Abstract
AIM: To identify factors predicting the clinical response of ulcerative colitis patients to granulocyte-monocyte apheresis (GMA).
METHODS: Sixty-nine ulcerative colitis patients (39 F, 30 M) dependent upon/refractory to steroids were treated with GMA. Steroid dependency, clinical activity index (CAI), C reactive protein (CRP) level, erythrocyte sedimentation rate (ESR), values at baseline, use of immunosuppressant, duration of disease, and age and extent of disease were considered for statistical analysis as predictive factors of clinical response. Univariate and multivariate logistic regression models were used.
RESULTS: In the univariate analysis, CAI (P = 0.039) and ESR (P = 0.017) levels at baseline were singled out as predictive of clinical remission. In the multivariate analysis steroid dependency [Odds ratio (OR) = 0.390, 95% Confidence interval (CI): 0.176-0.865, Wald 5.361, P = 0.0160] and low CAI levels at baseline (4 < CAI < 7) (OR = 0.770, 95% CI: 0.425-1.394, Wald 3.747, P = 0.028) proved to be effective as factors predicting clinical response.
CONCLUSION: GMA may be a valid therapeutic option for steroid-dependent ulcerative colitis patients with mild-moderate disease and its clinical efficacy seems to persist for 12 mo.
Keywords: Granulocyte-monocyte apheresis, Ulcerative colitis, Steroid therapy, Long-term follow-up, Predictive factors
INTRODUCTION
Ulcerative colitis (UC) is a chronic inflammatory bowel disease characterized by periods of remission and clinical relapses most likely related to a multifactorial dysfunction of the mucosal immune system[1].
The available pharmacological therapies are aimed at inducing remission and preventing relapses. In active UC, depending on the site of disease and on disease activity, the principle therapy is represented by oral and topical steroids with maintained remission in half of the examined patients (49%) at a 1 year follow-up. Steroids, which offer a good initial response rate, are however ineffective as maintenance therapy and well-known side-effects are observed in 22% of the patients who develop steroid dependency/resistance[2].
In those patients with chronic active disease, unresponsive to steroids, immunomodulators are largely used even though these treatments are limited by side-effects occurring in 6%-30% of cases as well as concerns over long-term toxicity[3]. In severe disease, as azathioprine has a delayed onset of efficacy, cyclosporine or tacrolimus may offer an alternative. The encouraging development of biologicals, such as infliximab (an anti-TNFα antibody showing effect in moderate to severe UC), is counterbalanced by the need for long-term efficacy and safety profile evaluations[4,5].
Granulocyte-monocyte apheresis (GMA), a selective apheresis technique, represents a recent alternative therapeutic option. Several open trials have been performed so far, showing efficacy rates up to 65%-75% associated with an excellent safety profile[6-13].
While its safety has been largely proven in the treatment of steroid refractory/dependent UC patients, few and conflicting results are available concerning the long-term follow-up and factors predicting clinical response, therefore current data do not help in identifying UC patients eligible for GMA treatment. The aim of the present study has thus been to single out factors predicting the clinical response to GMA.
MATERIALS AND METHODS
Patients
Between April 2005 and January 2008, a total of 69 patients with mild/moderate UC either steroid-dependent or resistant were selected for GMA treatment, from patients admitted to the GI Units of the Department of Clinical Sciences, Policlinico Umberto I and of Pisa Hospital.
The characteristics of the patient population (39 females, 30 males, mean age 42.36 years; range 27-75 years) are shown in Table 1. Mean disease duration was 41.34 mo (range 12-252 mo). Disease activity was mild/moderate in all the patients as established by a clinical activity index (CAI) value > 4; The mean clinical activity index (CAI) value was 9.10 (range 6-12; lower quartile = 8, median value = 9, upper quartile = 10, interquartile range = 2). Forty-seven patients presented left colitis and 22 pancolitis. Arthralgia was present in 15 patients.
Table 1.
