Abstract
AIM: To evaluate the potential usefulness of acoustic radiation force impulse (ARFI) images for evaluation of hepatocellular carcinomas (HCC)-associated radiofrequency ablation.
METHODS: From January 2010 to June 2010, a total of 38 patients with HCC including recurred HCCs after RFA underwent ARFI elastography. The brightness of tumor was checked and the shear wave velocity was measured for the quantification of stiffness. According to the brightness, the tumors were classified as brighter, same color and darker compared with adjacent parenchyma. Using the same methods, 8 patients with recurred HCCs after RFA state were evaluated about the brightness compared with adjacent RFA ablation area.
RESULTS: In the 38 patients with HCCs, 20 (52.6%) were brighter than surrounding cirrhotic parenchyma. Another 13 (34.2%) were darker. The others (5 cases, 13.2%) were seen as the same color as the adjacent liver parenchyma. Post-RFA lesions were darker than previous tumor and surrounding parenchyma in all 38 cases. However, recurred HCCs were brighter than the treated site in all 8 cases.
CONCLUSION: Using ARFI technique is helpful for differential diagnosis in order to detect recurred HCCs more easily in patients with confusing status.
Keywords: Hepatocellular carcinoma, Elastography, Radiofrequency ablation
INTRODUCTION
In recent decades, there has been an increasing interest in assessing the viscoelastic properties of tissues with ultrasound. Ultrasonography (US) tissue-strain analysis can be performed under compression using Hitachi Real-time Tissue elastography (HI-RTE, Hitachi Medical Systems Europe, Zurich, Switzerland), eSie Touch (Siemens, Erlangen, Germany) or Elasticity Imaging (Simems)[1-8].
Acoustic radiation force impulse (ARFI) imaging is a new ultrasound imaging modality to evaluation of tissue stiffness by radiation forced-based imaging method that is provided with conventional B-mode US.
In ARFI imaging, an initial ultrasonic pulse is transmitted at diagnostic intensity levels to obtain a baseline signal for later comparison. A short-duration (approximately 0.3 s), high intensity acoustic “pushing pulse” is then transmitted by the same transducer, and followed by a series of diagnostic intensity pulses, which are used to track the displacement of the tissue caused by the pushing pulse[9-11]. The response of the tissue to the radiation force is observed using conventional B-mode imaging pulses and it is possible to display the quantitative shear wave velocity (SWV, m/s) of ARFI displacement[12,13]. Because the velocity of the shear wave depends on the tissue stiffness, it is possible to apply ARFI technology to elastography[14-19].
Until now, there are no studies that have evaluated ARFI elastrography’s usefulness in differentiating HCC, post RFA HCC and recurred HCC after RFA and no studies have been reported on the quantification of tumor stiffness using shear wave velocity, which may help in the diagnosis.
The present study was performed to investigate the potential usefulness of ARFI elastrography for evaluation of HCC associated RFA, assuming that different features can be shown on ARFI elastrophy images according to the HCC, post RFA HCC and recurred HCC after RFA.
MATERIALS AND METHODS
Patients
Between January 2010 and June 2010, a total of 38 patients with viable HCC were evaluated by ARFI elastography. Eight patients had recurred HCC after RFA. Because every patient each had one HCC, a total of 38 HCCs were included in this study.
In cases of technical failure such as patient motion, the presence of a deep-seated lesion or the patient’s inability to hold their breath properly, recurred HCC after TACE or operation, and multiple HCCs were excluded from this study.
Mean tumor diameter was 2.4 cm (0.8-3.5 cm).
The diagnosis of all HCCs was based on the typical findings determined using either computed tomography (CT) or magnetic resonance (MR) imaging and/or biopsy. The following imaging findings were considered for the diagnosis of HCC: (1) tumors showing typical enhancement pattern (early enhancement on the arterial phase and wash-out on the portal and delayed phases); and/or (2) mass showing high signal intensity on the T2 weighted image.
