Abstract
AIM: To study the effect of salvianolate on expression of tumor necrosis factor (TNF)-α and interleukin (IL)-6 mRNA in small intestine of cirrhotic rats.
METHODS: Cirrhosis in rats was induced using CCl4 (0.3 mL/kg). Rats were randomly divided into non-treatment group, low-dose salvianolate (12 mg/kg) treatment group, medium-dose salvianolate (24 mg/kg) treatment group, and high-dose salvianolate (48 mg/kg) treatment group, and treated for 2 wk. Another 10 healthy rats served as a normal control group. Mortality of cirrhotic rats in each group was evaluated after treatment with salvianolate. Serum samples were taken from portal vein for the detection of endotoxin. Morphological changes in tissue samples from the ileocecum were observed under a light microscope. Expression of TNF-α and IL-6 mRNA in the small intestine of rats was analyzed by real-time reverse-transcriptase polymerase chain reaction.
RESULTS: The mortality of cirrhotic rats in the non-treatment group was 37.5%. No cirrhotic rat died in the high-dose salvianolate treatment group. The serum endotoxin level was significantly higher in the non-treatment group than in the salvianolate treatment and normal control groups. The intestinal mucosal and villous atrophy, necrosis and shedding of the intestinal mucosal epithelium, observed in the non-treatment group, were reversed in different salvianolate treatment groups. The TNF-α and IL-6 mRNA expression levels in small intestine were significantly lower in different salvianolate treatment groups than in the non-treatment group.
CONCLUSION: Salvianolate can reduce the endotoxin level, ameliorate the injury of intestinal mucosa, and inhibit the expression of TNF-α and IL-6 mRNA in small intestine of cirrhotic rats.
Keywords: Salvianolate, Cirrhosis, Endotoxin, Intestinal mucosa, Tumor necrosis factor-α, Interleukin-6
INTRODUCTION
In liver cirrhosis patients, disruption of intestinal barrier function (IBF) and increased intestinal permeability lead to bacterial translocation (BT) and endotoxemia[1-6], which increase susceptibility to infection, with spontaneous bacterial peritonitis (SBP) being the most frequent and severe[1,4]. Endotoxemia, resulting from BT[4], may provoke sustained activation of the immune system with release of proinflammatory cytokines, such as tumor necrosis factor (TNF)-α and interleukins (IL)-1, 6 and 8, and nitric oxide (NO), which in turn decrease IBF and increase severe complications[7,8]. Intestinal cytokines play an important role in the pathogenesis of intestinal injury and inflammation[9,10], especially TNF-α and IL-6, which may contribute to the systemic hemodynamic derangement of liver cirrhosis[11] and lead to liver failure[12]. Therefore, restoration of the intestinal barrier integrity and inhibition of the cytokine expression are the important goals in preventing intestinal endotoxemia. However, no effective remedy is available at present for the prevention and treatment of intestinal endotoxemia.
Radix Salviae Miltiorrhizae, a traditional Chinese medical herb known as “danshen”, has been widely used in treatment of various cardiovascular diseases[13,14]. Its extracts contain lipid-soluble diterpene quinones (tanshinones) and water-soluble phenolic acid derivatives, such as salvianolic acids A and B as well as lithospermic acid B[15]. Recent pharmacological studies showed that Salviae Miltiorrhizae (S. miltiorrhiza) can eliminate oxygen free radicals, enhance antioxidant activity, decrease serum levels of cytokines, and inhibit endotoxemia[16]. It has been demonstrated that S. miltiorrhiza can block the lethal toxicity of lipopolysaccharide (LPS) in mice by suppressing TNF-α release[17]. Salvianolate is a new water-soluble phenolic compound that is one of the most bioactive compounds in S. miltiorrhiza Bge. As far as we are know, no reports are available at present on the pharmacological activities of salvianolate in liver cirrhosis patients. TNF-α and IL-6 are the most frequent cytokines associated with liver dysfunction in cirrhosis patients[18], and show an increased local production in mesenteric lymph nodes in response to BT induced by intestinal injury[19].
The present study was designed to investigate the effect of salvianolate on endotoxin level in the portal vein and expression of TNF-α and IL-6 mRNA in small intestine of rats with CCl4-induced liver cirrhosis. Whether different doses of salvianolate can enhance the intestinal mucosal barrier function and prevent intestinal endotoxemia is also studied. The results of the present study provide a new strategy for the treatment of liver cirrhosis.