Characteristics of granulocyte-monocyte apheresis treated patients: Demographic and clinical data
| Sex | F/M | 39/30 |
| Age (yr) | mean (range) | 42.36 (27-75) |
| Duration of disease (mo) | mean (range) | 41.34 (12-262) |
| Location of disease | Left side/pancolitis | 47/22 |
| Steroid course | Dependent/resistant | 49/20 |
| CAI | mean (range) | 9.10 (6-13) |
| ESR (mm/h) | mean (range) | 33.09 (2-20) |
| CRP (mg/dL) | mean (range) | 2.04 (0.3-7.3) |
| WBC 103 | mean (range) | 10.69 (5.7-14) |
CAI: Clinical activity index; ESR: Erythrocyte sedimentation rate; CRP: C reactive protein; WBC: White blood cell.
All patients considered for GMA had failed to respond to mesalazine or sulphasalazine, and were under steroid treatment. Forty-nine patients had been found to be dependent on, and 20 resistant to, steroid treatment. The steroid-dependence/resistance have been respectively defined: relapse within 30 d after a complete tapering or at dose reduction impeding discontinuation of prednisolone treatment for more than one year; No response within 30 d at standard maximum daily dosage of prednisone[14].
The 20 patients resistant to steroids had started azathioprine (at a dose of 1.5-2.5 mg/kg per day) from 6 mo before enrolment.
Major exclusion criteria were severe cardiovascular disease (acute myocardial infarction, brain hemorrhage, severe arhythmia or heart failure) within the past 6 mo, severe renal failure, hypotension (< 80 mmHg systolic pressure), body weight < 35 kg, age < 12 years, pregnancy.
GMA treatment
After giving informed consent, all selected patients were submitted to GMA with Adacolumn (Otzuka Production, Milan, Italy). GMA was performed with the help of the Transfusional Unit of the two Institutions.
Each patient was submitted to 5-10 GMA sessions (1 session/wk), as a basic treatment course, according to the standard protocols[15]. Each GMA session lasted 60 min.
In particular, enrolled patients were divided into two groups: Presenting mild and moderate disease, respectively, according to their baseline CAI levels (4 < CAI < 7, 7 < CAI < 12 respectively). Out of 69, 29 patients with mild disease were submitted to a 5 session treatment, whereas 40 patients with moderate disease were treated with a further cycle of GMA.
During the GMA sessions, the treatment with oral and topical mesalazine and/or azathioprine was continued, at a stable dosage. Steroid tapering was started after the first session, according to the response to therapy. At the end of the treatment period, all concomitant therapies were carried on (oral and topical mesalazine, azathioprine).
Assessment of clinical response
Clinical efficacy was assessed at enrolment, within the first week after the last GMA session (short-term follow-up) and at 12 mo (long-term follow-up).
All patients were evaluated by clinical and laboratory assessment, including full blood count, erythrocyte sedimentation rate (ESR), C reactive protein (CRP).
Disease activity was evaluated by measuring the CAI according to the Rachmilewitz’s criteria[16]. Clinical remission was defined as a final CAI value equal or < 4. A partial response was defined as a reduction of CAI score from baseline. The increasing of CAI value was classified as no response.
To complete the clinical assessment, QoL was also evaluated by a questionnaire adapted to the Italian population[17], with 29 questions grouped into 4 domains: Intestinal symptoms, systemic symptoms, emotional and social functions. Mucosal lesions were measured using the endoscopic score of Rachmilewitz, which evaluates the granular pattern (no = 0, yes = 2), the characteristics of the vascular pattern (normal = 0, impaired = 1, absent = 2), fragility of the mucosa (no = 0, upon touch = 2, spontaneously = 4) and mucosal lesions (no = 0, minor = 2, severe = 4). Endoscopic remission was defined by a score < 2[16].
All responder patients underwent a clinical evaluation at 12 mo of follow-up. All the parameters collected were inserted in a standard database.
