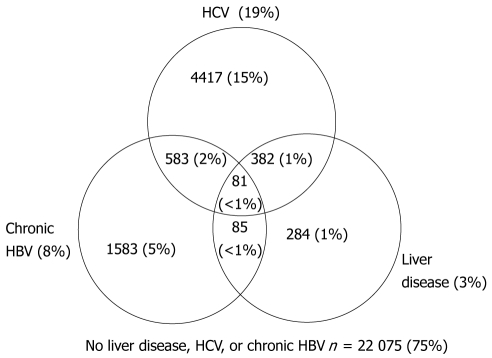
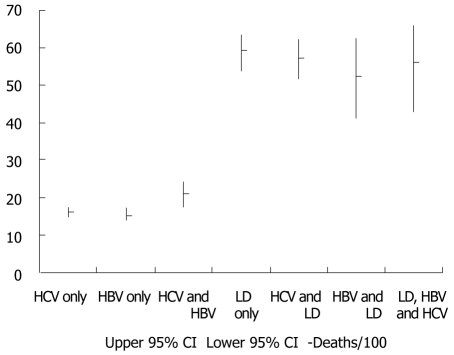
Figure 2 presents the cumulative number and proportions of individuals with HCV, HBV, liver disease, overlapping diagnoses, and none of these three conditions. Cumulative death rate per 100 persons and 95% confidence intervals for HCV, HBV, liver disease, and overlapping diagnoses are presented in Figure 3. Death rates were similar for individuals with only HBV and only HCV, but increased with dual HBV & HCV infection. The highest death rates occurred among individuals with liver disease regardless of HBV, HCV, and dual HBV/HCV infection status.
Figure 2.
Cumulative prevalence of hepatitis C virus, chronic hepatitis B virus , liver disease, and overlapping diagnoses among 29 490 individuals; Adult/adolescent Spectrum of human immunodeficiency virus-related Diseases Project, 11 U.S. metropolitan areas; 1998-2004. HCV: Hepatitis C virus; HBV: Hepatitis B virus.
Figure 3.
Cumulative death rate per 100 persons and 95% CI for hepatitis C virus , chronic hepatitis B virus , liver disease , and overlapping diagnoses among 29 490 individuals; Adult/adolescent spectrum of human immunodeficiency virus-related diseases project, 11 U.S. HCV: Hepatitis C virus; HBV: Hepatitis B virus; LD: Liver disease.
Multivariate analyses
Correlates of incident liver disease were examined by proportional hazards regression among 29 279 individuals (excluding the 212 with prevalent disease; Table 2). All demographic and clinical characteristics from Table 1 were included as covariates with the exception of AIDS as it was redundant with AIDS-defining CD4+ cell count categories. We also adjusted for intensity of health services utilization and year of enrollment. IDU and hemophiliacs had more than double the risk of liver disease relative to those without these risk factors (Table 5). Relative to Blacks, all other racial/ethnic groups had a two to three-fold higher risks of a liver disease diagnosis. Risk of liver disease also increased with increasing age, with AIDS-defining nadir CD4+ cell counts (< 200 cells/μL), alcohol use, and a prior diagnosis of HBV or HCV. Once these other factors were adjusted for, neither HAART in aggregate nor any specific antiretrovirals were associated with excess risk of liver disease.
Table 5.