MATERIALS AND METHODS
Animals
Ninety male Sprague-Dawley rats weighing 180-220 g were provided by Department of Animal Care, Zhejiang Traditional University (Hangzhou, China). Experimental animals were housed in individual cages at 22-25°C in a 12-h light/dark cycle with free access to standard laboratory diet and tap water.
Experimental protocol
The rats were randomly divided into normal control group (n = 10) and model group. Rats in the model group received subcutaneous injection of 40% CCl4 in a 2:3 mixture with olive oil (0.3 mL/kg), once a week for 12 wk. Liver cirrhosis was induced in 55 rats at the end of 12 wk, as shown by liver histological evaluation (Figure 1). The 55 rats in model group were further divided into non-treatment group (group B, n = 14), low-dose salvianolate (12 mg/kg) treatment group (group C, n = 14), medium-dose salvianolate (24 mg/kg) treatment group (group D, n = 14), and high-dose salvianolate (48 mg/kg) treatment group (group E, n = 13). Rats in group A were intraperitoneally (ip) injected with 5% glucose solution, once a week for 2 wk. Rats in groups C-E were ip injected with different doses of salvianolate dissolved in a 5% glucose solution, once a week for 2 wk. At the same time, 40% CCl4 was continued for an experimental period of 14 wk. At the end of the 14-wk experimental period, all rats were anesthetized with 3% chloral hydrate and dissected. Blood samples were taken from the portal vein and intestinal tissue for further analysis.
Figure 1.
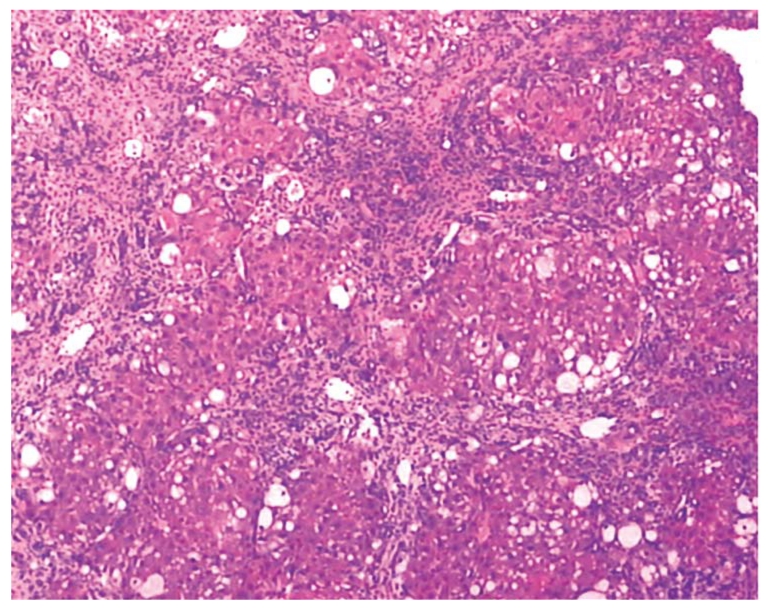
Liver histology in a cirrhotic rat model (HE staining, × 200).
Measurement of serum endotoxin level
Five milliliters of blood was taken from the portal vein and immediately put into a tube containing heparin. Plasma was taken after the blood was centrifuged at 3000 r/min for 1 min at 0°C. Endotoxin level in blood was measured by photometry, using a MB-80 microbiology kinetic rapid reader (Beijing, Gold Mountainriver Tech Development Co., Ltd, China).
Assessment of morphological changes in intestinal mucous membrane
At the end of the 14-wk experimental period, a horizontal incision was made along the mid-section to expose the abdominal cavities of all rats with their intestines excised. Ileal tissue samples were taken immediately and washed 3 times with cold physiological saline, fixed in a 10% formalin solution, dehydrated and embedded in paraffin. Each sample was cut into 4 μm-thick sections which were stained with hematoxylin and eosin (H and E), and examined under a light microscope (Olympus BX50; Tokyo, Japan).
Isolation and analysis of mRNA expression by real-time reverse transcriptase polymerase chain reaction
Total RNA was isolated from snap-frozen ileal tissue samples using the Trizol method (Invitrogen, Carlsbad, CA, USA) and treated with RNase-free water. Single-stranded cDNA was synthesized from the total RNA as follows. In brief, 1 μg RNA was pre-incubated with 1 μL oligo (dT)15 primer, and diethylpyrocarbonate (DEPC)-treated water was added to a total volume of 9.5 μL at 70°C for 10 min, and then rapidly chilled on ice. To the annealed primer/template, 4 μL 5 × RT (reverse transcriptase) buffer, 0.5 μL dNTP (10 mmol/L each), 25 U ribonuclease inhibitor (Takara, Dalian, China), 200 U Moloney murine leukaemia virus reverse transcriptase (Takara) and DEPC-treated water were added to a final volume of 20 μL. The reaction was incubated at 42°C for 1 h and terminated by placing it on ice after deactivation at 70°C for 10 min. The resultant cDNA was used as a template for subsequent polymerase chain reaction (PCR).