Predictive factors of clinical response
Demographic and clinical characteristics of enrolled patients were considered for statistical analysis as predictive factors of clinical response in the short-term follow-up. Steroid dependency, CAI, CRP and ESR values at baseline, use of immunosuppressant, duration of disease (mo), age (year) and extent of disease (left side, pancolitis), were analyzed. No complete laboratory and clinical data were available in the long-term follow-up, so that no data were analyzed to identify factors predicting a sustained response.
Statistical analysis
Univariate analysis of parameters considered at baseline was performed by using the t test and the McNemar test for paired data, whereas comparing the same parameters between responders and non-responders, univariate analysis was performed using the t test for unpaired data. The parameters proving significance (P < 0.05) on univariate analysis were entered into a multivariate logistic regression model with the calculation of relative risk as odds ratios (OR) with 95% confidence intervals (CI), in order to determine the independent contributors to remission.
RESULTS
Assessment of clinical response to GMA treatment
Twenty-nine mild UC patients (CAI < 7) were treated with 5 GMA sessions, and 40 moderate UC patients (CAI < 12) were treated with 10 GMA sessions, following standard protocols. All patients completed the GMA sessions. During the overall 545 sessions of apheresis no major side-effects were registered. Two patients experienced dizziness and headache and one patient experienced a transient episode of arrhythmia.
At 11 wk of follow-up, 40 patients showed a complete clinical remission associated with endoscopic response. The end of treatment corresponded to the complete tapering of steroids in all cases. A partial response was evidenced in 10, and no variation in 19 patients.
As far as the CAI was concerned, the mean value dropped from 9.1 (range 6-13) at enrolment to 4.2 (range 0-10) at the end of GMA treatment (P < 0.01). Indeed, a final CAI value < 4 was observed in 40 patients.
In consideration of the individual parameters contributing to the CAI evaluation, complete disappearance of urgency and tenesmus was observed in 38 out of 40 responder patients. In 5 out 15 patients, peripheral arthralgia disappeared. The mean QoL score significantly decreased from 37.9 (range 18-67) to 24.4 (range 7-42) (P < 0.01).
ESR, CRP and WBC decreased at the end of the GMA sessions as follows: mean ESR decreased from 33.9 mm/h (range 7-60 mm/h) to 20.1 mm/h (range 2-20 mm/h) (P < 0.01); Mean CRP decreased from 2.04 mg/dL (range 0.3-7.3 mg/dL) to 0.29 mg/dL (range 0.15-1.5 mg/dL) (P = NS); Mean WBC count decreased from 10.59 k/μL to 7.7 k/μL (range 5.7-14 k/μL) (P < 0.01).
The endoscopic evaluation improved greatly in 40/69 patients with a score ranging from a mean value of 8.08 (range 4-10) to 3.9 (range 1-8). Thirteen patients presented an improvement with the reduction of at least 1 grade of inflammation before the end of treatment. In 16 patients, the endoscopic aspect showed no change.
At 12 mo of follow-up, 46 out of 50 responder patients were evaluated; Thirty-two of them were still in clinical remission (CAI < 4). Twelve patients presented an early moderate relapse (CAI < 12) and started azathioprine (at a dose of 1.5-2.5 mg/kg per day). The remaining 2 patients showed a severe relapse (CAI > 13) and underwent surgery and biological therapy with infliximab, respectively.
Assessment of predictive factors of clinical response
At univariate analysis the differences between responders and non-responders was analyzed. Out of the considered parameters, only CAI (P = 0.039) and ESR (P = 0.017) levels at baseline were singled out as independent contributors to predict remission in the short-term follow-up, with low rates in the responders group (Table 2).
Table 2.