Correlates of liver disease, chronic hepatitis B virus, and hepatitis C virus by multivariate (proportional hazards) analyses. Adult/adolescent spectrum of human immunodeficiency virus-related diseases project, 11 U.S. metropolitan areas; 1998-2004
|
Proportional hazards ratio1-an estimate of relative risk (95% CI) |
|||
| Characteristic | Liver disease | Chronic HBV | HCV |
| Male gender | 1.2 (1.0-1.5) | 1.7 (1.4-2.0) | 1.3 (1.1-1.4) |
| White non-hispanic | 1.9 (1.6-2.3) | 1.0 (0.9-1.1) | 1.3 (1.1-1.4) |
| Hispanic | 2.2 (1.8-2.7) | 0.7 (0.6-0.8) | 1.4 (1.3-1.4) |
| Asian/pacific islander non-hispanic | 2.3 (1.1-5.0) | 1.0 (0.5-1.8) | 1.0 (0.6-1.7) |
| Native American non-Hispanic | 3.1 (1.5-6.3) | 0.3 (0.1-1.3) | 1.4 (0.9-2.1) |
| Each increase in decade of age relative to those 13-29 yr | 1.4 (1.3-1.5) | 1.0 (1.9-1.1) | 1.3 (1.2-1.3) |
| MSM | 0.9 (0.7-1.1) | 1.4 (1.2-1.7) | 0.7 (0.7-0.8) |
| IDU | 2.2 (1.8-2.6) | 1.2 (1.1-1.4) | 4.7 (4.4-5.1) |
| Hemophiliac | 2.6 (1.0-6.5) | 0.9 (0.3-2.8) | 7.0 (4.8-10.2) |
| HAART | 1.0 (0.8-1.2) | 0.1 (0.1-0.2) | 0.4 (0.4-0.4) |
| CD4 < 200 cells/microliter | 1.8 (1.2-2.6) | 3.9 (2.9-5.2) | 1.6 (1.4-1.9) |
| CD4 200-499 cells/microliter | 1.0 (0.7-1.5) | 2.0 (1.5-2.7) | 1.3 (1.1-1.5) |
| Alcohol use/problem drinking | 1.4 (1.1-1.6) | 0.7 (0.6-0.8) | 1.1 (1.0-1.2) |
| Chronic HBV | 1.4 (1.1-1.7) | N/A | 1.3 (1.1-1.4) |
| HCV | 1.6 (1.3-1.9) | 1.6 (1.4-1.8) | N/A |
Adjusted for each factor in the model plus intensity of health services utilization (number outpatient visits in a grouped linear variable) and study entry year. HAART: Highly active antiretroviral; CD4: CD4+ lymphocyte; N/A indicates not applicable; HBV: Hepatitis B virus; HCV: Hepatitis C virus; MSM: Male-male sex; IDU: Injection drug use.
Correlates of chronic HBV and HCV diagnoses were also examined by multivariate proportional hazards regression analyses, excluding those with prevalent chronic HBV (n = 1120) and HCV (n = 2334), respectively. IDU and hemophiliacs were 4.7-fold and seven-fold more likely to be diagnosed with HCV relative to those with other HIV risk factors. Men, Whites, Latinos, and those co-infected with HBV were all more likely to be diagnosed with HCV. HCV risk also increased with age among individuals 30 years of age and older relative to those less than 30 years of age. Factors associated with chronic HBV diagnosis include male gender, MSM, IDU, lower CD4+ cell count nadir, alcohol use, and co-infection with HCV.
Liver cancers
There were 25 diagnoses of liver cancer in the cohort. All of these patients were documented to have died, excluding three individuals lost to follow-up. Fifteen of the 25 had hepatocellular carcinoma, four had other specified morphologies (one diagnosis each of sarcoma, hepatoblastoma, lymphoma, and giant cell type), and six were unspecified. Of the 25, 13 were diagnosed with HCV and 10 with chronic HBV (this includes two diagnosed with both). Of the four diagnosed with neither virus, none had evidence of HBV or HCV screening documented in their medical chart.
DISCUSSION
We found that chronic HBV, HCV, and liver disease were frequent diagnoses among HIV-infected people followed in the ASD project from 1998 to 2004. Roughly one person out of a hundred people followed for a year was diagnosed with liver disease, two per 100 followed for a year were diagnosed with chronic HBV, and five out of a hundred were diagnosed with HCV. We observed relatively low rates of HBV and HCV screening, especially in earlier years of observation, despite consistent recommendations that all HIV-infected patients be screened for both infections in the guidelines for the treatment of HIV and prevention of opportunistic illness (including screening of HBV prior to administration of vaccine) covering 1998-2004[13]. Fortunately, over the observation period, increasing proportions of individuals were screened for HBV and HCV. Diagnoses of HBV and HCV also increased over the seven years of the study. Among individuals who died, liver disease was present at any point in nearly 10%, and liver disease was listed as a contributing factor or as a new or ongoing diagnosis within six months of death in 1% of deaths. The overall prevalence of liver disease was about 1% each year-this increased only slightly over the seven years. As expected, IDU was associated with all three outcomes examined by multivariate analyses (chronic HBV, HCV, and liver disease). Hemophilia, and thus an exposure to unscreened blood products, was most strongly associated with HCV, and alcohol use was associated with liver disease.