The PCR mixture contained 5 μL 10 × Taq buffer (Takara), 4 μL dNTP (10 mmol/L each), 2 μL gene-specific primers, 2.5 U Taq DNA polymerase (Takara) and 2 μL cDNA in a total volume of 50 μL. Thirty cycles of PCR amplification were performed with an initial incubation at 94°C for 3 min and a final extension at 72°C for 7 min. Each cycle consisted of denaturation at 94°C for 30 s, annealing at 53°C for 30 s, and extension at 72°C for 30 s. The primer sequences used for PCR are shown in Table 1.
Table 1.
Forward and reverse primers used in quantitative real-time polymerase chain reaction
| Gene | Primer sequence (5’-3’) | Annealing temperature (°C) | Product size (bp) |
| β-actin | 5’-ACTGCCGCATCCTCTTCCTC-3’ 5’-ACTCCTGCTTGCTGATCCACAT-3’ | 55 | 598 |
| Rat TNF-α | 5’-GGCAGGTCTACTTTGGAGTCATTGC-3’ | 58 | 318 |
| 5’-ACATTCGGGGATCCAGTGAGCTCCG-3’ | |||
| Rat IL-6 | 5’-GGATACCACCCACAACAG-3’ 5’-GGTCCTTAGCCACTCCTT-3’ | 58 | 451 |
TNF: Tumor necrosis factor; IL: Interleukin.
The quantities of cDNA that produced an equal amount of β-actin PCR product were used in PCR with the primers for IL-6 and TNF-α. Following reverse transcription polymerase chain reaction (RT-PCR), 5 μL samples of the amplified products was resolved by electrophoresis on 1% agarose gel and stained with ethidium bromide. The level of each PCR product was semi-quantitatively evaluated using a digital camera and an image analysis system (Vilber Lourmat, Marne La Vallée, France), and normalized to GAPDH.
DNA was amplified and detected by BioRad iCycler iQ PCR (BioRad Laboratories, California, USA) in a final volume of 20 μL, using SYBR green master mix reagent at a final concentration of 1 × (Applied Biosystems, Foster City, CA, USA). The PCR amplification conditions for DNA were 95°C for 3 min and 40 cycles at 95°C for 30 s at 55°C for 30 s, and at 72°C for 30 s. A melting curve analysis was carried out after amplification. The threshold cycle (Ct) values and baseline settings were determined by automatic analysis settings. Data were analyzed using the Opticon Monitor 3 software, which was supplied by The BioRad iCycler iQ PCR. Data about relative mRNA copies were expressed as relative quantification (RQ), which was calculated using the 2–ΔΔCt method, where ΔΔCt = ΔCt (cirrhosis group) - ΔCt (normal group), ΔCt = (Ctsample - Ctβ-actin).
Statistical analysis
Statistical analysis was performed with the SPSS version 13.0 (Chicago, IL, USA). Mortality of rats was compared using Fisher’s exact test. Endotoxin level was analyzed using Kruskal-Wallis H test. Results of quantitative RT-PCR were assessed by ANOVA. Data were expressed as mean ± SD. P < 0.05 was considered statistically significant.
RESULTS
Mortality
At the end of 14-wk experimental period, 6 rats in group B, 3 rats in groups B and C, and no rats in groups A and E died. The mortality rate of rats was significantly higher in group B than in groups A and E (P < 0.05, Table 2).
Table 2.
Mortality of rats in different groups
| Group | n | Mortality values |
| A | 10 | 0 (0/10)a |
| B | 14 | 37.5% (6/14) |
| C | 14 | 21.42% (3/14) |
| D | 14 | 21.42% (3/14) |
| E | 13 | 0 (0/13)a |
P < 0.05 vs non treatment group. A: Normal control group; B: Non treatment group; C: Low-dose salvianolate treatment group; D: Medium-dose salvianolate treatment group; E: High-dose salvianolate treatment group.