Univariate analysis of predictive factors of clinical response to granulocyte-monocyte apheresis: Age, White blood cells, erythrocyte sedimentation rate and clinical activity index score, C reactive protein, location duration of disease at baseline and use of immunosuppressant (mean ± SD)
| Responders (n = 40) | Non responders (n = 29) | P-value | |
| Age (yr) | 42.7 ± 13.8 | 41.9 ± 6.6 | 0.774 |
| WBC 103 | 10.2 ± 2.7 | 11.2 ± 2.2 | 0.106 |
| ESR (mm/h) | 30.6 ± 13.5 | 38.4 ± 12.5 | 0.017 |
| CAI | 8.6 ± 2.1 | 9.7 ± 2.2 | 0.039 |
| CRP (mg/dL) | 1.2 ± 1.2 | 1.4 ± 1.4 | 0.526 |
| Location of disease (left side/pancolitis) | 28/12 | 19/10 | 0.795 |
| Duration of disease (yr) | 11 | 6 | 0.581 |
| Use of immunosuppressant | 5 | 15 | 0.601 |
CAI: Clinical activity index; ESR: Erythrocyte sedimentation rate; CRP: C reactive protein; WBC: White blood cells.
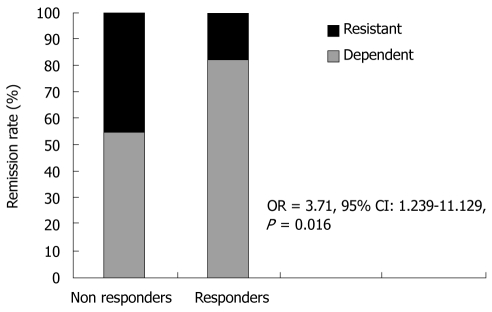
The logistic regression identified steroid dependency as an independent predictor of clinical response (OR = 3.71, 95% CI: 1.239-11.129, P = 0.016) (Figure 1).
Figure 1.
Correlation between clinical remission rate and steroid course. OR: Odds ratio; CI: Confidence interval.
Multivariate analysis revealed that steroid dependency (OR = 0.390, 95% CI: 0.176-0.865, Wald 5.361, P = 0.016) and low CAI levels at baseline (4 < CAI < 8) (OR = 0.770; 95% CI: 0.425-1.394, Wald 3.747, P = 0.028) were independent predictors of favorable clinical response in the short-term follow-up (Table 3).
Table 3.
Multivariate analysis of predictive factors of clinical response to granulocyte-monocyte apheresis: Steroid course and clinical activity index score at baseline
| Independent variables | OR | 95% CI | Wald | P-value |
| Steroid course | 0.390 | 0.176-0.865 | 5.361 | 0.016 |
| 4 < CAI < 8 | 0.770 | 0.425-1.394 | 3.747 | 0.028 |
| CRP (mg/dL) | 0.908 | 0.381-2.166 | 0.047 | 0.828 |
| ESR (mm/h) | 0.965 | 0.491-1.896 | 0.011 | 0.917 |
| Location of disease (Left side/pancolitis) | 0.822 | 0.462-1.462 | 0.446 | 0.504 |
| Duration of disease > 120 mo | 1.089 | 0.485-2.466 | 0.043 | 0.836 |
CAI: Clinical activity index; ESR: Erythrocyte sedimentation rate; CRP: C reactive protein; OR: Odds ratio; CI: Confidence interval.
DISCUSSION
GMA induced a complete response in 40 out of 69 patients with mild-moderate UC, with a significant reduction of CAI score levels, ESR, CRP, numbers of circulating neutrophils, as well as a significant improvement in QoL in accordance to the literature data[18-25]. Sustained remission was also observed in the follow-up in half of the treated patients.
A few heterogeneous studies have been carried out for identifying predictive factors of clinical response to GMA, but to our knowledge predictive factors of sustained remission have not been so far reported.
Moreover, small studies using Adacolumn (GMA) have reported that high CAI level at baseline represented a significant risk factor for GMA failure and that cumulative doses of steroids, previously used for UC treatment, were inversely related to clinical efficacy[21,24]. Conversely, a relatively large study, performed using Cellsorba device (leukocytapheresis), demonstrated that a steroid-dependent course and a high CRP concentration were independent predictors of favorable response[26].