Other researchers have found high rates of liver diseases and viral hepatitis among HIV-infected persons, especially HIV-infected hemophiliacs and injection drug users. The prevalence of HBV and HCV infection varies within HIV-infected cohorts largely due to the underlying prevalence of these infections in the general populations from which the cohorts are drawn and the proportion of IDU included. Relative to the 8% with chronic HBV and the 19% with HCV in our cohort, a New York City HIV clinic following 5639 people living with HIV from 1999 until 2007, found that 4% were HBV-infected and 25% were HCV infected[14]. The HIV Outpatient Survey (HOPS) conducted at 10 facilities in eight U.S. cities followed 7618 HIV-infected individuals 1996-2007 and found a 24% prevalence of HCV[15]. In a serological study of incarcerated individuals in three U.S. cities, of those who were HIV-infected, 38% were co-infected with HCV and 8% were HBsAg positive[16].
The D:A:D Study followed 23 441 individuals on three continents between 1999 and 2004, finding a 23% prevalence of HCV and a 7% prevalence of active HBV[17]. In this study, HCV was associated with a seven-fold excess risk of liver-related deaths (e.g. hepatocellular carcinoma, end stage liver disease, or hepatic failure) and active HBV was associated with a four-fold excess risk of liver-related deaths. This compares to our similar finding of liver related deaths occurring over four times more frequently among individuals infected with HCV or chronic HBV relative to uninfected individuals. In the Swiss HIV Cohort Study, 37% of 3111 people living with HIV were HCV-infected; this included 92% of IDU and 7% of those with other HIV risks[18]. In this study, 9% of HCV-co-infected individuals died relative to 4% of those without HCV. Of the deaths among HCV-infected individuals, 16% were possibly to definitely associated with end-stage liver disease relative to 5% of deaths among HIV-infected individuals without HCV[18]. In the Multicenter Cohort Study of MSM, about 8% of HIV-infected men followed between 1984 and 2000 were HBsAg positive, and liver-related mortality was about seven times higher (14 deaths per 1000 person-years) among HBsAg positive men relative to HBsAg negative men (two deaths per 1000 person-years)[19].
Of 755 people living with HIV evaluated following initiation of antiretrovirals at one Italian HIV clinic, 3% developed severe hepatotoxicity (4/100 person-years). Nearly all (96%) of these patients had evidence of HCV infection (relative to 67% without hepatotoxicity), 19% had evidence of HBV, and 19% had a history of alcohol abuse (relative to 7% and 13% prevalence of these diagnoses among those without hepatotoxicity, respectively)[20]. This is consistent with our finding of no excess risk of liver disease for individuals prescribed antiretrovirals once these other factors (HCV, HBV, alcohol use, etc.) were controlled for by multivariate analyses. In a Veteran’s Affairs cohort with comprehensive evaluations of 299 HIV-infected individuals over 6 mo, 27% had abnormal liver functions and for 51% of these, no underlying cause was established. Among the remainder, 30% had non-alcoholic fatty liver disease as a diagnosis, alcohol was attributed to 13%, chronic HBV to 9% and chronic active HCV to 5%[21].
Studies of HIV-infected hemophiliacs include a cohort study of 158 HIV-infected hemophiliacs followed in the pre- and post-HAART eras[22]. The predominant cause of non-AIDS mortality in both periods was end-stage liver disease (ESLD). Of 223 HIV-infected hemophiliacs without clinical AIDS, 9% of those co-infected with HCV developed liver failure after 10-20 years[23]. In Germany, 144 HIV and HCV co-infected hemophiliacs were examined, and the authors concluded that declining immune function may be associated with progression of liver failure[24].