A detailed medical history was obtained from all 38 patients. All patients had underlying liver disease which was established according to serum markers and liver biopsy as follows: chronic viral hepatitis was diagnosed as serum positive HBV-DNA and/or HCV-RNA with an elevated serum alanine amino transferase (ALT). Chronic alcoholic hepatitis was diagnosed as a history of long term alcohol consumption with an elevated serum ALT. Liver cirrhosis was diagnosed based on cross-sectional imaging findings and/or biopsy.
Image protocol and analysis
B-mode standard US scanning and ARFI elastography were performed using a SIEMENS Acuson S2000 using a 4-1 MHz curved array probe. Two experienced radiologists participated in the US scanning. Prior to RFA, the radiologists scanned the liver to locate the HCC detected on the other cross-sectional imaging technique, such as CT, MRI, and/or prior US. After fitting the ARFI image box to cover the lesion, an ARFI image was obtained with a corresponding B-mode image. The SWV was obtained from the HCC and hepatic parenchyma, three times. After RFA, ARFI images and SWV were obtained, as before RFA.
Two radiologists, who performed US, reviewed all of the B-mode and ARFI images. The stiffness of the pre- and post-RFA HCCs were analyzed on the ARFI images. The HCCs were categorized according to the brightness, darker (stiffer), same color (equally stiff), or brighter (softer), based on the HCCs brightness relative to the hepatic parenchyma on the ARFI images. The SWV of HCCs and hepatic parenchyma were averaged. These SWVs were used for quantification of stiffness.
Discrepancies between the two reviewers were resolved through consensus.
RESULTS
Table 1 summarized the stiffness of HCCs on the ARFI image. Before RFA, 20 HCCs (52.6%) appeared as a bright color, which meant the HCC were softer than hepatic background (Figure 1). When compared with the hepatic parenchyma, 13 HCCs (34.2%) had a darker appearance, which indicated HCCs were stiffer than surrounding hepatic parenchyma (Figure 2). The remaining HCCs (5 cases, 13.2%) were seen as the same color as the adjacent hepatic parenchyma, which indicated the HCC had the same stiffness as the hepatic background. After RFA, all HCCs (38 cases, 100%) revealed a darker color (Figure 3). All recurred HCC after RFA (8 cases), showed a bright appearance compared to the RFA site (Figure 4). This means that RFA ablation site was harder than before RFA, so the recurred HCC were softer than the surrounding area of the prior RFA site. 5 cases of recurred HCC after RFA were treated by re-RFA. The others were treated by TACE (2 cases), or operation (1 case).
Table 1.
Stiffness of hepatocellular carcinomas on acoustic radiation force impulse imaging
| Acoustic radiation force impulse imaging | Darker (stiffer) | Same color (equally stiff) | Bright (softer) |
| Pre-RFA HCCs (38) | 13 | 5 | 20 |
| Recurred HCCs (8) | 0 | 0 | 8 |
| Post-RFA HCCs (38) | 38 | 0 | 0 |
HCCs: Hepatocellular carcinomas; RFA: Radiofrequency ablation.
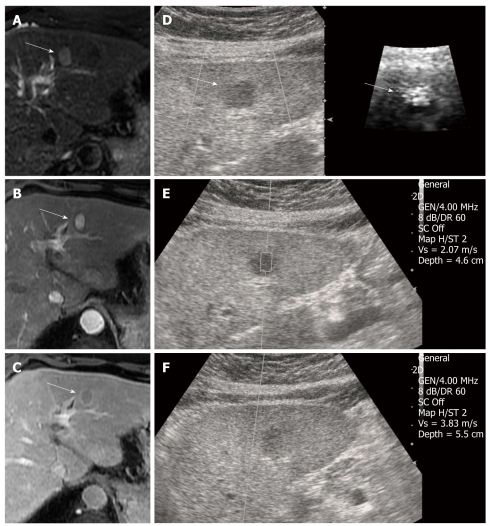
Figure 1.