Plasma endotoxin level
The plasma endotoxin level was < 20 pg/L in groups A and E, > 20 pg/L in 7 rats of group B, and significantly higher in the non-treatment group than in different salvianolate treatment groups and normal control group (P < 0.01). No marked difference was found in the plasma endotoxin level between the normal control and high-dose salvianolate treatment groups (Table 3).
Table 3.
Distribution of endotoxin levels in serum of different groups
| Group | n | Dose (mg/kg) |
Levels of endotoxin (pg/L) |
||||
| 0-1 | 1-10 | 10-20 | > 20 | Mean rank | |||
| A | 10 | - | 10 | 0 | 0 | 0 | 15.35b |
| B | 14 | - | 2 | 2 | 3 | 7 | 55.71 |
| C | 14 | 12 | 8 | 4 | 2 | 0 | 47.54a |
| D | 14 | 24 | 12 | 1 | 1 | 0 | 29.14b |
| E | 13 | 48 | 12 | 1 | 0 | 0 | 14.57b |
P < 0.05,
P < 0.01 vs non treatment group. A: normal control group; B: non treatment group; C: low-dose salvianolate treatment group; D: medium-dose salvianolate treatment group; E: high-dose salvianolate treatment group.
Histological changes in ileal tissue
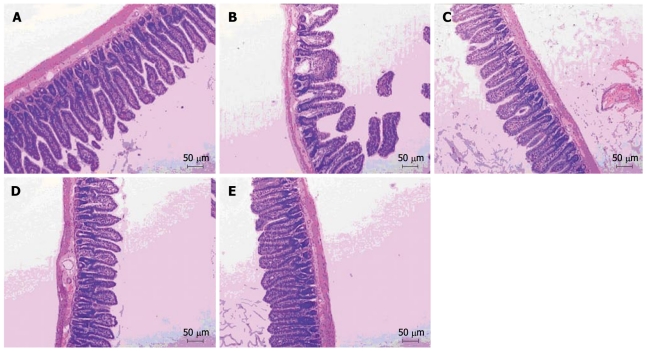
As shown in Figure 2, the intestinal mucosa in normal control group was intact and the villi were presented in an orderly fashion. No inflammatory cell infiltration occurred in the chorioepithelioma. In contrast, the intestinal mucosal villi in rats of the non-treatment group were atrophic, shorter and fractured. Some epithelial cells were necrotic. The mucous membrane showed signs of thinning. The intestinal mucosa was infiltrated with inflammatory cells (Figure 2B) and repaired gradually in different salvianolate treatment groups. The intestinal mucosal villi in rats of different salvianolate treatment groups were in good order and the mucous membrane became thicker. Inflammatory cell infiltration was decreased, especially in the high-dose salvianolate treatment group (Figure 2A-E).
Figure 2.
Mucosal morphology of ileal tissue in normal control group (A), non-treatment group (B), low-dose salvianolate treatment group (C), medium-dose salvianolate treatment group (D), and high-dose salvianolate treatment group (E) (HE staining, × 100).
Expression of TNF-α and IL-6 mRNA in small intestine
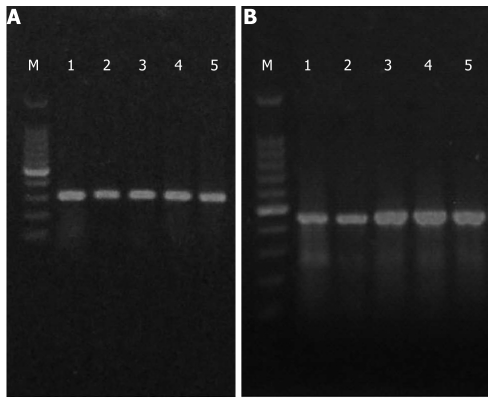
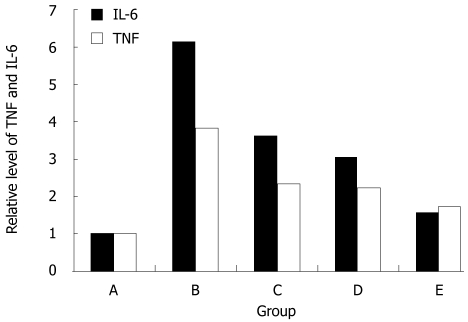
The bands (300-500 bp) of RT-PCR amplification products were visualized by 1% agarose gel electrophoresis (Figure 3). Real-time RT-PCR showed that the IL-6 and TNF-α mRNA expression levels were significantly higher in the non-treatment group than in the highest salvianolate dose (3.82 ± 1.30 vs 1.71 ± 0.27, 6.13 ± 4.13 vs 1.57 ± 0.31) treatment group (P < 0.01, P < 0.05, Figure 4).