The present data confirm that a steroid-dependent course is a predictor of clinical efficacy in GMA-treated patients. Thus, the present study is not in accordance with the previously reported negative association between remission and high cumulative dose of prednisone. Furthermore, these data support the use of standard treatment protocols in steroid-resistant patients, such as cyclosporine/tacrolimus, biological agents, immunosuppressant therapy, or surgery, rather than GMA, with regard to cost-effectiveness and time-saving.
Low baseline CAI scores were predictive of GMA treatment efficacy, thus confirming previously reported data and indicating GMA treatment only for mild-moderate disease.
Albeit a large multicenter, sham-controlled trial has been performed using GMA which did not support its effectiveness (response rate: 44% vs 39%, P = NS)[27], in that protocol duration of the disease was not indicated as well as the duration of concomitant medication. As suggested by the authors, unlike other studies, the study group did not include mainly steroid dependent/refractory patients, thus the need for further studies for better defining the issue is strongly suggested.
A recent meta-analysis, drawn from 7 randomized controlled trials, has shown homogeneous evidence that confirm GMA is much more effective in UC as compared to conventional therapy[28].
Despite limitations deriving from the retrospective nature of data analysis and the lack of long-term follow-up, the present study suggests that steroid dependency and mild disease represent good predictors of favorable clinical response to GMA treatment. The improvement in QoL related to the steroid-sparing effects was also relevant in the present series, suggesting an indication for GMA in pediatric UC patients, in whom steroid dependence is expected to interfere with a normal growth and development.
In conclusion, GMA may represent a useful therapeutic tool in steroid-dependent UC patients with mild to moderate disease, although further well designed sham-controlled studies are needed.
COMMENTS
Background
In a proportion of ulcerative colitis patients, the current pharmacological therapy is responsible for side-effects. A non-pharmacological approach, such as granulocyte-monocyte apheresis (GMA), proves effective in these patients and displays an excellent safety profile. Data are also available from studies involving pediatric patients showing good results, without side-effects. The efficacy of GMA has been documented by several studies, but few data are available on long-term follow-up and on factors predicting clinical response.
Research frontiers
This study showed that steroid dependency [Odds ratio (OR) = 4.097, P = 0.01] and low clinical activity index levels at baseline (OR = 0.745, P = 0.02) proved to be effective as factors predicting clinical response. GMA may be a valid therapeutic option for steroid-dependent ulcerative colitis patients with mild-moderate disease and its clinical efficacy appears to persist for 12 mo.
Innovations and breakthroughs
These findings support other published evidence on the association of clinical efficacy with an extremely favorable safety profile. The study also indicates that steroid dependency and low activity of the disease help identify those ulcerative colitis (UC) patients who are most likely to respond to treatment. Thus, GMA represents a promising non-pharmacological approach to UC, to be used when stable remission cannot be obtained by conventional therapy. Its use in pediatric UC patients, in whom steroid dependence is expected to interfere with a normal growth and development, could be an important challenge.
Peer review
The paper is generally well written and results analysed with appropriate statistical methods. The size of the cohort is fair and is enough to make some meaningful recommendations. The results are clearly tabulated and noted in the results section. The discussion is clearly written and the conclusions are appropriate to the findings.
Acknowledgments
Authors thank Dr. Patrizio Sala and Quirino Lai for statistical support.