Hepatocellular carcinoma in a 59-year-old man with underlying liver cirrhosis. A: T2-weighted magnetic resonance (MR) image shows a high signal intensity mass in segment 2 of the liver (arrow); B: On dynamic study, the mass revealed an arterial enhancement; C: Delayed wash-out, which is a typical finding of hepatocellular carcinoma (HCC) (arrow); D: On B-mode image, the HCC appears as a well defined, hypoechoic mass and the HCC have a bright (softer) color on the acoustic radiation force impulse (ARFI) image (arrows); E: The shear wave velocity (SWV) of HCC is 2.07 m/s; F: SWV of surrounding cirrhotic hepatic parenchyma measures 3.83 m/s. GEN: General; SC: Spatial compounding; Vs: Velocity.
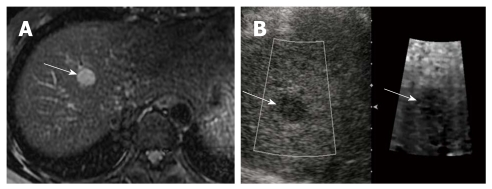
Figure 2.
Hepatocellular carcinoma in a 45-year-old woman with underlying liver cirrhosis. A: T2-weighted magnetic resonance image shows a high signal intensity hepatocellular carcinoma (HCC) in segment 4 of the liver (arrow); B: On a B-mode image, the HCC is seen as an ovoid, hypoechoic mass (arrow), that appears darker (stiffer) than hepatic background on the acoustic radiation force impulse image (arrow). The shear wave velocity of HCC and surrounding hepatic parenchyma were measured as 2.20 m/s and 1.57 m/s, respectively (not shown).
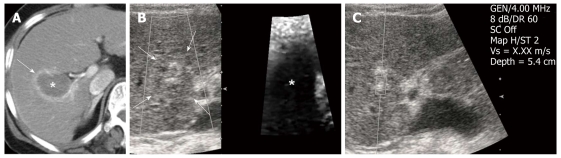
Figure 3.
Post-radiofrequency ablation hepatocellular carcinoma in a 71-year-old woman with underlying liver cirrhosis. A: Contrast-enhanced arterial phase computed tomography scan obtained immediately after radiofrequency ablation (RFA) shows the ablation zone of low attenuation (asterisk) with a surrounding ring of benign enhancement (arrows) in segment 5 of the liver; B: Next day, follow up B-mode image of post-RFA hepatocellular carcinoma (HCC) is seen as an ill-defined heterogeneous echogenic lesion (arrow) and the HCC appears darker on the acoustic radiation force impulse image (asterisk); C: The shear wave velocity of post-RFA HCC is uncheckable, shown as X.XX m/s. GEN: General; SC: Spatial compounding; Vs: Velocity.
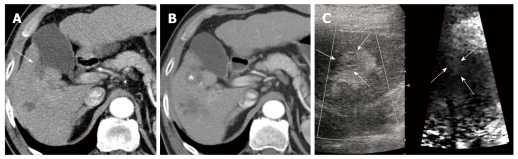
Figure 4.
Recurred hepatocellular carcinoma after radiofrequency ablation in a 65-year-old man with underlying liver cirrhosis. A: Follow-up post-radiofrequency ablation (RFA) computed tomography (CT) scan shows the ablation zone of low attenuation without residual or recurrent tumor in segment 5 of the liver (arrow); B: Contrast-enhanced CT scan obtained after 6 mo shows enhancing nodule in the ablated lesion (asterisk). The serum alpha-fetoprotein increased from 2.6 ng/mL to 16.8 ng/mL; C: On a B-mode image, prior RFA site and recurred hepatocellular carcinoma (HCC) are seen as homogeneous hyperechoic lesions and subtle heterogeneous echogenic nodules with a thin hypoechoic rim (arrows). The recurred HCC appears as a brighter color (softer) with a distinct border on the acoustic radiation force impulse image.
The tumor diagnosis of recurrence after RFA was based on the typical findings determined using either CT or MRI and serum tumor marker elevation (AFP and/or PIVKA).
Two cases showed typical imaging findings of tumor recurrence at CT, but serum tumor marker level was normal. But one showed typical imaging findings of tumor recurrence on MRI, another imaging study. The other recurred HCC was confirmed after surgery.