Figure 3.
Electrophoretograms of tumor necrosis factor-α (A) and IL-6 (B). M: Marker; lane 1: Normal control group; lane 2: Untreated group; lane 3: Low-dose salvianolate-treated group; lane 4: Medium-dose salvianolate-treated group; lane 5: High-dose salvianolate-treated group.
Figure 4.
Real-time reverse transcription polymerase chain reaction for the expression of relative mRNA level of TNF and IL-6 in normal control group (A), non-treatment group (B), low-dose salvianolate treatment group (C), medium-dose salvianolate treatment group (D), and high-dose salvianolate treatment group (E). TNF: Tumor necrosis factor; IL: Interleukins.
DISCUSSION
To the best of our knowledge, this is the first study to show that salvianolate can decrease the plasma level of endotoxin in the portal vein and restores intestinal mucosal injury in rats with CCl4-induced liver cirrhosis. During the development of cirrhosis, impaired intestinal mucosal barrier[10,20] and decreased function of hepatocytes and Kupffer cells can lead to invasion of enteric organisms/endotoxin in blood and formation of bacteremia and intestinal endotoxemia[21,22]. Endotoxin itself can destroy mitochondria and lysosomes in enteric epithelial cells, leading to cell autolysis. Bacteria and their products (e.g. LPS) can activate innate immune responses by triggering a complex gene program in intestinal epithelium[23], which can increase secretion of cytokines in the intestine and intestinal inflammatory disorders[24,25]. Ultimately, a vicious cycle can arise between intestinal endotoxemia and increased permeability of enteric mucosa, and lead to liver injury and sepsis, resulting in a high mortality[12,26]. In the present study, the plasma endotoxin level and mortality of cirrhotic rats were significantly higher in non-treatment group than in different salvianolate treatment groups.
In this study, the intestinal histopathological changes in cirrhotic rats were improved after treatment with salvianolate, indicating that salvianolate can protect intestine against villous atrophy, epithelial cell necrosis, and inflammatory cell infiltration. In parallel with these findings, endotoxemia was significantly reduced in rats after treatment with salvianolate, suggesting that salvianolate exerts its effect on the intestine by protecting the mucosal barrier integrity.
In this study, the altered IBF in rats with liver cirrhosis was found to be associated with the up-regulation of TNF-α and IL-6 mRNA expression levels in intestinal wall. It has shown that serum TNF-α and IL-6 expression levels are significantly increased in cirrhosis patients[21]. TNF-α is a cytokine that is mainly released by mononuclear cells in response to inflammatory stimuli. The gut and its associated lymphoid tissue, including mesenteric lymph nodes (MLN), have been shown to produce TNF-α in response to BT induced by intestinal injury[25,27]. More recently, increased TNF-α production by MLN with BT has been detected in cirrhotic rats[19]. It was reported that local production of TNF-α in MLN is increased in patients with advanced liver cirrhosis, especially ascites[19], which in common with experimental cirrhosis may also be induced by BT. It seems that enhanced TNF-α expression in gut of cirrhotic rats can result from increased intestinal leakiness, which allows penetration of bacteria and endotoxin into the gut wall. In return, TNF-α can affect the structure of intestinal mucosa, decrease the expression of tight junction (zona occludens 1), and change the morphology of colon in a mouse model of acute liver failure (ALF)[28]. It may also participate in the pathophysiology of SBP complicating ALF. In this context, the reduced intestinal TNF-α levels observed in rats with cirrhosis after treatment with salvianolate might reflect the improvement of IBF. The increased production of TNF-α in intestine is a crucial event that leads to loss of IBF and BT, and anti-TNF-α therapy may prevent BT and SBP.
IL-6 is a pleiotropic cytokine which can regulate the biological responses of several target cells, including hepatocytes. Similarly, a link between IL-6 and liver fibrosis/cirrhosis has also been reported[29,30]. Toda et al[21] reported that IL-6 has a direct mitogenic effect on hepatic stellate cells. The activation status of intestinal immune system cells is much higher than that of analogous peripheral cells. Porowski et al[31] reported that the intestine is an important source of IL-6 in patients with liver cirrhosis. Increased production of IL-6 is induced by LPS in intestinal injury[32,33]. In the present study, the intestinal IL-6 levels were higher in the non-treatment group and lower in different salvianolate treatment groups, suggesting that salvianolate has a direct anti-inflammatory effect on the intestine, and can thus prevent IBF by inhibiting the expression of TNF-α and IL-6 mRNA.