Relative to the increase in HCV screening that we observed (from 11% to 69%), the HOPS study documented a very similar trend-HCV screening increasing from 11% to 77% from 1996 to 2007[15]. However, even if patients are screened, few receive ongoing screening despite ongoing risk, fewer than half are evaluated, and a minority are treated, reflecting the many hurdles that HIV and HCV and HIV and HBV co-infected patients must surmount to be appropriately managed and treated for HBV and HCV when indicated[25,26].
Although the number of liver cancers we observed was small (n = 25), all 21 with hepatitis screening documented were HBV or HCV-infected. Our study was not designed to compare the occurrence of liver cancer among people living with HIV relative to the general population, but other researchers have found three to six fold excess risk of liver cancer associated with HIV infection[27,28].
Limitations of our analyses included that the data were collected solely by medical record review. Collecting data by chart review rather than targeted medical examinations, may have introduced errors. Exposures and diagnoses may have been missed, especially if an individual sought medical care at facilities other than those included in ASD. Other diseases recorded may have been in error, such as mistaking a “rule out” diagnosis with a true diagnosis. Further, the use of ICD-9 codes to define liver disease likely resulted in some losses of sensitivity and specificity (for example, including a code such as 571.4-chronic hepatitis-which might have included viral hepatitis infections without any known liver damage). Similarly ICD-9 code 571 includes both fatty liver and nonalcoholic steatohepatitis (NASH), which could not be distinguished from each other without additional information. Information on performance of liver biopsy was not collected, and biopsies were not likely to have been frequently performed, leaving us to rely upon broad clinical diagnoses rather than histological diagnoses. HBV and HCV were particularly difficult diagnoses to ascertain through chart review because of under-diagnosis-especially of asymptomatic infections, false negative screening tests due to low or transient antibody production[29-31], and abstractor (and practitioner) confusion regarding distinguishing markers of acute, chronic, and chronic active or chronically persistent infection. Observed trends in HBV and HCV diagnoses could have been caused by increases in screening, not increases in incidence and prevalence. Some known predisposing conditions of certain liver disease (such as obesity) were not routinely collected. Deaths and causes of death may have been under-ascertained as well, as some ASD sites linked to core surveillance and national death index data and others did not. Finally, although ASD data collection ended in 2004, we believe there have been no major changes in hepatitis screening, HBV vaccination, or liver disease trends among HIV-infected individuals and that our findings remain highly relevant today.
Despite its limitations, ASD provided a large single cohort in a contemporary HAART era, collected with a consistent protocol and including a wealth of data on the screening and diagnosis HBV and HCV and liver disease and mortality as outcomes. Although HIV case reporting was not required throughout the U.S. during the period of our study, the socio-demographic characteristics (age, gender, race-ethnicity, and HIV risk category) of 38 398 newly diagnosed HIV cases in 2004 for the 34 states and five dependent areas, with name-based reporting at that time, were highly similar to the characteristics of ASD, suggesting ASD was largely representative of national HIV cases at this time[32].
In conclusion, patients with HIV and liver disease had a much higher mortality compared to those with HIV without a liver disease diagnosis. Although HBV vaccination rates have improved and screening rates for HBV and HCV have climbed steadily, they are still inadequate, and efforts are needed to improve vaccination and screening rates. The high rates of incident HCV (5/100 person-years) indicate that individuals at risk should be screened and while remaining at risk, re-screened on a regular basis. Similarly, a sizable HBV incidence (2/100 person-years) supports improved screening and vaccination[33]. Further studies are needed to determine the optimal frequency of repeated HCV screening among those with ongoing risk, as well as a frequency to re-visit HBV vaccination among unvaccinated individuals. Until better data are available, annual screenings for HCV and HBV vaccination discussions are suggested. Treatment of HBV and HCV should be considered for all HIV co-infected individuals.