Table 2 summarized the SWV of HCCs and hepatic parenchyma. Cirrhotic liver parenchyma and non-cirrhotic liver parenchyma had a mean SWV of 2.62 m/s and 1.04 m/s, respectively. The mean SWVs of pre-RFA HCCs and recurred HCCs after RFA were 2.04 m/s and 2.02 m/s. All post-RFA HCCs were uncheckable, and their numerical value was shown ‘X.XX’. This nonnumerical value is due to abrupt tissue degeneration in the RFA site preventing a reliable reading.
Table 2.
Stiffness of hepatic parenchyma and hepatocellular carcinomas on shear wave velocity
| Mean shear wave velocity (m/s) | ||||
| Cirrhosis | Non-cirrhosis | Pre-RFA HCCs | Recurred HCCs | Post-RFA HCCs |
| 2.62 | 1.04 | 2.04 | 2.02 | uncheckable |
HCCs: Hepatocellular carcinomas; RFA: Radiofrequency ablation.
DISCUSSION
Recently, ARFI sonoelastography has been used to noninvasively generate internal mechanical excitation and has attracted great attention for its use in the measurement of tumor stiffness[20-21]. To our knowledge, no other investigators have evaluated the use of ARFI sonoelastography for the evaluation of post-RFA recurrent HCC. In our study, pre-RFA HCCs showed variable hardness as compared with the liver parenchyma on ARFI images. These results seem to be inconsistent with the results of Fahey’s study[22,23], in which all HCCs were softer than the surrounding liver. This discordancy was thought to be the difference in parenchymal hardness between chronic hepatitis and liver cirrhosis. Additionally, differences in severity of liver cirrhosis also might be contributing to the discrepancy.
Actually according to the other report[24], there was no statistically significant difference at the HCCs in terms of tumor stiffness suggested by ARFI elastography (P > 0.05).
In all post-RFA HCCs, thermal ablated necrotic lesions were seen as a darker color on the ARFI images and had uncheckable SWV. In the RFA site, the system constantly provided a nonnumerical value. It was considered an unknown mechanical error that was associated with immediate post-RFA state[25].
Although remarkable advances in surgical and imaging techniques have improved the prognosis of HCC patients, the high incidence of intrahepatic recurrence remains a major challenge in HCC therapy[26,27]. However, sometimes B-mode imaging for assessment of HCC recurrence is not satisfactory.
On ARFI imaging, most of the recurrent HCCs appeared brighter than the pre-RFA ablation sites. Moreover, most of the cases revealed distinct borders, unlike the B-mode images. The RFA ablation site comprised hard lesions that showed coagulative necrosis and fibrotic scarring. As a result, recurrent HCCs were found to be softer with improved contrast compared to the surrounding pre-RFA ablation area. Thus, we thought that the ARFI images were superior to the B-mode images for evaluating HCC recurrence after RFA and also useful to guide the second round of RFA for recurrent HCC.
COMMENTS
Background
Recently, acoustic radiation force impulse (ARFI) sonoelastography has been used to noninvasively generate internal mechanical excitation and has attracted great attention for its use in the measurement of tumor stiffness.
Research frontiers
To our knowledge, no other investigators have evaluated the use of ARFI sonoelastography for the evaluation of post-radiofrequency ablation (RFA) recurrent hepatocellular carcinoma (HCC).
Innovations and breakthroughs
The RFA ablation site was comprised of hard lesions that showed coagulative necrosis and fibrotic scarring. As a result, recurrent HCCs were found to be softer with improved contrast compared to the surrounding pre-RFA ablation area.
Applications
ARFI images are superior to B-mode images for evaluating HCC recurrence after RFA and also useful to guide the second round of RFA for recurrent HCC.
Terminology
ARFI imaging is a new ultrasonography (US) imaging modality used to evaluate tissue stiffness by a radiation force-based imaging method that is provided with conventional B-mode US.
Peer review
This is an interesting report of the effect of RFA on HCC by use of ARFI techniques.