In conclusion, salvianolate can reduce endotoxin level, restore intestinal mucosal injury, and inhibit expression of TNF-α and IL-6 in small intestine of cirrhotic rats. Clinical trials are needed to determine whether the aqueous extract from Radix Salviae Miltiorrhizae can favorably influence the natural history of liver cirrhosis, and reduce the risk of SBP and other septic complications in cirrhotic patients.
COMMENTS
Background
In liver cirrhosis, disruption of intestinal barrier function (IBF) leads to bacterial translocation and endotoxemia, which increase susceptibility to spontaneous bacterial peritonitis. Intestinal cytokines play an important role in the pathogenesis of IBF disruption and intestinal endotoxemia. Inhibition of cytokine gene expression in small intestine is an important goal in enhancing IBF in cirrhotic patients.
Research frontiers
Currently, no effective remedy is available for the prevention and treatment of IBF disruption in liver cirrhosis patients. Recent studies have shown that soluble phenolic acid derivatives can eliminate oxygen free radicals, enhance antioxidant activity, decrease serum levels of cytokines, and inhibit endotoxemia. In the present study, the authors demonstrated that salvianolate, a new water-soluble phenolic compound, could enhance IBF in cirrhotic rats.
Innovations and breakthroughs
Recent studies have highlighted the anti-inflammatory effects of soluble phenolic acid derivatives in Salvia miltiorrhiza Bge. The present study is the first to investigate the pharmacological activities of salvianolate in liver cirrhosis, showing that salvianolate decreases the plasma endotoxin level in the portal vein and restores intestinal mucosal injury in cirrhotic rats. The authors demonstrated that salvianolate could protect small intestine of cirrhotic rats by inhibiting tumor necrosis factor (TNF)-α and interleukin (IL)-6 gene expression and enhancing the intestinal mucosal barrier function, thus preventing intestinal endotoxemia.
Applications
By demonstrating the effects of salvianolate on expression of TNF-α and IL-6 mRNA in small intestine of cirrhotic rats, this study provides a new strategy for the treatment of liver cirrhosis. Salvianolate can be applied in clinical practice due to its potential pharmacological activities.
Terminology
Radix Salviae Miltiorrhizae is a traditional Chinese medical herb known as “danshen”.Salvianolate is a new water-soluble phenolic compound that is isolated from Radix Salviae Miltiorrhizae and one of the most bioactive compounds in S. miltiorrhiza Bge.
Peer review
The authors have illustrated the pharmacological activity of salvianolate using molecular biology techniques in an animal model of cirrhosis. The results of the study provide a new strategy for the treatment of liver cirrhosis. Further studies are needed to establish the mechanism of action of antifibrotic activity of salvianolate in cirrhotic rats.
Footnotes
Supported by Natural Science Foundation of Zhejiang Medical College 2009XZB06
Peer reviewers: Keiji Hirata, MD, Surgery 1, University of Occupational and Environmental Health, 1-1 Iseigaoka, Yahatanishi-ku, Kitakyushu 807-8555, Japan; Yu-Yuan Li, Professor, Department of Gastroenterology, First People’s Hospital of Guangzhou, 1 Panfu Road, Guangzhou 510180, Guangdong Province, China
S- Editor Sun H L- Editor Wang XL E- Editor Ma WH
References
- 1.Lata J, Stiburek O, Kopacova M. Spontaneous bacterial peritonitis: a severe complication of liver cirrhosis. World J Gastroenterol. 2009;15:5505–5510. doi: 10.3748/wjg.15.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang HY, Han de W, Su AR, Zhang LT, Zhao ZF, Ji JQ, Li BH, Ji C. Intestinal endotoxemia plays a central role in development of hepatopulmonary syndrome in a cirrhotic rat model induced by multiple pathogenic factors. World J Gastroenterol. 2007;13:6385–6395. doi: 10.3748/wjg.v13.i47.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellot P, Francés R, Such J. [Bacterial translocation in cirrhosis] Gastroenterol Hepatol. 2008;31:508–514. doi: 10.1157/13127094. [DOI] [PubMed] [Google Scholar]