Footnotes
Peer reviewers: John B Schofield, MB, BS, MRCP, FRCP, Department of Cellular Pathology, Preston Hall, Maidstone, Kent, ME20 7NH, United Kingdom; Francesco Manguso, MD, PhD, UOC di Gastroenterologia, AORN A. Cardarelli, Via A. Cardarelli 9, Napoli, 80122, Italy
S- Editor Wang JL L- Editor Logan S E- Editor Ma WH
References
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 2.Faubion WA Jr, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255–260. doi: 10.1053/gast.2001.26279. [DOI] [PubMed] [Google Scholar]
- 3.Masunaga Y, Ohno K, Ogawa R, Hashiguchi M, Echizen H, Ogata H. Meta-analysis of risk of malignancy with immunosuppressive drugs in inflammatory bowel disease. Ann Pharmacother. 2007;41:21–28. doi: 10.1345/aph.1H219. [DOI] [PubMed] [Google Scholar]
- 4.Hanai H. Positions of selective leukocytapheresis in the medical therapy of ulcerative colitis. World J Gastroenterol. 2006;12:7568–7577. doi: 10.3748/wjg.v12.i47.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen RD. Efficacy and safety of repeated infliximab infusions for Crohn's disease: 1-year clinical experience. Inflamm Bowel Dis. 2001;7 Suppl 1:S17–S22. doi: 10.1002/ibd.3780070505. [DOI] [PubMed] [Google Scholar]
- 6.Caprilli R, D'Ovidio V. Leukocytapheresis as promising therapy for inflammatory bowel disease. Dig Liver Dis. 2007;39:435–437. doi: 10.1016/j.dld.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Naganuma M, Funakoshi S, Sakuraba A, Takagi H, Inoue N, Ogata H, Iwao Y, Ishi H, Hibi T. Granulocytapheresis is useful as an alternative therapy in patients with steroid-refractory or -dependent ulcerative colitis. Inflamm Bowel Dis. 2004;10:251–257. doi: 10.1097/00054725-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Tomomasa T, Kobayashi A, Kaneko H, Mika S, Maisawa S, Chino Y, Syou H, Yoden A, Fujino J, Tanikawa M, et al. Granulocyte adsorptive apheresis for pediatric patients with ulcerative colitis. Dig Dis Sci. 2003;48:750–754. doi: 10.1023/a:1022892927121. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto T, Umegae S, Matsumoto K. Safety and clinical efficacy of granulocyte and monocyte adsorptive apheresis therapy for ulcerative colitis. World J Gastroenterol. 2006;12:520–525. doi: 10.3748/wjg.v12.i4.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandborn WJ. Preliminary data on the use of apheresis in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12 Suppl 1:S15–S21. doi: 10.1097/01.mib.0000195387.26892.22. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki Y, Yoshimura N, Saniabadi AR, Saito Y. Selective granulocyte and monocyte adsorptive apheresis as a first-line treatment for steroid naïve patients with active ulcerative colitis: a prospective uncontrolled study. Dig Dis Sci. 2004;49:565–571. doi: 10.1023/b:ddas.0000026299.43792.ae. [DOI] [PubMed] [Google Scholar]
- 12.Honma T, Sugimura K, Asakura H, Matsuzawa J, Suzuki K, Kobayashi M, Aoyagi Y. Leukocytapheresis is effective in inducing but not in maintaining remission in ulcerative colitis. J Clin Gastroenterol. 2005;39:886–890. doi: 10.1097/01.mcg.0000180638.59140.c5. [DOI] [PubMed] [Google Scholar]
- 13.Kruis W, Dignass A, Steinhagen-Thiessen E, Morgenstern J, Mössner J, Schreiber S, Vecchi M, Malesci A, Reinshagen M, Löfberg R. Open label trial of granulocyte apheresis suggests therapeutic efficacy in chronically active steroid refractory ulcerative colitis. World J Gastroenterol. 2005;11:7001–7006. doi: 10.3748/wjg.v11.i44.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munkholm P, Langholz E, Davidsen M, Binder V. Frequency of glucocorticoid resistance and dependency in Crohn's disease. Gut. 1994;35:360–362. doi: 10.1136/gut.35.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanai H. Leucocytapheresis for inflammatory bowel disease in the era of biologic therapy. Eur J Gastroenterol Hepatol. 2008;20:596–600. doi: 10.1097/MEG.0b013e3282f5e9f3. [DOI] [PubMed] [Google Scholar]