COMMENTS
Background
Despite widespread availability of antiretroviral treatment in the U.S., over 17 000 people living with human immunodeficiency virus (HIV) are estimated to die each year. Although increasing, the median age at death among HIV-infected people was 46 years in 2006, indicating most HIV deaths occur among relatively young individuals. Hepatitis and liver disease are major causes of premature death among people living with HIV. The authors conducted this review of liver disease and hepatitis screening and diagnoses to describe preventive care and the impact of these conditions among HIV-infected individuals receiving medical care in the U.S.
Research frontiers
In this report, the authors examined hepatitis and other hepatic diseases in a dynamic medical record review cohort of over 29 000 HIV-infected people. The authors found, over the study period of 1998 to 2004, improvements in hepatitis screening and hepatitis B vaccination, but the authors also saw there was much room for improvement in screening and vaccination coverage. On average, among 100 HIV-infected people followed for a year one person had a diagnosis of acute hepatitis B infection, two were diagnosed with chronic hepatitis B, five were diagnosed with hepatitis C, and one person with liver disease. Fewer than one-third (28% in 2003) of those without a documented diagnosis of hepatitis B had evidence of any hepatitis B vaccination.
Innovations and breakthroughs
The authors examined hematological diagnoses and risk factors in nearly 30 000 HIV infected individuals from a broad range of HIV risk groups and geographic locations within the U.S. Among other findings, the authors saw elevated death rates among people with chronic hepatitis B, hepatitis C, and liver disease, but mortality rates were elevated the most among individuals with liver disease, with or without chronic hepatitis B and hepatitis C. When the authors conducted a multivariate analysis, the authors saw that Blacks were at lower risk of liver disease than individuals with other racial/ethnic identities, older individuals, injection drug users, alcoholics/problem drinkers, and individuals with AIDS all had increased risks of liver disease. Antiretroviral use was not associated with liver disease.
Applications
The authors’ results highlight a need for improved hepatitis screening and vaccination. All HIV-infected individuals should be screened for hepatitis and, if at risk, vaccinated for hepatitis B. Uninfected individuals should be counseled in hepatitis risk reduction strategies, and individuals with chronic hepatitis infections should be assessed for treatment. It is likely that the authors’ estimates for hepatitis screening and vaccination are low, and that documentation of prior screening and vaccinations may have been missed, for example if they occurred at medical facilities not participating in ASD.
Peer review
A series of experiments are well-planned and well-performed and this manuscript is well written.
Footnotes
Peer reviewer: Dr. Kaye M Reid Lombardo, MD, Assistant Professor, Department of General Surgery, Mayo Clinic, 200 First St. SW, Rochester, MN 55 905, United States
S- Editor Tian L L- Editor Stewart GJ E- Editor Ma WH
References
- 1.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44:S6–S9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Lifson AR, Belloso WH, Carey C, Davey RT, Duprez D, El-Sadr WM, Gatell JM, Gey DC, Hoy JF, Krum EA, et al. Determination of the underlying cause of death in three multicenter international HIV clinical trials. HIV Clin Trials. 2008;9:177–185. doi: 10.1310/hct0903-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sulkowski MS. Viral hepatitis and HIV coinfection. J Hepatol. 2008;48:353–367. doi: 10.1016/j.jhep.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Tedaldi E, Peters L, Neuhaus J, Puoti M, Rockstroh J, Klein MB, Dore GJ, Mocroft A, Soriano V, Clotet B, et al. Opportunistic disease and mortality in patients coinfected with hepatitis B or C virus in the strategic management of antiretroviral therapy (SMART) study. Clin Infect Dis. 2008;47:1468–1475. doi: 10.1086/593102. [DOI] [PubMed] [Google Scholar]