Footnotes
Supported by Research Funds from Dong-A university
Peer reviewer: Toru Ishikawa, MD, Department of Gastroenterology, Saiseikai, Niigata Second Hospital, Teraji 280-7, Niigata 950-1104, Japan
S- Editor Sun H L- Editor O’Neill M E- Editor Ma WH
References
- 1.McLaughlin J, Renzi D, Parker K, Wu Z. Shear wave speed recovery using moving interference patterns obtained in sonoelastography experiments. J Acoust Soc Am. 2007;121:2438–2446. doi: 10.1121/1.2534717. [DOI] [PubMed] [Google Scholar]
- 2.Melodelima D, Bamber JC, Duck FA, Shipley JA. Transient elastography using impulsive ultrasound radiation force: a preliminary comparison with surface palpation elastography. Ultrasound Med Biol. 2007;33:959–969. doi: 10.1016/j.ultrasmedbio.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960–974. doi: 10.1053/j.gastro.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 5.Friedrich-Rust M, Ong MF, Herrmann E, Dries V, Samaras P, Zeuzem S, Sarrazin C. Real-time elastography for noninvasive assessment of liver fibrosis in chronic viral hepatitis. AJR Am J Roentgenol. 2007;188:758–764. doi: 10.2214/AJR.06.0322. [DOI] [PubMed] [Google Scholar]
- 6.Gheorghe L, Iacob S, Iacob R, Dumbrava M, Becheanu G, Herlea V, Gheorghe C, Lupescu I, Popescu I. Real time elastography - a non-invasive diagnostic method of small hepatocellular carcinoma in cirrhosis. J Gastrointestin Liver Dis. 2009;18:439–446. [PubMed] [Google Scholar]
- 7.Adamietz BR, Meier-Meitinger M, Fasching P, Beckmann M, Hartmann A, Uder M, Häberle L, Schulz-Wendtland R, Schwab SA. New diagnostic criteria in real-time elastography for the assessment of breast lesions. Ultraschall Med. 2011;32:67–73. doi: 10.1055/s-0029-1245821. [DOI] [PubMed] [Google Scholar]
- 8.Meier-Meitinger M, Häberle L, Fasching PA, Bani MR, Heusinger K, Wachter D, Beckmann MW, Uder M, Schulz-Wendtland R, Adamietz B. Assessment of breast cancer tumour size using six different methods. Eur Radiol. 2010 doi: 10.1007/s00330-010-2016-z. [DOI] [PubMed] [Google Scholar]
- 9.McAleavey SA, Menon M, Orszulak J. Shear-modulus estimation by application of spatially-modulated impulsive acoustic radiation force. Ultrason Imaging. 2007;29:87–104. doi: 10.1177/016173460702900202. [DOI] [PubMed] [Google Scholar]
- 10.Nightingale K, Soo MS, Nightingale R, Trahey G. Acoustic radiation force impulse imaging: in vivo demonstration of clinical feasibility. Ultrasound Med Biol. 2002;28:227–235. doi: 10.1016/s0301-5629(01)00499-9. [DOI] [PubMed] [Google Scholar]
- 11.Nightingale K, McAleavey S, Trahey G. Shear-wave generation using acoustic radiation force: in vivo and ex vivo results. Ultrasound Med Biol. 2003;29:1715–1723. doi: 10.1016/j.ultrasmedbio.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Fahey BJ, Nightingale KR, Nelson RC, Palmeri ML, Trahey GE. Acoustic radiation force impulse imaging of the abdomen: demonstration of feasibility and utility. Ultrasound Med Biol. 2005;31:1185–1198. doi: 10.1016/j.ultrasmedbio.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Nightingale K, Palmeri M, Trahey G. Analysis of contrast in images generated with transient acoustic radiation force. Ultrasound Med Biol. 2006;32:61–72. doi: 10.1016/j.ultrasmedbio.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Gallotti A, D'Onofrio M, Romanini L, Cantisani V, Pozzi Mucelli R. Acoustic Radiation Force Impulse (ARFI) ultrasound imaging of solid focal liver lesions. Eur J Radiol. 2011:[Epub ahead of print]. doi: 10.1016/j.ejrad.2010.12.071. [DOI] [PubMed] [Google Scholar]