- 4.Ribeiro TC, Chebli JM, Kondo M, Gaburri PD, Chebli LA, Feldner AC. Spontaneous bacterial peritonitis: How to deal with this life-threatening cirrhosis complication? Ther Clin Risk Manag. 2008;4:919–925. doi: 10.2147/tcrm.s2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.González Alonso R, González García M, Albillos Martínez A. [Physiopathology of bacterial translocation and spontaneous bacterial peritonitis in cirrhosis] Gastroenterol Hepatol. 2007;30:78–84. doi: 10.1157/13099277. [DOI] [PubMed] [Google Scholar]
- 6.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422–433. doi: 10.1002/hep.20632. [DOI] [PubMed] [Google Scholar]
- 7.Zhang HY, Han DW, Zhao ZF, Liu MS, Wu YJ, Chen XM, Ji C. Multiple pathogenic factor-induced complications of cirrhosis in rats: a new model of hepatopulmonary syndrome with intestinal endotoxemia. World J Gastroenterol. 2007;13:3500–3507. doi: 10.3748/wjg.v13.i25.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang HY, Han DW, Wang XG, Zhao YC, Zhou X, Zhao HZ. Experimental study on the role of endotoxin in the development of hepatopulmonary syndrome. World J Gastroenterol. 2005;11:567–572. doi: 10.3748/wjg.v11.i4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song HL, Lv S, Liu P. The roles of tumor necrosis factor-α in colon tight junction protein expression and intestinal mucosa structure in a mouse model of acute liver failure. BMC Gastroenterol. 2009;9:70. doi: 10.1186/1471-230X-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boroń-Kaczmarzka A, Hryniewicz A, Kemona A, Sokolewicz-Bobrowska E, Miegoć H. Morphologic changes of small intestine epithelium in the course of post-alcoholic liver cirrhosis. Drug Alcohol Depend. 1990;25:299–303. doi: 10.1016/0376-8716(90)90155-8. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-del-Arbol L, Urman J, Feranandez J, Gonzalez M, Navasa M, Gonescillo A, Albillos A, Jimenez W, Arroyo V. Systemic,renal and hepatic hemodynamic derangement in cirrhotic patients with spoutaneous bacterial peritonitis. Hepatology. 2003;38:1210–1218. doi: 10.1053/jhep.2003.50447. [DOI] [PubMed] [Google Scholar]
- 12.Han DW. Intestinal endotoxemia as a pathogenetic mechanism in liver failure. World J Gastroenterol. 2002;8:961–965. doi: 10.3748/wjg.v8.i6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yagi A, Fujimoto K, Tanonaka K, Hirai K, Takeo S. Possible active components of tan-shen (Salvia miltiorrhiza) for protection of the myocardium against ischemia-induced derangements. Planta Med. 1989;55:51–54. doi: 10.1055/s-2006-961824. [DOI] [PubMed] [Google Scholar]
- 14.Fung KP, Zeng LH, Wu J, Wong HN, Lee CM, Hon PM, Chang HM, Wu TW. Demonstration of the myocardial salvage effect of lithospermic acid B isolated from the aqueous extract of Salvia miltiorrhiza. Life Sci. 1993;52:PL239–PL244. doi: 10.1016/0024-3205(93)90471-e. [DOI] [PubMed] [Google Scholar]
- 15.Liu GT, Zhang TM, Wang BE, Wang YW. Protective action of seven natural phenolic compounds against peroxidative damage to biomembranes. Biochem Pharmacol. 1992;43:147–152. doi: 10.1016/0006-2952(92)90271-j. [DOI] [PubMed] [Google Scholar]
- 16.Guo J, Sun K, Wang CS, Fang SP, Horie Y, Yang JY, Liu YY, Wang F, Liu LY, Fan JY, et al. Protective effects of dihydroxylphenyl lactic acid and salvianolic acid B on LPS-induced mesenteric microcirculatory disturbance in rats. Shock. 2008;29:205–211. doi: 10.1097/SHK.0b013e318070c61a. [DOI] [PubMed] [Google Scholar]
- 17.Kim JS, Narula AS, Jobin C. Salvia miltiorrhiza water-soluble extract, but not its constituent salvianolic acid B, abrogates LPS-induced NF-kappaB signalling in intestinal epithelial cells. Clin Exp Immunol. 2005;141:288–297. doi: 10.1111/j.1365-2249.2005.02844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKay DM, Baird AW. Cytokine regulation of epithelial permeability and ion transport. Gut. 1999;44:283–289. doi: 10.1136/gut.44.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genescà J, Martí R, Rojo F, Campos F, Peribáñez V, Gónzalez A, Castells L, Ruiz-Marcellán C, Margarit C, Esteban R, et al. Increased tumour necrosis factor alpha production in mesenteric lymph nodes of cirrhotic patients with ascites. Gut. 2003;52:1054–1059. doi: 10.1136/gut.52.7.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palma P, Mihaljevic N, Hasenberg T, Keese M, Koeppel TA. Intestinal barrier dysfunction in developing liver cirrhosis: An in vivo analysis of bacterial translocation. Hepatol Res. 2007;37:6–12. doi: 10.1111/j.1872-034X.2007.00004.x. [DOI] [PubMed] [Google Scholar]