- 16.Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989;298:82–86. doi: 10.1136/bmj.298.6666.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin A, Leone L, Fries W, Naccarato R. Quality of life in inflammatory bowel disease. Ital J Gastroenterol. 1995;27:450–454. [PubMed] [Google Scholar]
- 18.Watanabe K, Oshitani N, Kamata N, Inagawa M, Yamagami H, Higuchi K, Arakawa T. Efficacy and endoscopic prediction of cytapheresis therapy in patients with refractory and steroid-dependent ulcerative colitis. Aliment Pharmacol Ther. 2006:24, 147–152. [Google Scholar]
- 19.Fukunaga K, Fukuda Y, Yokoyama Y, Ohnishi K, Kusaka T, Kosaka T, Hida N, Ohda Y, Miwa H, Matsumoto T. Activated platelets as a possible early marker to predict clinical efficacy of leukocytapheresis in severe ulcerative colitis patients. J Gastroenterol. 2006;41:524–532. doi: 10.1007/s00535-006-1789-y. [DOI] [PubMed] [Google Scholar]
- 20.D'Ovidio V, Aratari A, Viscido A, Marcheggiano A, Papi C, Capurso L, Caprilli R. Mucosal features and granulocyte-monocyte-apheresis in steroid-dependent/refractory ulcerative colitis. Dig Liver Dis. 2006;38:389–394. doi: 10.1016/j.dld.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Kanke K, Nakano M, Hiraishi H, Terano A. Clinical evaluation of granulocyte/monocyte apheresis therapy for active ulcerative colitis. Dig Liver Dis. 2004;36:811–817. doi: 10.1016/j.dld.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Takemoto K, Kato J, Kuriyama M, Nawa T, Kurome M, Okada H, Sakaguchi K, Shiratori Y. Predictive factors of efficacy of leukocytapheresis for steroid-resistant ulcerative colitis patients. Dig Liver Dis. 2007;39:422–429. doi: 10.1016/j.dld.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Hanai H, Watanabe F, Takeuchi K, Iida T, Yamada M, Iwaoka Y, Saniabadi A, Matsushita I, Sato Y, Tozawa K, et al. Leukocyte adsorptive apheresis for the treatment of active ulcerative colitis: a prospective, uncontrolled, pilot study. Clin Gastroenterol Hepatol. 2003;1:28–35. doi: 10.1053/jcgh.2003.50005. [DOI] [PubMed] [Google Scholar]
- 24.Cohen RD. Treating ulcerative colitis without medications--"look mom, no drugs!". Gastroenterology. 2005;128:235–236. doi: 10.1053/j.gastro.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 25.Habermalz B, Sauerland S. Clinical effectiveness of selective granulocyte, monocyte adsorptive apheresis with the Adacolumn device in ulcerative colitis. Dig Dis Sci. 2010;55:1421–1428. doi: 10.1007/s10620-009-0845-x. [DOI] [PubMed] [Google Scholar]
- 26.Sakata Y, Iwakiri R, Amemori S, Yamaguchi K, Fujise T, Otani H, Shimoda R, Tsunada S, Sakata H, Ikeda Y, et al. Comparison of the efficacy of granulocyte and monocyte/macrophage adsorptive apheresis and leukocytapheresis in active ulcerative colitis patients: a prospective randomized study. Eur J Gastroenterol Hepatol. 2008;20:629–633. doi: 10.1097/MEG.0b013e3282f5e9a4. [DOI] [PubMed] [Google Scholar]
- 27.Sands BE, Sandborn WJ, Feagan B, Löfberg R, Hibi T, Wang T, Gustofson LM, Wong CJ, Vandervoort MK, Hanauer S. A randomized, double-blind, sham-controlled study of granulocyte/monocyte apheresis for active ulcerative colitis. Gastroenterology. 2008;135:400–409. doi: 10.1053/j.gastro.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 28.Habermalz B, Sauerland S. Clinical effectiveness of selective granulocyte, monocyte adsorptive apheresis with the Adacolumn device in ulcerative colitis. Dig Dis Sci. 2010;55:1421–1428. doi: 10.1007/s10620-009-0845-x. [DOI] [PubMed] [Google Scholar]