- 5.Ribes J, Clèries R, Rubió A, Hernández JM, Mazzara R, Madoz P, Casanovas T, Casanova A, Gallen M, Rodríguez C, et al. Cofactors associated with liver disease mortality in an HBsAg-positive Mediterranean cohort: 20 years of follow-up. Int J Cancer. 2006;119:687–694. doi: 10.1002/ijc.21882. [DOI] [PubMed] [Google Scholar]
- 6.Hooshyar D, Hanson DL, Wolfe M, Selik RM, Buskin SE, McNaghten AD. Trends in perimortal conditions and mortality rates among HIV-infected patients. AIDS. 2007;21:2093–2100. doi: 10.1097/QAD.0b013e3282e9a664. [DOI] [PubMed] [Google Scholar]
- 7.Salmon-Ceron D, Lewden C, Morlat P, Bévilacqua S, Jougla E, Bonnet F, Héripret L, Costagliola D, May T, Chêne G. Liver disease as a major cause of death among HIV infected patients: role of hepatitis C and B viruses and alcohol. J Hepatol. 2005;42:799–805. doi: 10.1016/j.jhep.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, 2008 [Google Scholar]
- 9.Sulkowski MS. Management of hepatic complications in HIV-infected persons. J Infect Dis. 2008;197 Suppl 3:S279–S293. doi: 10.1086/533414. [DOI] [PubMed] [Google Scholar]
- 10.Thomas DL. The challenge of hepatitis C in the HIV-infected person. Annu Rev Med. 2008;59:473–485. doi: 10.1146/annurev.med.59.081906.081110. [DOI] [PubMed] [Google Scholar]
- 11.Farizo KM, Buehler JW, Chamberland ME, Whyte BM, Froelicher ES, Hopkins SG, Reed CM, Mokotoff ED, Cohn DL, Troxler S. Spectrum of disease in persons with human immunodeficiency virus infection in the United States. JAMA. 1992;267:1798–1805. [PubMed] [Google Scholar]
- 12.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 13.1997 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. USPHS/IDSA Prevention of Opportunistic Infections Working Group. MMWR Recomm Rep. 1997;46:1–46. [PubMed] [Google Scholar]
- 14.Kim JH, Psevdos G, Suh J, Sharp VL. Co-infection of hepatitis B and hepatitis C virus in human immunodeficiency virus-infected patients in New York City, United States. World J Gastroenterol. 2008;14:6689–6693. doi: 10.3748/wjg.14.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spradling PR, Richardson JT, Buchacz K, Moorman AC, Finelli L, Bell BP, Brooks JT. Trends in hepatitis C virus infection among patients in the HIV Outpatient Study, 1996-2007. J Acquir Immune Defic Syndr. 2010;53:388–396. doi: 10.1097/QAI.0b013e3181b67527. [DOI] [PubMed] [Google Scholar]
- 16.Hennessey KA, Kim AA, Griffin V, Collins NT, Weinbaum CM, Sabin K. Prevalence of infection with hepatitis B and C viruses and co-infection with HIV in three jails: a case for viral hepatitis prevention in jails in the United States. J Urban Health. 2009;86:93–105. doi: 10.1007/s11524-008-9305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber R, Sabin CA, Friis-Møller N, Reiss P, El-Sadr WM, Kirk O, Dabis F, Law MG, Pradier C, De Wit S, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 18.Greub G, Ledergerber B, Battegay M, Grob P, Perrin L, Furrer H, Burgisser P, Erb P, Boggian K, Piffaretti JC, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356:1800–1805. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 19.Thio CL, Seaberg EC, Skolasky R Jr, Phair J, Visscher B, Muñoz A, Thomas DL. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921–1926. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 20.Puoti M, Torti C, Ripamonti D, Castelli F, Zaltron S, Zanini B, Spinetti A, Putzolu V, Casari S, Tomasoni L, et al. Severe hepatotoxicity during combination antiretroviral treatment: incidence, liver histology, and outcome. J Acquir Immune Defic Syndr. 2003;32:259–267. doi: 10.1097/00126334-200303010-00004. [DOI] [PubMed] [Google Scholar]