- 21.Toda K, Kumagai N, Tsuchimoto K, Inagaki H, Suzuki T, Oishi T, Atsukawa K, Saito H, Morizane T, Hibi T, et al. Induction of hepatic stellate cell proliferation by LPS-stimulated peripheral blood mononuclear cells from patients with liver cirrhosis. J Gastroenterol. 2000;35:214–220. doi: 10.1007/s005350050333. [DOI] [PubMed] [Google Scholar]
- 22.Balzan S, de Almeida Quadros C, de Cleva R, Zilberstein B, Cecconello I. Bacterial translocation: overview of mechanisms and clinical impact. J Gastroenterol Hepatol. 2007;22:464–471. doi: 10.1111/j.1440-1746.2007.04933.x. [DOI] [PubMed] [Google Scholar]
- 23.Walton KL, Holt L, Sartor RB. Lipopolysaccharide activates innate immune responses in murine intestinal myofibroblasts through multiple signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2009;296:G601–G611. doi: 10.1152/ajpgi.00022.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ataseven H, Bahcecioglu IH, Kuzu N, Yalniz M, Celebi S, Erensoy A, Ustundag B. The levels of ghrelin, leptin, TNF-alpha, and IL-6 in liver cirrhosis and hepatocellular carcinoma due to HBV and HDV infection. Mediators Inflamm. 2006;2006:78380. doi: 10.1155/MI/2006/78380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Márquez-Velasco R, Massó F, Hernández-Pando R, Montaño LF, Springall R, Amezcua-Guerra LM, Bojalil R. LPS pretreatment by the oral route protects against sepsis induced by cecal ligation and puncture. Regulation of proinflammatory response and IgM anti-LPS antibody production as associated mechanisms. Inflamm Res. 2007;56:385–390. doi: 10.1007/s00011-007-6116-4. [DOI] [PubMed] [Google Scholar]
- 26.Guarner C, Soriano G. Bacterial translocation and its consequences in patients with cirrhosis. Eur J Gastroenterol Hepatol. 2005;17:27–31. doi: 10.1097/00042737-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Ozen S, Akisu M, Baka M, Yalaz M, Sozmen EY, Berdeli A, Kultursay N. Insulin-like growth factor attenuates apoptosis and mucosal damage in hypoxia/reoxygenation-induced intestinal injury. Biol Neonate. 2005;87:91–96. doi: 10.1159/000081897. [DOI] [PubMed] [Google Scholar]
- 28.Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367–G376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- 29.Sripa B, Mairiang E, Thinkhamrop B, Laha T, Kaewkes S, Sithithaworn P, Tessana S, Loukas A, Brindley PJ, Bethony JM. Advanced periductal fibrosis from infection with the carcinogenic human liver fluke Opisthorchis viverrini correlates with elevated levels of interleukin-6. Hepatology. 2009;50:1273–1281. doi: 10.1002/hep.23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girón-González JA, Martínez-Sierra C, Rodriguez-Ramos C, Macías MA, Rendón P, Díaz F, Fernández-Gutiérrez C, Martín-Herrera L. Implication of inflammation-related cytokines in the natural history of liver cirrhosis. Liver Int. 2004;24:437–445. doi: 10.1111/j.1478-3231.2004.0951.x. [DOI] [PubMed] [Google Scholar]
- 31.Porowski D, Niemczyk M, Ziółkowski J, Mucha K, Foroncewicz B, Nowak M, Pacholczyk M, Chmura A, Paczek L. Intestine as source of cytokines and growth factors. Transplant Proc. 2009;41:2989–2991. doi: 10.1016/j.transproceed.2009.07.083. [DOI] [PubMed] [Google Scholar]
- 32.Huber NL, Bailey SR, Schuster RM, Ogle CK, Lentsch AB, Pritts TA. Remote thermal injury increases LPS-induced intestinal IL-6 production. J Surg Res. 2010;160:190–195. doi: 10.1016/j.jss.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walton KL, Holt L, Sartor RB. Lipopolysaccharide activates innate immune responses in murine intestinal myofibroblasts through multiple signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2009;296:G601–G611. doi: 10.1152/ajpgi.00022.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]