- 15.Piscaglia F, Salvatore V, Di Donato R, D'Onofrio M, Gualandi S, Gallotti A, Peri E, Borghi A, Conti F, Fattovich G, et al. Accuracy of VirtualTouch Acoustic Radiation Force Impulse (ARFI) Imaging for the Diagnosis of Cirrhosis during Liver Ultrasonography. Ultraschall Med. 2011;32:167–175. doi: 10.1055/s-0029-1245948. [DOI] [PubMed] [Google Scholar]
- 16.Palmeri ML, Wang MH, Rouze NC, Abdelmalek MF, Guy CD, Moser B, Diehl AM, Nightingale KR. Noninvasive Evaluation of Hepatic Fibrosis using Acoustic Radiation Force-Based Shear Stiffness in Patients with Nonalcoholic Fatty Liver Disease. J Hepatol. 2011:[Epub ahead of print]. doi: 10.1016/j.jhep.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SU, Jang HW, Cheong JY, Kim JK, Lee MH, Kim DJ, Yang JM, Cho SW, Lee KS, Choi EH, et al. The usefulness of liver stiffness measurement using FibroScan in chronic hepatitis C in South Korea: a multicenter, prospective study. J Gastroenterol Hepatol. 2011;26:171–178. doi: 10.1111/j.1440-1746.2010.06385.x. [DOI] [PubMed] [Google Scholar]
- 18.Sporea I, Sirli R, Popescu A, Danilă M. Acoustic Radiation Force Impulse (ARFI)--a new modality for the evaluation of liver fibrosis. Med Ultrason. 2010;12:26–31. [PubMed] [Google Scholar]
- 19.Horster S, Mandel P, Zachoval R, Clevert DA. Comparing acoustic radiation force impulse imaging to transient elastography to assess liver stiffness in healthy volunteers with and without valsalva manoeuvre. Clin Hemorheol Microcirc. 2010;46:159–168. doi: 10.3233/CH-2010-1342. [DOI] [PubMed] [Google Scholar]
- 20.Heide R, Strobel D, Bernatik T, Goertz RS. Characterization of focal liver lesions (FLL) with acoustic radiation force impulse (ARFI) elastometry. Ultraschall Med. 2010;31:405–409. doi: 10.1055/s-0029-1245565. [DOI] [PubMed] [Google Scholar]
- 21.Nahon P, Kettaneh A, Lemoine M, Seror O, Barget N, Trinchet JC, Beaugrand M, Ganne-Carrié N. Liver stiffness measurement in patients with cirrhosis and hepatocellular carcinoma: a case-control study. Eur J Gastroenterol Hepatol. 2009;21:214–219. doi: 10.1097/MEG.0b013e32830eb8d7. [DOI] [PubMed] [Google Scholar]
- 22.Fahey BJ, Nelson RC, Hsu SJ, Bradway DP, Dumont DM, Trahey GE. In vivo guidance and assessment of liver radio-frequency ablation with acoustic radiation force elastography. Ultrasound Med Biol. 2008;34:1590–1603. doi: 10.1016/j.ultrasmedbio.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fahey BJ, Nelson RC, Bradway DP, Hsu SJ, Dumont DM, Trahey GE. In vivo visualization of abdominal malignancies with acoustic radiation force elastography. Phys Med Biol. 2008;53:279–293. doi: 10.1088/0031-9155/53/1/020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho SH, Lee JY, Han JK, Choi BI. Acoustic radiation force impulse elastography for the evaluation of focal solid hepatic lesions: preliminary findings. Ultrasound Med Biol. 2010;36:202–208. doi: 10.1016/j.ultrasmedbio.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Gallotti A, D'Onofrio M, Pozzi Mucelli R. Acoustic Radiation Force Impulse (ARFI) technique in ultrasound with Virtual Touch tissue quantification of the upper abdomen. Radiol Med. 2010;115:889–897. doi: 10.1007/s11547-010-0504-5. [DOI] [PubMed] [Google Scholar]
- 26.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999;229:216–222. doi: 10.1097/00000658-199902000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neeleman N, Andersson R. Repeated liver resection for recurrent liver cancer. Br J Surg. 1996;83:893–901. doi: 10.1002/bjs.1800830705. [DOI] [PubMed] [Google Scholar]