- 21.Crum-Cianflone N, Collins G, Medina S, Asher D, Campin R, Bavaro M, Hale B, Hames C. Prevalence and factors associated with liver test abnormalities among human immunodeficiency virus-infected persons. Clin Gastroenterol Hepatol. 2010;8:183–191. doi: 10.1016/j.cgh.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katsarou O, Touloumi G, Antoniou A, Kouramba A, Hatzakis A, Karafoulidou A. Progression of HIV infection in the post-HAART era among a cohort of HIV+ Greek haemophilia patients. Haemophilia. 2005;11:360–365. doi: 10.1111/j.1365-2516.2005.01109.x. [DOI] [PubMed] [Google Scholar]
- 23.Eyster ME, Diamondstone LS, Lien JM, Ehmann WC, Quan S, Goedert JJ. Natural history of hepatitis C virus infection in multitransfused hemophiliacs: effect of coinfection with human immunodeficiency virus. The Multicenter Hemophilia Cohort Study. J Acquir Immune Defic Syndr. 1993;6:602–610. [PubMed] [Google Scholar]
- 24.Rockstroh JK, Spengler U, Sudhop T, Ewig S, Theisen A, Hammerstein U, Bierhoff E, Fischer HP, Oldenburg J, Brackmann HH, et al. Immunosuppression may lead to progression of hepatitis C virus-associated liver disease in hemophiliacs coinfected with HIV. Am J Gastroenterol. 1996;91:2563–2568. [PubMed] [Google Scholar]
- 25.Scott JD, Wald A, Kitahata M, Krantz E, Drolette L, Corey L, Wang CC. Hepatitis C virus is infrequently evaluated and treated in an urban HIV clinic population. AIDS Patient Care STDS. 2009;23:925–929. doi: 10.1089/apc.2009.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jain MK, Opio CK, Osuagwu CC, Pillai R, Keiser P, Lee WM. Do HIV care providers appropriately manage hepatitis B in coinfected patients treated with antiretroviral therapy? Clin Infect Dis. 2007;44:996–1000. doi: 10.1086/512367. [DOI] [PubMed] [Google Scholar]
- 27.Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, Grigg R, Hylton T, Pawlish KS, McNeel TS, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 28.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52:611–622. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chamie G, Bonacini M, Bangsberg DR, Stapleton JT, Hall C, Overton ET, Scherzer R, Tien PC. Factors associated with seronegative chronic hepatitis C virus infection in HIV infection. Clin Infect Dis. 2007;44:577–583. doi: 10.1086/511038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen Stuart JW, Velema M, Schuurman R, Boucher CA, Hoepelman AI. Occult hepatitis B in persons infected with HIV is associated with low CD4 counts and resolves during antiretroviral therapy. J Med Virol. 2009;81:441–445. doi: 10.1002/jmv.21422. [DOI] [PubMed] [Google Scholar]
- 31.Thomson EC, Nastouli E, Main J, Karayiannis P, Eliahoo J, Muir D, McClure MO. Delayed anti-HCV antibody response in HIV-positive men acutely infected with HCV. AIDS. 2009;23:89–93. doi: 10.1097/QAD.0b013e32831940a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report, 2007. URL: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2007report/default.htm
- 33.Mofenson LM, Brady MT, Danner SP, Dominguez KL, Hazra R, Handelsman E, Havens P, Nesheim S, Read JS, Serchuck L, et al. Guidelines for the Prevention and Treatment of Opportunistic Infections among HIV-exposed and HIV-infected children: recommendations from -CDC, the National Institutes of Health, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. MMWR Recomm Rep. 2009;58:1–166. [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. HIV Surveillance Report, 2008; vol. 20. Published June 2010. Accessed 11/29/2010. Available at: URL: http://www.cdc.gov/hiv/surveillance/resources/reports/2008report/table12a.htm
- 35.Centers for Disease Control and Prevention. HIV Mortality (through 2006) slide set. Accessed 11/29/2010. Available from: URL: http://www.cdc.gov/hiv/topics/surveillance/resources/slides/mortality/slides/mortality.pdf